Abstract
Alterations in working memory, default-mode network (DMN), and dopamine transporter have all been proposed as endophenotypes for Attention-Deficit Hyperactivity/Disorder (ADHD). Despite evidence that these systems are interrelated, their relationship to each other has never been studied in the context of ADHD. In order to understand the potential mediating effects of task-positive and task-negative networks between DAT1 and diagnosis, we tested effects of genotype and diagnosis on regions of positive and negative BOLD signal change (as measured with fMRI) in 53 adults with ADHD and 38 control subjects during a working memory task. We also examined the relationship of these responses to ADHD symptoms. Our results yielded four principal findings: 1) association of 9R with adult ADHD, 2) marginal DAT1 association with task-related suppression in left medial PFC, 3) marginal genotype × diagnosis interaction in the dorsal anterior cingulate cortex, and 4) correlation of DMN suppression to ADHD symptoms. These findings replicate the association of the 9R allele with adult ADHD. Further, we show that DMN suppression is likely linked to DAT1 and to severity of inattention in ADHD. DMN may therefore be a target of DAT1 effects, and lie on the path between the gene and inattention in ADHD.
Keywords: fMRI, default-mode network, attention
1. Introduction
The “default-mode network” (DMN) describes a functionally connected set of brain regions that is more active at rest than during externally-oriented cognitive tasks (Buckner et al., 2008). It is a “task-negative” network, showing negative signal change during task as compared to resting baseline conditions. Further, as demands for attention to external stimuli increase, activation of DMN decreases and activation of “task-positive” networks increase, suggesting that resources are being allocated away from DMN and towards brain regions that support attention to task. Results from several studies suggest that deficits in attention to task can be linked to inadequate suppression of DMN during task performance (Weissman et al., 2006; Li et al., 2007; Eichele et al., 2008; Kelly et al., 2008). The behavioral consequences have been reflected in increased reaction time (RT) variability (Kelly et al., 2008), and self-reported mind wandering (Mason et al., 2007). Even on a trial-by-trial basis, reduction in task-related suppression of DMN regions has been found to temporally precede individual responses characterized by longer RTs (Weissman et al., 2006).
Working memory (WM) describes the set of cognitive processes that allow internal representations of stimuli in their absence, acting as a temporary workspace for incoming information to be held in mind, manipulated, or associated with memories that are accessed from long-term storage. WM is critical for higher order cognition as it allows active representations of information guiding conduct (e.g. rules) as well as providing a domain in which precepts can be considered against past emotions and experiences (Baddeley, 2003; D’Esposito, 2007).
Neuroimaging studies show that across WM tasks, a robust network of lateral frontal, posterior parietal, and anterior cingulate cortices are activated during working memory processes (Wager & Smith, 2003; Owen et al., 2005). Likewise, DMN is suppressed during working memory tasks (Whitfield-Gabrieli et al., 2009; Anticevic et al., 2010). Further, WM tasks are sensitive to DMN interference, with decreased task-related suppression predicting poorer performance in healthy adult subjects as well as participants at genetic risk for schizophrenia (Whitfield-Gabrieli et al., 2009; Anticevic et al., 2010). Therefore it is likely that adequate regulation of both “task-positive” and “task-negative” is necessary for successful performance. In a group of children with Attention-Deficit/Hyperactivity Disorder (ADHD), Fassbender et al (2009) found that task-related suppression of DMN during a serial addition task was decreased in ADHD subjects compared to controls, and that lack of suppression was associated with increased attentional fluctuations. These findings support the “Default-Mode Interference Hypothesis” (Sonuga-Barke & Castellanos, 2007), which maintains that ADHD-related inattention may be explained at least partially by periodic intrusions of DMN into cognitive and brain processes supporting externally-oriented tasks.
Although there is abundant support for a key role of dopamine in WM performance (Brozoski et al., 1979), it has not been tested whether these effects might be at least partially mediated via task-negative networks. However, mounting evidence supports a role for dopamine in DMN regulation, and specifically that the dopamine transporter protein (DAT) may be an important regulator of task-related suppression. In a recent Positron Emission Tomography/functional Magnetic Resonance Imaging (PET-fMRI) study, Tomasi et al (2009) found that DAT negatively predicted task-related suppression, in that adults with higher DAT binding showed less DMN suppression during a visual attention task. In addition, results from a pharmacological fMRI study of psychostimulants (e.g. methylphenidate/d-amphetamine), which work at least partially by blocking DAT function, showed that administration of the drug reversed a lack of task-related suppression that was found in children with ADHD at a medication naive baseline (Peterson et al., 2009).
Several imaging genetics studies have shown a relationship between a variable number tandem repeat (VNTR) in the 3′ untranslated region (UTR) of the gene that codes for DAT (DAT1 3′-UTR VNTR) and task-positive regions supporting WM. For example, the polymorphism has been shown in several studies of healthy adults by Bertolino et al (2006; 2008; 2009) and in healthy children by Stollstorff et al (2010) to have an effect on lateral prefrontal cortex activity during N-back tasks. The direction has been consistent throughout these reports: homozygosity for the 10-repeat allele predicts less activity in task-positive regions supporting WM. The effect of this gene variant on task-negative regions suppressed during working memory has not been investigated.
The effect of the DAT1 3′-UTR VNTR on brain activity in ADHD has been less consistent, with only three fMRI studies published to date (see Durston, 2010 for review). Inconsistencies are found in the left dorsal anterior cingulate cortex (dACC), where Bedard et al (2010) found that the 10R allele was associated with increased activation in children with ADHD, while Brown et al (2010) found that it was associated with decreased activity in this region in a sample of adults with ADHD. Further, while Durston et al (2008) found that the 10R/10R genotype predicted more activity in the cerebellar vermis of boys with ADHD, Brown et al (2010) found it predicted decreased vermis activity in an adult sample. The opposite brain effects in adult vs. child samples mirror those found in the genetic association literature, where meta-analysis supports an association of the 10R allele with ADHD in childhood (Faraone et al., 2005; Yang et al., 2007), and the 9R allele with ADHD in adulthood (Franke et al., 2010).
