Abstract
Background
Fibroblast heterogeneity is recognized and fibroblasts from diseased tissues, including asthma, have functional phenotypes that differ from normal. However, progenitor progeny relationships and the factors that control fibroblast differentiation are poorly defined.
Objective
To determine if interleukin-4 could alter the functional phenotype of fibroblasts during their differentiation from stem/progenitor cells.
Methods
Using a three-dimensional collagen gel system, we embryoid bodies derived from human embryonic stem cells and recovered spindle-shaped cells consistent with fibroblasts that had differentiated in the presence or absence of interleukin-4.
Results
Interleukin-4 induced fibroblast-like cells were more active in contracting collagen gels, in migration and in producing fibronectin than control (without interleukin-4) cells. Interleukin-4 induced cells demonstrated less expression of miR-155 that modulated contraction, migration and fibronectin production. These differences persisted in culture without further addition of interleukin-4, suggesting the differentiated phenotype might be a permanent alteration.
Conclusion
The current study demonstrates that interleukin-4 induces differentiation of stem/precursor cells into fibroblast-like cells that demonstrate a more “fibrogenic” phenotype, which is due to reduced expression of miR-155. These findings provide a novel mechanism for the persistent abnormalities in IL-4 related diseases and a novel target to regulate tissue remodeling by fibroblasts.
Keywords: embryonic stem cells, fibroblasts, IL-4, fibrosis, asthma, microRNA, miR-155, chemotaxis, collagen gel contraction, TGF-β
Introduction
Fibroblasts are ubiquitous mesenchymal cells that are believed to be responsible for the production and maintenance of extracellular matrix 1, 2. They are also potent sources of growth factors and can modulate the proliferation of epithelial and endothelial cells. In addition, fibroblasts respond vigorously to a variety of mediators that are released at sites of injury 3, 4. Thus, fibroblasts are thought to mediate repair directly by their actions on matrix and indirectly by their actions on other structural cells. Moreover, fibroblast heterogeneity has been described in several fibrotic disorders 5–7. Similarly, a number of studies have been demonstrated altered phenotypes of fibroblasts in airway diseases including asthma 8–10 and chronic obstructive pulmonary disease 11–14. Although their heterogeneity is widely recognized, the source(s) of fibroblasts and the genesis of the various subpopulations characterized by distinct phenotypes remain undefined. Potential sources are thought to be expansion of locally resident fibroblasts, differentiation from a local stem/precursor population and recruitment from circulating precursor populations 15–18. Importantly, the dynamic nature of the fibroblast population supports the concept that these cells can be targeted therapeutically.
We have established a reliable methodology that permits in vitro differentiation of fibroblast-like cells derived from human embryonic stem cells (hESCs). The current study was designed to determine if a specific cytokine could induce an altered phenotype of fibroblast-like cells derived from stem cells in this system. In the current study, we used interleukin-4 (IL-4), which is a profibrotic cytokine that is characteristically present in the airways in asthma, and demonstrate that IL-4 induces differentiation of hESCs into fibroblast-like cells with a more “fibrogenic” phenotype and that this is due to reduced expression of microRNA (miRNA) miR-155. This provides a novel target to regulate tissue remodeling by fibroblasts and a mechanism for asthma persistence despite anti-inflammatory therapy and long term “quiescence”.
Methods
Materials
Native type I collagen (rat tail tendon collagen [RTTC]) was extracted from rat-tail tendons using a previously published method 19. Details relating to commercially available reagents are provided in the Supplemental materials.
Human embryonic stem cell culture and differentiation in three-dimensional type I collagen gels
At the time of this study, the National Institutes of Health (NIH)-approved and University of Nebraska Medical Center (UNMC) Embryonic Stem Cell Research Oversight (ESCRO) and Institutional Review Board (IRB)-approved human embryonic stem cellline H9.2 (WiCell Research Institute, Madison, WI) was used. Undifferentiated hESCs were cultured on irradiated mouse embryonic fibroblasts in 6-well plates with hESCs culture medium containing 80% Dulbecco’s Modified Eagle Medium (DMEM)/F12, 20% KnockOut™ serum replacement, 1% non-essential amino acid, 1 mM L-glutamine, 0.1 mM 2-mercaptoethanol, and 4 ng/ml of basic fibroblast growth factor (bFGF). Colonies were mechanically dissected using finely pulled glass micropipettes (1.0mm OD; Clark Electromedical Instruments, Reading, UK) every 7 days and transferred to a freshly prepared MEF layer.
To prepare embryoid bodies (EBs), hESCs were treated with 1 mg/ml collagenase and cells were collected by centrifugation at 200 × g for 2 min. The pellet was resuspended in differentiation medium containing 90% DMEM/F12, 10% serum replacement, 1% non-essential amino acid, and 1 mM L-glutamine without 2-mercaptoethanol and bFGF 20. Cells were then placed into a bacteriological petri dish (Sarstedt, Nümbrecht, Germany) and cultured for 4–5 days. Floating EBs were collected into a 50 ml polypropylene conical tube (Falcon; Beckton-Dickinson Labware, Franklin Lakes, NJ) and precipitated without centrifugation.
Collagen gels containing EBs were prepared as described previously 21. The cultured gels, into which differentiated fibroblasts had migrated, were dissolved with 1 mg/ml collagenase at 37 °C in a 5% CO2 atmosphere for 1 h. The resulting cells were resuspended with DMEM containing 10% fetal bovine serum (10% FBS-DMEM) and centrifuged at 200 × g for 5 min. The cells, including the EBs, were cultured in a 100 mm tissue culture dish (Falcon) with 10% FBS-DMEM, 45 units/ml penicillin, 45 μg/ml streptomycin, and 1 μg/ml amphotericin B. When the fibroblasts, which grew as a monolayer, were near confluent, the cells were trypsinized and passaged in 10% FBS-DMEM. Contaminating EBs were lost with serial feeding.
Immunocytochemistry
Differentiated fibroblast-like cells were cultured until sub-confluence in 8-chamber slides (Nunc Inc, Naperville, IL) in 10% FBS-DMEM and fixed in 4% paraformaldehyde for 30 min. Cells were washed in phosphate buffered saline before permeabilization and following each subsequent step. Blocking was performed using by horse serum for 1 h. Permeabilization was performed in 0.1% sodium citrate buffer with 0.1% Triton at 4 °C for 5 min. Cells were incubated sequentially with monoclonal anti-vimentin (1:200 dilution), anti-alpha smooth muscle actin (α-SMA; 1:50 dilution), anti-pan cytokeratin (1:200) or anti-desmin (1:20) antibodies at 4 °C overnight. Cells were then incubated with fluorescein isothiocyanate stain-green immunofluoresence (FITC) conjugated anti-mouse IgG antibody and stained with propidium iodide after washing. Stained cells were visualized and photographed using a Nikon Eclipse TE300 microscope (Nikon, Tokyo, Japan) equipped with a DP71 digital camera (Olympus, Tokyo, Japan).
