Abstract
Generalized social phobia (GSP) involves the fear of being negatively evaluated. Previous work suggests that self-referentiality, mediated by medial prefrontal cortex (MFPC), plays an important role in the disorder. However, it is not clear whether this anomalous MPFC response to self-related information in patients with GSP concerns an increased representation of their own or others’ opinions. In this paper we examined whether GSP is associated with increased response to own (1st person) or other individuals’ (2nd person) opinions relative to healthy individuals. Unmedicated individuals with GSP (n=15) and age, IQ, and gender-matched comparison individuals (n=15) read 1st (e.g., I’m ugly), and 2nd (e.g., You’re ugly) person viewpoint comments during fMRI. We observed significant group-by-viewpoint interactions within ventral MPFC. Whereas the healthy comparison individuals showed significantly increased (or less decreased) BOLD responses to 1st relative to 2nd person viewpoints, the patients showed significantly increased responses to 2nd relative to 1st person viewpoints. The reduced BOLD responses to 1st person viewpoint comments shown by the patients correlated significantly with severity of social anxiety symptom severity. These results underscore the importance of dysfunctional self-referential processing and MPFC in GSP. We believe that these data reflect a reorganization of self-referential reasoning in the disorder with a self-concept perhaps atypically related to the view of others.
Keywords: Social Phobia, fMRI, self-referentiality, medial prefrontal cortex, amygdala
1. INTRODUCTION
Generalized Social Phobia (GSP) involves a strong and persistent fear of social or performance situations that involves the potential for negative evaluation. It is a relatively common disorder associated with a high risk for alcohol and drug abuse, depression, and suicide (Kaufman and Charney 2000; Kessler 2003; Beesdo, Bittner et al. 2007).
FMRI studies in GSP have focused on examining responses to social stimuli. In particular, extensive work has examined the response to facial expressions, including harsh (Phan, Fitzgerald et al. 2006), angry (Stein, Goldin et al. 2002; Straube, Kolassa et al. 2004; Straube, Mentzel et al. 2005; Blair, Geraci et al. under revision), fearful (Stein, Goldin et al. 2002; Blair, Shaywitz et al. 2008; Blair, Geraci et al. under revision), disgusted (Amir, Klumpp et al. 2005), happy (Straube, Mentzel et al. 2005) and neutral (Birbaumer, Grodd et al. 1998; Stein, Goldin et al. 2002) expressions. This work finds increased amygdala response in GSP (Stein, Goldin et al. 2002; Straube, Mentzel et al. 2005; Phan, Fitzgerald et al. 2006) though dorsal medial prefrontal cortex (MPFC) has also been implicated (Stein, Goldin et al. 2002; Straube, Kolassa et al. 2004; Amir, Klumpp et al. 2005; Blair, Shaywitz et al. 2008).
However, the response to facial expressions in both healthy individuals (Pessoa, McKenna et al. 2002; Pessoa, Padmala et al. 2005; Mitchell, Nakic et al. 2007) and patients (Pine, Klein et al. 2005; McClure, Monk et al. 2007) is under considerable attentional control; increased attention to other features in the environment reduces the representation of facial expressions and thus the emotional response to them (Pessoa and Ungerleider 2004; Mitchell, Nakic et al. 2007; Luo, Holroyd et al. 2010). Moreover, the intent and self-relevance of facial expressions can be difficult to ascertain (i.e., “Are you laughing at me or smiling with me? Angry with me or somebody else?”). Given that fears of other people’s scrutiny and judgment lies at the core of GSP (First, Spitzer et al. 1995), aberrant processing of self-referential information may play a role in the disorder.
In the first study to directly examine self-referential processing in GSP using fMRI, patients were assessed when processing self vs. other-referential criticism (e.g., “You’re ugly” vs. “He’s ugly”) and praise (e.g., “You’re beautiful” vs. “He’s beautiful”) (Blair, Geraci et al. 2008). This study revealed selectively increased BOLD responses within both the amygdala and dorsal MPFC in patients with GSP to self-referential criticism. In short, these data indicated that the heightened sensitivity to self-referential criticism in patients with GSP reflects dysfunction in dorsal MPFC as well as the amygdala (see also recent work by Goldin and colleagues that examines emotion regulation in social phobia to criticism [Goldin, Manber-Ball et al. 2009; Goldin and Gross 2010]). The MPFC finding is of particular relevance given the extensive data implicating dorsal as well as ventral MPFC in self-referential processing (Johnson, Baxter et al. 2002; Fossati, Hevenor et al. 2003; Phan, Taylor et al. 2004; Seger, Stone et al. 2004; Mitchell, Banaji et al. 2005; Moran, Macrae et al. 2006); for review see (Gillihan and Farah 2005; Northoff, Heinzel et al. 2006; Schmitz and Johnson 2007; Legrand and Ruby 2009; van der Meer, Costafreda et al. 2010). Specifically, both regions show increased responses during self-related as opposed to non-self related reasoning. Moreover, in addition to the MPFC, recent work has also highlighted the role of the amygdala in the response to praise and criticism in healthy individuals (Frewen, Dozois et al. 2010). Taken together, these findings suggest that MPFC might make independent contributions to GSP but may also interact as part of a circuit.
The goal of the current study was to extend our earlier work (Blair, Geraci et al. 2008) and to specifically examine whether patients with GSP show aberrant responding to self-referential comments depend on whether the origin of those comments is another individual (e.g., hearing “You’re ugly”) or the self (e.g., thinking “I’m ugly”). Our aim is to go beyond describing the regions implicated in GSP and rather to probe the nature of information-processing perturbations that occur within these regions. Without this information, it will be difficult to optimize treatments for this disorder, particularly when they are targeted towards specific cognitive processes. As such, we used a novel verbal-comment paradigm to implement a 2(Viewpoint: 1st or 2nd Person) by 3(Valence: negative, neutral and positive) by 2(Group: GSP, healthy comparison) design.