Working memory, task-related suppression of DMN, and alterations in DAT protein levels, have all been proposed independently as endophenotypes for ADHD. These proposals are supported by the following evidence: (1) Deficits in WM performance are related with a large effect size to ADHD in childhood (Willcutt et al., 2005) and adulthood (Hervey et al., 2004), and alterations in neural circuitry supporting WM have been found in adults and children ADHD (Vance et al., 2007; Wolf et al., 2009; Bayerl et al., 2010; Valera et al., 2010); (2) Adult subjects with ADHD show alterations in DMN connectivity (Castellanos et al., 2008; Uddin et al., 2008), and in childhood ADHD subjects show reduced task-related suppression of DMN (Fassbender et al., 2009; Peterson et al., 2009); (3) Meta-analyses have supported an association between alleles in DAT1 3′-UTR VNTR and ADHD (Yang et al., 2007; Gizer et al., 2009; Franke et al., 2010), molecular imaging studies have shown altered levels of DAT availability in ADHD subjects (see Krause, 2008 for review), and administration of methylphenidate works at least partially by blocking DAT (Volkow et al., 1998). As reviewed above, all three of these systems are interrelated, however, their relationship to each other has not been studied together in ADHD.
We tested the effect of DAT1 3′-UTR VNTR variation on both task-positive and task-negative networks associated with WM, and how these networks relate to ADHD in adulthood. To this end, we evaluated genotype effects in task-positive and task-negative regions of interest (ROIs) in a group of adult ADHD and control participants while they performed an N-back WM task. To understand the relationship of these brain response patterns to ADHD, we tested diagnosis effects in the ROIs, and the relationship of activity and suppression during our N-back task to ADHD symptoms. Based on the literature reviewed above, we hypothesized that adult ADHD would be associated with the 9R allele, decreased activation in task positive regions, and decreased task-related suppression of DMN. Further, given the established link between decreased task-related suppression and inattention to task, we hypothesized that task-related suppression in DMN would be negatively related to DSM-defined symptoms of inattention.
2. Methods
This study is a secondary data analysis, integrating neuroimaging data collected in one study (MH062152) which lasted from 2001–2008, and genetic data collected in MH062152, or in one of four other studies (MH57934, HD37694, MH064019, HD36317). Previous imaging reports have been published on subsets of this sample (Valera et al., 2005; Seidman et al., 2006; Makris et al., 2007; Biederman et al., 2008; Monuteaux et al., 2008; Brown et al., 2010; Valera et al., 2010), but none examining the effect of a gene variant on brain activity during the N-back task. All studies were conducted in accordance with the Declaration of Helsinki and the standards established by the Partners Healthcare Human Research IRB. Written informed consent was obtained from all participants. Participants include all individuals for whom both the N-back fMRI task and DNA were collected, and who met the inclusion criteria for this analysis.
2.1. Participants
Participants were recruited from Massachusetts General Hospital (MGH) clinics and advertisements posted in the Boston area. Exclusion criteria were an estimated full scale IQ <80; lifetime history of psychosis; recent alcohol or substance dependence or abuse; inadequate command of the English language; sensorimotor handicaps. Additional inclusion criteria were age 18–53, self-identified as Caucasian, and carriage of two common DAT1 3′VNTR alleles (9-repeat and/or 10-repeat). The resulting sample included 91 adult participants: 53 with ADHD, and 38 controls. Participants with ADHD were told to withhold taking their psychostimulant medication for at least 24 hours previous to the scan. Of the 53 participants with ADHD, 23 (43.4%) reported taking stimulants near the time of scan but had washed out, 20 participants with ADHD (37.7%) had never taken psychostimulants, and the remaining 9 (17.0%) participants had taken psychostimulants at some point in the past.
2.2. Assessment methods
To assess for psychiatric diagnoses we administered the Structured Clinical Interview for DSM-IV (SCID-I; First et al., 1997). To assess ADHD, we used a module derived from the Schedule of Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E; Orvaschel & Puig-Antich, 1987). This module systematically acquires information on all DSM-IV ADHD symptoms, domains of impairment and age at onset. Previous work has shown that retrospective childhood diagnoses of ADHD can be made in a reliable and valid manner using this method (Biederman et al., 1990; Faraone et al., 2000). An ADHD diagnosis was made if the DSM-IV criteria were met in childhood and persisted into adulthood. At the time of the clinical interview, the distribution of ADHD subtypes was: combined, N=17 (32%); inattentive, N=18 (34%); hyperactive/impulsive, N=1(2%); and remitted, N=17 (32%). Subjects with remitted ADHD were below the DSM-IV 6-symptom threshold at the time of the interview but met criteria including childhood onset and persistence into adulthood. Block Design and Vocabulary subtests from the Wechsler Adult Intelligence Scale-III (WAIS-3; Wechsler, 1997) were used to estimate IQ. Academic achievement was assessed with Reading and Arithmetic subtests from the Wide-Range Achievement Test (WRAT-3; Jastak & Jastak, 1993).
2.3. Genotyping methods
Genotyping of the DAT1 3′-UTR VNTR was conducted at the MGH Psychiatric and Neurodevelopmental Genetics Unit using the same protocol as described in several previous reports from our group (Mick et al., 2006; Brown et al., 2010). Briefly, Genomic DNA (5 ng) was amplified in a 7 ml reaction using HotStarTaq DNA Polymerase (0.2 U), the proprietary HotStarTaq Buffer (1_), dNTPs (200 mM), and the marker specific primers (0.2 mM). Primers were ordered from Applied BioSystems and are as follows: SLC6A3-F 6FAMTGTGGTGTAGGGAACGGCCTGAG, SLC6A3-R CCTCCTGGAGGTCACGGCTCAAGG. The SLC6A3-R primer also contains the proprietary tail. For amplification, samples were heated at 92°C for 9 min to activate the HotStarTaq Polymerase. This is followed by 12 cycles of denaturation for 30 sec at 93°C, annealing for 30 sec beginning at 64.5°C and dropped 0.5°C every cycle, and primer extension at 72°C for 30 sec; 37 cycles of denaturation for 30 sec at 93°C, annealing for 30 sec at 58°C, and primer extension at 72°C for 30 sec; 72°C for 1 hr. Amplified products were pooled and combined with size standard (LIZ-250) before being analyzed on an ABI-3730. GeneMapper v3.5 was used to analyze the raw results from the ABI3730, however, a genotype was not considered final until two technologists had independently checked (and corrected) the GeneMapper results and were in agreement.
2.4. fMRI paradigm
We used a variant of the sequential letter visual “N-back” task as described in previous publications from our group (Valera et al., 2005; Whitfield-Gabrieli et al., 2009; Valera et al., 2010). Briefly, the task contained intermittent blocks of the 2-back working memory condition, the 0-back vigilance condition, and a baseline fixation condition. N-back stimuli were generated using MacStim software running on a Mac iBook G4, and projected onto a screen situated in the rear of the magnet bore. Stimuli were viewed through a mirror attached to the head coil. Responses were collected using an MRI safe button box.
