Abstract
Background
A doctor diagnosis of asthma is associated with increased morbidity (pain and acute chest syndrome, ACS) among children with sickle cell disease (SCD). An association between IgE levels and asthma and morbidity has not been investigated in children with SCD.
Objective
We tested the hypothesis that elevated total and allergen-specific IgE levels are associated with asthma and SCD morbidity in children with SCD.
Methods
A cross-sectional study of children with SCD who participated in the Silent Cerebral Infarct Trial was conducted. Logistic regression and negative binomial regression were used to investigate potential associations of total and allergen-specific IgE levels with asthma diagnosis and SCD morbidity, both confirmed by medical record review. Elevation of total IgE was defined as age- and sex-adjusted IgE exceeding 90th percentile compared to a non-atopic reference population. IgE antibody positivity to Altermaria alternata (mold), Blatella germanica (cockroach), and Dermatophagoides pteronyssinus (dust mite) was assessed by ImmunoCAP analysis.
Results
Children with SCD (140 asthmatics, 381 non-asthmatics) were evaluated. Elevations in total IgE (p = 0.04) and IgE antibody specific for Altermaria alternata (p = 0.0003), Blatella germanica (p = 0.008), and Dermatophagoides pteronyssinus (p = 0.01) were associated with asthma. ACS (p = 0.048) but not pain (p = 0.20) was associated with total IgE, but neither were associated with specific IgE levels.
Conclusions
Significantly increased levels of total and allergen-specific IgE levels are associated with asthma in SCD. High IgE levels are a risk factor for ACS and not pain rates.
Keywords: Total IgE, allergen-specific IgE, asthma risk indicator, acute chest syndrome, pain, sickle cell disease, hemoglobinopathies
INTRODUCTION
Among children with sickle cell disease (SCD), pulmonary disease is a major cause of both morbidity and mortality (1–4). Asthma is a common and complex chronic disorder of the airways characterized by variable and recurring symptoms, airflow obstruction, bronchial hyper-responsiveness, and underlying inflammation (5). Among children with SCD, clinical diagnosis of asthma is associated with an increased rate of acute chest syndrome (ACS) (6–14) and pain (11). The causal path for why a clinical diagnosis of asthma is associated with an increased incidence of ACS and pain in patients with SCD is currently unknown.
A physician diagnosis of asthma is based on the presence of many factors, including audible wheezing, obstruction on spirometry testing with reversibility after administration of bronchodilator, and elevated total and/or specific IgE levels. The relationship between IgE elevation and asthma has been extensively documented. At least 80% and 50% of asthmatic children and adults, respectively, have elevated total serum IgE levels (15). Among children examined for asthma, an elevated IgE level is among the strongest risk factors for asthma (16–17).
To date, the relationship between IgE levels and SCD-related morbidity has not been assessed. Given the strong relationship between asthma and IgE levels, and the established association between asthma and SCD-related morbidity (6–14), two hypotheses were tested: 1) elevated IgE levels (total and allergen-specific IgE) are associated with a parental report of a physician diagnosis of asthma; and 2) elevated IgE levels are associated with an increased incidence of ACS or pain episodes in children with SCD.
METHODS
Patient Population
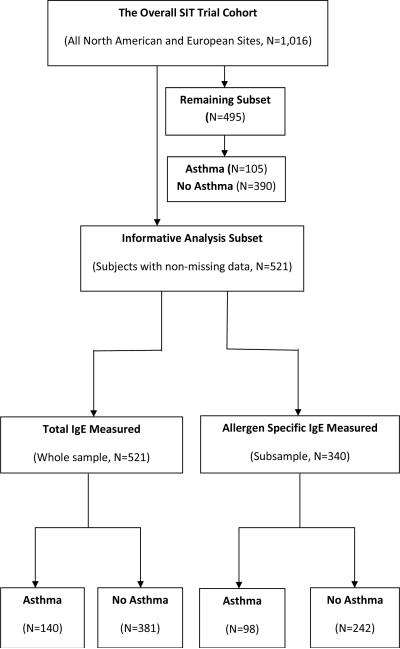
The Silent Cerebral Infarct Transfusion (SIT) Trial includes 29 North American (US and Canada) and European (UK and France) clinical sites, a clinical coordinating center and a statistical and data coordinating center, to test the following hypothesis: prophylactic blood transfusion therapy in children with SCD will result in at least an 86% reduction in the rate of subsequent overt strokes or new or progressive cerebral infarcts as defined by magnetic resonance imaging of the brain. Details of the recruitment were described in the study design manuscript for the SIT Trial that was recently published (18). Briefly, from 2004 to 2010, patients with hemoglobin SS (94%) or SB zero (6%), the most severe types of SCD, were recruited between the ages of 5 and 14 years. This was an unselected cohort of children with the exception that they did not have any evidence of overt stroke, were not on regular blood transfusion therapy, and were not receiving hydroxyurea therapy. A priori we have no reasons to believe that these exclusion criteria resulted in any ascertainment bias, because asthma status was not a consideration for selection. A total of 1016 children were recruited to this trial, of which we only had sufficient resources to evaluate the first 521 participants with the subsequently recruited 495 participants untested (Figure 1). Sample characteristics of age, sex, hemoglobinopathy diagnosis, white blood cell count, hemoglobin, fetal hemoglobin, household smoking, asthma status, clinical site and parental asthma for the first 521 children were not statistically significantly different from those in the entire cohort (p > 0.05, Table 1). Similarly, sample characteristics between the analysis subset (n=340) for allergen-specific IgE and the entire cohort were statistically non-significant (details not given in Table 1). This study was approved by the Institutional Review Board of all participating sites, and informed consent was obtained from each participant.
