Abstract
Human and animal studies indicate that drugs of abuse affect males and females differently, but the mechanism(s) underlying sex differences are unknown. The nucleus accumbens (NAc) is central in the neural circuitry of addiction and medium spiny neurons (MSNs) in the NAc show drug-induced changes in morphology and physiology including increased dendritic spine density. We previously showed in drug-naïve rats that MSN dendritic spine density is higher in females than males. In this study, we investigated sex differences in the effects of cocaine on locomotor activity as well as MSN dendritic spine density and excitatory synaptic physiology in rats treated for 5 weeks followed by 17-21 days of abstinence. Females showed a greater locomotor response to cocaine and more robust behavioral sensitization than males. Spine density was also higher in females and, particularly in the core of the NAc, the magnitude of the cocaine-induced increase in spine density was greater in females. Interestingly, in cocaine-treated females but not males, cocaine-induced behavioral activation during treatment was correlated with spine density measured after treatment. Miniature EPSC (mEPSC) frequency in core MSNs also was higher in females, and increased with cocaine in both the core and shell of females more than males. We found no differences in mEPSC amplitude or paired-pulse ratio of evoked EPSCs, suggesting that sex differences and cocaine effects on mEPSC frequency reflect differences in excitatory synapse number per neuron rather than presynaptic release probability. These studies are the first to demonstrate structural and electrophysiological differences between males and females that may drive sex differences in addictive behavior.
1. INTRODUCTION
Human and animal studies show that drugs of abuse affect males and females differently (reviewed by Becker and Hu, 2008). For example, studies with rats show that females acquire self-administration (Lynch and Carroll, 1999) and develop conditioned place preference (Russo et al., 2003) to cocaine in fewer sessions than males. Females also show a greater locomotor response and stronger sensitization to repeated cocaine exposure (Sircar and Kim, 1999; Festa and Quinones-Jenab, 2004). Circulating estradiol promotes locomotor activation in response to cocaine in females, though underlying sex differences persist after gonadectomy (Hu and Becker, 2003).
The nucleus accumbens (NAc) is integral to the development and expression of addiction-related behaviors (Wheeler and Carelli, 2009). The principal neurons of the NAc are GABAergic medium spiny neurons (MSNs), which receive excitatory input from the prefrontal cortex (PFC), hippocampus, amygdala, and thalamic nuclei (Groenewegen et al., 1999). Repeated exposure to drugs such as cocaine produces changes in MSN dendritic morphology (reviewed by Robinson and Kolb, 2004), synaptic and intrinsic physiology (reviewed by Kalivas and Volkow, 2005), and gene expression (reviewed by Dietz et al., 2009) that may be related to the process of addiction. For example, repeated cocaine treatment followed by abstinence increases MSN dendritic spine density (Robinson and Kolb, 1999; Li et al., 2004), surface expression of AMPA receptors (Boudreau and Wolf, 2005; Boudreau et al., 2007), and AMPA/NMDA ratios (Kourrich et al., 2007). Cocaine also alters intrinsic excitability of MSNs (Zhang et al., 1998; Dong et al., 2006; Kourrich and Thomas, 2009). Many of these changes persist after drug withdrawal suggesting long-lasting plasticity of excitatory synaptic connectivity. Such plasticity could be related to long-term changes in behavior, such as vulnerability to relapse, that are a hallmark of addiction (Kalivas, 2009).
Previously, we found that MSNs in females have higher dendritic spine density and more large spines than in males (Forlano and Woolley, 2010), suggesting sexual dimorphism in the neural circuitry of addiction that could be related to females’ greater responses to drugs of abuse. Here we used morphological analyses of dendritic spines and whole-cell patch-clamp electrophysiology in male and female rats to investigate 1: whether cocaine-induced changes in MSN dendritic spines are paralleled by changes in MSN sensitivity to excitatory synaptic input, and 2: whether cocaine-induced changes in MSN dendritic spines and synaptic physiology differ between males and females. Our findings show that morphological sex differences in the core of the NAc are paralleled by functional sex differences, and that sex differences persist in cocaine-treated animals with females showing larger effects of cocaine than males.
2. MATERIALS AND METHODS
2.1 Animal treatment and behavior monitoring
All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Northwestern University Institutional Animal Care and Use Committee. Male and female Sprague-Dawley rats (Harlan, Indianapolis, IN), 47-54 days old at the beginning of treatment, were used for all experiments. Animals were separated by sex, housed in groups of two to three per cage, and kept under a 12-hour light:dark cycle. At least one week after arrival, each rat was assigned to one of four treatment groups: saline males (n = 30), saline females (n = 30), cocaine males (n = 29), or cocaine females (n = 28). On each testing day, rats were placed individually in an open field activity chamber (16.5 in. × 16.5 in. × 20 in.) and allowed to acclimate for 30 min. while their locomotor activity was tracked and recorded with LimeLight software (Actimetrics, Wilmette, IL). After the acclimation period, each animal was injected (i.p.) with either saline (0.5 ml) or cocaine HCl dissolved in saline (15 mg/kg) and post-injection locomotor activity was recorded for an additional 1 hr. This was repeated 5 days a week for 5 weeks, followed by 17-21 days of abstinence. This treatment regimen and dose of cocaine was based on the original demonstrations that chronic administration of psychostimulants increases MSN dendritic spine density (Robinson and Kolb, 1997, 1999). All video files were coded and locomotor activity was assayed by total distance traveled during each monitoring period, as measured by the LimeLight program. In the subset of 5 animals in each group used for analysis of dendritic spines (see below), further behavioral analyses were performed on data from the first 30 min. after injection. The proportion of time spent in the center (inner ¼) vs. the periphery (outer ¾) of the open field was calculated by Limelight, and recorded videos were scored for rearing bouts (defined as the front of the body lifting up with both front paws off of the floor of the chamber). In a subset of females (12 saline, 12 cocaine), estrous cycles were monitored by vaginal lavage throughout the treatment and abstinence periods.
2.2 DiI-labeling and morphological analysis of dendritic spines
After 17 days of abstinence, 5 rats per group were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and perfused with 200 ml of cold, 2% paraformaldehyde in 0.1M phosphate buffer (PB), pH 7.2. Brains were harvested, coded, and coronal serial sections (250 μm) through the NAc were cut with a vibrating tissue slicer. Slices were stored at 4°C in 0.03% sodium azide in 0.1M PB until DiI labeling.