On the basis of our earlier data, which found these stimuli to elicit GSP-related perturbations in dorsal and ventral MPFC (Blair, Geraci et al. 2008; Blair, Geraci et al. in press), we hypothesize that GSP involves hyper-responsiveness to social feedback from others. Thus, we expect patients with GSP to show (to 2nd person point-of-view comments), increased responses within MPFC (i.e., there will be a group-by-viewpoint interaction). This might be particularly marked for others’ criticism (i.e., there will be a group-by-viewpoint-by-valence interaction). Alternatively, given that GSP is associated with an increased level of self-criticism (Cox, Walker et al. 2002; Cox, Fleet et al. 2004) it could be hypothesized that it involves hyper-responsiveness to self-referential comments whether these are generated internally, or externally (i.e., there will be a main effect of group within MPFC).
2. MATERIALS AND METHODS
2.1. Subjects
This study included 15 patients with GSP and 15 healthy comparison (HC) individuals, group-matched on age, gender, and IQ (see Table 1). Subjects were recruited from NIMH Institutional-Review-Board (IRB) approved advertisements. Subjects with GSP met criteria for GSP according to the DSM-IV (1994) criteria based on the Structural Clinical interview for DSM-IV Axis I disorders (SCID) (First, Spitzer et al. 1997) and a confirmatory clinical interview by a board-certified psychiatrist (DSP). No GSP patient had another Axis-1 diagnosis; all were currently medication-free >6 months. Specifically, 14 of the 15 patients reported that they were medication naïve, and two reported past medication use (one patient reported taking Xanax for 6 months 22 years ago and Zoloft for 2 months 9 years ago). No patient reported receiving past CBT. HCs were excluded if they had a history of any psychiatric illness. All subjects were in good physical health, as confirmed by a complete physical exam, and provided written informed consent. Further, as part of the assessment, all subjects completed the Liebowitz Social Anxiety Scale - Self Report (LSAS-SR), and the Inventory of Depressive Symptomatology – Self Report (IDS-SR). In addition, for the patients with GSP, the level of overall social, occupational and psychological functioning was assessed by the Global Assessment of Functioning (GAF). Scores on these measures characterized the GSP group as having moderate levels of social anxiety with some associated impairment in functioning; Table 1.
Table 1.
Subject Characteristics: S.D. in Brackets ().
| Patients with GSP (N = 15) | Healthy subjects (N = 15) | P | |
|---|---|---|---|
| Age | 30.3 (8.49) | 31.1 (6.37) | n.s. |
| Gender | 8 M/7 F | 9 M/6 F | n.s. |
| Race | |||
| Caucasian | 9 | 9 | - |
| African-American | 5 | 5 | |
| Asian | 1 | 1 | |
| IQ | 115.4 (12.54) | 111.9 (8.9) | n.s. |
| LSAS | 67.7 (21.82; range 40–120) | 17.5 (11.81; range 0–23) | p<0.001 |
| IDS | 5.1 (4.78) | 9.2 (6.30) | ns |
| GAF | 61.3 (4.50) | - | |
Key to Table 1: M = Male; F = Female; LSAS = Liebowitz Social Anxiety Scale; IDS = Inventory of Depressive Symptomatology; GAF = Global Assessment of Functioning
2.2. Task
Subjects viewed comments that varied according to whether they were 1st person viewpoints (e.g., “I’m beautiful), or 2nd person viewpoints (e.g., “You’re beautiful”). Thirty-two negative (e.g., idiot, ugly, hated), thirty-two neutral (e.g., human, average, OK), and thirty-two positive comments (e.g., genius, beautiful, loved), matched on number of letters and words were used. Thus, the task involved a 3(Valence: Negative, Neutral, Positive) by 2(Viewpoint: 1st Person, 2nd Person) design. In addition, the comments could be negative (e.g., I’m an idiot; You’re an idiot), neutral (e.g., I’m a human; You’re a human), or positive (I’m a genius; You’re a genius). The 3rd person point of view was not included as our earlier study had shown that patients with GSP do not show increased responses to self-referential comments originating from a 3rd person perspective. The valence words used (available per request from the corresponding author) were identical to those used in the Blair et al. (2008) study. Prior to scanning, subjects were told that they would view different comments that were always about them, but that they would appear either as 1st or 2nd person viewpoints. For the 2nd person comments they were told to imagine that they were coming from somebody whose opinion they really care about. Following our earlier design, for each comment and regardless of speaker, subjects simply pressed a button with their left hand when they had read each comment. Each comment was presented for 2500ms with a 500ms inter-stimulus interval and was presented in a fully randomized order within each run. In addition, for each run, 44 trial-length fixation points were presented between the stimuli (four at the beginning and end, and thirty-six randomized throughout the run), providing an implicit baseline against which the other events could be contrasted.
There were two runs. In addition to 44 null events, each run included sixteen negative 1st person, sixteen negative 2nd person, sixteen neutral 1st person, sixteen neutral 2nd person, sixteen positive 1st person, and sixteen positive 2nd person comments, resulting in a total of 96 comments per run. In short, subjects received 32 comments of each of the 6 conditions.
Following EPI acquisition, subjects rated each individual comment on a 7-point Likert scale, according to how the comments made them feel where 1=Extremely unhappy, 4=Neither unhappy nor happy, and 7=Extremely happy.
