Abstract
BACKGROUND
Identification of neuronal progenitor/stem cells in the postnatal gut suggests the development of transplantation approaches to enteric nervous system (ENS) diseases. Many clinical applications would require engrafting large segments of postnatal gut in vivo. We investigated the ability of unselected gut cells vs. selected enteric neural crest stem cells (eNCSCs) to engraft and differentiate in the postnatal gut in the Hirschsprung Disease (HD, ednrbsl/sl) rat.
METHODS
Total intestinal cells or eNCSCs (α4 integrin+, p75++) from embryonic day (E)14.5 rats carrying a marker transgene (hPAP) were injected intraperitoneally (IP) into neonatal HD rats and their healthy littermates. The entire gut was systematically analyzed 3 weeks later for hPAP+ cells between the serosal surface and the muscularis mucosae. Engrafted cells were examined for HuC/D, S-100B, NPY, nNOS, and VIP expression.
KEY RESULTS
0/33 rats injected with unselected cells had hPAP+ cells in the ENS that expressed neuronal or glial markers. 5/11 healthy and 4/5 HD rats injected with eNCSCs showed widespread but low density engraftment in the ENS with cells expressing neuronal or glial markers. Neurons expressed nNOS and VIP. There was no engraftment in the colon of either HD or WT rats.
CONCLUSIONS & INFERENCES
eNCSCs will engraft diffusely throughout the postnatal gut of HD rats and differentiate into neurons and glia. Engraftment is not uniform, likely related to age-dependent changes in the gut mesenchyme. IP injection is easily performed in sick neonates and may be developed as a technique to supply exogenous ENS cells to the diseased postnatal gut.
Keywords: Enteric Nervous System (ENS), transplantation, submucosal plexus, myenteric plexus, endothelin-B receptor (ednrb), R26-hPAP transgenic rat
The identification of cell populations within the postnatal intestine that migrate into recipient gut and differentiate into neurons and glia has led to keen interest in developing neural crest stem cell/enteric nervous system (ENS) progenitor-based therapies for enteric neuropathies. The best characterized of these disorders is Hirschsprung disease (HD), in which there is an absence of the ENS in a distal segment of gut. Mutations in multiple genes are associated with HD, most commonly the genes encoding the ret proto-oncogene and the endothelin-B receptor, ednrb. In animal models, the phenotype is shown to result from a failure of neural crest (NC)-derived cells to colonize the distal gut (1).
A variety of intestinal cell selection techniques for ENS-progenitor transplantation (reviewed in (2) and(3)) are reported. Neurosphere like body (NLB) culture is a frequently used method to obtain a population of cells enriched in ENS-progenitors. NLBs can be cultured from postnatal mucosal biopsies (4) and have been used to supply ENS cells that alter the function of distal colon explants (5).
Other groups obtain a population of cells enriched in ENS precursors by immunologic selection. Anitha et al reported that conditionally immortalized (6) gut cells immunoselected for expression of the low-affinity neurotrophin receptor, p75, engraft near an intramural injection site, form neurons, and alter motility of the adult mouse colon. An enriched population of post-migratory (enteric) Neural Crest Stem Cells (eNCSCs) can be isolated from the gut by fluorescent cell sorting for α4 integrin expression and high expression of p75 (7, 8). These cells are present in the postnatal gut and in the proximal intestine of the HD rat (ednrbsl/sl) (9, 10). They have successfully engrafted the HD rat and wildtype (WT) colon in explant culture, forming neurons but not glia (10, 11).
Several important issues remain in the quest for cell replacement ENS therapies: (i) Most stem cell therapy for enteric neuropathies will be carried out postnatally. The postnatal gut provides a markedly different environment for colonization than the embryonic gut and several studies indicate that colonization of the more mature colon is less efficient (12, 13). (ii) The gut is not a homogeneous tube. Enteric neuropathies or ENS injuries can occur in regions of the GI tract that provide significantly different environments for engraftment. (iii) Treatment of some ENS neuropathies may require engraftment of cells into areas containing endogenous NC-derived cells. The effect of the presence of these cells on engraftment is not fully explored. (iv) Treatment of human enteric neuropathies or ENS injuries may require large scale engraftment of cells over a large area of gut. Engraftment through direct intramural injection would be difficult to apply clinically to these disorders.
Martucciello et al reported in vivo transplantation of premigratory NC cells after intraperitoneal (IP) injection in adult mice. They observed intestine specific tropism of the injected NC cells. Direct intramural injection of the cells resulted in clumps of cells whereas IP injection resulted in a more diffuse engraftment throughout the intestine distal to the stomach. Injected cells were not found outside of the GI tract (15).