Coating of tungsten particles with DiI was performed as described previously (Forlano and Woolley, 2010). A Gene Gun powered by helium gas (90-110 psi) was used for ballistic delivery of coated particles onto tissue sections. Labeled tissue was stored in 0.1M PB at 4°C overnight in the dark to allow DiI diffusion throughout cellular membranes. Within 7 days of labeling, tissue was imaged with a spinning disc laser confocal system (PerkinElmer) on a Leica microscope equipped with a 100X oil immersion objective. The number of complete MSNs labeled per animal was not sufficient for a rigorous analysis of the entire dendritic tree. However, because we showed previously that MSN dendritic length and branching do not differ between males and females, whereas spine density and morphology do, we quantified dendritic spine density and morphology in the current study. Image stacks of 8-10 dendritic segments in both the core and shell of the NAc were collected using 0.2 μm z steps. We focused on thin dendrites (0.9-1.2 μm diameter), characteristic of those distal to the soma because this is the region of the MSN dendritic tree in which spine density is most sensitive to psychostimulants (Robinson and Kolb, 1999; Li et al., 2003). The location of each imaged segment was mapped onto diagrams of coronal brain sections from the Paxinos and Watson rat brain atlas (1998; Fig. S1). Confocal image stacks were examined using Volocity software (Improvision-PerkinElmer). Dendritic spines were counted by scrolling through the z plane along ≥20 μm length of each segment. For branched spines, each termination resulting in a spine head was counted as an individual spine. Spine number was divided by segment length measured in 3 dimensions and spine density was expressed as spines / 10 μm. The same dendritic segments along which spine density was measured were also evaluated for spines with large (0.7 - 0.9 μm) or giant (≥ 1.0 μm) heads (Forlano and Woolley, 2010) and branched spines. The diameter of a spine head was measured in the optical section in which it was greatest. From these data, we calculated the density and percentage of large or giant spines along each segment. We also calculated the density and percentage of branched spines along each segment, counting by the number of heads on branched spines (rather than the number of branch points). All imaging and spine analysis was performed by an experimenter blind to the animal’s sex and treatment. A post-hoc power analysis indicated that our sample size was adequate to detect a spine density difference between groups of the magnitude shown by Robinson and Kolb (1999).
2.3 Electrophysiology
After 17-21 days of abstinence, the remaining rats (25 saline males, 25 saline females, 24 cocaine males, and 23 cocaine females) were prepared for acute brain slice recording. Rats were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and perfused with ice-cold oxygenated sucrose artificial cerebrospinal fluid (s-ACSF) containing (in mM): 75 NaCl, 75 sucrose, 25 NaHCO3, 15 dextrose, 2 KCl, 1.25 NaH2PO4, 3 MgCl2, 0.5 CaCl2, 2.4 Na pyruvate, 1.3 ascorbic acid; osmolality 300 mmol/kg; pH 7.4. Unless otherwise indicated, all reagents were obtained from Sigma. Brains were removed and blocked either parasagittally or in an oblique coronal plane to preserve cortical inputs to the NAc core or shell respectively (Yang et al., 1996). Slices (300 μm) were collected with a vibratome and then incubated at 35°C in oxygenated s-ACSF for 30 min, during which s-ACSF was gradually replaced with regular ACSF containing (in mM): 126 NaCl, 26 NaHCO3, 10 dextrose, 3 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2; osmolality 300 mmol/kg; pH 7.6. After this, slices were kept at room temperature for up to 4 hr after the animal was killed. For recording, slices were transferred to a Zeiss Axioscope equipped with DIC optics, a video camera and a 40X water-immersion lens. Slices were superfused with warm (34°C) oxygenated ACSF containing the GABAA receptor antagonist SR 95531 (2 μM, Tocris). Whole-cell patch-clamp recordings were made using glass electrodes (4-5 MΩ) filled with a solution containing (in mM): 115 K gluconate, 20 KCl, 10 phosphocreatine, 10 HEPES, 2 EGTA, 2 MgATP, 0.3 NaGTP, with 0.3% biocytin; osmolality, 300 mmol/kg; pH, 7.3. Signals were amplified and filtered (2 kHz) with either an Axopatch 200B or Multiclamp 700B amplifier. First, membrane potential was recorded in current-clamp and −600 to +600 pA 100 ms current steps were applied to identify MSNs, recognized by their high resting membrane potential (−73 to −89 mV), inward rectification, slow ramping subthreshold depolarization in response to current injections, and prominent spike after-hyperpolarization (Fig 5C; O’Donnell and Grace, 1993; Belleau and Warren, 2000). Then, spontaneous and evoked EPSCs were recorded in voltage-clamp. A concentric bipolar electrode was placed just outside the NAc to elicit paired pulses at 50, 100, and 250 ms ISI with at least 6 sweeps each. The inter-sweep interval was 10 seconds, which did not produce any short-term facilitation between sweeps. Tetrodotoxin (TTX, 1 μM, Tocris) was then applied and cells were monitored until action potentials were completely eliminated, after which mEPSCS were recorded for at least 5 min. The holding potential in all experiments was −70 mV, making the recorded EPSCs likely to be the result of current flow through AMPA receptors. Dopamine levels in slices were not controlled. Electrophysiological data were analyzed using IgorPro 5.01 software running Neuromatic (www.neuromatic.thinkrandom.com). Miniature EPSCs were detected automatically by Neuromatic using a threshold detection algorithm based on Kudoh and Taguchi (2002). The detection threshold was set at 3X standard deviation of noise, with a 2 ms onset window from baseline and a 5 ms peak window. After the event detection algorithm was run, all traces were visually examined to confirm the detection of mEPSCs, including overlapping currents and rejection of current fluctuations that did not show a characteristic EPSC asymmetry between rise time and decay time. When overlapping currents were evident, the baseline of the second current was adjusted to its onset.
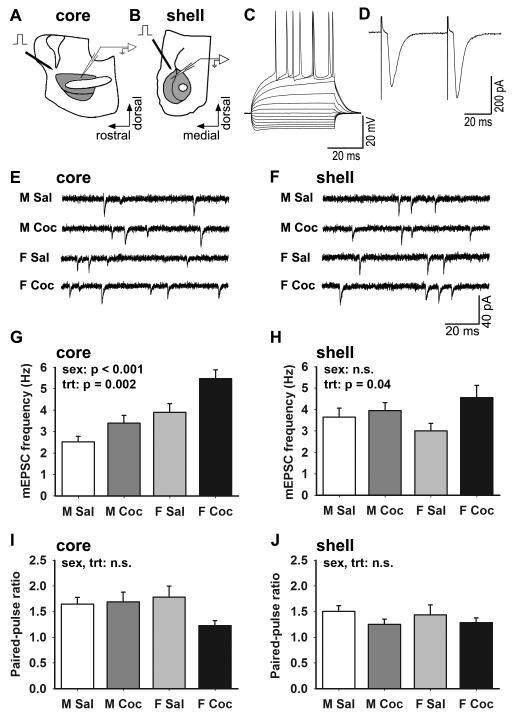
Figure 5.
Sex and cocaine effects on MSN physiology. (A, B) Schematics showing slice orientation and electrode placement for recording in the core (A) and shell (B). (C) Representative current-clamp recording of MSN responses to current injections. (D) Representative voltage-clamp recording of MSN EPSCs evoked at a 50 ms interstimulus interval (ISI). (E, F) Representative voltage-clamp recordings of mEPSCs recorded in TTX from MSNs in the core (E) and shell (F) for each group. (G, H) Mean ± SEM mEPSC frequency in core (G) and shell (H) MSNs. In the core, mEPSC frequency was sexually dimorphic and increased by cocaine. In the shell, mEPSC frequency was not sexually dimorphic, but was increased by cocaine with the effect driven by females. (I, J) Mean ± SEM paired-pulse ratio (PPR) for core (I) and shell (J) MSNs at a 50 ms ISI. There was no effect of sex or cocaine on PPR in either subregion. M Sal = saline-treated males (sample size for mEPSCs: n = 15 core, n = 17 shell; PPR: n = 8 core, n = 11 shell), M Coc = cocaine-treated males (mEPSCs: n = 16 core, n = 13 shell; PPR: n = 4 core, n = 7 shell), F Sal = saline-treated females (mEPSCs: n = 14 core, n = 21 shell; PPR: n = 5 core, n = 8 shell), F Coc = cocaine-treated females (mEPSCs: n = 18 core, n = 12 shell; PPR: n = 5 core, n = 9 shell).