Stimuli were sequences of white capital letters on a black background, presented centrally (200 ms duration, 1800 ms inter-stimulus interval) in pseudo-random order. Participants were instructed to respond to every stimulus using a response box, pressing one button to signal targets and another to signal non-targets. In the 0-back condition, the target was the letter “X” (23% of trials), and all other stimuli were non-targets. In the 2-back condition, the target was any letter identical to the letter that preceded it two trials back, “2-back” (26% of trials).
Participants were administered three runs of the task, each lasting 5.6 minutes. Each run of trials incorporated a block design with 12 epochs: 1) three 36-second epochs of 0-back; 2) three 36-second epochs of 2-back; and 3) six 20-second epochs of “fixation”. Each of the three runs contained alternating 0-back and 2-back blocks, each preceded by a fixation block. Two condition orders were constructed, one beginning with fixation and then 0-back, one beginning with fixation and then 2-back. Condition order was randomized across runs and subjects. Percent of correct responses (accuracy), mean RT for correct responses (speed), and intra-subject standard deviation (variability) were used as performance measures.
2.5. Demographic and behavioral data analysis
Demographic and behavioral data were analyzed in PASW Statistics 18.0 ©. 2 × 2 ANOVAs with group (ADHD vs. controls) and genotype (9R-carriers vs. 10R-homozygotes) as fixed factors were run on demographic, clinical, and N-back performance data. We created genotype groups based on carriage of the 9R allele for several reasons. First, our sample only included 8 participants with two 9R alleles -- 7 ADHD, 1 Control -- Ns too small for a random effects analysis. Second, this grouping has been the convention for previous imaging genetics studies not just in ADHD (Durston et al., 2005; Durston et al., 2008; Bedard et al., 2010), but also in normal controls (Schott et al., 2006; Dreher et al., 2009). Finally, the 9R allele appears to have a dominant effect on DAT availability (van Dyck et al., 2005; van de Giessen et al., 2009).
2.6. fMRI acquisition and analysis
Imaging data was collected using a Siemens Sonata 1.5T full-body scanner at the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging. fMRI was performed with an echo-planar imaging pulse sequence (21 axial slices, TR=2000ms, 5mm thickness, 1mm interslice interval, TE=40ms, flip angle=90°, 168 volumes/run).
fMRI data were analyzed with Statistical Parametric Mapping (SPM2, Wellcome Department of Cognitive Neurology, London). Preprocessing included correction for head motion, spatial normalization, and spatial smoothing with a Gaussian filter (8-mm full width at half maximum). We dropped any single runs that contained more than 3mm of scan-to-scan head motion, stimulus correlated motion of r ≥ 0.5, or accuracy of less than 68% on the WM task. This resulted in dropping single runs from 7 controls and 9 participants with ADHD, and 2 runs from 2 ADHD participants. However, because all participants were administered 3 runs, we were able to retain these subjects by including remaining runs in our analysis.
After preprocessing, statistical analysis was performed at the single-subject level. Each epoch of trials was modeled using a boxcar function convolved with a canonical hemodynamic response function. Low-frequency components of the blood-oxygen-level-dependent (BOLD) signal were modeled as confounding covariates. Our contrast of interest was the activation associated with the 2-back condition using the 0-back condition as a control baseline, as in previous studies (Cohen et al., 1994; Valera et al., 2005; Valera et al., 2010). Individual contrast maps were submitted to a second-level analysis in which subjects were treated as random effects.
To test main effects and interactions in our contrast of interest, we ran a 2 × 2 ANOVA with diagnosis and genotype as fixed factors. A voxel-level threshold of p < 0.005 uncorrected with an extent threshold of K > 5 voxels was used to define significant clusters within our two a priori ROIs which were used as search areas (see below for ROI definition). Cluster-level p-values reported are corrected for the number of voxels across the entire respective ROI.
To test the relationship between ADHD symptoms and brain response, we ran bivariate Pearson correlations between the number of symptoms endorsed on the structured interview with mean signal change during the 2-back condition in each of our four correlation ROIs (see below), in the ADHD subjects only. Given the previously identified relationship between inattention and DMN suppression, we also specifically explored the relationship between number of symptoms endorsed on the inattentive subscale and brain response in the correlation ROIs.
2.7. Regions of interest
Anatomical regions of interest (ROIs) were created from the AAL library (Tzourio-Mazoyer et al., 2002) as found in WFU Pickatlas v 2.4 (Maldjian et al., 2003). The task-positive ROI was constructed to include areas found in meta-analysis to be maximally activated during N-back tasks (Owen et al., 2005). This ROI included a union of the AAL-defined supplementary motor area (SMA), middle cingulum (dACC), cerebellar vermis, lateral superior and middle frontal gyri (DLPFC), inferior parietal lobules, caudate, and putamen. The task-negative ROI was constructed to include mePFC (containing anterior cingulum, frontal medial orbital, frontal superior medial), posterior cingulum, and precuneus (Buckner et al., 2008).
For brain-symptom correlations in the ADHD subjects, we created four independent functionally defined ROIs derived from regions of peak signal change across the 53 ADHD subjects. These ROIs were defined as 5mm spheres surrounding voxels of peak activation and peak suppression in the anterior and posterior brain during the 2-back vs. 0-back contrast. Beta values describing the mean signal change in the 5mm spheres were used for correlations. Voxels of peak suppression identified were in left subgenual anterior cingulate (sACC [−3 36 −9]) and left posterior cingulate (PCC [−3 −18 42]). Voxels of peak activation were identified in R DLPFC [39 36 30] and L Inferior Parietal Lobule [−30 −66 51].
3. Results
3.1. Genotype results
Of the 91 participants, 46 were homozygous for the 10R allele, and 45 were 9R-carriers (8 were 9R/9R, 37 were 9R/10R). This is not an unexpected distribution of alleles for a mixed Caucasian sample (Kang et al., 1999), and there was no evidence that these data were not in Hardy-Weinberg equilibrium (p = 0.89). As can be seen in Table 1, 12 of 38 controls (32%), and 33 of 53 ADHD participants (62%) were 9R-carriers. There was a statistically significant difference (χ2 = 8.34, p = 0.004), indicating that the 9R allele was more frequent in the ADHD sample.
Table 1.