Martucciello’s results suggested that IP injection would allow us to transplant eNCSCs to a large segment of gut in a manner that would be tolerated by neonatal HD rats. These rats have aganglionosis that frequently extends proximal to the ileocecal junction, they typically die of intestinal obstruction by the age of weaning, and they do not tolerate laporatomy (for direct intramural injection) well. We hypothesized that post-migratory gut NC cells would migrate into the intestine and that selected eNCSC would be enriched in this type of cell. Further, we hypothesized that these cells would survive in the recipient gut, contribute to the ENS, and show preferential engraftment of aganglionic bowel.
MATERIALS AND METHODS
Animals
Ednrbsl/sl rats carry a naturally occurring null-allele of the endothelin-B receptor (16). The rats used in this study are on an inbred albino Wistar-Kyoto (WKY) genetic background. The intestinal phenotype was not altered from previous reports by the change in genetic background (16, 17). Rats carrying the human placental alkaline phosphatase gene under the transcriptional control of the Rosa26 promoter (R26-hPAP transgenic) were provided by Dr. Eric Sandgren and backcrossed to the WKY strain (18). All animal procedures were approved the Committee on Use and Care of Animals at The University of Michigan. All rats were housed as specific-pathogen free in temperature-and humidity-controlled environments, with free access to standard rat chow and a 12:12-hr light:dark cycle.
Collection and isolation of cells for transplantation
R26-hPAP pregnant rats were euthanized at embryonic day (E) 14.5 and the pup intestine (from the esophagogastric junction through descending colon) isolated and dissociated as previously described (8). Cells were stained with antibodies to p75 (192I Clone) and α4 integrin (Beckton-Dickinson, San Jose, CA; Mα4-1 clone directly conjugated to phycoerythrin). Cell sorting and analysis were performed on a FACSVantage dual-laser flow-cytometer (Beckton-Dickinson) as previously described. Alpha4 integrin+ cells in the top 2% of p75 expression were selected as eNCSC based on previous work by these authors and others and used immediately for IP injection (8, 10). Dead cells were eliminated from sorts as 7-amino-actinomycin D positive.
Recipient rats
HD rats have aganglionosis that extends from the rectum to at least the ascending colon and often extending into the distal ileum. They develop intestinal obstruction in the neonatal period and do not live beyond 5 weeks. They are referred to as HD rats throughout this report. Ednrbsl/+ rats are indistinguishable from WT rats visually (because they are on an albino background), in lifespan, and in fertility. Ednrb genotype was determined by PCR analysis of a biopsy specimen as previously described (19).
The entire litter from a heterozygous mating was injected IP with either freshly dissociated gastrointestinal cells (1 × 105 to 18 × 105; average 6 × 105 per pup, n=33) or freshly sorted eNCSCs (1.5 × 104 to 8 × 104; average 4.4 × 104 cells per pup, n=18) in the first week of life (range 2–7 days). Pups were returned to their nursing mothers after the procedure and euthanized in the 4th week of life for histological analysis.
Histologic analysis
Rats were perfused with PBS followed by 4% paraformaldehyde prior to collection of anterior abdominal wall and peritoneum, liver, both kidneys, and the GI tract. The stomach, small intestine, and colon (including the rectum) were separated, rinsed with PBS, and fixed with 4% paraformaldehyde at 4°C for 3–6 hours. Tissues were equilibrated with 20% sucrose overnight, The entire tissue was “Swiss-rolled” according to the technique described by Moolenbeek (20), quick frozen in OCT, and sectioned (5–6 micrometers). Sections were allowed to dry completely, washed with PBS, then incubated with blocking buffer (1% goat serum, 1% BSA, 0.5% triton X-100 in PBS) for one hour at room temperature followed by mouse IgG2a anti-hPAP (SIGMA, A2951, 1:500) co-incubated with either mouse IgG2b anti-human neuron-HuC/D (Moleculer Probes A21271, 5 microliter/mL) or mouse IgG1 anti-glial S-100B (SIGMA S2532, 1:500) overnight at 4°C. Secondary antibodies were FITC conjugated goat anti-mouse IgG2a (Southern Biotech, 1080-02), R-PE conjugated goat anti-mouse IgG2b (Southern Biotech, 1090-09), or R-PE conjugated goat anti-mouse IgG1 (Southern Biotech 1070-02).
Adjacent sections of intestinal segments demonstrating cell bodies between the serosa and the muscularis mucosa and co-expressing hPAP and HuC/D were further examined for co-expression of hPAP and NPY (Bachem T4070.0050, 1/1000), VIP (Bachem T-4246.0050, 1/1000) or nNOS (Fisher AB5380MI, 1/1000).
Statistics
Results are presented as mean ± SEM and p<0.05 is considered significant. Data were analyzed using GraphPad Prism version 5a for Mac OS X (GraphPad Software, San Diego, CA). The contingency affect of genotype on engraftment was calculated by chi-squared test for trend. Comparisons between two groups were made using the nonparametric Mann-Whitney test.