After recording, sections were fixed in 4% paraformaldehyde in PB at 4°C for ≥24 hr. Biocytin was visualized using the ABC technique (Vector Elite kit, Vector Laboratories). Slices were rinsed in PB, permeabilized with 0.2% Triton-X, and blocked with 1% bovine serum albumin (BSA). The slices were then incubated overnight in a solution containing the conjugated avidin-biotin complex, 0.2% Triton-X, and 1% BSA in PB. Following several rinses, biocytin was visualized with diaminobenzidine enhanced with 0.01% nickel chloride, oxidized with 0.3% H2O2. Slices were then rinsed, mounted onto gelatin-coated slides, and coverslipped with Mowiol. The locations of filled cells were mapped onto diagrams of the NAc from the Paxinos and Watson rat brain atlas (1998; Fig S2).
2.4 Statistical analysis
Data are expressed as mean ± SEM. Statistical tests were performed in SPSS (IBM Corporation, Somers, NY) and SigmaPlot (Systat Software Corporation, San Jose, CA). For behavioral and morphological data, initial analyses were performed with 3-way ANOVAs with sex, treatment, and time (for behavior) or sex, treatment, and subregion (for morphology) as factors. When 3-way ANOVAS showed an interaction effect, analyses were broken down into multiple 2-way ANOVAs. Also, in cases where a significant interaction was detected, Tukey’s tests were used to perform post-hoc comparisons. For electrophysiological experiments, data from the core and shell were collected on separate days from separate animals, so analyses were performed by 2-way ANOVAs for each subregion. Pearson correlations were calculated for spine density and behavioral data within animals. In all analyses, significance was set at α = 0.05.
3. RESULTS
3.1 Chronic cocaine-induced locomotor activation is greater in females than males
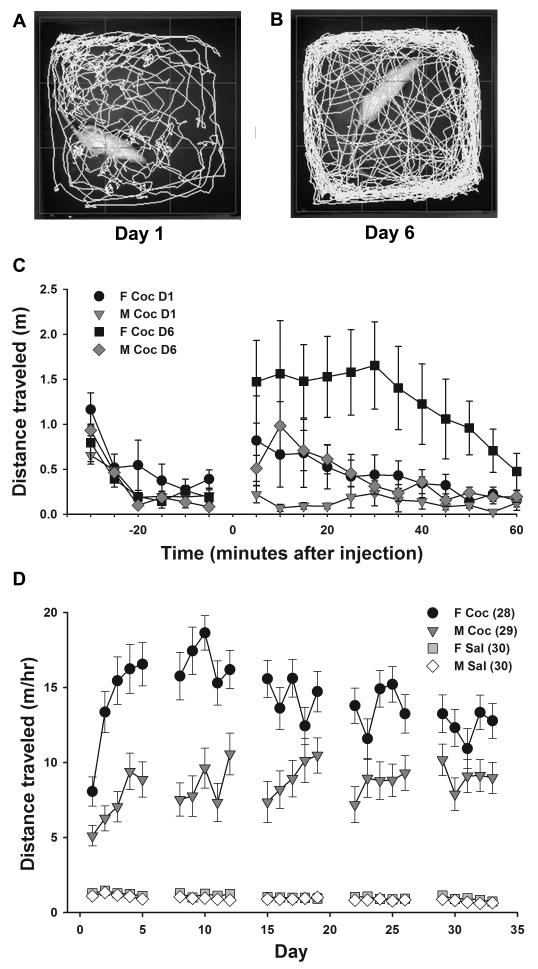
To investigate sex differences in the behavioral response to chronic cocaine exposure, we monitored locomotor behavior in male and female rats injected with cocaine (n = 29 males, n = 28 females) or saline vehicle (n = 30 males, n = 30 females) once daily on a 5-days-on, 2-days-off schedule, for 5 weeks (Robinson and Kolb, 1999). On each injection day, we measured the locomotor activity (Fig. 1A, B) of each rat during a 30 min. habituation period and for 1 hr after injection. Detailed analysis of locomotor activity on the first and sixth injection days for the 5 male and 5 female cocaine-treated animals in which dendritic spine density/morphology were analyzed showed that rats rapidly acclimated to the open field, and that cocaine-induced locomotor activity peaked shortly after injection, remained high for about 30 min., and then gradually subsided (Fig. 1C). Females showed more activity on both days, and activity was higher on the sixth injection day compared to the first in both males and females indicating sensitization in both sexes.
Figure 1.
Cocaine-induced locomotor activation and behavioral sensitization are greater in females than males. (A, B) 5-minute activity traces for one female rat beginning 15 min. after cocaine injection on the first (A) and sixth (B) injection day. (C) Mean ± SEM distance traveled in 5-min. bins for a subset (n = 5 per group) of cocaine-treated females and males on Day 1 and Day 6 of treatment, showing the 30-min. habituation period before injection and the 1 hr observation period after injection, illustrating the pattern of cocaine-induced locomotor behavior. (D) Mean ± SEM daily cumulative distance traveled during 1 hr after injection for all animals across all 5 weeks of treatment. Females showed more cocaine-induced locomotor activity throughout treatment, with greatest the sex difference during the first 2 weeks. M Sal = saline-treated males, M Coc = cocaine-treated males, F Sal = saline-treated females, F Coc = cocaine-treated females; numbers in parentheses indicate sample sizes in each group.
An initial 3-way repeated measures ANOVA of daily post-injection locomotor activity for all animals across all 5 weeks (Fig. 1D) showed effects of treatment (F1,108 = 829.2, p < 0.001), sex (F1,108= 71.15, p < 0.001), and time (F24,108 = 5.17, p < 0.001), as well as significant interactions among all three factors. Overall, females showed greater activity in response to cocaine than males throughout the treatment period. On day 1, cocaine-treated males showed 460% greater activity than control males (5.11 ± 0.67 m/hr compared to 1.12 ± 0.90 m/hr), whereas cocaine-treated females showed 634% greater activity than control females (8.06 ± 0.97 m/hr compared to 1.27 ± 0.96 m/hr). Both males and females sensitized to repeated cocaine treatments, but females sensitized more rapidly and to a greater extent. Females reached their peak activity, 1483% over controls, during week 2, whereas males did not peak until the end of week 3, at 1036% over controls (Fig. 1D). Stronger sensitization in females was evident in a within-groups analysis that showed a significant effect of injection day in cocaine-treated females (F1,25 = 92.7, p = 0.011), but not in males (F1,28 = 0.86, p = 0.6). Interestingly, cocaine-treated females’ activity declined slightly from week 3 to week 5. In a subset of females (12 saline, 12 cocaine) whose estrous cycles we monitored throughout the treatment period, this decline in cocaine-induced activity corresponded with an increase in cycle disruptions in cocaine-treated females, whereas saline-treated females showed no change in cyclicity or activity levels (data not shown); this is consistent with previous studies showing that repeated cocaine administration disrupts the estrous cycle (King et al., 1993). Despite this decline, however, cocaine-induced locomotor activity remained higher in females than in males throughout the 5-week treatment period. In 9/12 cocaine-treated females, estrous cylicity resumed during the post-treatment abstinence period.