Collagen gel contraction assay
Collagen gels contraction assays were performed as described previously 21.
Chemotaxis assay
Cell migration was assessed using the Boyden blindwell chamber (Neuro Probe Inc., Gaithersburg, MD) as previously described 22.
Proliferation assay
Cells were transferred to 12-well plates (2 × 104 cells/well) in 10% FBS-DMEM and cultured. Cell numbers from three separate wells were determined using a Coulter electronic cell counter (Beckman Coulter Inc., Fullerton, CA).
Measurement of TGF-β1, fibronectin and PGE2
To evaluate mediator production, cells were cultured until sub-confluent. Cells were then treated with or without transforming growth factor (TGF)-β1 (100 pM) in serum-free DMEM for 48 h. Quantification of TGF-β1 was performed by enzyme-linked immunosorbent assay (ELISA) 21. Fibronectin was assayed with an inhibition ELISA that specifically detects human but not bovine fibronectin 23. Prostaglandin (PG) E2 production was determined by enzyme immunoassay (EIA; Cayman Chemical, Ann Arbor, MI) following the manufacturer’s instructions.
Western blot analysis
α-SMA was quantified by western blot as described 24.
MicroRNA preparation and microarray assay
MiRNAs were extracted using mirVana miRNA Isolation Kit (Ambion, Applied Biosystem, Austin, TX) following the manufacturer’s instructions. MiRNA expression was profiled by using miRCURY LNA microRNA Array (v. 11.0, Exiqon, Vedbaek, Denmark) following the manufacturer’s instructions. Raw data were analyzed by using GenePix Pro 6.1 (Molecular Devices, Sunnyvale, CA).
Real time PCR
Real time PCR for miR-155 quantification was performed with commercially available reagents (see Supplemental Materials for details).
Transfection of microRNA Mimic or Inhibitor
MicroRNA mimic and inhibitor were transfected into cells using lipofectamine (see Supplemental Materials for details).
Wound closure assay
In vitro wound closure was performed and quantified as described 25. To explore the effect of miR-155 mimic or inhibitor on this assay, the transfection was conducted on the second day of culture.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). All data were analyzed using the GraphPad Prism 4 (GraphPad Software, San Diego, CA). Analysis of variance was performed with the use of the non-parametric Kruskal-Wallis test. When applicable, the Mann-Whitney U test was used for comparisons between groups. For all comparisons, three different batches of control and IL-4 induced cells were evaluated, and significance was determined using separate experiments performed on different occasions. A p value of < 0.05 was considered significant.
Results
IL-4 induced fibroblast-like cells demonstrate a “fibrogenic” phenotype
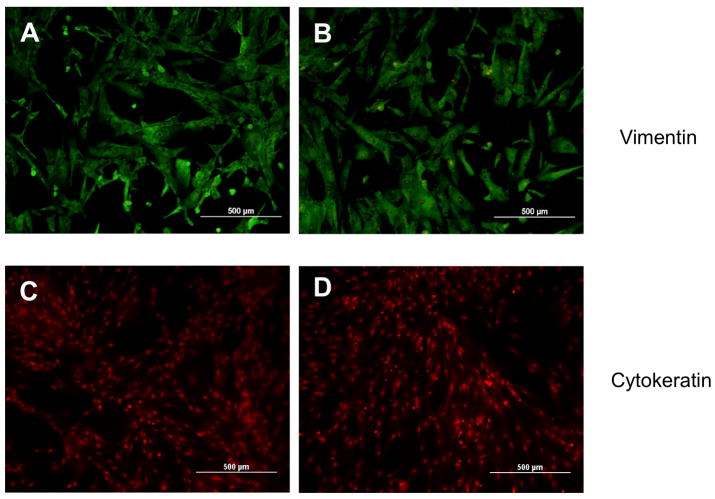
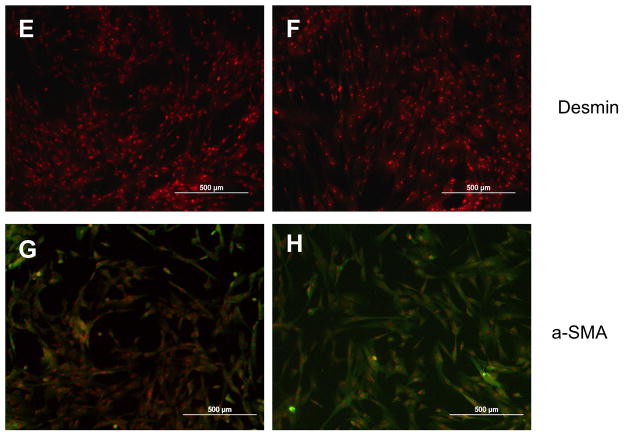
Using three-dimensional type I collagen gel culture, fibroblast-like spindle shaped cells were differentiated from hESCs. The presence of IL-4 during the differentiation did not visibly affect the outgrowth of fibroblast-like cells (Suppl. Fig. 1A, B). When subsequently cultured in monolayer, fibroblast-like cells obtained both in the presence and absence of IL-4 cells demonstrated a characteristic spindle shaped morphology (Suppl. Fig. 2A, B). To evaluate the phenotypes of the fibroblast-like cells derived from hESCs, immnocytochemistry was performed using several cytoskeletal markers. Both IL-4 induced and spontaneously differentiated (control) cells were entirely positive when stained for vimentin (Fig. 1A, B), but completely negative for cytokeratin (Fig. 1C, D) and desmin (Fig. 1E, F). Expression of α-SMA was less extensive in either cell type compared to vimentin expression. However, IL-4 induced cells showed higher intensity of α-SMA staining compared to control cells (Fig. 1G, H). Expression of Thy-1 (CD90) was also less extensive compared to vimentin and no difference between control and IL-4 induced cells was observed (Suppl. Fig. 3A, B). Similarly, both control and IL-4 induced cells strongly expressed collagen type I (Suppl. Fig. 3C, D), but were negative by immunocytochemistry for CD34, CD45 and CD11b (Suppl. Fig. 3E–J).