Figure 1.
Flow diagram describing samples of the SIT Trial study cohort.
Table 1.
Comparisons of sample characteristics (mean ± SD, or median with range, or frequency) between the analysis data and the entire cohort with no statistically significant demographic or clinical differences
| Variables | Analysis data (n=521) | Entire cohort (n=1016) | P-value |
|---|---|---|---|
| Age (years) | 9.1 ± 2.1 | 9.1 ± 2.4 | 0.95 |
| Sex: | |||
| Male | 271 | 521 | 1.00 |
| Female | 250 | 480 | |
| Hemoglobin type: | |||
| SS | 470 | 883 | 0.74 |
| SB zero | 31 | 64 | |
| White blood count (/mm3) | 12000 (1080–147000) | 12000 (1055–151000) | 0.76 |
| Hemoglobin (g/dL) | 8.0 ± 1.0 | 8.1 ± 1.0 | 0.21 |
| Fetal hemoglobin (%) | 11 (1–98) | 11 (0–98) | 0.98 |
| Household smoking: | |||
| Yes | 121 | 252 | 0.45 |
| No | 381 | 716 | |
| Patient asthma: | |||
| Yes | 140 | 245 | 0.32 |
| No | 381 | 756 | |
| Clinical site: | |||
| North America | 460 | 877 | 0.34 |
| Europe | 61 | 138 | |
| Parental asthma: | |||
| Yes | 112 | 190 | 0.22 |
| No | 397 | 794 | |
| ACS rate (/100 patient-years) | |||
| Asthma | 19 (14–25); p=0.02* | 22 (18–27); p<0.0001# | 0.68 |
| No-asthma | 12 (10–15) | 12 (10–14) | |
| Pain rate (/100 patient-years) | |||
| Asthma: | 67 (54–83); p=0.046* | 73 (61–87); p<0.0176# | 0.14 |
| No-asthma: | 51 (45–59) | 57 (52–63) | |
In the first 521 participants, a doctor diagnosis of asthma was associated with increased incidence rates of ACS and pain episodes. After final adjustment for age, hemoglobin F and baseline hemoglobin levels, ACS incidence rates were 19 (95% CI 14 – 25) and 12 (95% CI 10 – 15) episodes per 100 patient-years among children with and without an asthma diagnosis, respectively (p = 0.02); pain rates were 67 (95% CI 54 – 83) and 51 (95% CI 45 – 59) episodes per 100 patient-years among children with and without an asthma diagnosis, respectively (p = 0.046).
In the entire cohort, a doctor diagnosis of asthma was associated with increased incidence rates of ACS and pain episodes. After final adjustment for age, hemoglobin F and baseline hemoglobin levels, ACS incidence rates were 22 (95% CI 18 – 27) and 12 (95% CI 10 – 14) episodes per 100 patient-years among children with and without an asthma diagnosis, respectively (p < 0.0001); pain rates were 73 (95% CI 61 – 87) and 57 (95% CI 52 – 63) episodes per 100 patient-years among children with and without an asthma diagnosis, respectively (p = 0.0176).
Definitions of ACS, pain and asthma
An entry criterion of the study was an ongoing relationship with the physicians on the local hematology service. Each site investigator agreed to this criterion, and the vast majority of all admissions were at the tertiary care facility, with the hematology service following the child. The criterion was instituted because patients could have been randomly allocated to receive blood transfusion at least monthly, thus requiring the tertiary care hospital to be the place of enrollment. Because the rare admissions that did not occur at the tertiary care center were not counted, there could be an under ascertainment of events, with a potential bias toward the null hypothesis. An ACS episode was defined as a pulmonary process that required admission, and was defined at the local site by site investigators after a review of the medical records. A priori (before the start of enrollment), investigators at all sites agreed to case definition of a painful event (a painful episode requiring hospitalization and treatment with opiates that could not be attributable to a cause other than SCD) and ACS (a clinical diagnosis designated locally). Pneumonia was indistinguishable from ACS, and was thus considered an episode of ACS in this analysis. Hospitalizations in individuals that represented the highest 10% of pain and ACS episodes were reconfirmed with local sites to ensure accuracy. Since all the site investigators agreed that they could accurately identify all of the episodes in their hospitals within three years, all ACS and pain episodes in the three years prior to signing the informed consent were recorded (each participant contributed 3 patient-years in this study).