Our behavioral data are consistent with previous studies showing a sex difference in behavioral responses to cocaine, and show that the difference in locomotor activity persists throughout a chronic 5-week treatment. After a 17-21 day abstinence period following saline or cocaine treatment, all animals were subsequently used for either morphological or electrophysiological studies.
3.2 Dendritic spine density in the NAc is sexually dimorphic and increases with chronic cocaine
The sex difference in the behavioral effects of cocaine could reflect differences in the neural circuitry involved in drug addiction. Chronic cocaine exposure has been shown to increase the density of dendritic spines on MSNs in the NAc (Robinson and Kolb, 1999; a study using females), and our previous studies in drug-naïve rats showed that MSN dendritic spine density is greater in females than in males (Forlano and Woolley, 2010). To test whether the effect of cocaine on spine density is sexually dimorphic, we used diolistics to label NAc-containing brain slices from a subset of our behaviorally characterized saline- and cocaine-treated male and female rats (n=5 each group) after 17 days of abstinence from treatment. We imaged 8-10 distal dendritic segments in the NAc core (Fig. 2A-D) and shell of each animal using confocal microscopy, and counted spines from 3-dimensional image stacks. An initial 3-way ANOVA was significant overall (F1,4 = 6.24, p < 0.001) and showed effects of treatment (F1,4 = 12.61, p = 0.001), sex (F1,4 = 4.82, p = 0.036), and subregion (F1,4 = 20.31, p < 0.001). Given the difference between subregions, we conducted subsequent analyses on the core and shell separately.
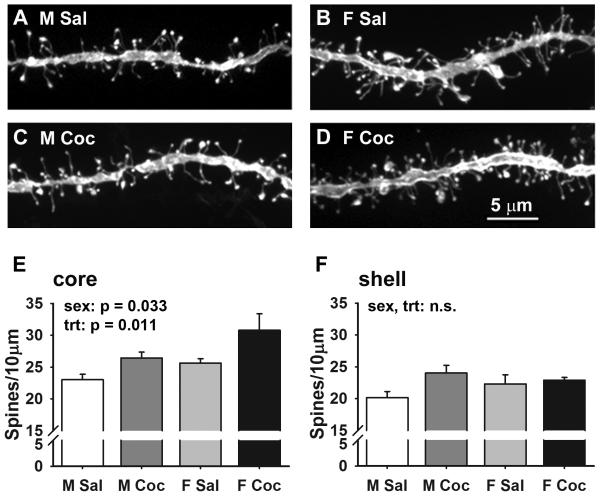
Figure 2.
Sex and cocaine effects on MSN dendritic spine density. (A-D) Projected confocal images of representative DiI-labeled dendritic segments from MSNs in the core of the NAc. (E, F) Mean ± SEM dendritic spine density in the core (E) and shell (F). Spine density is both sexually dimorphic and increased by cocaine in the core, but there were no significant sex or cocaine effects in the shell. M Sal = saline-treated males, M Coc = cocaine-treated males, F Sal = saline-treated females, F Coc = cocaine-treated females; n = 5 animals per group.
In the core, MSN spine density was both sexually dimorphic and increased by cocaine (Fig. 2E; 2-way ANOVA: sex F1,16 = 5.44, p = 0.033; treatment F1,16= 8.32. p = 0.011; no interaction). Core spine density was greater in females than males in both saline- (by 11%) and cocaine-treated (by 16%) animals. Cocaine increased spine density in both males and females, and while there was no statistical interaction between sex and cocaine treatment, the cocaine-induced increase was more robust in females (20% increase) compared to males (15% increase). In the shell, there were no overall effects of sex or cocaine on spine density, though there was a trend toward a cocaine-induced increase in spine density (F1,16 = 4.29, p = 0.055) driven by an effect apparent in males but not in females (Fig. 2F). Considering all groups combined, spine density was slightly lower overall in the shell, 22.3 ± 0.6 on average, than the core, 26.4 ± 0.9 on average, as we (Forlano and Woolley, 2010) and others (Meredith et al., 1992) have reported. Plotting the locations of imaged dendritic segments in core and shell confirmed a similar distribution of segments among treatment groups and revealed no systematic differences in spine density within the core or shell (Fig. S1).
These results demonstrate that the sex difference in dendritic spine density evident in the NAc core of drug-naïve animals persists after chronic cocaine treatment. Cocaine-treated females had the highest spine densities in the core, paralleling their high level of behavioral activation.
3.3 Dendritic spine morphology in the NAc is sexually dimorphic but not affected by chronic cocaine
In addition to differences in the density of dendritic spines, differences in dendritic spine morphology may be related to sex differences in addiction-related behaviors. For example, we previously reported that females have more large dendritic spines than males (Forlano and Woolley, 2010), possibly reflecting differences in the history of activity at NAc synapses. Additionally, Robinson and Kolb (1999) reported that chronic cocaine treatment increases the number of branched dendritic spines on MSN dendrites. Thus, we analyzed both the density and proportion of dendritic spines with large (0.7 - 0.90 μm) or giant (≥ 1.0 μm) heads (Fig. 3A) and the density and proportion of branched dendritic spines (Fig. 3D) along the same segments used for spine density analysis. Results were the same for large and giant spines considered separately, so the categories were combined.
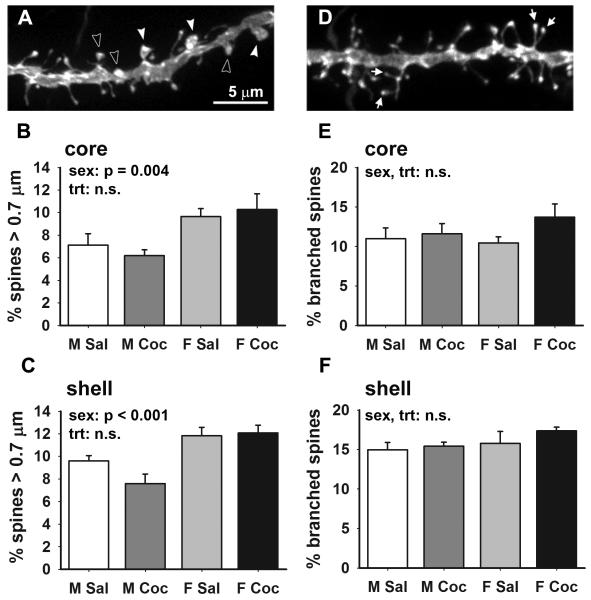
Figure 3.