RESULTS
All pups survived the injection and return to their nursing mother. Thirty-three pups were injected with hPAP+, unselected, dissociated gut cells and 16 pups were injected with hPAP+ eNCSCs (Table 1). They were euthanized during the third week after IP injection and abdominal tissues examined closely for hPAP+ cells. No hPAP+ cells were found in sections of anterior abdominal wall or peritoneal membrane. Similarly, no hPAP+ cells were detected in the kidneys or liver. Scattered hPAP+ cells were noted in the GI tract of animals receiving unselected cells. These cells were primarily located in the epithelium and lamina propria throughout the intestine, but rarely noted in the region of the myenteric or submucosal plexus. No colocalization of hPAP with the neuronal marker HuC/D or the glial marker S-100B was observed (data not shown).
Table 1.
| Number of pups with hPAP+ cells expressing a neuronal or glial marker in the ENS/number of pups injected (%) | ||
|---|---|---|
| Ednrb genotype of recipient | Unselected cells injected | eNCSC injected |
| +/+ | 0/6 (0) | 2/2 (100) |
| sl/+ | 0/16 (0) | 3/9 (30) |
| sl/sl (HD rat) | 0/11 (0) | 4/5 (80) |
Exogenous (hPAP+) cells were also noted in the GI tract of pups injected with eNCSCs. 10 ± 2% of engrafted cells were located in the epitheilum or lamina propria of the gut. Of these, 5.5±0.8% co-expresssed HuC/D and none co-expressed S-100B. Compared to pups injected with approximately 10 times as many unselected cells, more hPAP+ cells were noted in the region of the submucosal or myenteric plexus in pups injected with eNCSCs.
In 9 of the 16 eNCSC injected pups, hPAP colocalized with HuC/D or S-100B in cells within or adjacent to enteric ganglia (Table 1). Exogenous HuC/D or S-100B positive cells were observed most frequently in the distal small intestine. Using a single “Swiss-roll” section showing the entire length of the distal small intestine, all ganglia in the submucosal and myenteric plexus were identified and counted using HuC/D or S-100B labeling. Individual labeled cells in the region of the submucosal or myenteric ganglia were also counted. The percentage of these ganglia/cells in which hPAP colocalized with HuC/D or S-100B in the 5 pups (2 WT, 1 heterozygous, 2 HD) showing highest density of engraftment is shown in Figure 1A. Exogenous cells expressed the neuronal marker more frequently than the glial marker (p<0.05 for both submucosal and myenteric plexuses) and were equally likely to be found in the submucosal or myenteric region. We did not find a difference in the likelihood of engraftment of hPAP+ cells between the 3 genotypes (p=0.08) or the quality of engraftment and there was no clear relationship between the number of cells injected or the day of injection with successful engraftment. Representative examples of exogenous cells expressing HuC/D or S-100B in the stomach, distal small intestine, and cecum in 3 different HD injected pups are shown in Figures 2, 3, and 4 respectively. Exogenously-derived neurons and glia were found both individually in close proximity to ganglia and intermingled with endogenous cells within ganglia. The majority of engrafted neurons were found in the small intestine with highest density in the distal small intestine. Exogenously derived neurons were relatively rare in the stomach and cecum (Figure 1B).
Figure 1.
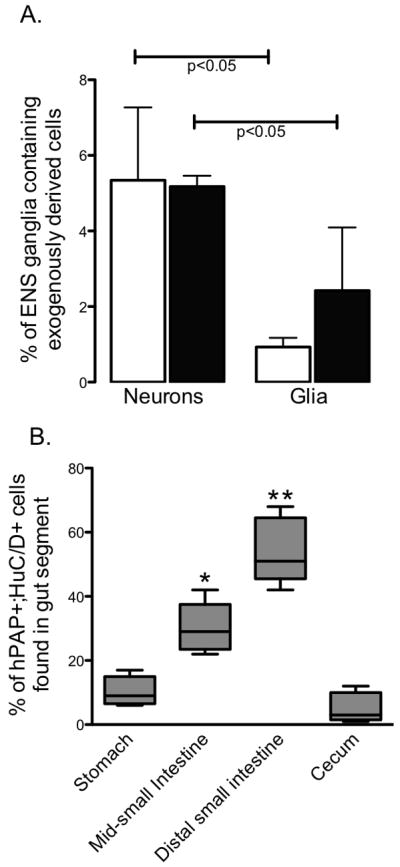
(A) Plexus localization and phenotype of engrafted cells. Selected eNCSCs transgenically expressing hPAP were IP injected into rat pups in the first week of life. All HuC/D+ (neurons) or S-100B+ (glia) ganglia or individual cells outside of ganglia were counted in a single, full-length section of the distal small intestine in 5 pups. The percentage of these cells also hPAP+ in the region of the submucosal plexus (white bars) or myenteric plexus (black bars) is shown. (B) Distribution of engrafted neurons throughout the gut. The gut was divided into 4 segments (stomach with the proximal third of the small intestine, mid-small intestine, distal third of the small intestine, and cecum with colon) and all hPAP+;HuC/D+ expressing ganglia or individual cells outside of ganglia were counted in a single, full-length section in 5 pups. Boxes represent the percentage of all hPAP+;HuC/D+ cells in each animal found in the indicated gut segment. Whiskers indicate minimum and maximum value. * p<0.05 compared to stomach, distal small intestine and cecum; ** p<0.05 compared to stomach, mid-small intestine and cecum
Figure 2. IP injection of isolated eNCSCs results in scattered neuronal and glial engraftment in the stomach ENS.