A diagnosis of asthma was considered separate from ACS and pneumonia when the patient was admitted to the hospital for an exclusive treatment of an asthma exacerbation. It was based on an affirmative answer to the following question to the parent: “Does the patient currently carry a diagnosis of asthma?” The use of asthma medication was also recorded. When a diagnosis of asthma was made and no asthma medication was recorded in the SIT Trial data base, we reconfirmed the diagnosis of asthma with a review of the medical records by the site coordinator (confirmation criteria of any hospital admissions, ED visits or medications - Advair, Flovent, Montelukast - for asthma). Similarly, if the patient was recorded as having prescriptions for inhaled corticosteroids, bronchodilators, or a cysteinyl leukotriene receptor antagonist, but the parent did not state that the child had asthma, the site coordinator was required to recheck the medical records for a diagnosis of asthma. Thus, the resulting designation was a parental report of a physician diagnosis of asthma, with support from the medical record review, or more simply referred to as a “physician diagnosis of asthma”. An assumption was made that asthma is a chronic condition and lifetime-long illness (5). Exposure to tobacco smoke was based on an affirmative answer to the following question: “Does either the parent or the primary caretaker identify anyone living in the home who smokes or smoked tobacco products in the last 3 years either inside or outside the home?”
All laboratory values and clinical parameters were checked for outliers and missing data. Specifically, based on the distribution, each value below the 5th percentile and above the 95th percentile was re-confirmed for accuracy at the local site, and when discrepant, was changed accordingly. For all missing key variables, additional contact was made with the local site coordinator, to determine if the data were not available or simply not recorded.
Total and allergen-specific IgE measurements
Plasma was obtained from EDTA-treated blood from 521 children with either hemoglobin SS or SB zero participating in the SIT trial. Blood was spun at 3000 RPM (1900 × g) for 8 min to pellet the cells and the plasma was gently removed. For the first 459 samples, the blood was diluted with Hank's Balanced Salt Solution for a procedure involving the isolation of lymphocytes, prior to centrifugation and removal of the plasma. For that subset of samples, a dilution factor of 3.3 was determined by comparing the total IgE measurements from undiluted and diluted plasma control samples (n = 6). Using that factor, an adjustment was made to the IgE calculations for the diluted samples.
Total and allergen-specific IgE levels in the plasma were measured using the Phadia ImmunoCAP 250 Autoanalyzer (Kalamazoo, MI, USA). Total plasma IgE was reported in kU/L, where 1 U = 2.44 ng/mL of IgE, based on the World Health Organization IgE Standard 75/502. The concentration of IgE in plasma is highly age-dependent; mean plasma IgE levels progressively increase in healthy children up to the age of 10 to 15 years, and then decline from the second through the eighth decades of life (19–20). For this reason, the total plasma IgE has been reported together with the number of standard deviations and percentile in which the subject's total IgE fell as compared to an African American population-based, age-adjusted non-atopic mean (19–20).
IgE antibody measurements specific for mold (Altemaria alternata), cockroach (Blatella germanica), or dust mite (Dermatophagoides pteronyssinus) were performed. The rationale for selection of the three specific allergens includes: 1) Altermaria alternate sensitivity is reportedly associated with the most severe outcomes from asthma (21); Alternaria was selected because we were evaluating ACS, a severe outcome in SCD. 2) Based on reports in the Inner City Asthma Consortium, asthma in African American populations because Alternaria is related to the presence of sensitivity to cockroach allergen (22–23). 3) Overall asthma is known to be associated with sensitivity to indoor, and thus perennial, allergens such as this species of dust mite (22–23). At the time of the analysis, the FDA accepted analytical sensitivity of the IgE antibody ImmunoCAP assay was 0.35 kUA/L. Due to the dilution factor resulting from the specimen handling, the actual IgE antibody positive/negative cut-point for this study was set at 1 kUA/L. Total serum IgE levels in the undiluted and diluted groups were similar to each other. Specifically, the proportions of patients that had an IgE level greater than the 90th percentile were not statistically significantly (p = 0.08) different in the diluted (52%) and non-diluted (39%) groups (data not shown). Allergen-specific IgE antibody levels were only measured in the first 340 (65% of the analysis data) participants, because of limited test resources and the sample was representative of the entire population.
Statistical analysis
All data analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). Student's t-test was used to compare mean differences. Association between binary IgE and asthma status was assessed using logistic regression. Relationship between quartile IgE Z-scores (i.e., age-adjusted and standardized total IgE residuals with mean of zero and standard deviation of one) and asthma status was assessed using Fisher's exact probability test and Cochran-Armitage trend test. Associations between binary IgE or asthma and SCD related morbidity were assessed using negative binomial regression model and Poisson regression model. Since a negative binomial regression model can be superior to a Poisson regression model in correction for over-dispersed count data such as ACS and pain episodes, the former was used to report final model test p-values and parameter estimates in this analysis. Covariates included in the full regression model were age, sex, white blood cell count, hemoglobin, and fetal hemoglobin (logit transformation of fetal hemoglobin was used to approximate normality), site (North American vs. European) and parental asthma. Only statistically significant covariate terms remained in the final regression models. P < 0.05 was used to flag statistical significance.