Sex and cocaine effects on MSN dendritic spine morphology. (A) Projected confocal image of a DiI-labeled dendritic segment illustrating large (0.7-0.9 μm diameter, open arrowheads) and giant (≥1.0 μm diameter, filled arrowheads) spine heads. (B, C) Mean ± SEM percentage of spines with large or giant heads in the core (B) and shell (C). Females had more large and giant spines in both the core and shell , but there was no effect of cocaine. (D) Projected confocal image of a DiI-labeled dendritic segment illustrating branched spines (arrows). (E, F) Mean ± SEM percentage of branched spines in the core (E) and shell (F). Scale bar in A applies to A and B. Branched spines were neither sexually dimorphic nor specifically affected by cocaine either subregion. M Sal = saline-treated males, M Coc = cocaine-treated males, F Sal = saline-treated females, F Coc = cocaine-treated females; n = 5 animals per group.
We found that the density of large and giant dendritic spines is greater in females than males in both the core and shell, with no effect of cocaine on spine head size. In the core, the densities of large/giant spines were (in spines / 10 μm): 1.2 ± 0.2 in saline males, 1.6 ± 0.2 in cocaine males, 2.4 ± 0.1 in saline females, 2.8 ± 0.2 in cocaine females. In the shell, the densities of large/giant spines were: 1.9 ± 0.1 in saline males, 1.7 ± 0.2 in cocaine males, 2.5 ± 0.01 in saline females, and 2.7 ± 0.1 in cocaine females. However, because interpretation of differences in large/giant spine density is confounded by the fact that spine density overall is greater in females and increased by cocaine (in the core), we performed statistical analyses on the percentage of spines that were large/giant (Fig. 3B, C), which is a better indicator of effects on large/giant spines specifically. Two-way ANOVAs showed sex differences in spine head size in the core (F1,16 = 11.6, p = 0.004) and shell (F1,16 = 22.12, p < 0.001), with no effect of cocaine in the core or shell and no interaction effects (all p values > 0.1). Combining saline- and cocaine-treated groups, the proportion of large spines was 50% higher in females than males in the core and 39% higher in the shell.
When we analyzed the density and proportion of branched spines, we found no specific sex difference or effect of cocaine. In the core, the densities of branched spines were: 2.5 ± 0.3 in saline males, 3.0 ± 0.3 in cocaine males, 2.8 ± 0.2 in saline females, and 4.0 ± 0.3 in cocaine females. In the shell, the densities of branched spines were: 3.2 ± 0.3 in saline males, 3.7 ± 0.2 in cocaine males, 3.5 ± 0.4 in saline females, and 4.1 ± 0.2 in cocaine females. Thus, branched spine density was greater in females and increased by cocaine, particularly in the core. But because core spine density overall is affected by sex and cocaine, these differences mirrored effects on spine density in general. Considering the percentage of spines with branched morphology, there were no sex or cocaine-related differences or interaction effects in either the core (2-way ANOVA, all p values > 0.1; Fig. 3E) or shell (2-way ANOVA, all p values > 0.1; Fig. 3F).
3.4 Behavioral activation and NAc spine density are correlated in cocaine-treated females
We found that cocaine-induced locomotor activity, which has been attributed more to function of the NAc core than the shell (Pulvirenti et al., 1994; Ito et al., 2004), is highly sexually dimorphic. Furthermore, our morphological analyses indicated that the cocaine effect on dendritic spine density is stronger in the core than shell. To further test the relationship between the sex difference in cocaine-induced behavior and the cocaine-induced increase in spine density, we more closely examined behavior in the 5 animals in each group that were used for morphological analysis and compared spine density and behaviors on an animal-by-animal basis. These analyses indicated that, specifically for females, those animals that showed the most robust behavioral responses to cocaine were those with the greatest cocaine-induced increase in MSN dendritic spine density. This relationship was not observed in males.
Plotting cocaine-induced locomotor behavior across the 5 weeks of treatment specifically for animals used in spine density analysis showed the same sexually dimorphic activity pattern as the larger behavioral data set (Fig. 4A). With this smaller dataset, a 3-way repeated measures ANOVA showed no significant effect of week but did yield between-subject effects of treatment (F1,16 = 62.52, p < 0.001), sex (F1,16 = 11.74, p = 0.003), and an interaction between sex and treatment (F1,16 = 9.99, p = 0.006) such that the sex difference was significant only in cocaine-treated animals (p < 0.001) but not saline-treated animals (p > 0.7, Tukey’s post-hoc test).
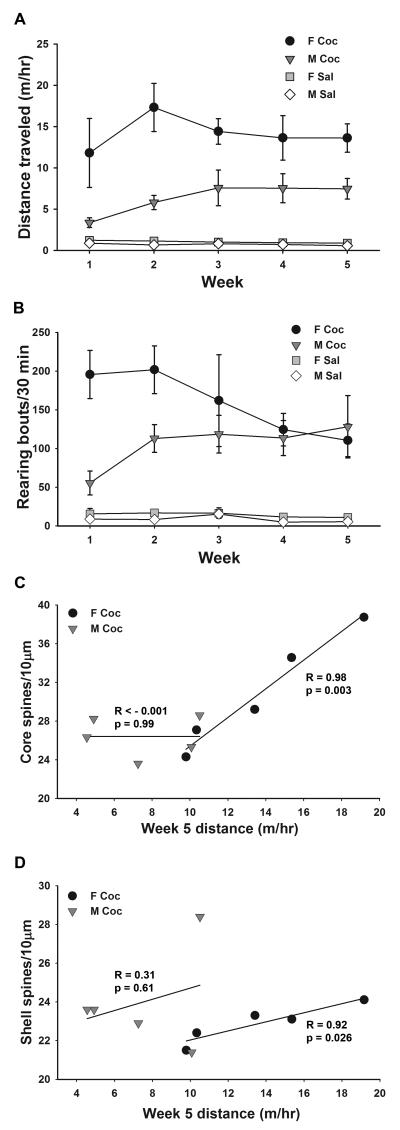
Figure 4.
MSN dendritic spine density is tightly correlated with locomotor activity in cocaine-treated females, but not males. (A) Mean ± SEM weekly cumulative distance traveled in the 1 hr after injection for the subset of rats used for spine density/morphology analysis. (B) Mean ± SEM weekly rearing behavior in the first 30 min. after injection for the same rats as in (A). (C, D) Dendritic spine density in both the core (B) and shell (C) of cocaine-treated females but not males correlated with average distance traveled in the last week of treatment i. M Sal = saline-treated males, M Coc = cocaine-treated males, F Sal = saline-treated females, F Coc = cocaine-treated females; n = 5 animals per group.
Rearing behavior was also affected by cocaine in a sexually dimorphic manner (Fig. 4B). We counted bouts of rearing during the first 30 min. after injection, when activity levels were highest (see Fig. 1C). As expected (Roy et al., 1978), cocaine dramatically increased rearing. Interestingly, the pattern of rearing behavior across weeks differed between males and females. In females, cocaine-induced rearing was particularly high during weeks 1 and 2, and then decreased slightly in weeks 3 through 5; in contrast, rearing in cocaine-treated males was lower in week 1 and then increased so that there was no sex difference by week 3. A 3-way ANOVA of rearing data showed a significant interaction between sex, treatment, and week overall (F4,16 = 15.29, p = 0.001). Two-way ANOVAs within each week confirmed sex by treatment interactions specifically in weeks 1 (F1,16 = 15.94, p = 0.001) and 2 (F1,16 =5.97 p = 0.027), in which sex differences were present in animals treated with cocaine (p < 0.001) but not saline (p > 0.9, Tukey’s post-hoc test).