Demographic, Cognitive and Performance Data
| Controls | ADHD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9R-carriers (N = 12) | 10R/10R (N = 26) | 9R-carriers (N = 33) | 10R/10R (N = 20) | Statistic | ||||||
| Demographic and Achievement Data | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | |
| Age | 30.7 | 9.1 | 31.6 | 11.2 | 33.7 | 10.5 | 37.6 | 11.2 | 1.6 | 0.207 |
| IQ estimate | 119.6 | 9.2 | 115.3 | 11 | 115.7 | 14.4 | 116.9 | 12.9 | 0.37 | 0.779 |
| WRAT Reading | 112.9 | 7.4 | 107.4 | 7.6 | 106.5 | 9.1 | 106.4 | 7.9 | 2.04 | 0.114 |
| WRAT Math | 105.9 | 15.6 | 108.8 | 10.3 | 100.7 | 12.5 | 101.4 | 12.3 | 2.4 | 0.074 |
| N | % | N | % | N | % | N | % | χ2 | p | |
| Sex (% Females) | 6 | 50 | 16 | 61.5 | 16 | 48.5 | 10 | 50 | 1.14 | 0.777 |
| % Left Handed | 1 | 8.3 | 3 | 13 | 4 | 12.1 | 2 | 10 | 0.16 | 0.984 |
| Lifetime Psychiatric Comorbidities | ||||||||||
| N | % | N | % | N | % | N | % | χ2 | p | |
| Depressiona | 2 | 16.7 | 1 | 3.8 | 5 | 15.2 | 4 | 20.0 | 3.03 | 0.387 |
| Anxietyb | 1 | 8.3 | 2 | 7.7 | 9 | 27.3 | 4 | 20.0 | 4.68 | 0.197 |
| Dependencec | 0 | 0.0 | 3 | 11.5 | 3 | 9.0 | 3 | 15.0 | 2.01 | 0.571 |
| N-Back Performance Datad | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | |
| 0 Back - % Correct | 97.9 | 1.7 | 97.5 | 5.2 | 96.9 | 4.7 | 97.4 | 2.2 | 0.19 | 0.90 |
| 0 Back – RT (ms) | 526 | 67 | 558 | 89 | 556 | 111 | 527 | 73 | 0.75 | 0.52 |
| 0 Back – SD (ms) | 129 | 39 | 124 | 32 | 145 | 45 | 135 | 36 | 1.56 | 0.21 |
| 2 Back - % Correct | 88.8 | 8.7 | 89.9 | 6.5 | 86.9 | 7.9 | 87.9 | 6.7 | 0.85 | 0.47 |
| 2 Back – RT (ms) | 811 | 140 | 799 | 127 | 815 | 178 | 785 | 157 | 0.16 | 0.92 |
| 2 Back – SD (ms) | 248 | 72 | 237 | 53 | 251 | 56 | 245 | 68 | 0.3 | 0.82 |
| 2 Back – 0 Back RT | 284 | 107 | 240 | 86 | 258 | 121 | 258 | 123 | 0.44 | 0.72 |
As suggested by others (M. M. Weissman et al., 1984), the diagnosis of major depression was made only if the depressive episode was associated with marked impairment;
Since many anxiety disorders are measured by our structured interviews, we aggregated them into a binary measure coded positive if two or more anxiety disorders were endorsed and negative otherwise. We previously found this summary variable to measure a meaningful anxiety syndrome (Mennin et al., 2000);
Lifetime dependence was found only for alcohol and marijuana;
Due to a technical error, n-back performance data was lost for a single ADHD 9R-carrier.
3.2. Demographic and clinical variables
No significant differences were found between the four groups on any of the demographic, cognitive or comorbidity variables (see Table 1).
ADHD participants did not differ by genotype on any of the clinical variables measured including age of onset, ADHD symptoms in childhood, or symptoms reported at interview (see Table 2). Twelve 9R-carriers (36.4%) and eleven 10R-homozygotes (55.5%) reported taking psychostimulants near the time of scan, but had washed out for at least 24 hours before the MRI scan. Fifteen 9R carriers (45.5%) and five 10R homozygotes (25%) had never taken psychostimulants. The remaining participants had taken psychostimulants at some point in the past. Proportions of subjects based on psychostimulant history did not differ between genotype groups (see Table 2).
Table 2.
Clinical and Medication History in ADHD Subjects
| 9R-carriers (N = 33) | 10R/10R (N = 20) | Statistic | ||||
|---|---|---|---|---|---|---|
| Clinical Data from Structured Interview | ||||||
| Mean | SD | Mean | SD | t | p | |
| Age of ADHD Onset | 4.81 | 2.49 | 5.00 | 2.43 | 0.27 | 0.791 |
| Inattentive Sxs – Childhood | 7.91 | 1.18 | 7.65 | 1.79 | 0.58 | 0.568 |
| Inattentive Sxs – Recent | 5.85 | 2.33 | 5.45 | 2.48 | 0.59 | 0.559 |
| Hyperactive Sxs – Childhood | 6.24 | 2.32 | 6.00 | 2.81 | 0.34 | 0.735 |
| Hyperactive Sxs - Recent | 4.45 | 2.82 | 4.30 | 2.58 | 0.20 | 0.843 |
| Psychostimulant Historya | ||||||
| N | % | N | % | |||
| Near time of scan | 12 | 36.4 | 11 | 55.0 | ||
| Only in past | 5 | 15.2 | 4 | 20.0 | ||
| Medication Naïve | 15 | 45.5 | 5 | 25.0 | ||
χ2 (2) = 2.52, p = 0.284; Participants prescribed stimulant medication near time of scan were washed out for at least 24 hours.
3.3. N-back behavioral performance
There were no significant differences between the four groups on the N-back measures including accuracy, speed, or variability (see Table 1).
3.4. Main effects of task
As expected, the N-back task showed positive activations in a robust network of cortical, subcortical, and cerebellar areas, as well as deactivation across the DMN. Figure 1 and Table 3 display the significant clusters across the entire sample (N = 91).
Figure 1.
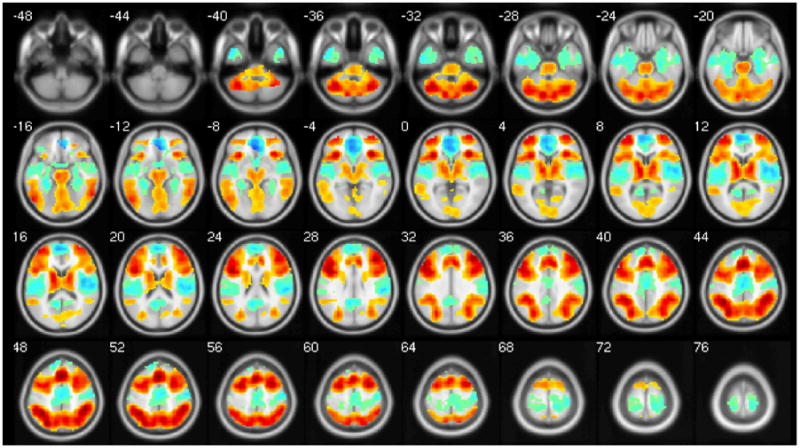
Main effect of Task (2-Back > 0-Back). Hot colors show areas with positive BOLD signal change, and cool colors show areas with negative BOLD signal change.