RESULTS
Demographics
A total of 521 children with SCD had complete clinical data and measured total IgE levels for this analysis (see Figure 1 and Table 2). One hundred and forty of 521 (27%) children met the criteria of having a physician diagnosis of asthma, with statistically significantly (p = 0.01) higher asthma prevalence in males (32%, 86 of 271) than in females (22%, 54 of 250), i.e., males accounted for 61% (86 of 140) asthmatics and 49% (185 of 381) non-asthmatics. Hemoglobin SS distribution was not statistically significantly different (p = 0.41) in asthma group (96%, 131 of 137) and non-asthma group (93%, 339 of 364). There were no statistically significant (p > 0.05) mean age (9.0 ± 2.1vs. 9.1 ± 2.1 years), white blood count (11450, 1300 – 23000 vs. 12000, 1080 – 147000 /mm3), hemoglobin (8.1 ± 0.9 vs. 8.0 ± 1.0 g/dL), or fetal hemoglobin (12%, 2% – 69% vs.11%, 1% – 98%) differences between asthma and non-asthma groups, respectively. There was no statistically significant difference (p = 0.91) of household smoking rates in asthma group (24%, 33 of 140) vs. non-asthma group (23%, 88 of 381). North American sites accounted for 96% (134 of 140) asthmatics and 86% (326 of 381) non-asthmatics (p = 0.001). Prevalence of parental asthma was statistically significantly (p < 0.0001) higher in the asthma group (41%, 58 of 140) than that in the non-asthma group (14%, 54 of 381). Details of sample characteristics are given in Table 2.
Table 2.
Clinical and laboratory findings in 521 children in the SIT trial with a total plasma IgE measure (a subset of 340 children with allergen specific IgE antibody measured, of which 98 had asthma, for a prevalence of asthma similar to the overall cohort, 29% vs. 27%)
| Variables* | N | Asthma | Non-asthma | P |
|---|---|---|---|---|
| Age (years) | 521 | 9.0 ± 2.1 (5.4–13.9) | 9.1 ± 2.1 (5.1–14.9) | 0.79 |
| Sex: Male | 271 | 86 | 185 | 0.01 |
| Sex: Female | 250 | 54 | 196 | |
| Hemoglobin SS | 470 | 131 | 339 | 0.41 |
| Hemoglobin SB zero | 31 | 6 | 25 | |
| White blood count (/mm3) | 518 | 11450 (1300–23000) | 12000 (1080–147000) | 0.67# |
| Hemoglobin (g/dL) | 520 | 8.1 ± 0.9 (5.5–10.7) | 8.0 ± 1.0 (5.9–11.7) | 0.32 |
| Fetal hemoglobin (%) | 521 | 12 (2 #x2013; 69) | 11 (1 #x2013; 98) | 0.44# |
| Smoking: Yes | 121 | 33 | 88 | 0.91 |
| Smoking: No | 400 | 107 | 293 | |
| Site: North America | 460 | 134 | 326 | 0.001 |
| Site: Europe | 61 | 6 | 55 | |
| Parent asthma: Yes | 112 | 58 | 54 | <0.0001 |
| Parent asthma: No | 409 | 82 | 327 | |
| Plasma IgE (kU/L) | 521 | 141 (4 – 4006) | 82 (6 – 3802) | 0.0005# |
| IgE percentile: ≥ 90th | 261 | 81 | 180 | 0.04 |
| IgE percentile: < 90th | 260 | 59 | 201 | |
| IgE percentile: ≥ 98th | 132 | 50 | 82 | 0.001 |
| IgE percentile: < 98th | 389 | 90 | 299 | |
| Anti-A. alternata: Positive | 74 | 34 | 40 | 0.0004 |
| Anti-A. alternata: Negative | 266 | 64 | 202 | |
| Anti-B. germanica: Positive | 46 | 21 | 25 | 0.01 |
| Anti-B. germanica: Negative | 294 | 77 | 217 | |
| Anti-D. pteronyssinus: Positive | 98 | 29 | 42 | 0.02 |
| Anti-D. pteronyssinus: Negative | 242 | 69 | 200 |
Mean ± SD with range for age and hemoglobin; median with range for white blood count, fetal hemoglobin and plasma IgE.
P-values were based on student's t-tests of log-scale mean differences for white blood count, fetal hemoglobin and plasma IgE.