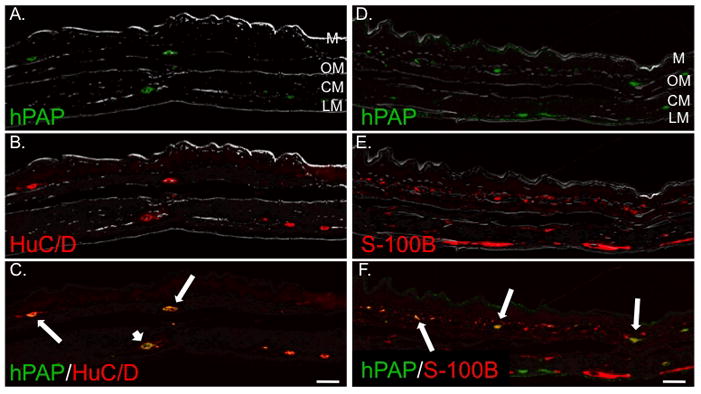
Gastric body sections 20 days after IP injection of a 2 day-old HD pup with 1.5 × 104 hPAP+ eNCSCs. All sections are oriented with the mucosa at the top of the photo. Bright field views are overlayed to aid localization within the stomach wall: LM, longitudinal muscle layer; CM, circular muscle layer; OM, oblique muscle layer; and M, mucosa. Sections were labeled immunohistochemically for hPAP (A and D), HuC/D (B), or S-100B (E). Co-labeling is illustrated in panels C and F. Long arrows indicate co-expression of the indicated markers in the region of the submucosal plexus. The short arrow indicates co-expression of hPAP and HuC/D in the region of the myenteric plexus. Bar = 100 μm.
Figure 3. IP injection of isolated eNCSCs results in widespread neuronal and glial engraftment in the distal small intestine ENS.
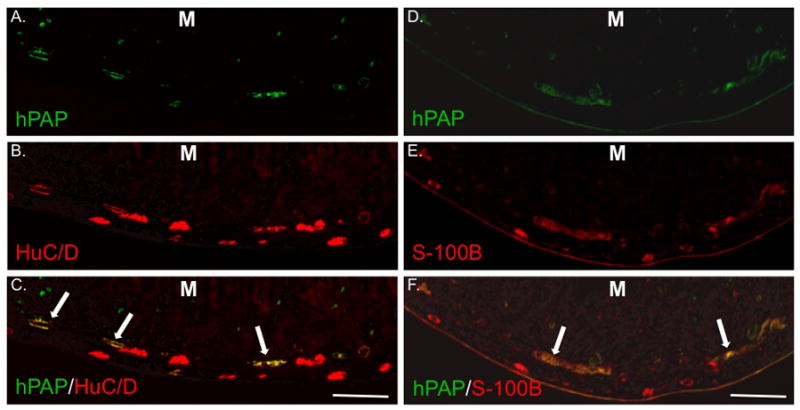
Sections from the distal third of the small intestine 21 days after IP injection of a 7 day-old HD pup with 5 × 104 hPAP+ eNCSCs. All sections are oriented with the mucosa (M) at the top of the photo. Sections were labeled immunohistochemically for hPAP (A and D), HuC/D (B), or S-100B (E). Co-labeling is illustrated in panels C and F. Long arrows indicate co-expression of markers in the region of the submucosal plexus. Bar = 200 μm.
Figure 4. IP injection of isolated eNCSCs results in rare, scattered neuronal and glial engraftment in the cecal ENS.