The NAc is connected to limbic areas that are known to play important roles in stress and anxiety-related behavior (Groenewegen et al., 1996; LeDoux, 2000), and activity in the shell can modulate anxiety (Sturm et al., 2003). As an indication of anxiety-like behavior, we analyzed the proportion of time after cocaine or saline treatment that each animal spent in the center of the activity chamber compared to the periphery (Prut and Belzung, 2003). This analysis revealed that while cocaine-treated animals spent more time in the center vs. periphery than saline-treated controls did, there were no overall sex differences and little change in this measure across the 5 week treatment period (3-way repeated measures ANOVA: sex F1,16 = 0.004, p = 0.95; treatment F1,16 = 31.04, p < 0.001; no effect of week; no interaction; data not shown).
One unexpected finding that arose from analysis of behavior in the same animals used for morphological studies was that, specifically in females, rearing behavior and locomotor activity during treatment were correlated with dendritic spine density measured after treatment. Females that reared the most early in the treatment period were those that showed the highest levels of locomotor activity late in the treatment period, and these females also had higher spine densities in the core and the shell. Thus rearing behavior in the first week was correlated with spine density in females for both the core (R = 0.87, p = 0.05) and shell (R = 0.99, p < 0.002), but not in males for either the core (R = 0.36, p = 0.55) or shell (R = −0.19, p = 0.76; data not shown). A very similar pattern was found for locomotor activity during the last 2 weeks of treatment. Plotting each animal’s average locomotor activity from week 5 of treatment vs. that animal’s spine density in the core (Fig. 4C) or shell (Fig. 4D) showed strong linear correlations in cocaine-treated females, but not in males; the same was true for locomotor activity in week 4 vs. spine density. A correlation between activity and spine density in cocaine-treated females was evident in both core spine density (week 4: R = 0.94, p = 0.016; week 5: R = 0.98, p = 0.003) and shell spine density (week 4: R = 0.89, p = 0.045; week 5: R = 0.92, p = 0.02), although the slope of the relationship was steeper in the core, consistent with its higher spine density. In cocaine-treated males, spine density did not correlate with locomotor activity in the core (week 4: R = −0.29, p = 0.64; week 5: R < 0.01, p = 0.99) or the shell (week 4: R = −0.76, p = 0.14; week 5: R = 0.31, p = 0.61). Unlike for rearing and locomotor activity, there were no correlations between time spent in the center vs. periphery of the activity chamber and spine density in either the core or shell (data not shown). Also, there were no correlations between the low levels of rearing or locomotor activity in saline-treated females or males and their spine densities (data not shown).
3.5 The frequency of mEPSCs in the NAc core is sexually dimorphic and increases with chronic cocaine
We next investigated whether sex and cocaine effects on dendritic spine density and morphology are paralleled by differences in MSN synaptic physiology. We made whole-cell patch-clamp recordings from MSNs in acute slices from the remainder of the rats used for behavioral analysis. Slices were cut in either the parasagittal (Fig. 5A) or oblique coronal (Fig. 5B) planes in an effort to preserve inputs from the PFC into the NAc core or shell respectively (Yang et al., 1996). MSNs were identifiable on the basis of their small round somata, high resting membrane potential (−73 to −89 mV), inward rectification, slow ramping subthreshold depolarization in response to current injections, and prominent spike after-hyperpolarization in current clamp recordings (Fig. 5C; O’Donnell and Grace, 1993; Belleau and Warren, 2000). We then switched to voltage clamp to record synaptically evoked EPSCs by stimulating just outside the NAc in the path of incoming PFC axons. When connections from the PFC were maintained sufficiently to evoke an EPSC, we recorded pairs of EPSCs at intervals of 50 (Fig. 5D), 100, and 250 ms to measure paired-pulse ratio (PPR). Finally, we applied TTX to the slice and recorded mEPSCs (Fig. 5E, F) to measure mEPSC frequency, amplitude, and kinetics. The most striking sex and cocaine effects we observed were in mEPSC frequency in the core, in which group differences closely paralleled group differences in the density of dendritic spines.
Core mEPSC frequency was both sexually dimorphic and increased by cocaine in both males and females (Fig. 5G; 2-way ANOVA: sex F1,59 = 21.01, p < 0.001; treatment F1,59 = 10.52, p = 0.002; no interaction). Overall, core mEPSC frequency was 61% greater in females than males, and the cocaine-induced increase in females (41%) was slightly greater than in males (36%). In the shell, there was no sex difference in mEPSC frequency (2-way ANOVA: F1,59 = 0.004, p > 0.9) but there was an effect of cocaine (F1,59 = 4.43, p = 0.04; Fig. 5H). Cocaine-treated animals showed 24% greater mEPSC frequency in the shell than saline animals. The cocaine-induced increase was driven by the females (51%) rather than the males (8%), but the interaction between sex and treatment was not statistically significant (F1,59 = 1.68, p = 0.19). Thus, particularly in the core, the relationships in mEPSC frequency among groups corresponded well with those in spine density. As was the case for spine density, plotting the locations of recorded cells in core and shell confirmed a similar distribution of cells among treatment groups and revealed no systematic differences in mEPSC frequency within the core or shell (Fig. S2).
Corresponding sex and cocaine effects on dendritic spine density and mEPSC frequency in the core suggested that the number of excitatory synapses per MSN is sexually dimorphic and affected by cocaine. Differences in release probability also can affect mEPSC frequency, however. To investigate differences in release probability, we measured PPRs in the subset of cells in which we could evoke an EPSC (in the core, n = 8 male saline, 4 male cocaine, 5 female saline, 5 female cocaine; in the shell, n = 11 male saline, 7 male cocaine, 8 female saline, 9 female cocaine). This analysis showed no significant differences in PPR at a 50 ms ISI in either the core (2-way ANOVA: all p values > 0.1; Fig. 5I) or the shell (2-way ANOVA: all p values > 0.4; Fig. 5J); PPR at 100 and 250 ms ISI also were not different among groups (all p values > 0.1; data not shown). The lack of sex difference or cocaine effect in PPR indicates that the differences in mEPSC frequency we observed are due primarily to differences in the number of excitatory synapses per neuron. However, we did observe a trend toward an interaction at the 50 ms ISI in the core (2-way ANOVA: F1,18 = 3.3, p = 0.086), in which cocaine-treated females had a nonsignificantly lower PPR than other groups. Thus, it remains possible that a higher probability of release could contribute to greater mEPSC frequency in the core of cocaine females.
In contrast to mEPSC frequency, we found no sex differences, cocaine effects, or interaction effects on mEPSC amplitude (Fig. S3) or kinetics (Fig. S4) in either the core or shell. Additionally, while estrous cyclicity in cocaine-treated females typically recovered within 2 weeks after treatment, there was no relationship apparent between stage of the cycle and any aspect of MSN electrophysiology (not shown).