In a population where we define normal as less than the 90th percentile, we would expect only 10% to be greater than 90th percentile by definition; however, in the SCD population, about 50% (261 of 521) of the population has a total IgE level greater than the 90th percentile when compared to the general population (Table 2). Likewise, when compared to the general population, total IgE levels were also much higher, with 25% (132 of 521) subjects in the SCD population having total IgE levels greater than the 98th percentile compared to the expected 2% subjects (Table 2). In addition, the percentiles of the Z-score categories defined in this analysis data are 53rd percentile for the lowest Z-score group (< −0.8), 80th for the low Z-score group (−0.8 to < 0), 93rd for the high Z-score group (0 to < 0.6), and 98th for the highest Z-score group (≥ 0.6). Further, there were also no statistically significant differences in mean total IgE levels (p = 0.73), mean IgE percentiles (p = 0.48), and proportions of subjects with greater than 90th percentile (p = 1.00) between household smoking and non-smoking groups (data not given in Table 2).
Asthma and SCD-related morbidity
A doctor diagnosis of asthma was associated with increased incidence rates of ACS and pain episodes. After final adjustment for age, hemoglobin F and baseline hemoglobin levels, ACS incidence rates were 19 (95% CI 14 – 25) and 12 (95% CI 10 – 15) episodes per 100 patient-years among children with and without an asthma diagnosis, respectively (p = 0.02, Table 3); pain rates were 67 (95% CI 54 – 83) and 51 (95% CI 45 – 59) episodes per 100 patient-years among children with and without an asthma diagnosis, respectively (p = 0.046, Table 4).
Table 3.
Results for associations between total plasma IgE, allergen specific IgE, asthma and incidence rates of ACS
| Variables | Mean ACS rate (per 100 patient years) | 95% CI | P |
|---|---|---|---|
| Asthma | 19 | 14 – 25 | 0.02 |
| Non-asthma | 12 | 10 – 15 | |
| Total IgE percentile: ≥ 90th | 17 | 14 – 21 | 0.048 |
| Total IgE percentile: < 90 | 12 | 9 – 15 | |
| Anti-A. alternate: Positive | 19 | 13 – 29 | 0.26 |
| Anti-A. alternate: Negative | 15 | 12 – 19 | |
| Anti- B. germanica: Positive | 19 | 11 – 31 | 0.48 |
| Anti- B. germanica: Negative | 15 | 13 – 19 | |
| Anti-D. pteronyssinus: Positive | 19 | 13 – 29 | 0.29 |
| Anti-D. pteronyssinus: Negative | 15 | 12 – 19 |
Table 4.
Results for associations between total IgE, allergen specific IgE, asthma and incidence rates of pain episodes that required hospitalization
| Variables | Mean pain rate (per 100 patient years) | 95% CI | P |
|---|---|---|---|
| Asthma | 67 | 54 – 83 | 0.046 |
| Non-asthma | 51 | 45 – 59 | |
| IgE percentile: ≥ 90th | 52 | 44 – 61 | 0.20 |
| IgE percentile: < 90 | 60 | 51 – 71 | |
| Anti-A. alternata: Positive | 56 | 41 – 77 | 0.82 |
| Anti-A. alternata: Negative | 59 | 50 – 69 | |
| Anti- B. germanica: Positive | 71 | 49 – 104 | 0.26 |
| Anti- B. germanica: Negative | 56 | 48 – 66 | |
| Anti-D. pteronyssinus: Positive | 60 | 44 – 82 | 0.86 |
| Anti-D. pteronyssinus: Negative | 58 | 49 – 68 |
Total IgE / allergen-specific IgE and asthma
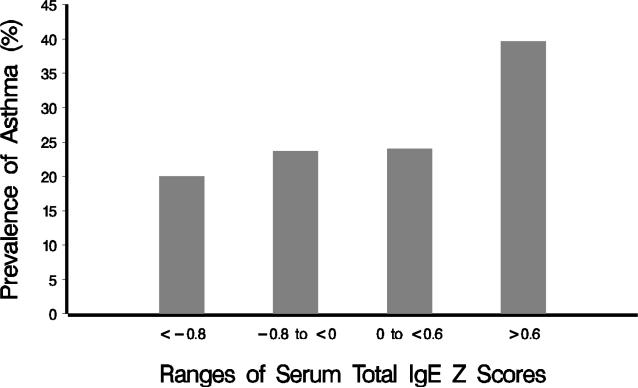
There was a statistically significant association of elevated IgE with increased risk of asthma diagnosis (p < 0.05, Table 2). There was an incremental increase in the percentages of children with asthma, and the pattern of increasing prevalence with each quartile was statistically significant (Fisher's exact test p = 0.002, Cochran-Armitage trend test p = 0.001, Figure 2). Allergen-specific IgE antibody positivity rates in children with SCD were statistically significantly higher in asthma group vs. non-asthma group, and individuals with at least a single positive allergen-specific IgE antibody test were at increased odds for a physician diagnosis of asthma (p < 0.05, Table 2).
Figure 2.
Asthma prevalence among children within each of the four different IgE categories by age-adjusted/-standardized (mean 0, standard deviation 1) IgE Z scores of < −0.8, −0.8 to < 0, 0 to < 0.6, and ≥ 0.6. There was a statistically significant increasing trend of asthma prevalence with each quartile (Cochran-Armitage trend test p = 0.001; Fisher's exact p = 0.002).