Figure 1. Representative immunocytochemistry of differentiated fibroblast-like cells.
(A) Vimentin in control cells. (B) Vimentin in IL-4 induced cells. (C) Cytokeratin in control cells. (D) Cytokeratin in IL-4 induced cells. (E) Desmin in control cells. (F) Desmin in IL-4 induced cells. (G) α-SMA in control cells. (H) α-SMA in IL-4 induced cells.
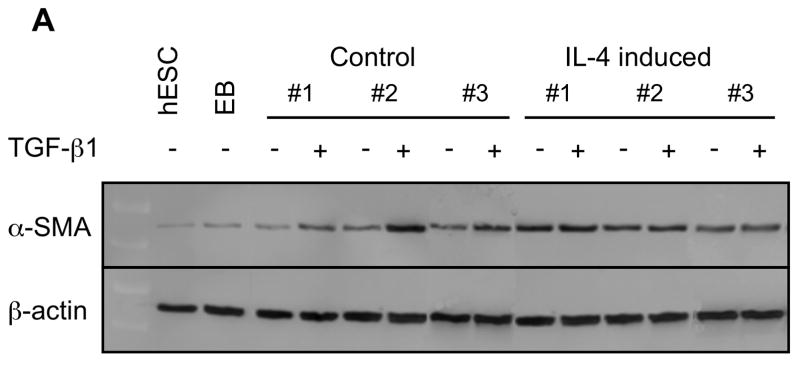
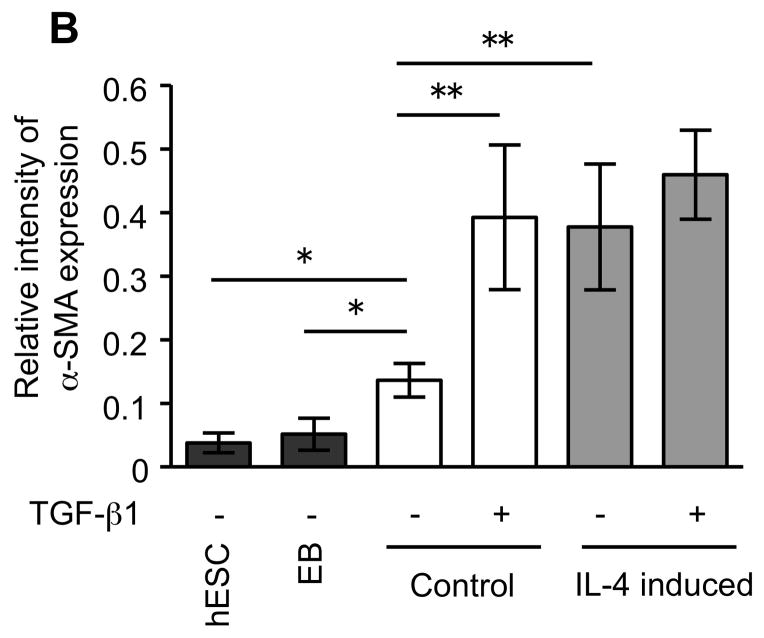
To further confirm the difference of α-SMA expression between control and IL-4 induced fibroblast-like cells, western blot analysis was performed. Both control and IL-4 induced cells demonstrated significantly higher α-SMA expression than the undifferentiated hESCs and EBs (p =0.02 each; Fig. 2A, B). IL-4 induced cells showed α-SMA expression to a greater degree compared to control cells (277% of control, p = 0.008). Following TGF-β1 treatment, α-SMA expression increased in control fibroblast-like cells, but not in IL-4 induced cells.
Figure 2.
α-SMA expression in undifferentiated hESCs, EBs and differentiated fibroblast-like cells. (A) Representative western blots for α-SMA. β-actin expression is shown as a loading control. (B) Densitometric analysis of α-SMA expression expressed relative amount to β-actin. Three different batches of control and IL-4 induced cells (indicated by #1, #2 and #3 in Panel A), each prepared from hECSs on separate occasions, were each evaluated three times. Values are mean ± SD. * p < 0.05, ** p < 0.01.
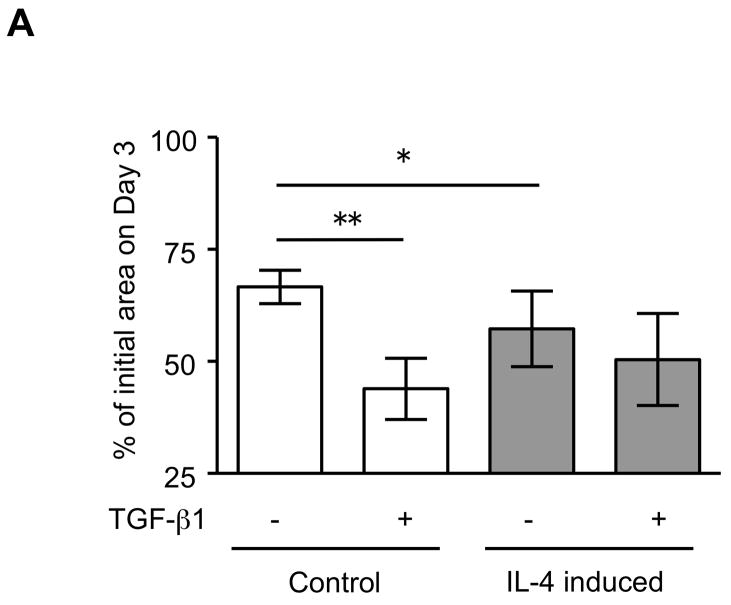
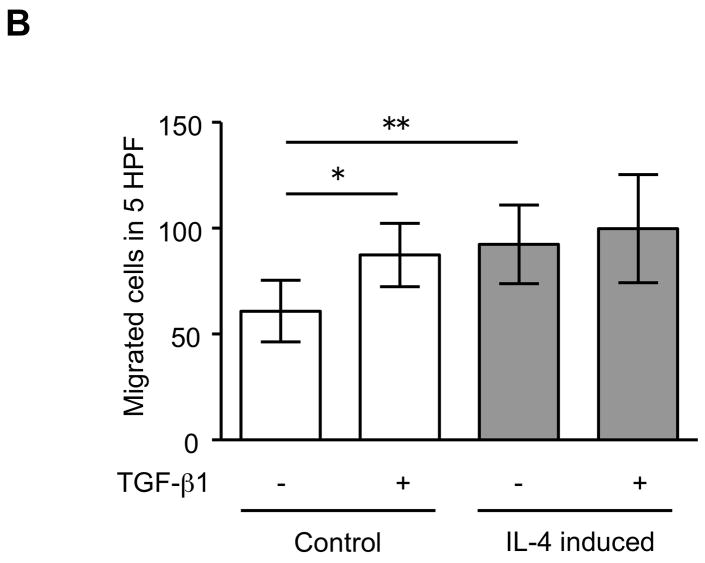
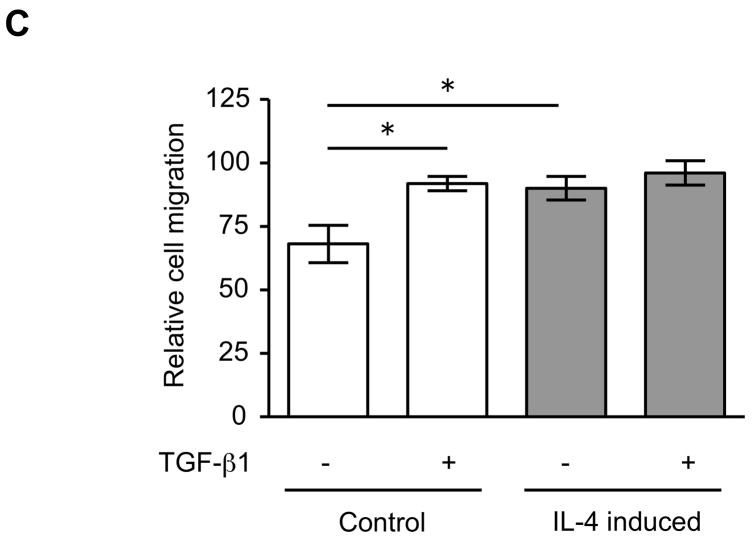
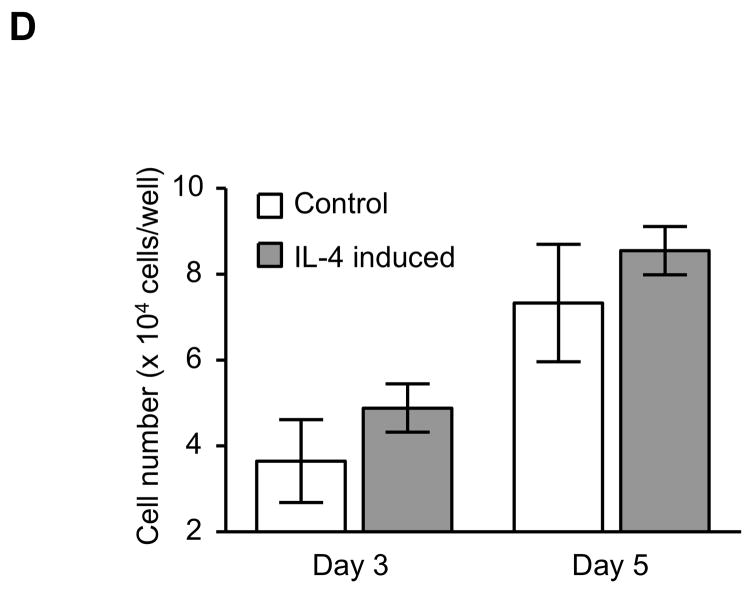
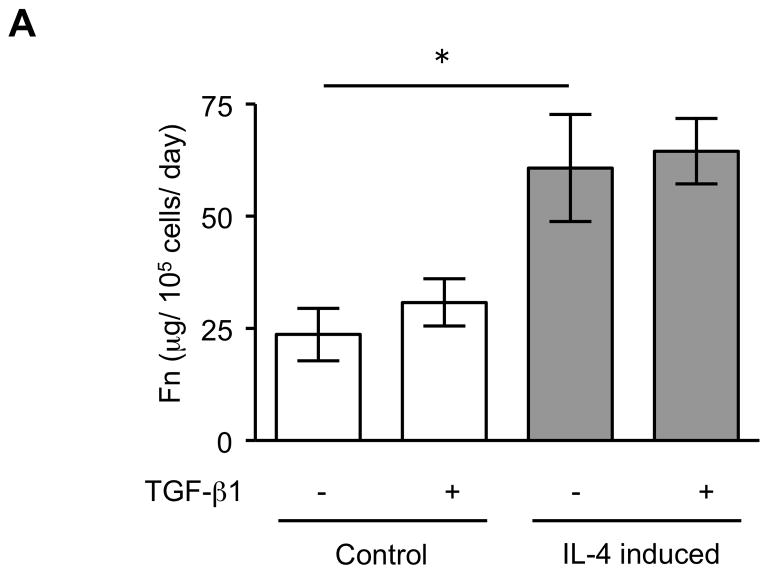