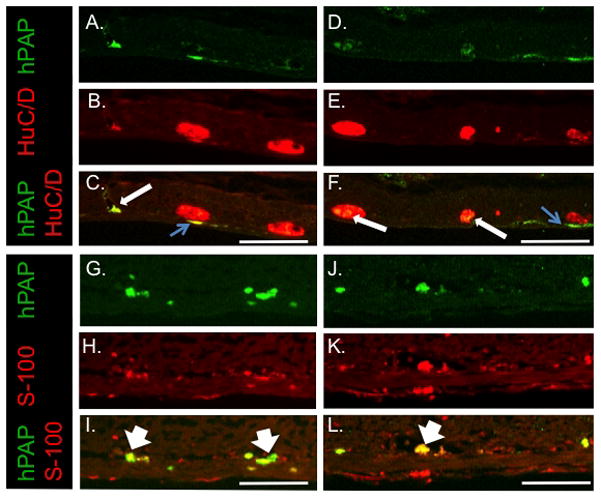
Sections from cecum 22 days after IP injection of a 2 day-old HD pup with 5 × 104 hPAP+ eNCSCs. All sections are oriented with the mucosa at the top of the photo. Sections were labeled immunohistochemically for hPAP (A, D, G and J), HuC/D (B and E), or S-100B (H and K). Co-labeling is illustrated in panels C, F, I and L. Long arrows indicate isolated exogenously-derived neurons and exogenous-derived neurons participating in ganglia of the myenteric plexus with endogenous neurons. Thin blue arrows demonstrate hPAP+ between myenteric ganglia and the longitudinal muscle layer, a location consistent with the germinal niche. Wide arrows indicate exogenously derived glia in the region of the submucosal plexus. Bar = 200 μm.
We did not see exogenous cells develop neuronal or glial phenotypes in the aganglionic colon of the HD rats. Rare, exogenously-derived HuC/D positive cells were identified in the aganglionic distal small intestine of 2 HD rats (Figure 5).
Figure 5. IP injection of isolated eNCSCs results in scattered engraftment of neurons in the aganglionic distal small intestine of the HD rat.
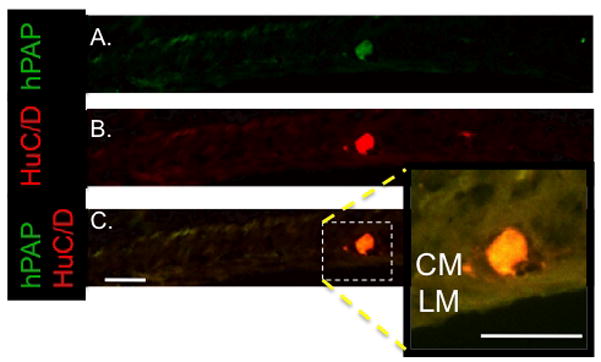
Representative section from a HD rat without endogenous neural cells in the distal small intestine 20 days after IP injection of with 1.5 × 104 hPAP+ eNCSCs All sections are oriented with the mucosa at the top of the photo. The section is labeled immunohistochemically for hPAP (A) and HuC/D (neuronal marker) (B) expression. Co-expression is illustrated in (C) demonstrating an isolated collection of exogenously-derived neurons (enlarged in the inset photo) in the region of the myenteric plexus. CM = circular muscle layer; LM = longitudinal muscle layer; bar = 100 μm.
Immunohistochemistry for nNOS, NPY, and VIP was conducted on intestinal sections from pups with exogenous cells expressing HuC/D. Approximately 50% of the engrafted HuC/D positive cells expressed one of these markers. Of these, the majority were nNOS+. No colocalization of hPAP and NPY was observed (Table 2). Exogenous cells expressing neuronal markers did not generally clump together, but tended to reside outside but in close proximity to ganglia or they contributed 1–2 cells to a ganglion. Fifty percent of the exogenous cells expressing S-100B grouped together and diffusely populated ganglia containing endogenous neurons (Figure 6).
Table 2.
| Percentage of hPAP+ cells in the distal small intestine ENS expressing neurotransmitter | |
|---|---|
| nNOS | 37±15 |
| VIP | 8±7 |
| NPY | 0 |
Figure 6. Engrafted neurons participate in ganglia with endogenous neurons and express VIP and nNOS while engrafted glia diffusely populate ganglia.