2.3. fMRI Parameters
Whole-brain blood oxygen level dependent (BOLD) fMRI data were acquired using a 1.5 Tesla GE MRI scanner. Following sagital localization, functional T2* weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence (matrix=64×64mm, repetition time (TR)=3000ms, echo time (TE)=30ms, field-of-view (FOV)=240mm (3.75×3.75×4mm voxels). Images were acquired in 31 contiguous 4mm axial slices per brain volume, with each run lasting 7 minutes. In the same session, a high-resolution T1-weighed anatomical image was acquired to aid with spatial normalization (three-dimensional Spoiled GRASS; TR=8.1ms; TE=3.2ms, flip angle=20°; FOV=240 mm, 124 axial slices, thickness=1.0mm; 256×256 acquisition matrix).
Data were analyzed as an event related design within the framework of the general linear model using Analysis of Functional Neuroimages (AFNI) (Cox 1996). Both individual and group-level analyses were conducted. The first four volumes in each scan series, collected before equilibrium magnetization was reached, were discarded. Motion correction was performed by registering all volumes in the EPI dataset to a volume collected close to acquisition of the high resolution anatomical dataset.
The EPI datasets for each subject were spatially smoothed (isotropic 6mm kernel) to reduce variability among individuals and generate group maps. Next, the time series data were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run and multiplying the result by 100, producing regression coefficients representing percent-signal change. Regressors for six comment categories (1st Person-Negative, 1st Person-Neutral, 1st Person-Positive, 2nd Person-Negative, 2nd Person-Neutral, 2nd Person-Positive) were created by convolving the train of stimulus events with a gamma-variate haemodynamic response function. Linear regression modeling was performed using these regressors plus regressors for a first-order baseline drift function. This produced for each voxel and each regressor, a beta coefficient and its associated t-statistic.
Voxel-wise group analyses involved transforming single subject beta coefficients into the standard coordinate space of Talairach and Tournoux(Talairach and Tournoux 1988). Subsequently, a 2(Group: GSP, HC) by 2(Viewpoint: 1st Person, 2nd Person) by 3(Valence: Negative, Neutral, Positive) ANOVA was performed. To correct for multiple comparisons for the whole-brain analysis, we adjusted initial results first emerging at uncorrected whole-brain threshold of p<0.005. To do so, we performed a spatial clustering operation using AlphaSim to generate a whole-brain corrected threshold corresponding to a corrected p-value of .05 (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). This procedure relied on 1,000 Monte Carlo simulations taking into account the entire EPI matrix with a map-wise false-positive probability of p<0.05 corrected for multiple comparisons. Subsequently, to facilitate interpretation of results from our main ANOVA, the average percent signal change was measured within each of the functional regions of interest (ROI) that were identified by this ANOVA. This generated mean values for each event type in each subject, which was extracted and submitted to post-hoc group-level statistics performed within SPSS.
3. RESULTS
3.1. Behavioral Data
Ratings data collected after scanning were analyzed by a 2(Group: GSP, HC) by 2(Viewpoint: 1st Person, 2nd Person) by 3(Valence: Negative, Neutral, Positive) ANOVA. There were significant main effects of viewpoint and valence. 2nd person viewpoints were rated significantly more positively than 1st person viewpoints (F(1,28)=7.26; p<0.05) and positive comments significantly more positively and negative comments significantly more negatively, than neutral comments (F(1,29)=390.29 & 379.34 respectively; p<0.001). There was also a significant viewpoint-by-valence interaction (F(2,56)=7.26; p<0.001). Positive 2nd person viewpoints were rated significantly more positively than 1st person viewpoints (F(1,28)=17.46; p<0.001). However, ratings of negative and neutral comments did not significantly differ whether they were to 2nd or 1st person viewpoints (F(1,29)=1.15 & 0.97 respectively; ns). There was no significant main effect of group (F(1,28)=2.54; ns) or group-by-viewpoint interaction (F(1,28)<1; ns). There were also no significant group-by-valence or group-by-viewpoint-by-valence interactions; Table 3.
Table 3.
Ratings and RTs for the six comment categories. S.D. in Brackets ().
| GSP | HC | |||
|---|---|---|---|---|
| Viewpoint | Ratings | RTs | Ratings | RTs |
| 2nd Person Negative | 2.26 (0.51) | 1245.42 (251.55) | 2.23 (0.48) | 992.12 (338.00) |
| 2nd Person Neutral | 4.24 (0.27) | 1257.44 (266.37) | 4.44 (0.35) | 956.07 (342.08) |
| 2nd Person Positive | 5.59 (0.31) | 1225.73 (287.00) | 5.82 (0.59) | 964.29 (360.00) |
| 1st Person Negative | 2.28 (0.43) | 1235.16 (267.00) | 2.28 (0.53) | 940.40 (325.88) |
| 1st Person Neutral | 4.28 (0.29) | 1232.56 (245.94) | 4.45 (0.33) | 954.10 (344.33) |
| 1st Person Positive | 5.44 (0.36) | 1204.43 (322.01) | 5.63 (0.57) | 909.01 (323.98) |
Reaction time (RT) data collection during scanning was similarly analyzed using a 2(Group: GSP, HC) by 2(Viewpoint: 1st Person, 2nd Person) by 3(Valence: Negative, Neutral, Positive) ANOVA. There were significant main effects of viewpoint and group. RTs to 2nd person viewpoints were significantly greater than RTs to 1st person viewpoints (F(1,28)=4.67; p<0.05), and RTs by patients with GSP were significantly greater than RTs by the healthy comparison individuals (F(1,28)=6.27; p<0.005). There was no significant group-by-viewpoint interaction (F(1,28)<0.47; ns), and there were no significant emotion main effects, group-by-valence, or group-by-viewpoint-by-valence interactions; Table 3.
We next examined whether there was a relationship between the behavioral data, both in terms of ratings and RTs, and the severity of social anxiety in the patients with GSP as indexed by the LSAS. There were no significant correlation between LSAS scores and ratings for any of the six conditions (Pearson’s r(13) = range ± 0.004 to 0.256; ns). However, in terms of the RT data, there was a significant negative correlation between LSAS scores and the 2nd person negative viewpoints specifically (Pearson’s r(13) = 0.532; p<0.05).