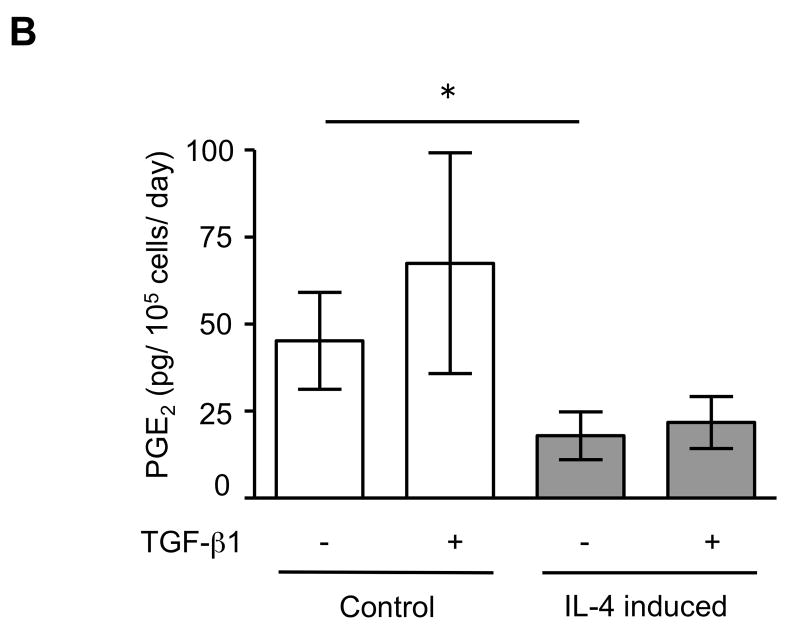
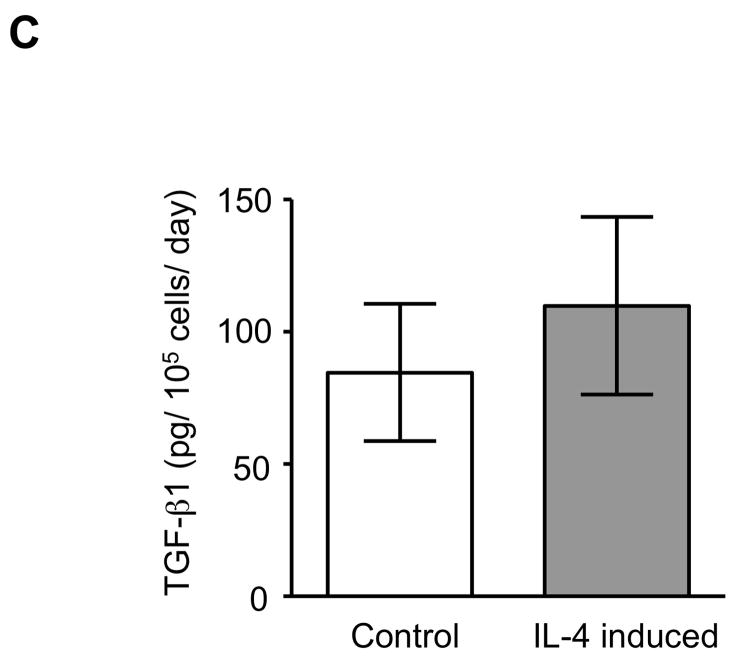
The expression of α-SMA is increased in fibroblasts present in areas of fibrosis 26. This suggested that the increased expression of α-SMA expression in fibroblasts that had differentiated in the presence of IL-4 could be a feature of a distinct functional phenotype. To further evaluate this possibility, we next performed several functional assays associated with repair and fibrosis including contraction of three-dimensional collagen gels, chemotaxis, wound closure and proliferation. IL-4 induced cells were more active in contracting collagen gels and in chemotaxis than control cells (128 and 152% of control, p = 0.02 and 0.009; Fig. 3A, B). Exogenous TGF-β1 significantly stimulated contractile and chemotactic activities in control, but not in IL-4 induced cells. The wound closure assay also indicated that IL-4 induced cells migrated significantly more in the absence of TGF-β1 treatment (p = 0.002; Fig. 3C and Suppl. Fig. 4). Control cells but not IL-4 induced cells clearly showed enhanced on migration when treated with exogenous TGF-β1. With regard to proliferation, IL-4 induced cells showed a 1.3-fold (day 3) and 1.2-fold (day 5) increase in cell numbers but these differences did not differ significantly from control cells (Fig. 3D). To further define the functional differences between control and IL-4 induced cells, we evaluated several secreted factors, fibronectin, PGE2 and TGF-β1, that are believed to differ in cells from fibrotic compared to control tissues. IL-4 induced cells produced more fibronectin (260% of control, p = 0.002; Fig. 4A) and, in contrast, less PGE2 (35% of control, p = 