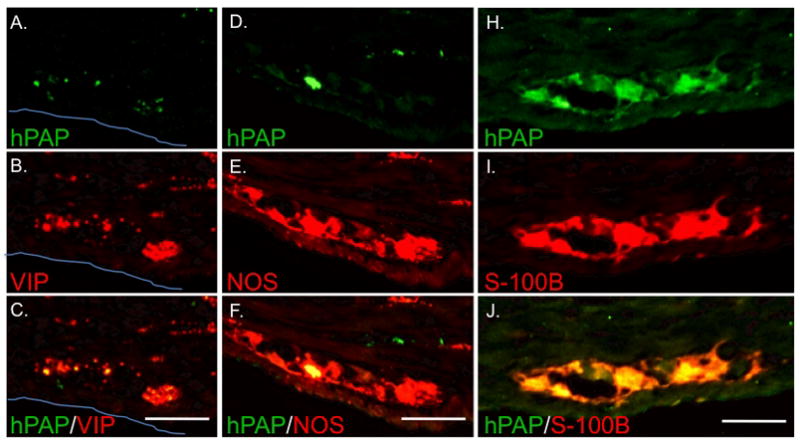
Views of individual myenteric ganglia in the distal small intestine of a HD rat injected with 1.5 × 104 hPAP+ eNCSCs on day-of-life 2. Expression of hPAP (A, D, and H) was often noted in a small subset of cells within individual ganglia. Immunohistochemical labeling for VIP (B) and NOS (E) demonstrates the presence of both endogenous neurons and exogenous neurons within the ganglia (C and F). The glial marker S-100B most often labeled cells throughout the ganglia (I) suggesting a more clustered engraftment pattern. All sections are oriented with mucosa toward the top of the photo. The blue line indicates the serosal surface in A–C. Bar = 50 μm.
DISCUSSION
We examined the engraftment and developmental potential of exogenously supplied eNCSCs in healthy and HD rats. The flow-cytometric selection of NCSCs from the embryonic gut enriches for a population of cells that, when injected IP in young animals, migrate into the gut, localize at low density within or in close proximity to submucosal and myenteric ganglia, and differentiate into neurons and glia. Some cells also take up residence in the mucosa. The exogenous cells survive 3 weeks and contribute neurons to the ganglia in the distal small intestine. The maximal percentage of ganglia containing engrafted cells we observed was 11% of ganglia of the submucosal plexus and 6% of the myenteric plexus. Engraftment was not affected by the ednrb genotype of the recipient gut. Neuronal NOS and VIP expression was observed in the neurons derived from exogenous cells. Engraftment was seen in the stomach, small intestine, and cecum but was not observed in the more distal colon of healthy or HD rats. Contrary to our hypothesis, the absence of endogenous NC-derived cells had no affect on engraftment success.
Not all of the injected eNCSC localized to the ENS. “Ectopic” migration/localization of eNCSCs transplanted into the NC migration pathway in embryonic chicks is reported by Mosher et al (11). These authors speculated that the ectopic migration of heterotopically and heterochronically transplanted cells reflected responses to signals that these cells would not normally encounter (11). With IP injection into the neonatal rat, we observed exogenous gut-derived cells only in the gut. Because those cells that did not localize to the region of the ENS did not develop neuronal or glial markers, we cannot distinguish whether they are non-NC-derived cells unfortunately included in our selected population or correctly selected NC-derived cells following postnatal localization/differentiation cues they do not usually encounter. A large proportion of cells selected in this manner may not be capable of forming neurons or glia. When cultured in vitro at low density, 50% of plated cells do not form colonies and ~15% of the colonies do not stain with a neuronal or glial marker(8). Therefore, we know very little about a substantial proportion of the cells we injected and how these cells respond to the environment of the peritoneal cavity. The exogenous cells that localized adjacent to myenteric ganglia may represent a less well defined NC-derivative such as the recently identified NC-derived cell near the ganglia of the proximal foregut of the postnatal mouse (21) or the putative NC stem cells that reside in germinal niches between the myenteric ganglia and longitudinal muscle in the adult mouse. These cells may or may not express HuC/D, divide very slowly, migrate into the adjacent ganglia, differentiate into mature neurons, and significantly contribute to the abundance of ENS neurons in the adult. Activation of the 5-HT4 receptor appears to stimulate this process that takes up to 24 weeks in the mouse (22). Interestingly, we frequently observed hPAP+ cells in this location, some of which also expressed HuC/D (see Figure 4C) and some of which did not (Figure 4F). We did not study their expression of stem cell markers or their behavior beyond the 3 week time point, so cannot know whether we supplied exogenous NC stem cells to the germinal niches of the myenteric plexus. It certainly is an exciting possibility however, as this may point to a transplantation technique that would augment the ability of the adult ENS to repair itself.
We found the highest density of engrafted neurons and glia in the distal small intestine. We speculate that tropism for the distal small bowel is in response to signals present in the perinatal gut, which are not present during embryonic NC colonization of the developing gut. For example, Mosher et al reported that ~50% of eNCSCs isolated using the same technique express the RET receptor (11) which others have shown is critical for the organization of Peyer’s patches in older embryos (23). Thus, the RET+ cells we injected could be following the organizational cues for this lymphoid tissue, which is particularly dense in the distal small bowel.
Kruger et al reported injection of freshly isolated eNCSCs into the wall of E14.5 rat embryonic colon with subsequent 5 days explant culture. Intramural injection resulted in 150 to 200 microns of engraftment within the colon (10). In this respect, the current technique has advantages and disadvantages. Intramural injection results in a high density of donor neurons with a very limited spacial distribution, but IP injection results in a low density of donor neurons (and glia) spread from the stomach to the cecum. The further refinement of cell selection techniques, the deployment of chemoattractants to specific regions of the gut, or the use of agents to promote proliferation of the cells in vivo, may allow for targeted repair of relatively large segments of ENS using IP injection.