Total IgE elevation and SCD-related morbidity
Total IgE level elevation among children with SCD was statistically significantly associated with an increased incidence of ACS episodes (negative binomial regression p = 0.0476, Poisson regression p = 0.0137), but there was no evidence that elevated levels were also statistically significantly associated with an increased incidence of pain episodes (negative binomial regression p = 0.3324, Poison regression p = 0.0938). After adjusting for the covariate effects of hemoglobin F and hemoglobin levels, ACS incidence rates were 17 (95% CI 14 – 21) and 12 (95% CI 9 – 15) episodes per 100 patient-years among children with and without IgE elevation, respectively (negative binomial p = 0.048, Table 3); pain rates were 52 (95% CI 44 – 61) and 60 (95% CI 51 – 71) events per 100 patient-years among children with and without an elevated total IgE level per year, respectively (negative binomial p = 0.20, Table 4). The difference in incidence rates of pain was 52 − 60 = −8 (95% CI −21 to 5) events per 100 patient-years (data not shown in Table 4). Site (p = 0.87 for ACS and 0.14 for pain) and parental asthma (p = 0.32 for ACS and 0.60 for pain) were not statistically significant predictors, they were not included in the final model.
DISCUSSION
Previously, our group (6, 11, 14) and others (7–10, 12–13) have demonstrated that children with SCD and asthma are at increased risk for ACS (6–14) and pain (11) episodes when compared to children with SCD alone; however, there has been limited evaluation of asthma risk factors. In the current analysis, we tested two hypotheses – first, whether the asthma risk factors of total and allergen-specific IgE are associated with a physician diagnosis of asthma; second, whether total and allergen-specific IgE are associated with increased risk of ACS and pain episodes. We provide significant evidence that elevated total and specific IgE levels are associated with a physician diagnosis of asthma in children with SCD. Further, we found new evidence that total IgE levels were associated with ACS rates (p = 0.048, Table 3) but not with pain (p = 0.20, Table 4). Lastly, we validated the findings from others by showing that a physician diagnosis of asthma is associated with an increased incidence rate of pain (11) and ACS (6, 11, 14) among children in the SIT Trial.
An unexpected finding in this cohort of children with SCD was that 50% of the children had a total IgE level greater than the 90th percentile for normal after adjustment for age. Several plausible explanations exist as to why total IgE levels are elevated in the entire cohort. Individuals with SCD are noted to have an inflammatory state, with elevation of both non-specific inflammatory markers (such as C-reactive protein) and those associated with evidence of vascular injury (such as vascular cell adhesion molecule-1) (24). Perhaps, the elevation of total IgE in SCD, both in children with and without asthma, is an indication of inflammation that occurs in SCD (24). Our study design could not test this hypothesis. Despite the overall high proportion of children with elevated IgE levels, there was still a highly significant association between IgE levels and asthma, suggesting that asthma is a distinct co-morbid condition among patients with SCD.
While the association of total IgE and allergen-specific IgE antibody positivity with risk of a physician asthma diagnosis was observed, an elevated IgE compared to the general population cannot be used to determine the diagnosis of asthma among children with SCD because approximately 50% of the children with SCD will have elevated IgE levels greater than 90% of the expected, age-adjusted level. As expected, among patients with a total IgE level greater than 90th percentile of the normal population, their baseline prevalence of asthma was 31%, not much different than the entire cohort rate of asthma, 27%. These asthma prevalence data are compatible with estimates (22% probable, 14% possible, 3% previous, yielding 39% combined) from a large survey in elementary schoolchildren of African-Americans (25). Similar asthma prevalence was found in our recent study in St. Louis city schools that further suggests that the prevalence of asthma has been stable from early 1990s to mid-2000s (26). If the threshold is increased to greater than the 98th percentile of the normal population, then the prevalence of asthma increases to 38%. Thus, among patients with SCD, simply having an elevated total IgE level (greater than the 90th percentile) alone in comparison to the age-matched non-atopic counterparts in African-American children is not sufficient evidence that a child has a diagnosis of asthma. Given the above discussions, an IgE level above 98th percentile is suggestive as a potential and clinically useful biomarker of asthma risk for children with SCD who deserve a more comprehensive pulmonary and allergy evaluation.
As can be expected in all studies, this analysis is subject to limitations. Firstly, the entire cohort was not tested for IgE levels; although, our data suggest that the sample was representative. Secondly, although entry into the trial did not require pre-selection for severity of SCD, this analysis may still be sensitive to potential selection bias, as children who participated in this trial could be typically healthier, with more motivated or better educated parents; selection bias is common to almost all clinical trials. Despite this limitation, we believe that we have provided reasonable evidence in supporting our original hypotheses.