0.002; Fig. 4B). Control cells showed trends towards greater increases of both fibronectin and PGE2 production following TGF-β1 stimulation (p = 0.13 each), whereas there was no response to TGF-β1 stimulation in IL-4 induced cells. IL-4 induced cells also demonstrated a trend toward a greater release of endogenous TGF-β1, but this was not statistically significant (p = 0.11; Fig. 4C).
Figure 3. Physiological differences between control and IL-4 induced fibroblast-like cells derived from hESCs.
(A) Collagen gel contraction. The size of gels was measured on day 3 and shown as percentage of initial area. (B) Chemotaxis towards fibronectin. Chemotaxis was assessed by counting the number of migrated cells in five high-power fields (HPF). (C) Migration of fibroblast-like cells in the wound closure assay. The cell migration is expressed as a percentage of the cell occupied area in the initial wound area. (D) Cell proliferation. Cells were plated at a density of 2 × 104 cells/well in and cultured. Cell numbers were determined on days 3 and 5 using an electronic cell counter. For all experiments, three different batches of control and IL-4 induced cells were evaluated on three separate occasions, each performed in triplicate. Values are mean ± SD. * p < 0.05, ** p < 0.01.
Figure 4. Production of biomarkers from control and IL-4 induced fibroblast-like cells derived from hESCs.
(A) Fibronectin production. (B) PGE2 production. (C) TGF-β1 production. For the measurement of TGF-β1, only media without TGF-β1 were used. Three different batches of control and IL-4 induced cells were evaluated on three separate occasions, each performed in triplicate. Values are mean ± SD. * p < 0.01.