While donor cells injected directly into the colon wall survive and differentiate (10, 11), they do not appear to migrate into the mature colon. We did not observe neuronal or glia engraftment in the colon beyond the cecum. Recently age-dependent changes in the colon are reported to be crucial for enteric neural crest cell invasion (12, 13). Our findings – the failure of exogenous eNCSC to migrate into either the HD or WT colon - are consistent with studies suggesting a temporal change in permissiveness to NC invasion within the colonic microenvironment that is not restricted to the ednrb-deficient gut. Interestingly, invasion into the postnatal small intestine does not appear to be similarly affected.
While Hotta et al found that donor NC-derived cells migrate into an aneural segment of embryonic colon more efficiently than an embryonic colon segment that already contains ENS precursors (12), we observed no engraftment advantage for the colon of the HD rat. It may be that the age-related inhibition of migration completely masks a small migration advantage in the anural HD colon or that the donor cells actually migrate into the colon but do not survive. The current experiments cannot answer these possibilities. Anitha et al reported survival and functional engraftment of NC-derived cells when they were injected directly into the colon of the nNOS−/− mouse, in which endogenous NC-derived cells are present (14). These findings suggest that migration of NC cells into the mature colon may be inhibited, but survival within this environment is possible.
The differentiation of NCSCs is highly influenced by the local microenvironment (7) and perhaps their migration history. When injected into the neural crest migration pathway of the chick and allowed to migrate into the gut, they form neurons and glia. When they migrate to other locations in the peripheral nervous system they show a propensity to form neurons over glia, but local signals can promote gliogenesis from these cells. Interestingly, when injected directly into the colon, they only form neurons (11, 14). Compared to other studies, we observed (i) a higher percentage of cells expressing neither the neuronal or glial marker and (ii) the absence of NPY expression. These differences may be related to the method of transfer, the age and location of the gut, or the wide range of other variables that affect the expression of specific neurotransmitters in the GI tract (24–27).
This study has the major limitation that we did not document that the engrafted cells result in a higher density of ganglia or alter GI tract neuromuscular function. It is possible that engraftment of the exogenous eNCSC in the first week alters the development of the endogenous NC-derived cells of the gut resulting in no functional consequences. Further refinement of this technique to produce a higher density engraftment is underway so that functional studies can be done.
In summary, selection of eNCSCs from the gut enriches for a population of cells that gives rise to neurons and glia spread from the stomach to the cecum when given as a single IP injection in the first week of life in the rat. Refinement of the selection process, the development of cell population expansion techniques, and/or manipulation of the recipient GI tract may allow for an engraftment targeted not only to regions of the GI tract but also in cell fate determination.
Acknowledgments
Acknowledgements and disclosures:
Dr. Tsai organized, conducted and interpreted results of the majority of described experiments. Ms. Murakami assisted in the acquisition of data and managed the animal resources. Dr. Gariepy directed the experimental design/direction, the overall interpretation of results and prepared the manuscript. We would like to thank Dr. Eric Sandgren for generously sharing the R26-hPAP transgenic rats. This work was supported by R21 DK073900 from the National Institutes of Health, USA, C. Gariepy PI, the Department of Pediatrics at The University of Michigan, and The Research Institute at Nationwide Children’s Hospital.
Footnotes
Studies performed in the Department of Pediatrics, University of Michigan and The Nationwide Children’s Research Institute
Competing Interests: The authors have no competing interests.