3.2. EPI Data
BOLD response data were analyzed by a 2(Group: GSP, HC) by 2(Viewpoint: 1st Person, 2nd Person) by 3(Valence: Negative, Neutral, Positive) ANOVA. Two critical statistical maps generated tests of our two sets of hypotheses: the map for group-by-viewpoint interactions, assessing whether patients with GSP show a differential response to 2nd person viewpoints, and the map for the main effect of group, assessing whether patients with GSP show an increased response to self-referential comments, irrespective of viewpoint, relative to healthy comparison individuals. We first focus on these to critical maps. However, we also do consider the statistical map for the group-by-valence interaction given our previous findings that patients with GSP showed elevated responses for self-referential criticism but not praise or neutral comments. No regions survived correction for multiple comparisons from the group-by-viewpoint-by-valence interaction statistical map. Finally, data on the main effects of viewpoint and valence are presented in the Supplemental Data Section. In addition, the data pertaining to the results from a 2(Viewpoint: 1st Person, 2nd Person) by 3(Valence: Negative, Neutral, Positive) ANOVA involving the healthy comparison individuals only is presented in Supplemental Data.
The group-by-viewpoint interaction identified a ventral region of MPFC (BA 10); see Table 2 and Figure 1. While the HCs showed significantly greater BOLD responses within this region to 1st person, relative to 2nd person viewpoints (F(1,14)=9.87; p<0.05; η2p=0.416), the patients with GSP showed the opposite pattern, significantly greater BOLD responses to 2nd, relative to 1st person viewpoints (F(1,14)=7.07; p<0.05; η2p=0.336). Moreover, patients with GSP showed greater BOLD responses to 2nd person viewpoints relative to the HCs (F(1,29)=3.53; p<0.07; η2p=0.112); see Figure 1a.
Table 2.
Significant areas of activation for the group-by-viewpoint interaction, group main effect, and group-by-valence interaction †
| REGION | BA | Mm3 | X | Y | Z | F-value |
|---|---|---|---|---|---|---|
| Group-by-viewpoint interaction | ||||||
| R ventral MPFC | 10 | 1291 | 1 | 48 | 10 | 18.81 |
| L middle occipital gyrus | 18 | 1279 | -29 | -86 | 6 | 29.87 |
| R superior temporal gyrus | 39 | 866 | 42 | -55 | 27 | 15.91 |
| R precuneus | 7 | 2349 | 2 | -65 | 37 | 16.29 |
| Group main effect (GSP > HC for all) | ||||||
| L dorsal MPFC | 9 | 2730 | -9 | 54 | 24 | 33.38 |
| R lateral middle frontal cortex** | 9/10 | 1524 | 29 | 46 | 25 | 16.79 |
| L lateral middle frontal cortex** | 9 | 6938 | -41 | 37 | 31 | 25.28 |
| R medial frontal gyrus | 6 | 2147 | 9 | -5 | 54 | 17.09 |
| R amygdala* | 85 | 29 | -7 | -15 | 4.76 | |
| R cingulate gyrus | 24/32 | 1293 | 18 | 5 | 42 | 17.59 |
| R postcentral gyrus | 3/4 | 27745 | 34 | -28 | 46 | 56.92 |
| R middle temporal gyrus | 22 | 1530 | 46 | -38 | -2 | 17.69 |
| L middle occipital gyrus | 2749 | -32 | -66 | 17 | 25.19 | |
| L inferior parietal lobule | 40 | 2749 | -43 | -24 | 29 | 24.19 |
| Group-by-valence interaction | ||||||
| L lateral middle frontal cortex** | 9 | 1980 | -43 | 23 | 35 | 14.56 |
| L MPFC | 10 | 1606 | -10 | 35 | -9 | 11.25 |
All activations are effects observed in whole brain analyses significant at p < 0.005 corrected for multiple comparisons (significant at p < 0.05) except
significant at p < 0.05. x,y,z coordinates and F-values are reported for the voxel with peak affect size.
Extending into MPFC.
Figure 1. Interactions of group-by-viewpoint.
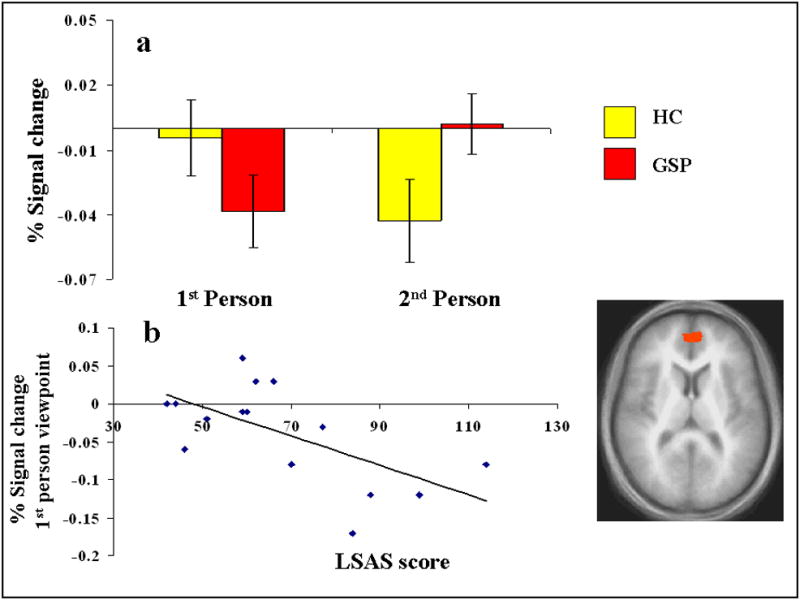
right MPFC (1, 48, 10): (a) BOLD responses to 1st and 2nd person viewpoints, and (b) severity of symptomatology and BOLD response to 1st person viewpoints in the patients with GSP.