Table 3.
Main effect of task in whole brain (2-Back > 0-Back; N = 91).
| Cluster-Level | Voxel-Level (max vox) | |||||
|---|---|---|---|---|---|---|
| Significant Clusters | K (cluster extent) | Corrected p-value | x | y | Z | T |
|
Task-Positive Cluster | ||||||
| Pre-SMA; stretching to bilateral Mid & Inf Frontal Gyri, Sup & Inf Parietal Lobule, dACC, Ant & Post Lobe of Cerebellum, Precuneus, Thalamus, Striatum, and Mid Occipital Gyrus | 35961 | p < 0.001 | 0 | 21 | 48 | 17.64 |
| Task-Negative Cluster | ||||||
| sACC; stretching to bilateral Medial Frontal Gyrus, Insula, Post Cingulate, Precuneus, Angular/Supramarginal Gyrus, Postcentral Gyrus, Paracentral Lobule, Sup & Mid Temporal Gyrus, and Parahippocampal Gyrus | 11461 | p < 0.001 | −3 | 36 | −9 | 14.27 |
Clusters listed are those with p<.05 cluster-level corrected for whole brain. Coordinates are in MNI. Anatomical regions defined using Talaraich Daemon atlas, with max vox as first region listed. P-values are cluster-level corrected for whole brain.
3.5 Effects of genotype and diagnosis in task-negative ROI
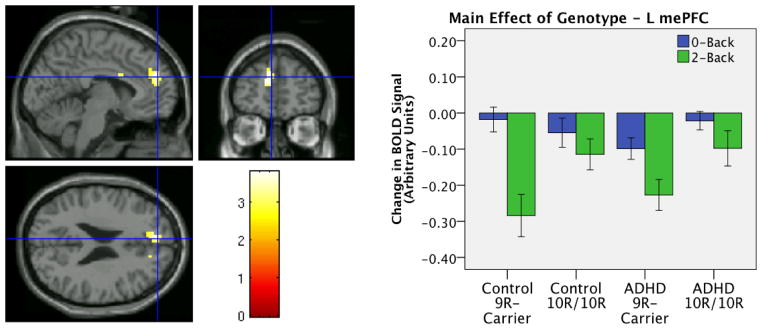
Searching across the task-negative ROI we found a marginal main effect of genotype in the left mePFC (peak voxel −12 51 18, k = 82, cluster-level corrected p=.055). Examination of beta values from this cluster shows that this effect was due to more task-related suppression in the 9R-carriers compared to 10R-homozygotes (see Figure 2). No main effects of diagnosis and no interactions were found in the task-negative ROI.
Figure 2.
Effects in Task Negative ROI: Trend effect of genotype found in left medial prefrontal cortex (peak voxel −12 51 18, k = 82, p-corrected = 0.055). Bars show average signal change across cluster.
3.6. Effects of genotype and diagnosis in task-positive ROI
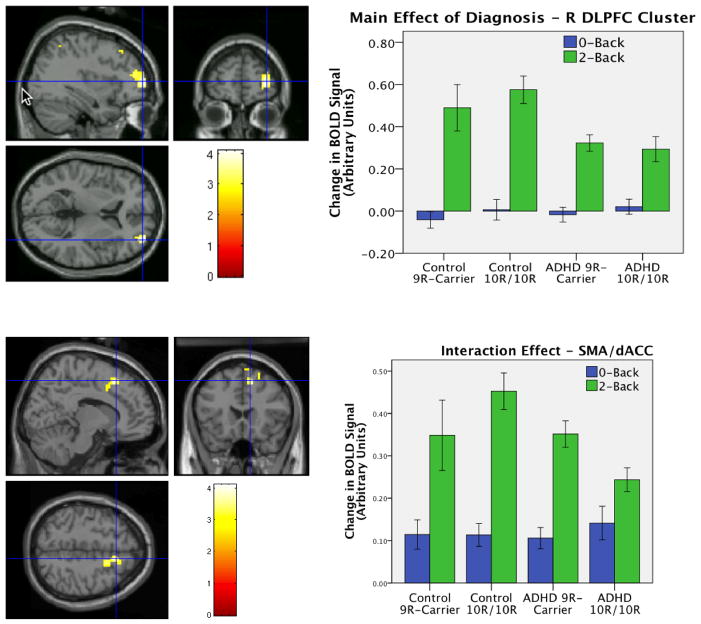
Searching across the task-positive ROI we found a main effect of diagnosis in the R DLPFC (peak voxel [36 57 6], K = 113, cluster-level corrected p = 0.047). Examination of beta values from this cluster shows that this effect was due to reduced BOLD signal change in the ADHD compared to control participants (see Figure 3 top). We also found a marginal interaction effect in the dACC/pre-supplementary motor area (pre-SMA; peak voxel [12 21 51], k = 94, cluster-level corrected p = 0.081; see Figure 3 bottom) indicating that 9R genotype predicted decreased activity in this region in the controls and increased activity in the ADHD group. No main effects of genotype were found in the task-positive ROI.
Figure 3.
Effects in task-positive ROI: Main effect of diagnosis (top) found in right DLPFC (peak voxel 36 57 6, k = 113, p-corr = 0.047) and trend interaction effect (bottom) in left dACC/pre-SMA (peak voxel 12 21 51, k = 94, p-corr = 0.081). Bars show average signal change across cluster.
3.7. Relationship of ADHD symptoms to brain response
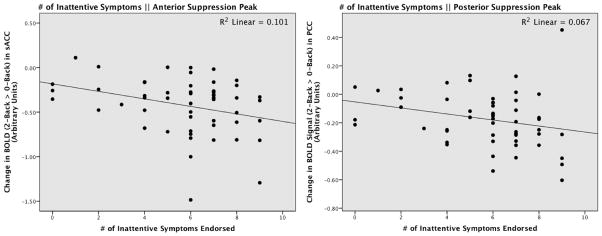
We found a marginally significant negative relationship of suppression in sACC (r = −0.237, p = 0.088) and suppression in PCC (r = −0.241, p = 0.082) to total number of ADHD symptoms endorsed on the structured interview. Total symptoms endorsed were not related to either of the task-positive correlation ROIs (all p’s > 0.72). Suppression in sACC was significantly related to (r = −0.318, p = 0.020), and suppression in PCC was marginally related to (r = −0.260, p = 0.060) number of inattentive symptoms within the ADHD group, suggesting that greater default suppression predicted more inattentive symptoms (see Figure 4). We did not find any evidence that either of the task-positive peaks were related to inattentive symptoms (R DLPFC: r = 0.012, p = 0.931; L Inf Par Lobule: r = 0.055, p = 0.695).