The observation that elevated total IgE levels are associated with ACS is important, but may be confounded by the significant overlap in clinical findings between an asthma exacerbation and ACS, such as wheezing, low grade fever, and new radiodensity on a chest radiograph (3). Unrecognized environmental factors associated with both asthma and ACS may also render confounding effects. One such environment factor, environmental tobacco smoke, was assessed and not found to be a significant predictor of either asthma or SCD morbidity. Smoking status was reportedly associated with increased IgE levels in a normal adult Swiss population (27). No supportive evidence of household smoking and IgE level elevation was found in the present study cohort of children with SCD. Given the existing literature on parental environmental tobacco smoke exposure and lung function in their children (28–29), we believe that a more thorough evaluation is warranted, with direct and longitudinal measurements of recent tobacco exposure, before the association between environmental tobacco smoke exposure and an increase in ACS incidence rates can be excluded. Other environmental risk factors such as air pollution may be influential and associated with a diagnosis of asthma or an increased incidence of ACS that remain to be measured and modeled in future surveys.
Previously, we identified that asthma was associated with the incidence rate of pain episodes in children with SCD (11). In the current analysis, we found that IgE elevation was associated with a diagnosis of asthma, but not with incidence rate of pain episodes, in children with SCD (see Table 4). As is always the case, the negative result can either be a false or true negative one. We believe that elevated IgE levels are not truly associated with pain episodes for two reasons. First, our observation that patients with elevated IgE levels (greater than the 90th percentile) appeared to have a lower, although statistically non-significant (p = 0.20), mean pain rate estimate, when compared with patients without elevated IgE levels (less than 90th percentile). The direction of difference in the incidence rates of pain (52 − 60 = −8 events per 100 patient-years; Table 4) appeared to be in the opposite direction when compared to what was postulated. Second, the difference in incidence rates of pain was not statistically significantly different from zero (95% CI ranged from −21 to 5 events per 100 patient-years; data not given in Table 4); the true difference in the pain incidence rates between the two populations appears to be modest, if at all.
Conclusions
This study provides evidence that a physician diagnosis of asthma among children with SCD is associated with elevation of total IgE levels and the presence of allergen-specific IgE antibody. These findings also demonstrate that an elevation in total serum IgE levels is associated with an increase in the incidence of ACS episodes among children with SCD. Future work is required to determine the mechanism for these associations.
Clinical implications.
An elevated IgE level is a risk factor for acute chest syndrome but not pain in children with sickle cell disease.
Capsule summary.
A doctor diagnosis of asthma is a risk factor for increased morbidity in sickle cell disease. This study demonstrates that elevated total IgE levels are associated with sickle cell disease-related morbidity.
Acknowledgments
Declaration of all sources of funding: This study is supported by funding from the National Institute of Neurological Disorders and Stroke U01 NS042804 and the National Heart, Lung, and Blood Institute RO1-HL079937.
Abbreviations used
- SCD
Sickle cell disease
- ACS
Acute chest syndrome
- IgE
Immunoglobulin E
- SIT
The Silent Cerebral Infarct Transfusion (SIT) Trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powars D, Chan L, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine. 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. PMID: 16267411. [DOI] [PubMed] [Google Scholar]
- 2.Platt O, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. PMID: 7993409. [DOI] [PubMed] [Google Scholar]
- 3.Vichinsky E, Neumayr L, Earles A. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. New Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. PMID: 10861320. [DOI] [PubMed] [Google Scholar]
- 4.Strunk RC, Brown MS, Boyd JH, Bates P, Field JJ, DeBaun MR. Methacholine challenge in children with sickle cell disease: a case series. Pediatr Pulmonol. 2008;43:924–929. doi: 10.1002/ppul.20884. PMID: 18671275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007 doi: 10.1016/j.jaci.2007.09.043. Full report. [DOI] [PubMed] [Google Scholar]
- 6.Boyd J, Moinddin A, Strunk R, DeBaun M. Asthma and acute chest in sickle cell disease. Pediatr Pulmonol. 2004;38:229–232. doi: 10.1002/ppul.20066. PMID: 15274102. [DOI] [PubMed] [Google Scholar]
- 7.Palma-Carlos AG, Palma-Carlos ML, Costa AC. Minor hemoglobinopathies: a risk factor for asthma. Eur Ann Allergy Clin Immuno. 2005;37:177–182. PMID: 15984316. [PubMed] [Google Scholar]