The group main effect identified one region with peak activation in dorsal MPFC (this region did not overlap with the more ventral MPFC region identified by the group-by-viewpoint interaction) and two more laterally distributed regions, both with peak activation within dorsal regions of lateral middle frontal cortex extending into MPFC (BA 9/10); see Table 2 and Figure 2. In all three regions, the patients with GSP showed a significantly greater BOLD response relative to the HCs (F(1,28)=15.71–31.17; p<0.001; η2p range=0.359 to 0.527). The group-by-valence interaction identified one region with peak activation in MPFC and one region with peak activation within lateral middle frontal cortex extending into MPFC; Table 2. In both regions, the patients with GSP showed a significantly greater BOLD response compared to the HCs for positive (F(1,29)=29.55 & 14.18 respectively; p<0.005; η2p =0.513 & 0.336) and negative (F=(1,29)=11.97 & 17.73 respectively; p<0.005; η2p =0.299 to 0.388) relative to neutral comments.
Figure 2. Main effects of group.
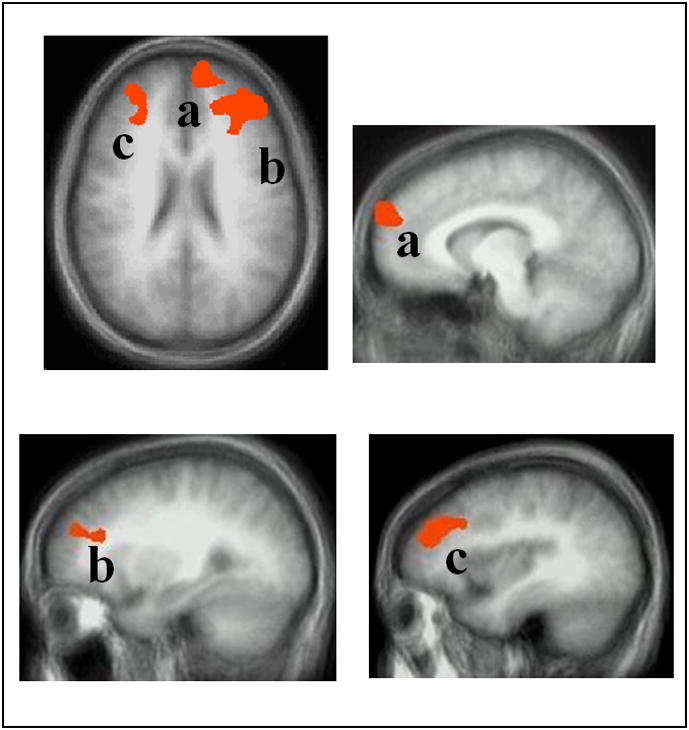
regions of MPFC and lateral middle frontal cortex in axial and sagittal view: (a) left MPFC (-9, 54, 24), (b) right MPFC/ lateral middle frontal cortex (29, 46, 25), and (c) left MPFC/ lateral middle frontal cortex (-41, 37, 31). In all regions, the patients with GSP showed increased BOLD responses compared to the healthy comparison individuals.
3.3. EPI Correlational Analysis
Using correlational analysis involving the GSP group only, we examined whether the increased responses to 2nd person viewpoints in GSP related to severity of symptomatology. Specifically, we tested whether there was a significant relationship between severity of social anxiety on the LSAS-SR and BOLD responses to 2nd person viewpoints, relative to 1st person viewpoints (i.e., 2nd > 1st), within the MPFC activation identified by the group-by-viewpoint interaction. In addition we tested whether there was a significant relationship between BOLD responses to 2nd and 1st person viewpoints separately within the MPFC activations identified by the group main effect and LSAS scores.
The analysis revealed a significant negative correlation within the MPFC activation identified by the group-by-viewpoint interaction (Pearson’s r(13) =-.532; p<0.05). Of note, this relationship appeared to be driven by the activation to “I” comments with follow-up analysis showing a significant negative correlation between LSAS scores and activation to “I” comments (Pearson’s r(13) =-.645; p<0.01; see Figure 1b.), but no significant correlation with “You” comments (Pearson’s r(13) =-.199; p=0.477). Although there was a trend towards significant positive correlations with LSAS scores and activation within the lateral middle frontal regions (extending into MPFC) identified by the group main effect (Pearson’s r(13) =.058-.504; ns), none of the correlations reached significance. All correlations were positive.
In accordance with our a priori hypotheses regarding MPFC pathologies in GSP, we focused on correlations involving MPFC. However, we also conducted explorative analyses involving the other regions identified by our analysis (Table 2) to inform about potential significant relationships between symptomatology level and activations for future research. For all regions, there was a negative correlation between symptomatology level and BOLD response to 1st relative to 2nd person viewpoints, however, none reached significance (Pearson’s r(13) =-.041-.471; ns). Finally, we also conducted analyses involving the two MPFC regions identified by the group-by-valence interactions. None of the correlations involving BOLD responses to positive relative to neutral statements were significant (Pearson’s r(13)=±.027 to .108; ns); however, there was a significant positive correlation for BOLD responses to negative relative to neutral statements and LSAS scores within the MPFC BA 10 region (Pearson’s r(13)=.569; p<0.05).
4.1. COMMENT
In this paper we examined the neural response to self-referential 1st and 2nd person viewpoints in GSP. We asked whether patients with GSP show increased BOLD responses to comments about themselves depended upon whether they concern another person’s point of view or the self view. Our results indicated a complex pathophysiology in patients with GSP with more ventral regions of MPFC showing heightened responsiveness to others’ viewpoint of the self relative to HCs. In contrast, more dorsal and lateral regions of middle frontal cortex showed a generally heightened responsiveness to self-referential comments irrespective of viewpoint. Proximal to both of these regions, patients with GSP showed greater responses to self-referential criticism and praise relative to neutral viewpoints compared to the HCs. Symptom levels, as indicated by the LSAS, showed an inverse relationship with activity to 1st person viewpoints within the region of ventral MPFC identified by the group-by-viewpoint interaction.