References
- 1.Laranjeira C, Pachnis V. Enteric nervous system development: Recent progress and future challenges. Auton Neurosci. 2009 Nov 17;151(1):61–9. doi: 10.1016/j.autneu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Hotta R, Natarajan D, Thapar N. Potential of cell therapy to treat pediatric motility disorders. Semin Pediatr Surg. 2009 Nov;18(4):263–73. doi: 10.1053/j.sempedsurg.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Thapar N. New frontiers in the treatment of Hirschsprung disease. J Pediatr Gastroenterol Nutr. 2009 Apr 1;48( Suppl 2):S92–4. doi: 10.1097/MPG.0b013e3181a15d62. [DOI] [PubMed] [Google Scholar]
- 4.Metzger M, Caldwell C, Barlow AJ, Burns AJ, Thapar N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. 2009 Jun 1;136(7):2214–25. e1–3. doi: 10.1053/j.gastro.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 5.Metzger M, Bareiss P, Danker T, Wagner S, Hennenlotter J, Guenther E, et al. Expansion and Differentiation of Neural Progenitors Derived From the Human Adult Enteric Nervous System. Gastroenterology. 2009 Jun 21; doi: 10.1053/j.gastro.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, et al. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5096–100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000 Oct 1;20(19):7370–6. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002 Aug 15;35(4):643–56. doi: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 9.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002 Aug 15;35(4):657–69. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruger GM, Mosher JT, Tsai Y-H, Yeager KJ, Iwashita T, Gariepy CE, et al. Temporally distinct requirements for endothelin receptor B in the generation and migration of gut neural crest stem cells. Neuron. 2003 Dec 4;40(5):917–29. doi: 10.1016/s0896-6273(03)00727-x. [DOI] [PubMed] [Google Scholar]
- 11.Mosher JT, Yeager KJ, Kruger GM, Joseph NM, Hutchin ME, Dlugosz AA, et al. Intrinsic differences among spatially distinct neural crest stem cells in terms of migratory properties, fate determination, and ability to colonize the enteric nervous system. Dev Biol. 2007 Mar 1;303(1):1–15. doi: 10.1016/j.ydbio.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotta R, Anderson RB, Kobayashi K, Newgreen DF, Young HM. Effects of tissue age, presence of neurones and endothelin-3 on the ability of enteric neurone precursors to colonize recipient gut: implications for cell-based therapies. Neurogastroenterol Motil. 2010 Mar 1;22(3):331–e86. doi: 10.1111/j.1365-2982.2009.01411.x. [DOI] [PubMed] [Google Scholar]
- 13.Druckenbrod NR, Epstein ML. Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development. 2009 Sep 1;136(18):3195–203. doi: 10.1242/dev.031302. [DOI] [PubMed] [Google Scholar]
- 14.Anitha M, Joseph I, Ding X, Torre ER, Sawchuk MA, Mwangi S, et al. Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology. 2008 May 1;134(5):1424–35. doi: 10.1053/j.gastro.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martucciello G, Brizzolara A, Favre A, Lombardi L, Bocciardi R, Sanguineti M, et al. Neural crest neuroblasts can colonise aganglionic and ganglionic gut in vivo. European journal of pediatric surgery: official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift für Kinderchirurgie. 2007 Feb 1;17(1):34–40. doi: 10.1055/s-2007-964952. [DOI] [PubMed] [Google Scholar]
- 16.Gariepy CE, Cass DT, Yanagisawa M. Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc Natl Acad Sci USA. 1996 Jan 23;93(2):867–72. doi: 10.1073/pnas.93.2.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagahama M, Tsutsui Y, Ozaki T, Hama K. Myenteric and submucosal plexuses of the congenital aganglionosis rat (spotting lethal) as revealed by scanning electron microscopy. Biol Signals. 1993 May–Jun;2(3):136–45. doi: 10.1159/000109485. [DOI] [PubMed] [Google Scholar]
- 18.Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999 Oct 1;214(1):128–38. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- 19.Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest. 1998 Sep 15;102(6):1092–101. doi: 10.1172/JCI3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moolenbeek C, Ruitenberg EJ. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim. 1981 Jan 1;15(1):57–9. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 21.Walters LC, Cantrell VA, Weller KP, Mosher JT, Southard-Smith EM. Genetic Background Impacts Development Potential of Enteric Neural Crest-Derived Progenitors in the Sox10Dom Model of Hirschsprung Disease. Hum Mol Genet. 2010 Aug 25; doi: 10.1093/hmg/ddq357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M-T, Kuan Y-H, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009 Aug 5;29(31):9683–99. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veiga-Fernandes H, Coles MC, Foster KE, Patel A, Williams A, Natarajan D, et al. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature. 2007 Mar 29;446(7135):547–51. doi: 10.1038/nature05597. [DOI] [PubMed] [Google Scholar]
- 24.Moriez R, Abdo H, Chaumette T, Faure M, Lardeux B, Neunlist M. Neuroplasticity and neuroprotection in enteric neurons: role of epithelial cells. Biochem Biophys Res Commun. 2009 May 8;382(3):577–82. doi: 10.1016/j.bbrc.2009.03.073. [DOI] [PubMed] [Google Scholar]
- 25.di Giancamillo A, Vitari F, Bosi G, Savoini G, Domeneghini C. The chemical code of porcine enteric neurons and the number of enteric glial cells are altered by dietary probiotics. Neurogastroenterol Motil. 2010 Sep;22(9):e271–8. doi: 10.1111/j.1365-2982.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 26.Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010 May 1;138(5):1772–82. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 27.Chaumette T, Lebouvier T, Aubert P, Lardeux B, Qin C, Li Q, et al. Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental Parkinsonism. Neurogastroenterol Motil. 2009 Feb 1;21(2):215–22. doi: 10.1111/j.1365-2982.2008.01226.x. [DOI] [PubMed] [Google Scholar]