Figure 4.
Scatterplots showing relationship of suppression in the sACC (Left: r = −0.318, p = 0.020), and PCC (Right: r = −0.260, p = 0.060) to number of Inattentive Symptoms endorsed at structured interview.
4. Discussion
Our study of the relationship of DAT1 genotype and adult ADHD to task-positive and task-negative working memory networks yielded four principal findings: 1) we replicated the association of the 9R allele to adult ADHD, 2) we found that 9R-carriers showed marginally greater task-related suppression in mePFC compared to 10R-homozygotes; 3) we found a marginal genotype × diagnosis interaction in the dACC/pre-SMA, and 4) suppression in peak regions of the task-negative network predicted a greater number of ADHD inattentive symptoms.
Our first novel finding was a marginal relationship between DAT1 genotype and task-related suppression in mePFC (p-corrected = 0.055). These data introduce a novel target for DAT1 and suggest that gene effects on behavior and diagnosis may be at least partially mediated by DMN. The effect of DAT1 variation on DMN has not, to our knowledge, been studied before in any population. Our findings are, however, consistent with a recent PET-fMRI study by Tomasi et al (2009). They found that lower striatal DAT binding levels predicted greater DMN suppression in healthy adults, and we found that a genotype shown to result in lower striatal DAT expression (Heinz et al., 2000; Mill et al., 2002; Brookes et al., 2007) also predicted greater suppression of DMN. Together, the two findings suggest that genotypic variance in DAT1 may affect DMN via striatal DA expression.
Our second novel finding was a relationship of task-related suppression of DMN to inattentive ADHD symptoms in adult patients. Based on previous literature supporting a negative relationship between mind-wandering/task-inappropriate thoughts and DMN suppression, we hypothesized that less suppression would predict more inattentive symptoms, but in fact we found that it predicted fewer. Importantly, this finding need be interpreted in the context of a lack of relationship between inattentive symptomology and performance on the n-back task (all r’s < 0.2, all p’s > 0.16), meaning that patients with a greater number of inattentive symptoms performed equally as well on the task as those ADHD participants with fewer inattentive symptoms. The correlation therefore suggests that the more inattentive ADHD subjects needed a greater magnitude of DMN suppression in order to perform the task as well as those participants with fewer symptoms. These findings extend the relationship of task-related suppression to a clinical inattentive phenotype measured outside the scanner, but suggest that these relationships must be interpreted in the context of task performance.
Additionally, we found that the 9R allele was associated with adult ADHD as well as marginally associated with increased DMN suppression; therefore it is not surprising that DMN suppression would predict ADHD severity in adults. These data converge with data from the child literature, which finds an association of both the 10R allele and decreased DMN suppression with ADHD. Thus, it is possible that DMN suppression is directionally linked to the two common DAT1 alleles, and that while the 10R childhood ADHD risk allele (Yang et al., 2007; Gizer et al., 2009) may predispose towards a lack of DMN suppression (as seen in neuroimaging studies of ADHD children; (Fassbender et al., 2009; Peterson et al., 2009)), the 9R adult ADHD risk allele (Franke et al., 2010) may predispose towards increased task related DMN suppression (as suggested by the current study). This relationship therefore poses task-related suppression of DMN on the path from the DAT1 risk allele to expression of symptoms in both child and adult ADHD.
In this adult sample, we did not find a diagnosis effect anywhere in our task-negative ROI, contrary to previous reports showing decreased task-related suppression in children with ADHD (Fassbender et al., 2009; Peterson et al., 2009). The lack of findings may be related to the high performance rates of both our groups on the fMRI task. Both ADHD and control groups performed the task well, and to equal levels. It may therefore be that task-related suppression is only altered in ADHD when task demands surpass an attentional or difficulty threshold. For instance, Fassbender et al (2009) found that mePFC was significantly less suppressed in those ADHD children who showed greater RT variability. Since we did not find differences in RT variability between the diagnosis groups, it may be that the task parameters associated with DMN alterations in ADHD were not captured by our task, which may be a function of age (e.g., task more difficult for children). Future studies should test the effect of varying task demands on DMN response in ADHD.
Contrary to findings by Bertolino et al. (2006; 2008; 2009), who found a main effect of DAT1 in DLPFC during N-back tasks, we did not find a main effect of DAT1 anywhere in our task-positive ROI. This discrepancy may be due to a lack of power in our group of control participants carrying the 9R allele, which only contained 12 subjects, or because of our use of ADHD patients and control subjects as opposed to control subjects only. The marginal genotype × diagnosis interaction effect did however suggest that genotype effects were in different directions in the ADHD and control samples. In ADHD the dACC/pre-SMA was found to be hypoactive in the 10R/10R group, consistent with a previous study in an overlapping sample (Brown et al., 2010) during a different (interference) task. These findings suggest that in task-positive medial wall regions, DAT1 genotype effects may differ in the context of other ADHD-related risk factors.
Our finding of ADHD “hypofrontality” is consistent with many previous neuroimaging studies in both adults and children with ADHD including our own (Valera et al., 2005; Valera et al., 2010), and with dominant theories about the neuroanatomical underpinnings of the disorder (see Paloyelis et al., 2007 for review). The current sample overlaps with an initial report of brain activity during the n-back task by Valera et al (2005) which includes only 27 (30%) of participants in the current report), and with a subsequent report (Valera et al., 2010) which added additional subjects (includes 64 (70%) of participants in current report). In these studies, R DLPFC was found respectively to be marginally and significantly less active in the adults with ADHD, and thus our DLPFC findings are not new. However, the three overlapping reports strongly support DLPFC alterations in adults with ADHD, particularly in its role supporting working memory.
We replicated the meta-analytic study of Franke et al (2010), finding an association between the 9R allele and ADHD in adults. These findings are contrary to the association of the 10R allele to ADHD in children, which have also been confirmed with meta-analysis (Faraone et al., 2005; Yang et al., 2007; Gizer et al., 2009). As mentioned above, it should be noted however that this discrepancy is consistent with our principal brain findings: in ADHD children DMN suppression (which we found to be linked to DAT1) has been found to be lower in ADHD (Fassbender et al., 2009; Peterson et al., 2009), whereas in our adult sample DMN suppression was found to be increased with ADHD severity.