In our earlier study, patients with GSP showed a significantly increased response within MPFC relative to HCs to self-referential criticism but not (3rd person) criticism directed at others. In the current study, our primary goal was to extend those findings by more precisely determining the dysfunctional properties in GSP within MPFC to different origins of self-referential comments; specifically whether they originated from another individual or the self. In our earlier study, the patients showed heightened responsiveness in a relatively dorsal and lateral region of middle frontal cortex (with peak activations at x,y,z = 18,49,33 & 16,35,48, although extending across the midline and down to 6mm above the AC line) to self-referential criticism but not criticism directed at others. In our current study, our group main effect identified regions proximal to those identified previously x,y,z = -9,54,24 & 29,46,25 (extending down to 18mm above the AC line), with the patients with GSP showing increased responses relative to the HCs. In short, these data suggest that these dorsal and lateral regions of MPFC show elevated responsiveness in patients with GSP to self-referential comments irrespective of whether they reflect 1st or 2nd person viewpoints.
Notably, there was a significant group-by-valence interaction proximal to the dorsal MPFC regions identified by the group main effect. In contrast to our earlier study where the patients with GSP showed heightened activity within MPFC to only self-referential criticism, here they showed heightened activity to both self-referential criticism and self-referential praise (there was no significant group-by-valence-by valence interaction within this region). It is possible that this broadening MPFC response reflect changes in attentional task parameters, with the increased number of self-referential negative comments (33%: negative “I” and “You”) generating less of a “pop-out” effect than in our previous study where only one in six comments (16%: negative “You”) were self-referential negative. It should also be noted that work with HCs has documented that both criticism and praise can elicit embarrassment (Oatley, Keltner et al. 2006). As such, and given the propensity for embarrassment, and the tendency to negatively interpret and worry about positive social events (Alden and Wallace 1995; Wallace and Alden 1997; Alden, Taylor et al. 2008), we might expect GSP to be associated with increased responses across social valence under certain experimental conditions.
Within the social neuroscience literature, a distinction has been made between ventral and dorsal regions of MPFC with ventral MPFC particularly associated with (degree of) self-related processing (e.g., Seger, Stone et al. 2004; Mitchell, Banaji et al. 2005; Mitchell, Macrae et al. 2006; Moran, Macrae et al. 2006), particularly in emotional related aspects of self-related processing (see meta-analytic review by der Meer et al, 2010) and dorsal MPFC more associated with mentalizing (the representation of other individuals’ beliefs and intentions) (Fletcher, Happe et al. 1995; Brunet, Sarfati et al. 2000; Gallagher, Happe et al. 2000; Mitchell, Macrae et al. 2006). Further, a recent study indicated that dorsal MPFC might be particularly associated with self-related processing that involves personal semantic facts, suggesting an autobiographical component to the dorsal/ventral subdivide (Moran, Heatherton et al. 2009). Thus, this work provides a framework in which we can consider the current findings. It suggests that the increased activation in dorsal MPFC across viewpoints in GSP may reflect an increased propensity to evaluate self-referential comments (cf. Northoff 2006 review on self-referential/evaluative processing), irrespective of whether these comments are generated by others or the self, with respect to autobiographical information. Such a suggestion, while speculative, is in line with suggestions that individuals with GSP show an increased tendency to engage in detailed reviews of events following a social interaction (Clark and Wells 1995) and that social anxiety is generated and maintained by retrospective rumination (Rapee and Heimberg 1997). However, further work is clearly needed to explore the potential relationship between self-referential processing and retrospective rumination.
In addition to the dorsal and lateral region of middle frontal cortex, we observed a group-by-viewpoint interaction within a more ventral region of MPFC (x,y,z = 1,48,10). We also observed a group-by-valence interaction within ventral MPFC (-10, 35, -9). Importantly, a region of ventral MPFC proximal to this has been associated with (degree of) self-related processing in healthy individuals (Phan, Taylor et al. 2004; Seger, Stone et al. 2004; Mitchell, Banaji et al. 2005; Mitchell, Macrae et al. 2006; Moran, Macrae et al. 2006). Moreover, activation within this region is particularly noted for for emotional aspects of self-referential processing (see van der Meer, Costafreda et al. 2010). In line with the importance of this region for emotional aspects of self-referential processing, the patients with GSP showed significantly increased activity relative to HCs, for both positive and negative comments. With respect to self-referential processing, the current study HCs showed an increased activation to 1st (“I”) relative to 2nd (“You”) viewpoints within this region. Previously, an EEG study by Esslen also reported increased activity to presentations of ”I” relative to “He/She” trait adjectives in this region in healthy individuals (Esslen, Metzler et al. 2008). This is consistent with repeated findings that ventral MPFC shows greater activation to self- as opposed to other-referential reasoning (see meta-analytic review by der Meers et al 2010; though for a notable exception to this conclusion, see Ochsner et al. 2005). Critically, in contrast to HCs, patients with GSP did not show increased activation to 1st (“I”) relative to 2nd (“You”) viewpoints within this region. Instead, they showed significantly greater activation (or less deactivation) to “You” relative to “I” comments. Moreover, their activation to “I” comments correlated negatively with their level of social symptomatology. We believe that these data suggest a profound reorganization of self-referential reasoning in GSP. While a detailed computational account of self-referential reasoning remains to be provided, it appears to involve matching information to the individual’s “self-concept” (Northoff, Heinzel et al. 2006). While for healthy individuals this appears to be particularly related to self-generated viewpoints (“am I really like this?”), in GSP this may be particularly related to others’ viewpoints (“am I really like what this other person considers me to be?”). Moreover, the finding of an inverse relationship between symptom levels and activity to 1st person viewpoints within ventral MPFC allows the speculative suggestion that not only do patients with GSP reflect more on the self through the eyes of others but also that they reflect less on the self through self-evaluation. In this regard, it is notable that work has shown a relationship between self-clarity and social anxiety (Stopa 2009), and that individuals high on social anxiety not only show reduced confidence in their judgments of self-descriptive words but also take longer making those judgments (Wilson and Rapee 2006; Stopa 2009). Indeed, in the current study we found that the patients with GSP took longer responding to the statements relative to the healthy comparison individuals, consistent with the other behavioral data suggesting increased difficulty with self-evaluation. Of course these suggestions are speculative. However, they make precise predictions with respect to future work.