4. DISCUSSION
We investigated cellular correlates of the sexually dimorphic behavioral response to chronic cocaine exposure. Consistent with previous studies using shorter treatments (van Haaren and Meyer, 1991; Hu and Becker, 2003), females showed more cocaine-induced locomotor activation and stronger behavioral sensitization to cocaine. Subsequent analyses showed both sex differences and cocaine effects on excitatory synaptic input to MSNs. Particularly in the core, dendritic spine density was higher in females, and while cocaine increased spine density in both males and females, the magnitude of cocaine’s effect was greater in females. Although we did not analyze dendritic length in this study, we showed previously that neither dendritic length nor any aspect of dendritic branching in MSNs is sexually dimorphic (Forlano and Woolley, 2010). Thus sex and cocaine effects on dendritic spine density are likely to reflect differences in dendritic spine number per MSN. Whole-cell patch-clamp recording supported this idea. We found that, particularly in the core, sex and cocaine effects on spine density were paralleled by effects on mEPSC frequency with no differences in PPR. Results were slightly different in the shell, where sex differences are less robust (Forlano and Woolley, 2010). Cocaine increased mEPSC frequency in the shell of both males and females, but the effect on spine density was only a statistical trend. Perhaps most interestingly, we found that specifically in females, rearing behavior and locomotor activity during treatment was correlated with dendritic spine density at the end of treatment (measured after post-treatment abstinence). These studies indicate that the increase in MSN dendritic spine density after chronic cocaine exposure is paralleled by increased excitatory synaptic input to MSNs, and demonstrate cellular correlates of females’ greater behavioral sensitivity to cocaine.
4.1 Sex differences in behavioral responses to cocaine
Sensitization of locomotor activity is a relatively simple way to assay neural adaptations to repeated cocaine exposure. Numerous studies indicate that mechanisms involved in behavioral sensitization overlap with mechanisms related to addiction such as acquisition of self-administration (Piazza et al., 1989), motivation to consume drugs (Lorrain et al., 2000), and escalation of drug-taking (Ferrario and Robinson, 2007). Our observation that females show greater behavioral responses to cocaine, including more and more rapid sensitization, corresponds with previous results in humans and animals (reviewed by Quinones-Jenab, 2006; Evans and Foltin, 2010) and suggests that neural adaptations to cocaine may be amplified in females.
Cocaine-induced locomotor activity in females decreased slightly after ~2 weeks, in parallel with disruptions of the estrous cycle. Others have shown that removal of circulating estradiol in females decreases cocaine-induced locomotor activity and sensitization (Sell et al., 2002; Hu and Becker, 2003), though a smaller sex difference persists in gonadectomized animals. Thus it is likely that circulating estradiol potentiates the brain’s sensitivity to cocaine. It is worth noting, however, that we found no relationship between stage of the cycle and any measure of MSN electrophysiology.
4.2 Subregional variation in the effects of cocaine
We found that cocaine increased measures of excitatory synaptic input to MSNs in both the core and shell, but effects were stronger in the core. Sex differences also were stronger in the core. There is evidence that the core plays a greater role than the shell in modulating motor activity (Pulvirenti et al., 1994; Ito et al., 2004), which is consistent with our observation that sex differences in cocaine-induced locomotor activity were paralleled by synaptic sex differences in the core but not shell.
Going beyond a simple core-shell distinction, the NAc is known to be anatomically (Meredith et al., 1992; Brog et al., 1993; Groenewegen et al., 1999) and neurochemically (Voorn et al., 1989) heterogeneous, and may be organized into ensembles of functionally related neurons (Heimer et al., 1997). However, because we found sex and cocaine effects using DiI labeling and whole-cell recording, both of which sample cells essentially randomly, it is unlikely that these effects are restricted to any functional category of MSNs. One caveat is that we failed detect an effect of cocaine on spine density in the shell of females, which has been observed before with a longer abstinence period (Robinson and Kolb, 1999). The role of abstinence after drug treatment in rewiring neural circuitry of the NAc is unknown, but abstinence has been shown to modulate some of cocaine’s electrophysiological effects differently in core vs. shell MSNs (Kourrich and Thomas, 2009). Thus it is possible that differences in the abstinence period account for the differences in our results in the shell. Differences in abstinence also might account for the fact that we found no specific effect of cocaine on branched spines, whereas Robinson and Kolb (1999) did. Alternatively, this could be related to measuring spines in DiI- versus Golgi-labeled tissue and/or counting spines in stacks of confocal images versus with brightfield microscopy.
4.3 Plasticity of AMPAR-mediated synaptic transmission
The cocaine-induced increase in MSN spine density suggests structural reorganization of neural circuitry in the NAc (Robinson and Kolb, 2004), and the corresponding increase in mEPSC frequency indicates that cocaine-induced spines support enhanced AMPAR-mediated synaptic transmission. These findings nicely parallel a series of studies by Wolf and colleagues demonstrating that repeated cocaine treatment followed by a 1-3 week abstinence period increases surface expression of AMPARs (Boudreau and Wolf, 2005; Boudreau et al., 2007; 2009). Our findings suggest that at least some of the additional AMPARs at the surface are localized to spine synapses, likely on new dendritic spines. The failure of cocaine to increase spine head size or mEPSC amplitude both argue against addition of AMPARs to existing synapses, though we cannot rule out that possibility. Indeed, in contrast to our findings, Kourrich et al (2007) reported an increase in mEPSC amplitude as well as frequency in shell MSNs after 5 days of cocaine treatment followed by abstinence. Wolf’s and Kourrich’s studies were all done in males, however. The sex differences we observed predict that, at least in the core, surface expression of AMPARs on MSNs might be greater in females and that cocaine might increase surface AMPARs to a greater extent in females.
Spine head size may be related to the history of synaptic activity and/or synaptic strength (e.g. Matsuzaki et al., 2004; reviewed by Alvarez and Sabatini, 2007). Although females had more large spines than males (regardless of cocaine treatment), mEPSC amplitude was not sexually dimorphic, arguing against stronger synapses in females. Instead, the sex difference in spine head size might reflect a difference in relative proportion of inputs from different brain areas (French and Totterdell, 2004), which in turn could influence behavioral responses to cocaine.
4.4 Dendritic spines and locomotor activity
One unanticipated finding was that, specifically in females, spine density measured after 17 days of abstinence from treatment was tightly correlated with cocaine-induced locomotor activation during treatment. Although we measured spine density after an abstinence period, locomotor sensitization to repeated psychostimulant treatment is known to persist for weeks at a level similar to that observed at the end of treatment (Downs and Eddy, 1932; Paulson et al., 1991; reviewed by Pierce and Kalivas, 1997); therefore it is likely that locomotor activity at the end of our treatment was comparable to what would be observed with a cocaine challenge after abstinence. Because the role of abstinence in producing the cocaine-induced increase in spine density is unknown, it is not clear whether spine density was elevated at the time locomotor activity was measured or whether it increased afterward, during the abstinence period. If spine density were increased by cocaine during treatment, this would mean that females with higher dendritic spine density show higher levels of cocaine-induced activity. Alternatively, if abstinence is required for the increase in spine density, it could be that females with particularly strong behavioral responses to cocaine also respond more strongly to abstinence, producing a greater increase in MSN spine density during the abstinence period. We are currently investigating the role of abstinence from treatment in increasing MSN spine density in both males and females.