Limitations of our study include an unbalanced design, so that although we had a large sample size (N = 91), power was reduced by our smallest cell (N=12). Further, even though genotypes did not statistically differ in terms of ADHD medication history, the mixed history in our sample may be a confound given the effect of psychostimulants on DAT, and the unknown effect of previous psychopharmacological treatment on brain function. We also included ADHD subjects with varied DSM-IV subtypes and participants who had recently remitted from ADHD. Future studies might replicate results with a medication naïve sample, and test the effect of persistence and/or DSM-IV subtypes on brain data and the link to genes. Because data was collected and analyzed over an extended period (2001–2008), future studies should replicate the findings with newer methods. For instance, our paradigm employed a classic block design which has limitations including the inability to model individual responses, and we chose a univariate analytical approach which does not explore connectivity within or across components as can be done with an Independent Components Analysis. Finally, readers should take caution in interpreting the genotype and interaction effects as both of these findings were only marginally significant after correction for multiple comparisons across the entire respective ROI.
Despite these considerations, we found that the 9R allele was associated with both the diagnosis of ADHD and marginally with increased suppression in DMN in adults, the latter of which was associated with a greater number of inattentive symptoms. These findings therefore suggest that task-related suppression of DMN might act as an intermediate phenotype between DAT1 and ADHD. Further, the differences in direction between our DMN findings in this adult sample and those found previously in children mirror the DAT1 gene effects in adults vs. children with ADHD. While the childhood ADHD profile is associated with 10R-homozygosity and decreased task-related suppression in ADHD, our study in adults suggests associations to both the 9R allele and increased DMN suppression. These findings therefore introduce not only a novel neural target for DAT1, but provide a basis for future longitudinal studies to investigate differences in gene and brain effects between individuals with and without persistent forms of ADHD.
Acknowledgments
The authors would like to thank Sharmila Bandyopadhyay, Marlene Oscar Berman, Denise Boriel, Katie Crum, Kalika Kelkar, Alexandra Lomedico, Ksenija Marinkovic, Snezana Milanovic, Michael Schiller, Heidi Thermenos, Michael Vitulano, and our research study volunteers for their generous assistance.
Funding/Support: This research was supported by grants from: NIMH MH 62152 (LJS), MH 57934 (SF), MH 071535 (EV), HD37694 (SF), MH64019 (TS), HD36317 (JB); Boston University School of Medicine, Division of Graduate Medical Sciences Graduate Student Research Fellowship (AB); the National Alliance for Research on Schizophrenia and Depression Distinguished Investigator Award (JB); Janssen Pharmaceuticals (JB); the March of Dimes Foundation (LJS), the Mental Illness and Neuroscience Discovery (MIND) Institute (LJS); the Kimmerly-Neil Fund for the Study of Cognition and Psychiatric Disorders in Children; the Pediatric Psychopharmacology Council Fund; and the National Center for Research Resources (P41RR14075).
Footnotes
Financial Disclosures
Dr. Ariel Brown reports no conflicts of interests.
Dr. Joseph Biederman is currently receiving research support from the following sources: Elminda, Janssen, McNeil, and Shire. In 2010, Dr. Joseph Biederman did not receive any outside income. In 2009, Dr. Joseph Biederman received a speaker’s fee from the following sources: Fundacion Areces, Medice Pharmaceuticals, and the Spanish Child Psychiatry Association. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, Alza, AstraZeneca, Bristol Myers Squibb, Celltech, Cephalon, Eli Lilly and Co., Esai, Forest, Glaxo, Gliatech, Janssen, McNeil, Merck, NARSAD, NIDA, New River, NICHD, NIMH, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, The Prechter Foundation, Shire, The Stanley Foundation, UCB Pharma, Inc. and Wyeth.
Dr. Eve M. Valera has received travel support and honoraria from the McNeil and Janssen divisions of Ortho-McNeil-Janssen Pharmaceuticals and anticipates receiving honoraria from Galenea Pharmaceuticals.
Dr. Nikos Makris reports no conflicts of interest.
Dr. Alysa Doyle reports no conflicts of interest.
Dr. Susan Whitfield-Gabrieli reports no conflicts of interest.
Dr. Eric Mick receives research support from the following sources: Ortho-McNeil Janssen Scientific Affairs, Pfizer, Shire Pharmaceuticals, and has been an advisory board member for Shire Pharmaceuticals.
Dr. Thomas Spencer has received research support from, has been a speaker for or on a speaker bureau or has been an Advisor or on an Advisory Board of the following sources: Shire Laboratories, Inc, Eli Lilly & Company, Glaxo-Smith Kline, Janssen Pharmaceutical, McNeil Pharmaceutical, Novartis Pharmaceuticals, Cephalon, Pfizer and the National Institute of Mental Health.
Dr. Stephen Faraone has, in the past year received consulting fees and has been on Advisory Boards for Eli Lilly, Ortho-McNeil and Shire Development and has received research support from Shire and the National Institutes of Health. In previous years, Dr. Faraone has received consulting fees or has been on Advisory Boards or has been a speaker for the following sources: Shire, McNeil, Janssen, Novartis, Pfizer, Ortho-McNeil and Eli Lilly. In previous years he has received research support from Eli Lilly, Shire, Pfizer and the National Institutes of Health.