It is worth briefly noting that in our earlier study (Blair, Geraci et al. 2008), the patients showed not only showed heightened MPFC but also amygdala activity to self-referential criticism but not criticism directed at others. As noted above, the current study revealed that proximal regions of MPFC to those found in our earlier study showed a main effect of group. We suggested that this reflected elevated responsiveness in patients with GSP within these dorsal and lateral regions of MPFC to self-referential comments irrespective of whether they reflect 1st or 2nd person viewpoints. Notably, the amygdala also showed a main effect of group in the current study. These data would be consistent with patients with GSP showing greater emotional responsiveness to self-referential comments irrespective of whether they reflect 1st or 2nd person viewpoints. Overall, the amygdala results from the two studies are also consistent with the data from a recent study by Northoff et al. which showed that the amygdala response to IAPS pictures increases as a function of self-relatedness (Northoff, Schneider et al. 2009). I.e., within this framework we would expect to see the similar within-group amygdala activation to the viewpoints in the current study because all viewpoints were about the self, but not in the past study where the viewpoints could either be about the self or somebody else.
It is also worth briefly considering the current results in the context of recent studies investigating self referential processing in patients with major depressive disorder (MDD); (Grimm, Ernst et al. 2009; Lemogne, le Bastard et al. 2009; Yoshimura, Okamoto et al. 2010). In these studies, patients and healthy comparison individuals judged whether negative and positive words/ images were self-related. All three of these studies reported aberrant activity within more dorsal regions of frontal cortex that were relatively proximal to those regions implicated in the group main effect in the current study. In two cases, activity was increased with respect to self-referential processing in patients with MDD (Lemogne, le Bastard et al. 2009; Yoshimura, Okamoto et al. 2010) and in the third it was decreased (Grimm, Ernst et al. 2009). This may indicate that the aberrant responsiveness within the more dorsal regions of frontal cortex seen in the main group effect in the current study is less specific to GSP (though it is critical to remember that there are substantial processing differences between responding to comments made about you in the 1st/2nd person as opposed to judging whether a word/ image relates to you). In contrast, aberrantly increased responsiveness to self referential statements within ventral MPFC was only seen in one of these studies (Yoshimura, Okamoto et al. 2010). This suggests that the pathology within ventral MPFC may be far more specific to GSP. This is particularly notable when it is considered that the patients with MDD were showing an exaggeration of the appropriate recruitment of this region when judging the self relatedness of negative words (Yoshimura, Ueda et al. 2009). In contrast, in the current study, the patients with GSP were showing increased responsiveness within this region to 2nd person comments relative to 1st person comments; i.e., a pattern of response that was notably divergent with that seen in the healthy participants (who showed greater responsiveness to 1st person relative to 2nd person comments; see Supplemental Data).
Several caveats should be considered with respect to this study. First, the sample size, while comparable with the previous literature, is relatively small. However, it should be noted in this respect that group differences were highly significant. In addition, and as noted by one of our reviewers, our sample is strengthened by the fact that all of our patients were medication free, rendering our results free from the “noise” associated with medication use in patient groups. Second, there may have been group differences in agreement with the comments. For example, subjects might differentially agree with the comments “I’m ugly” as opposed to “I’m beautiful”. However, it is important to remember that: (i) the groups did not differ in their ratings of the happiness induced by the comments; (ii) there was a significant main effect of group and significant group-by-viewpoint and group-by-valence interactions. Similarly, the current study did not investigate group differences in participant’s judgments regarding the relatedness of the comments to the self. If the hypothesis developed above is correct, healthy participants should relate comments more to the self when these comments reflect 1st person viewpoints while patients with GSP should relate comments to the self more when these comments reflect 2nd person viewpoints. Data on judgments of self relatedness would allow this issue to be investigated. We are currently collecting such data in our on-going work. Third, it is possible that the two groups differed in MPFC response during fixation point (baseline) trials and that the patients with GSP were engaging in higher levels of self-referential processing even during baseline. However, while this possibility warrants future examination, it remains clear that the patients with GSP were showing anomalous MPFC and middle frontal cortex activity to self-referential task stimuli.
In summary, in this study we found further support of a pivotal role of MPFC and disrupted self-referential processing in GSP. Specifically, our results suggest a more complex pathophysiology that involves functional (disorder-specific) specifications within MPFC, with increased autobiographical-related processing in dorsal regions of MPFC, and decreased self-concept related processing in ventral regions of MPFC. Therapeutically, our results suggest a continued targeting of the excessive preoccupation in GSP of other people’s judgments (Stopa 2009), but also encourages an increased attention to the patients’ own self-concept independent of others’ view.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health.