MiR-155 modulates differentiated fibroblast-like cell phenotype
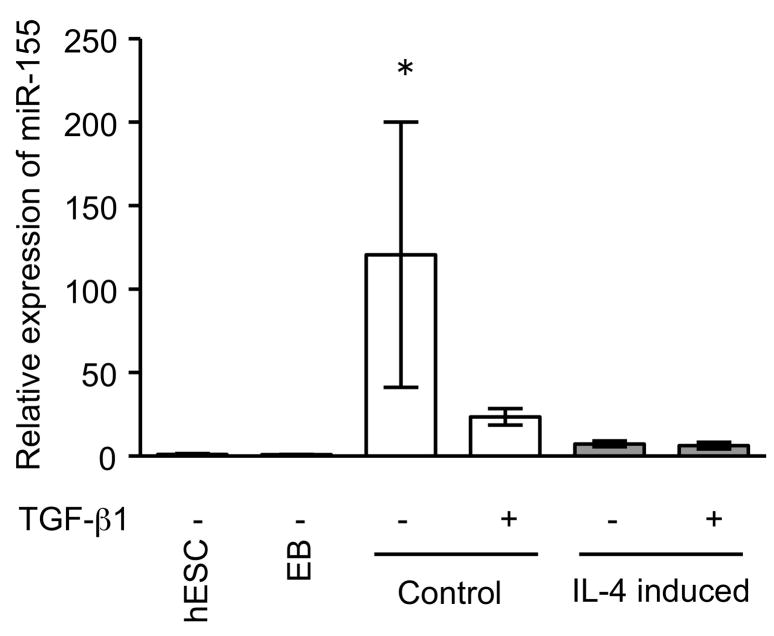
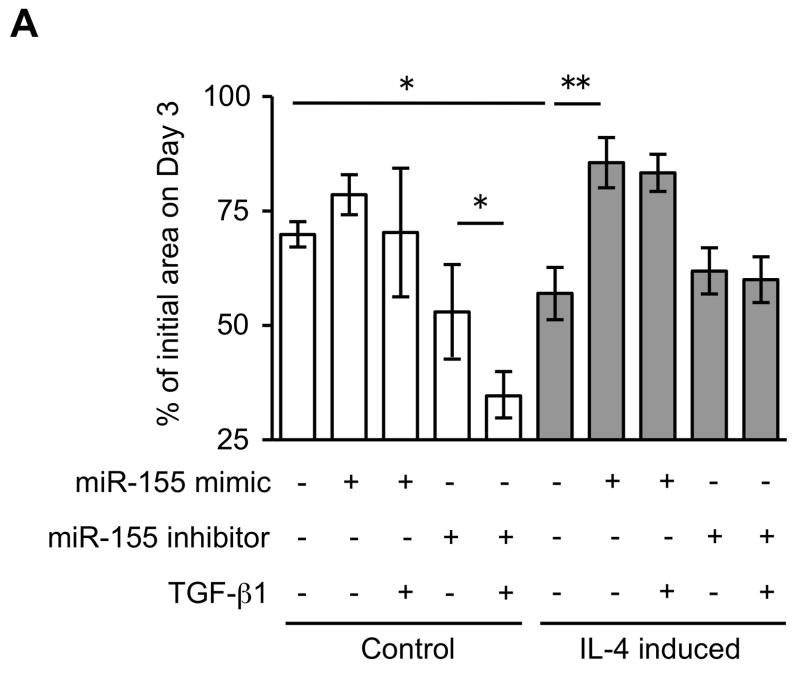
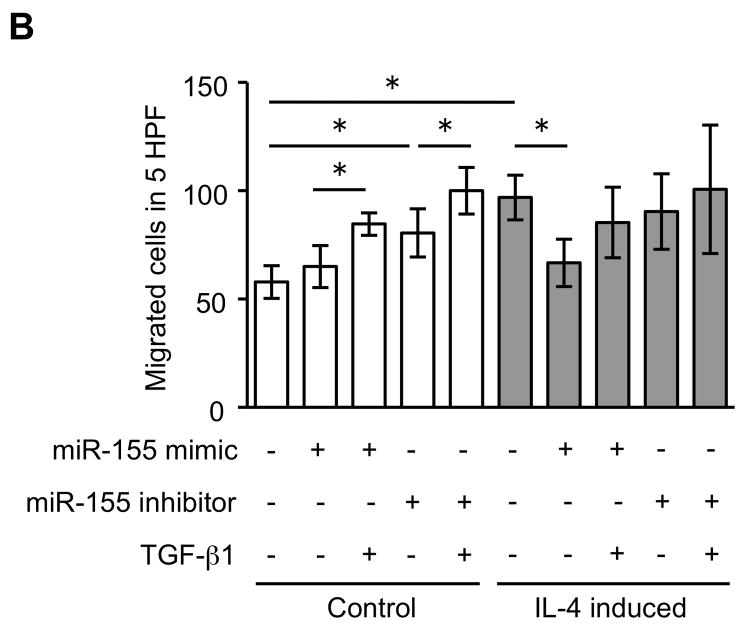
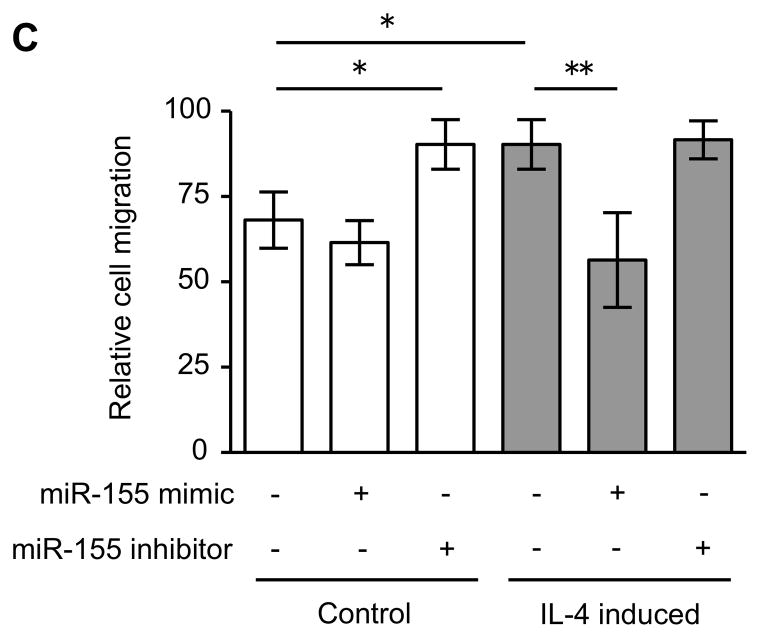
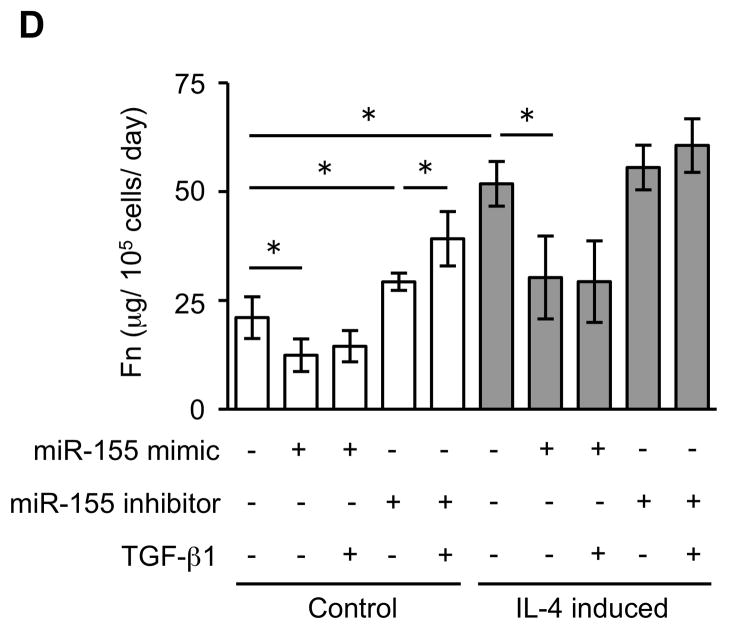
Since miRNAs are known to play a role in development and cell differentiation, we suspected that altered miRNA expression induced by IL-4 may contribute to the altered phenotype of the fibroblast-like cells derived from hESCs. Therefore, we used microarray analysis to assess the miRNA profile of these cells using two different batches each of control and IL-4 induced cells. These two comparisons of miRNA profile indicated that miR-155 was under-expressed in IL-4 induced cells compared to control cells, and thus, miR-155 was considered to be a candidate for differential modulation of the differentiated fibroblast-like cell phenotype (Suppl. Fig. 5). To further confirm the different expression of miR-155 in the two cell types, we next quantified miR-155 expression in undifferentiated hESCs, EBs and differentiated fibroblast-like cells by real time PCR. Undifferentiated hESCs and EBs had essentially undetectable miR-155 (Fig. 5). In contrast, miR-155 was readily detected in differentiated fibroblasts. Importantly, IL-4 induced cells expressed miR-155 levels that were 17 fold lower (p < 0.002) compared to control induced cells. In addition, TGF-β1 significantly reduced the expression of miR-155 in control cells, but had no effect on the IL-4 induced cells. To confirm a mechanistic role for miR-155 in the altered phenotype of IL-4 induced cells, we transfected control and IL-4 induced cells with miR-155 mimic or inhibitor. In control cells, which showed a high level of miR-155 expression, an miR-155 inhibitor enhanced collagen gel contraction (p = 0.13; Fig. 6A), chemotaxis (p = 0.002; Fig. 6B), wound closure (p = 0.02; Fig. 6C and Suppl. Fig. 6) and fibronectin production (p = 0.002; Fig. 6D). In contrast, an miR-155 mimic inhibited contraction (p = 0.002), chemotaxis (p = 0.002), wound closure (p = 0.008) and fibronectin production (p = 0.002) in IL-4 induced cells which demonstrated low miR-155 expression. Neither cell type demonstrated significant changes in PGE2 or TGF-β1 production or α-SMA expression in response to miR-155 (Suppl. Fig. 7A–C). Also miR-155 had no effect on the response to exogenous TGF-β1 in either type of differentiated fibroblast-like cell.
Figure 5. MiR-155 expression in undifferentiated hESCs, EBs and differentiated fibroblast-like cells.
Cell layers of differentiated cells were extracted after 48 h treatment with or without TGF-β1 (100 pM). MiR-155 expression by real time PCR is normalized to the amount of rRNA and expressed as 2−ΔΔCT values. * p < 0.01, compared with other groups.
Figure 6. MiR-155 regulates the differentiated fibroblast-like cell phenotype.
(A) Collagen gel contraction on day 3. (B) Chemotaxis towards fibronectin. (C) Cell migration by wound closure assay. (D) Fibronectin production. Three different batches of control and IL-4 induced cells were evaluated on three separate occasions in all experiments. Values are mean ± SD. * p < 0.05, ** p < 0.01.
Discussion