Sex differences in glutamatergic and/or dopaminergic transmission in response to cocaine may underlie the sex-specific correlations between spine density and locomotor activity. Glutamate signaling in the NAc has been shown to play a critical role in psychostimulant-induced locomotor sensitization (Pierce et al., 1996; Bell et al., 2000; Ghasemzadeh et al., 2003), although there is also evidence for an uncoupling of cocaine-induced AMPAR plasticity and locomotor sensitization (Boudreau et al., 2007; Bachtell and Self, 2008). Our finding that locomotor activation and dendritic spine density are correlated in females argues for a positive relationship between glutamatergic synaptic activity in the NAc and locomotor activation, at least under some circumstances. To date, most studies of glutamatergic mechanisms of addiction have been performed in males without attention to possible sex differences. In contrast, sex differences in dopaminergic signaling in the NAc have been more extensively studied (reviewed by Becker and Hu, 2008). One possibility is that these pathways are more tightly coupled in females, accounting for the sex specific correlation. Alternatively, such pathways may converge in animals with particularly high levels of locomotor activity, which is more common in females.
4.5 Possible molecular mechanisms of sexually dimorphic responses to cocaine
While we did not directly test the effects of gonadal steroids on cocaine-induced behavior in this study, many previous studies have shown that estradiol underlies greater behavioral responses to cocaine in females (reviewed by Festa and Quinones-Jenab, 2004). Interestingly, there is substantial overlap among plasticity-related signaling molecules known to be downstream of estradiol and those implicated in the NAc response to cocaine. For example, BDNF expression is upregulated by estradiol (Scharfmann and MacLusky 2006, Fan et al 2008), BDNF levels in the NAc are increased by exposure to and abstinence from psychostimulants (reviewed by Russo et al., 2009), and BDNF enhances locomotor activity in response to psychostimulants (reviewed by McGinty et al., 2010). CaMKII is linked to both BDNF and estradiol, and transient overexpression of CaMKIIα in the NAc increases locomotor responses to psychostimulants (Loweth et al., 2010). Finally, ΔFosB is of great interest because it accumulates in the NAc with repeated drug treatment (Nestler, 2008), modulates the expression of genes involved in spine formation (reviewed by Lai and Ip, 2009; Maze et al., 2010) and increases locomotor responses to cocaine (Zachariou et al., 2006). Although there are no published reports of sex differences in ΔFosB expression after cocaine treatment, there is a putative ERE on the FosB gene (Bourdeau et al., 2004) and FosB is upregulated by estrogen receptor activation in other cell types (Chen et al., 2009). When examining the roles of BDNF, CaMKII, and/or ΔFosB in the behavioral and neural responses to cocaine, it will be important to take sex differences and estradiol effects on these pathways into account. Such studies could provide a mechanistic basis for variation in sensitivity to cocaine between males and females, as well as increasing insight into the development and expression of long-term drug-induced plasticity in the reward circuitry.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by NIH R01 DA020492 to CSW, T32 HD007068, and F32 DA025453 to AMW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Self DW. Renewed cocaine exposure produces transient alterations in nucleus accumbens AMPA receptor-mediated behavior. J Neurosci. 2008;28:12808–12814. doi: 10.1523/JNEUROSCI.2060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol. 2000;84:2204–2216. doi: 10.1152/jn.2000.84.5.2204. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME. Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. J Neurochem. 2009;110:363–377. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau V, Deschenes J, Metivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18:1411–1427. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Chen J, An BS, Cheng L, Hammond GL, Leung PC. Gonadotropin-releasing hormone-mediated phosphorylation of estrogen receptor-alpha contributes to fosB expression in mouse gonadotrophs. Endocrinology. 2009;150:4583–4593. doi: 10.1210/en.2009-0455. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Dietz KC, Nestler EJ, Russo SJ. Molecular mechanisms of psychostimulant-induced structural plasticity. Pharmacopsychiatry. 2009;42(Suppl 1):S69–78. doi: 10.1055/s-0029-1202847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, Malenka RC. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- Downs AW, Eddy NB. The effect of repeated doses of cocaine on the rat. J Pharmacol Exp Ther. 1932;46:199–200. [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Robinson TE. Amphetamine pretreatment accelerates the subsequent escalation of cocaine self-administration behavior. Eur Neuropsychopharmacol. 2007;17:352–357. doi: 10.1016/j.euroneuro.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Woolley CS. Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol. 2010;518:1330–1348. doi: 10.1002/cne.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Quantification of morphological differences in boutons from different afferent populations to the nucleus accumbens. Brain Res. 2004;1007:167–177. doi: 10.1016/j.brainres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Permenter LK, Lake R, Worley PF, Kalivas PW. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur J Neurosci. 2003;18:1645–1651. doi: 10.1046/j.1460-9568.2003.02880.x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- King TS, Canez MS, Gaskill S, Javors MA, Schenken RS. Chronic cocaine disruption of estrous cyclicity in the rat: dose-dependent effects. J Pharmacol Exp Ther. 1993;264:29–34. [PubMed] [Google Scholar]
- Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh SN, Taguchi T. A simple exploratory algorithm for the accurate and fast detection of spontaneous synaptic events. Biosens Bioelectron. 2002;17:773–782. doi: 10.1016/s0956-5663(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Lai KO, Ip NY. Recent advances in understanding the roles of Cdk5 in synaptic plasticity. Biochim Biophys Acta. 2009;1792:741–745. doi: 10.1016/j.bbadis.2009.05.001. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Arnold GM, Vezina P. Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res. 2000;107:9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- Loweth JA, Singer BF, Baker LK, Wilke G, Inamine H, Bubula N, Alexander JK, Carlezon WA, Jr., Neve RL, Vezina P. Transient overexpression of alpha-Ca2+/calmodulin-dependent protein kinase II in the nucleus accumbens shell enhances behavioral responding to amphetamine. J Neurosci. 2010;30:939–949. doi: 10.1523/JNEUROSCI.4383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, Nestler EJ. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr., Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Agolia R, Arts MP, Groenewegen HJ, Zahm DS. Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat. Neuroscience. 1992;50:149–162. doi: 10.1016/0306-4522(92)90389-j. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse. 1993;13:135–160. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th Edition Academic Press; San Diego: 1998. [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Berrier R, Kreifeldt M, Koob GF. Modulation of locomotor activity by NMDA receptors in the nucleus accumbens core and shell regions of the rat. Brain Res. 1994;664:231–236. doi: 10.1016/0006-8993(94)91977-1. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V. Why are women from Venus and men from Mars when they abuse cocaine? Brain Res. 2006;1126:200–203. doi: 10.1016/j.brainres.2006.08.109. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Roy SN, Bhattacharyya AK, Pradhan S, Pradhan SN. Behavioural and neurochemical effects of repeated administration of cocaine in rats. Neuropharmacology. 1978;17:559–564. doi: 10.1016/0028-3908(78)90148-x. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67:281–290. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. J Pharmacol Exp Ther. 1999;289:54–65. [PubMed] [Google Scholar]
- Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkotter J. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium-binding protein. J Comp Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56(Suppl 1):149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Seamans JK, Gorelova N. Electrophysiological and morphological properties of layers V-VI principal pyramidal cells in rat prefrontal cortex in vitro. J Neurosci. 1996;16:1904–1921. doi: 10.1523/JNEUROSCI.16-05-01904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ. Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J Neurosci. 1998;18:488–498. doi: 10.1523/JNEUROSCI.18-01-00488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.