Dr. Larry Seidman reports no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nature Review Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Bayerl M, Dielentheis TF, Vucurevic G, Gesierich T, Vogel F, Fehr C, Stoeter P, Huss M, Konrad A. Disturbed brain activation during a working memory task in drug-naive adult patients with ADHD. Neuroreport. 2010;21:442–446. doi: 10.1097/WNR.0b013e328338b9be. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Schulz KP, Cook EH, Jr, Fan J, Clerkin SM, Ivanov I, Halperin JM, Newcorn JH. Dopamine transporter gene variation modulates activation of striatum in youth with ADHD. Neuroimage. 2010;53:935–942. doi: 10.1016/j.neuroimage.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, Nardini M, Weinberger DR, Dallapiccola B. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. Journal of Neuroscience. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Di Giorgio A, Blasi G, Sambataro F, Caforio G, Sinibaldi L, Latorre V, Rampino A, Taurisano P, Fazio L, Romano R, Douzgou S, Popolizio T, Kolachana B, Nardini M, Weinberger DR, Dallapiccola B. Epistasis between dopamine regulating genes identifies a nonlinear response of the human hippocampus during memory tasks. Biological Psychiatry. 2008;64:226–234. doi: 10.1016/j.biopsych.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Di Giorgio A, Blasi G, Romano R, Taurisano P, Caforio G, Sinibaldi L, Ursini G, Popolizio T, Tirotta E, Papp A, Dallapiccola B, Borrelli E, Sadee W. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. Journal of Neuroscience. 2009;29:1224–1234. doi: 10.1523/JNEUROSCI.4858-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Knee D, Munir K. Retrospective assessment of DSM-III attention deficit disorder in nonreferred individuals. Journal of Clinical Psychiatry. 1990;51:102–106. [PubMed] [Google Scholar]
- Biederman J, Makris N, Valera EM, Monuteaux MC, Goldstein JM, Buka S, Boriel DL, Bandyopadhyay S, Kennedy DN, Caviness VS, Bush G, Aleardi M, Hammerness P, Faraone SV, Seidman LJ. Towards further understanding of the co-morbidity between attention deficit hyperactivity disorder and bipolar disorder: a MRI study of brain volumes. Psychological Medicine. 2008;38:1045–1056. doi: 10.1017/S0033291707001791. [DOI] [PubMed] [Google Scholar]
- Brookes KJ, Neale BM, Sugden K, Khan N, Asherson P, D’Souza UM. Relationship between VNTR polymorphisms of the human dopamine transporter gene and expression in post-mortem midbrain tissue. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2007;144B:1070–1078. doi: 10.1002/ajmg.b.30572. [DOI] [PubMed] [Google Scholar]
- Brown AB, Biederman J, Valera EM, Doyle AE, Bush G, Spencer T, Monuteaux MC, Mick E, Whitfield-Gabrieli S, Makris N, LaViolette PS, Oscar-Berman M, Faraone SV, Seidman LJ. Effect of dopamine transporter gene (SLC6A3) variation on dorsal anterior cingulate function in attention-deficit/hyperactivity disorder. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2010;153B:365–375. doi: 10.1002/ajmg.b.31022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Science. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Forman SD, Braver TS, Casey BJ, Serven-Schrelber D, Noll DC. Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Human Brain Mapping. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S. Imaging genetics in ADHD. Neuroimage. 2010;53:832–838. doi: 10.1016/j.neuroimage.2010.02.071. [DOI] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, Steenhuis MP, Minderaa RB, Buitelaar JK, Kahn RS, van Engeland H. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Molecular Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Mulder MJ, Casey BJ, Ziermans TB, Vessaz MN, Van Engeland H. Dopamine transporter genotype conveys familial risk of attention-deficit/hyperactivity disorder through striatal activation. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:61–67. doi: 10.1097/chi.0b013e31815a5f17. [DOI] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, von Cramon DY, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Feighner JA, Monuteaux MC. Assessing symptoms of attention deficit hyperactivity disorder in children and adults: which is more valid? Journal of Consulting and Clinical Psychology. 2000;68:830–842. [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Research. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press; Washington, D.C: 1997. [Google Scholar]
- Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti-Hummer A, Heine M, Jacob CP, Lesch KP, Casas M, Ribases M, Bosch R, Sanchez-Mora C, Gomez-Barros N, Fernandez-Castillo N, Bayes M, Halmoy A, Halleland H, Landaas ET, Fasmer OB, Knappskog PM, Heister AJ, Kiemeney LA, Kooij JJ, Boonstra AM, Kan CC, Asherson P, Faraone SV, Buitelaar JK, Haavik J, Cormand B, Ramos-Quiroga JA, Reif A. Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology. 2010;35:656–664. doi: 10.1038/npp.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18:485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Jastak J, Jastak S. Wide Range Achievement Test. 3. Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Kang AM, Palmatier MA, Kidd KK. Global variation of a 40-bp VNTR in the 3′-untranslated region of the dopamine transporter gene (SLC6A3) Biological Psychiatry. 1999;46:151–160. doi: 10.1016/s0006-3223(99)00101-8. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Krause J. SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder. Expert Review of Neurotherapeutics. 2008;8:611–625. doi: 10.1586/14737175.8.4.611. [DOI] [PubMed] [Google Scholar]
- Li CS, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38:640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cerebral Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Biederman J, Spencer T, Faraone SV, Sklar P. Absence of association with DAT1 polymorphism and response to methylphenidate in a sample of adults with ADHD. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2006;141B:890–894. doi: 10.1002/ajmg.b.30376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. American Journal of Medical Genetics. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Monuteaux MC, Seidman LJ, Faraone SV, Makris N, Spencer T, Valera E, Brown A, Bush G, Doyle AE, Hughes S, Helliesen M, Mick E, Biederman J. A preliminary study of dopamine D4 receptor genotype and structural brain alterations in adults with ADHD. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2008;147B:1436–1441. doi: 10.1002/ajmg.b.30870. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children: Epidemiologic Version. Nova University; Fort Lauderdale, FL: 1987. [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional MRI in ADHD: a systematic literature review. Expert Review of Neurotherapeutics. 2007;7:1337–1356. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Plessen KJ, Yu S. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. American Journal of Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein HG, Tischmeyer W, Gundelfinger ED, Heinze HJ, Duzel E. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. Journal of Neuroscience. 2006;26:1407–1417. doi: 10.1523/JNEUROSCI.3463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience and Biobehavioral Review. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Stollstorff M, Foss-Feig J, Cook EH, Jr, Stein MA, Gaillard WD, Vaidya CJ. Neural response to working memory load varies by dopamine transporter genotype in children. Neuroimage. 2010;53:970–977. doi: 10.1016/j.neuroimage.2009.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Telang F, Wang GJ, Chang L, Ernst T, Fowler JS. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4:e6102. doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP. Network homogeneity reveals decreased integrity of default-mode network in ADHD. Journal of Neuroscience Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Valera EM, Brown A, Biederman J, Faraone SV, Makris N, Monuteaux MC, Whitfield-Gabrieli S, Vitulano M, Schiller M, Seidman LJ. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. American Journal of Psychiatry. 2010;167:86–9. doi: 10.1176/appi.ajp.2009.09020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- van de Giessen EM, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. Journal of Nuclear Medicine. 2009;50:45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. Journal of Nuclear Medicine. 2005;46:745–751. [PubMed] [Google Scholar]
- Vance A, Silk TJ, Casey M, Rinehart NJ, Bradshaw JL, Bellgrove MA, Cunnington R. Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Molecular Psychiatry. 2007;12:826–832. 793. doi: 10.1038/sj.mp.4001999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. American Journal of Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive Affective and Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale III [manual] 3. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Plichta MM, Sambataro F, Fallgatter AJ, Jacob C, Lesch KP, Herrmann MJ, Schonfeldt-Lecuona C, Connemann BJ, Gron G, Vasic N. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Human Brain Mapping. 2009;30:2252–2266. doi: 10.1002/hbm.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Chan RC, Jing J, Li T, Sham P, Chen RY. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3′-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2007;144:541–550. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]