The present study demonstrates that, in a three-dimensional collagen gel culture system in which fibroblast-like cells differentiate from hESC-derived EBs, the presence of IL-4 during the differentiation process resulted in cells with an altered phenotype. Several functional and biochemical assays indicated that IL-4 induces differentiation of cells with a more “fibrogenic” phenotype compared with control cells which are differentiated in collagen gels without cytokines. Further, the expression of microRNA miR-155 is markedly reduced in cells that differentiated in the presence of IL-4. In support of a mechanistic role for reduced miR-155 expression in the altered functional phenotype, the introduction of an miR-155 mimic to IL-4 induced cells reversed several of “fibrogenic” phenotypes toward the “control” phenotype. Conversely, an miR-155 inhibitor induced control cells to more closely resemble IL-4 induced cells. Taken together, these results suggest that IL-4 alters the differentiation of fibroblast-like cells from hESCs and that this results in reduced expression of miR-155, which, in turn, causes the cells to demonstrate features that resemble cells from fibrotic tissues.
Fibroblasts are heterogeneous mesenchymal cells that play important roles in the production and maintenance of extracellular matrix and regulate other structural cells 1, 2, 27. These cells are routinely cultured as adherent cells and demonstrate a characteristic spindle shaped morphology. However, while fibroblast heterogeneity is clearly recognized, there are no specific markers to distinguish distinct sub-populations of fibroblasts. In the current study, we demonstrated that the differentiated cells stained positively for vimentin, a non-specific mesenchymal marker, and failed to stain both for cytokeratin, an epithelial marker, and desmin, a marker of muscle cells. Both control and IL-4 induced cells showed positive expression of α-SMA, which is often used as a marker of myofibroblasts. However, α-SMA fails to distinguish between myofibroblasts and smooth muscle cells 15. While desmin is a marker of smooth muscle cells, it can be lost after smooth muscle cells are maintained in culture 28. Thy-1 (CD90) is an outer membrane leaflet glycoprotein that is expressed on subsets of neural cells, lymphocytes, and fibroblasts 29. The current study showed positive staining for Thy-1 on both control and IL-4 induced fibroblast-like cells. Most normal lung fibroblasts show positive expression of Thy-1, while the majority of myofibroblasts from fibrotic sites are negative 30. Similarly, fibroblasts are positive for collagen type I, as were both control and IL-4 induced cells. Fibrocytes, which are thought to be a circulating precursor of fibroblasts, are also positive for collagen I, but are also positive for CD34, CD45 and CD11b, while being typically negative for α-SMA and Thy-1 31. Hence, the markers used identify our cells as mesenchymal cells, and indicate that differ from both myofibroblasts and from fibrocytes. However, the use of surface markers to identify subpopulations of mesenchymal cells is not fully developed, and we have opted to refer to the spindle shaped differentiated cells as “fibroblast-like” cells. We feel several other observations support our conservative approach to identifying our mesenchymal cells. First, in contrast to staining for vimentin, desmin and keratin, the immunohistochemical staining of our fibroblast-like cells was not uniform for Thy-1, CD34, CD45 and CD11b suggesting that cell population that results from the differentiation process may be heterogeneous. Further, in vivo, it is likely that many cytokines and growth factors contribute to the differentiation process, thus it is unlikely that the differentiation of our cells exactly recapitulates in vivo differentiation. Nevertheless, the key finding reported in the current manuscript is that, using the three dimensional culture system, we were able to demonstrate that the presence of the cytokine IL-4 can alter the final differentiated phenotype of the resulting “fibroblast-like” cells. We also have similar results with “fibroblast-like” cells from murine ESCs in the presence and absence of IL-4 (data not shown).