- 8.Nordness ME, Lynn J, Zacharisen MC, Scott PJ, Kelly KJ. Asthma is a risk factor for acute chest syndrome and cerebral vascular accidents in children with sickle cell disease. Clin Mol Allergy. 2005;3:2. doi: 10.1186/1476-7961-3-2. PMID: 15663785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60:206–210. doi: 10.1136/thx.2004.029165. PMID: 15741436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant R. Asthma in the pediatric sickle cell patient with acute chest syndrome. J Pediatr Health Care. 2005;19:157–162. doi: 10.1016/j.pedhc.2004.12.003. PMID: 15867831. [DOI] [PubMed] [Google Scholar]
- 11.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108:2923–2927. doi: 10.1182/blood-2006-01-011072. PMID: 16690969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sylvester KP, Patey RA, Broughton S, Rafferty GF, Rees D, Thein SL, et al. Temporal relationship of asthma to acute chest syndrome in sickle cell disease. Pediatr Pulmonol. 2007;42:103–106. doi: 10.1002/ppul.20430. PMID: 17186507. [DOI] [PubMed] [Google Scholar]
- 13.Duckworth L, Hsu L, Feng H, Wang J, Sylveter JE, Kissoon N, et al. Physician-diagnosed asthma and acute chest syndrome: association with NOS polymorphisms. Pediatr Pulmonol. 2007;42:332–338. doi: 10.1002/ppul.20582. PMID: 17351927. [DOI] [PubMed] [Google Scholar]
- 14.Bernaudin F, Strunk RC, Kamdem A, Arnaud C, An P, Torres M, et al. Asthma is associated with acute chest syndrome, but not with an increased rate of hospitalization for pain among children in France with sickle cell anemia: a retrospective cohort study. Haematologia. 2008;93:1917–1918. doi: 10.3324/haematol.13090. PMID: 18815195. [DOI] [PubMed] [Google Scholar]
- 15.Wahn U, Chuchalin A, Kowalski M. Prediction and early diagnosis. Chem Immunol Allergy. 2004;84:128–134. doi: 10.1159/000081461. PMID: 15496769. [DOI] [PubMed] [Google Scholar]
- 16.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. PMID: 2911321. [DOI] [PubMed] [Google Scholar]
- 17.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relationship between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. PMID: 1891008. [DOI] [PubMed] [Google Scholar]
- 18.Casella JF, King AA, Barton B, White DA, Noetzel MJ, Ichord RN, et al. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatr Hematol Oncol. 2010;27:69–89. doi: 10.3109/08880010903360367. PMID: 20201689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbee RA, Halonen M, Lebowitz M, Burrows B. Distribution of IgE in a community population sample: correlations with age, sex, and allergen skin test reactivity. J Allergy Clin Immunol. 1981;68:106–11. doi: 10.1016/0091-6749(81)90167-6. PMID: 7251998. [DOI] [PubMed] [Google Scholar]
- 20.Saarinen UM, Saarinen UM, Juntunen K, Kajosaari M, Björkstén F. Serum immunoglobulin E in atopic and non-atopic children aged 6 months to 5 years: a follow-up study. Acta Paediatr Scand. 1982;71:489–494. doi: 10.1111/j.1651-2227.1982.tb09457.x. PMID: 7136662. [DOI] [PubMed] [Google Scholar]
- 21.O'Holleran MT, Yunginger JW, Offord KP, Somers MJ, O'Connell EJ, Ballard DJ, et al. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. New England Journal of Medicine. 1991;324:359–363. doi: 10.1056/NEJM199102073240602. PMID: 1987459. [DOI] [PubMed] [Google Scholar]
- 22.Huss K, Adkinson NF, Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001;107:48–54. doi: 10.1067/mai.2001.111146. PMID: 11149990. [DOI] [PubMed] [Google Scholar]
- 23.Matsui EC, Sampson HA, Bahnson HT, Gruchalla RS, Pongracic JA, Teach SJ, et al. on behalf of the Inner-city Asthma Consortium. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy 2010 Jun 17. doi: 10.1111/j.1398-9995.2010.02412.x. [Epub ahead of print]. PMID: 20560910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129–151. PMID: 15280088. [PubMed] [Google Scholar]
- 25.Webber MP, Carpiniello KE, Oruwariye T, Appel DK. Prevalence of asthma and asthma-like symptoms in inner-city elementary schoolchildren. Pediatr Pulmonol. 2002;34:105–111. doi: 10.1002/ppul.10146. PMID: 12112776. [DOI] [PubMed] [Google Scholar]
- 26.Nelson KA, Meadows L, Yan Y, Schootman M, Strunk RC. Asthma prevalence in low-income urban elementary school students in St. Louis, 1992 and 2004. J Pediatr. 2009;154:111–115. doi: 10.1016/j.jpeds.2008.07.017. PMID:18760422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wüthrich B, Schindler C, Medici TC, Zellweger JP, Leuenberger P. IgE levels, atopy markers and hay fever in relation to age, sex and smoking status in a normal adult Swiss population. SAPALDIA (Swiss Study on Air Pollution and Lung Diseases in Adults) Team. Int Arch Allergy Immunol. 1996;111:396–402. doi: 10.1159/000237398. PMID:8957114. [DOI] [PubMed] [Google Scholar]
- 28.Moshammer H, Hoek G, Luttmann-Gibson H, Neuberger MA, Antova T, Gehring U, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173:1255–1263. doi: 10.1164/rccm.200510-1552OC. PMID: 16484675. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Wypij D, Gold DR, Speizer FE, Ware JH, Ferris BG, Jr, et al. A longitudinal study of the effects of parental smoking on pulmonary function in children 6–18 years. Am J Respir Crit Care Med. 1994;149:1420–1425. doi: 10.1164/ajrccm.149.6.8004293. PMID: 8004293. [DOI] [PubMed] [Google Scholar]