Footnotes
The authors have no conflicts of interest or financial disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alden LE, Taylor CT, et al. Social anxiety and the interpretation of positive social events. Journal of Anxiety Disorders. 2008;22(4):577–590. doi: 10.1016/j.janxdis.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Alden LE, Wallace ST. Social phobia and social appraisal in successful and unsuccessful social interactions. Behavior Research and Therapy. 1995;33(5):497–505. doi: 10.1016/0005-7967(94)00088-2. [DOI] [PubMed] [Google Scholar]
- Amir N, Klumpp H, et al. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57(9):975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64(8):903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, et al. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9(6):1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Blair KS, Geraci M, et al. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008;65(10):1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, et al. Social norm processing in adult Social Phobia: Atypically increased ventromedial frontal cortex responsiveness to unintentional (embarrassing) transgressions. The American Journal of Psychiatry. in press doi: 10.1176/appi.ajp.2010.09121797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, et al. The Neural Response to Emotional Expressions is Developmental Stable in Social Phobia. Archives of General Psychiatry under revision [Google Scholar]
- Blair KS, Shaywitz J, et al. Response to emotional expressions in Generalized Social Phobia (GSP) and Generalized Anxiety Disorder (GAD): Evidence for separate disorders. The American Jouranl of Psychiatry. 2008;165(9):1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet E, Sarfati Y, et al. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11(2):157–166. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social phobia: diagnosis, assessment, and treatment. New York, NY: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- Cox BJ, Fleet C, et al. Self-criticism and social phobia in the US national comorbidity survey. Journal of Affective Disorders. 2004;82(2):227–234. doi: 10.1016/j.jad.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Cox BJ, Walker JR, et al. Self-criticism in generalized social phobia and response to cognitive-behaviour treatment. Behavior Therapy. 2002;33:479–491. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Esslen M, Metzler S, et al. Pre-reflective and reflective self-reference: a spatiotemporal EEG analysis. Neuroimage. 2008;42(1):437–449. doi: 10.1016/j.neuroimage.2008.01.060. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, et al. Structured Clinical Interview for DSM-IV. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- First MB, Spitzer RL, et al. Structured Clinical Interview for DSM-IV ± Patient Edition (SCID-P) Washington, DC: American Psychiatric Press Inc; 1995. [Google Scholar]
- Fletcher PC, Happe F, et al. Other minds in the brain: a functional imaging study of ”theory of mind“ in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, et al. In search of the emotional self: an FMRI study using positive and negative emotional words. The American Journal of Psychiatry. 2003;160(11):1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJ, et al. Neuroimaging social emotional processing in women: fMRI study of script-driven imagery. Social, Cognitive & Affective Neuroscience. 2010 doi: 10.1093/scan/nsq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, et al. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychological Bulletin. 2005;131(1):76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10(1):83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, et al. Neural Mechanisms of Cognitive Reappraisal of Negative Self-Beliefs in Social Anxiety Disorder. Biologial Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Ernst J, et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Human Brain Mapping. 2009;30(8):2617–2627. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, et al. Neural correlates of self-reflection. Brain. 2002;125(Pt 8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depression and Anxiety. 2000;12(Suppl 1):69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The impairments caused by social phobia in the general population: implications for intervention. Acta Psychiatrica Scandinavica Supplement. 2003;(417):19–27. doi: 10.1034/j.1600-0447.108.s417.2.x. [DOI] [PubMed] [Google Scholar]
- Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychological Review. 2009;116(1):252–282. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, et al. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social, Cognitive & Affective Neuroscience. 2009;4(3):305–312. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Holroyd T, et al. Emotional automaticity is a matter of timing. Journal of Neuroscience. 2010;30(17):5825–5829. doi: 10.1523/JNEUROSCI.BC-5668-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Monk CS, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Nakic M, et al. The impact of processing load on emotion. Neuroimage. 2007;34(3):1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, et al. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17(8):1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, et al. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, et al. Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Social Neuroscience. 2009;4(3):197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, et al. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18(9):1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, et al. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Schneider F, et al. Differential parametric modulation of self-relatedness and emotions in different brain regions. Human Brain Mapping. 2009;30(2):369–382. doi: 10.1002/hbm.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley K, Keltner D, et al. Understanding emotions. Cambridge: Blackwell; 2006. [Google Scholar]
- Pessoa L, McKenna M, et al. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences U S A. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, et al. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28(1):249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Neuroimaging studies of attention and the processing of emotion-laden stimuli. Progress in Brain Research. 2004;144:171–182. doi: 10.1016/S0079-6123(03)14412-3. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, et al. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, et al. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Pine DS, Klein RG, et al. Face-emotion processing in offspring at risk for panic disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(7):664–672. doi: 10.1097/01.chi.0000162580.92029.f4. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behavior Research and Therapy. 1997;35(8):741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews. 2007;31(4):585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Stone M, et al. Cortical Activations during judgments about the self and an other person. Neuropsychologia. 2004;42(9):1168–1177. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, et al. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59(11):1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stopa L. Why is the Self Important in Understanding and Treating Social Phobia? Cognitive Behavior Therapy. 2009:1. doi: 10.1080/16506070902980737. [DOI] [PubMed] [Google Scholar]
- Straube T, I, Kolassa T, et al. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56(12):921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, et al. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52(3):163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- van der Meer L, Costafreda S, et al. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews. 2010;34(6):935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Wallace ST, Alden LE. Social phobia and positive social events: The price of success. Journal of Abnormal Psychology. 1997;106(3):416–424. doi: 10.1037//0021-843x.106.3.416. [DOI] [PubMed] [Google Scholar]
- Wilson JK, Rapee RM. Self-concept certainty in social phobia. Behavior Research and Therapy. 2006;44(1):113–136. doi: 10.1016/j.brat.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Okamoto Y, et al. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. Journal of Affect Disorders. 2010;122(1–2):76–85. doi: 10.1016/j.jad.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Ueda K, et al. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain and Cognition. 2009;69(1):218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