IL-4, a cytokine characteristically released by T-helper type 2 cells, is known as a profibrotic cytokine. It can directly affect various functions of fibroblasts, including cytokine and adhesion molecule expression, extracellular matrix protein synthesis and α-SMA expression 32–34. In a prior study, we demonstrated that IL-4 also inhibits PGE2 production. Since PGE2 functions as an endogenous inhibitor of fibroblast repair functions, this is another mechanism by which IL-4 can augment fibroblast-mediated repair 35. The current study extends these studies in an important and very distinct way: it shows that IL-4 may also result in differentiation of functionally distinct fibroblast populations from precursors. By this mechanism, IL-4 could result in a persistent effect, in contrast to the transient effects observed when IL-4 is added to differentiated fibroblast cultures.
The current study also demonstrates at least one mechanism by which IL-4 causes the “fibrogenic” phenotype. Specifically, IL-4 induced cells under-express the microRNA miR-155. MiRNAs are a novel family of small approximately 19- to 25-nucleotide single-stranded, noncoding RNAs. They are expressed in developmental stage, tissue or cell type specific manners, cause posttranscriptional gene repression by increasing mRNA degradation or by inhibiting translation 36. This is achieved by direct binding between their 5′ region and the 3′ untranslated region (UTR) of target mRNAs. MiRNAs may also indirectly alter gene expression by epigenetic mechanisms 37, 38. MiRNAs have been associated with diverse biological processes, including development, cellular differentiation and the pathogenesis of various diseases.
MiR-155 represents as a typical multifunctional miRNA 39. To date, evidence supports a role for miR-155 in haematopoiesis, inflammation and immunity. Over-expression of miR-155 has been found to be associated with a variety of malignant tumors 40, rheumatoid arthritis 41, cardiovascular diseases 42, 43 and viral infections 44–46. A recent study demonstrated that mice deficient for miR-155 are immunodeficient and display increased lung airway remodeling 47. Consistent with this, the current study shows that IL-4 induced cells, which expressed lower levels of miR-155, performed more robustly in in vitro models of tissue repair and remodeling and, importantly, an miR-155 mimic could, at least partially, modulate these functions toward control cells. Thus, the current study also demonstrates that, in addition to its other suggested functions, miR-155 may be an important modulator of tissue repair and remodeling.
The current study does not define the specific targets of miR-155 in regulation of the fibroblast-like cell functions. Although, in this study, fibronectin production from the differentiated fibroblast-like cells was modulated by miR-155, no homology between miR-155 and the fibronectin gene was revealed by in silico assessment. A large number of predicted targets of miR-155 can be obtained from the online databases (991 from MicroCosm, 1985 from TargetScan and 199 from PicTar), and many could interact either directly or indirectly with the functions assessed. However, not all in silico predicted targets have been found to be responsive to the predicted miRNA when tested experimentally 48. In this context, a limited number of target genes have been experimentally validated for direct binding to miR-155 39. It has been reported that angiotensin II type 1 receptor (AT1R) is regulated by miR-155 in human lung fibroblasts and that TGF-β1 treatment resulted in the decreased expression of miR-155 and the increased expression of AT1R 42. Consistent with this, the current study demonstrated reduced expression of miR-155 by exogenous TGF-β1 in control fibroblast-like cells. Since angiotensin II has been associated with fibrotic vascular remodeling, this study is also consistent with a profibrotic role for reduced miR-155 expression. In contrast, Pottier et al have reported that miR-155 is up-regulated in a murine model of lung fibrosis, and that it increases human fibroblast motility and targets keratinocyte growth factor (KGF, FGF-7) directly 49. Similarly, Kong et al demonstrated increased expression of MiR-155 in response to TGF-β in a murine mammary epithelial cell line 50. To what degree these results depend on species, tissue of origin and model systems is unknown. Thus, additional investigation will be required to define the specific role of miR-155 in tissue fibrosis.
The current study also demonstrates that IL-4 induced fibroblast-like cells have diminished responses to TGF-β1. TGF-β1 is a widely recognized activator of fibroblasts, promoting myofibroblast development by inducing expression of α-SMA and extracellular matrix 51, 52. TGF-β1 is also known to stimulate fibroblast contraction and migration 53, 54. In a prior study, our group demonstrated that fibroblasts from patients with chronic obstructive pulmonary disease are also less responsive to TGF-β1, which is due to the increased expression of the inhibitory protein of TGF-β signaling pathway, Smad7 13. However, we could not detect altered expression of Smad7 in control versus IL-4 induced fibroblast-like cells (data not shown). In the current study, however, the reduced response to exogenous TGF-β1 in differentiated fibroblast-like cells was independent of miR-155. Thus, the mechanism for the altered signaling among these two types of fibroblast-like cells remains undefined.
The current study evaluated differentiation of fibroblast-like cells from EBs derived from hESCs. When this occurred in the presence of the cytokine IL-4, cells with a more fibrotic phenotype resulted. There are no consensus criteria for distinct discrimination between fibroblasts and myofibroblasts. α-SMA is a well-known marker of used to delineate myofibroblasts, however, some populations of fibroblasts are positive for α-SMA and some myofibroblasts are negative. Since the current study showed that IL-4 induced cells had stronger α-SMA expression than control cells, they could have been described as “myofibroblast-like” cells. More importantly, these differences persisted in culture in the absence of IL-4, consistent with an altered differentiation and this appears to be mediated, in part, by miR-155.
IL-4 is a mediator characteristically present in the airways in asthma. In addition, fibroblasts cultured from the airways of asthmatics have an altered, more fibrotic phenotype 8–10. The current study suggests that one mechanism for the accumulation of these functionally altered populations of cells is their differentiation in the presence of inflammatory cytokines. It is likely that many inflammatory cytokines in addition to IL-4 could alter the differentiation of fibroblasts. In preliminary studies, we evaluated several cytokines such as TGF-β1 and IL-13. All appeared to alter the differentiation of fibroblast-like cells in our system. As the effects of IL-4 appeared to be relatively marked and consistent, we pursed IL-4 in detail. Thus, our model system, which assessed only IL-4, is unlikely to recapitulate the entire milieu present in a disease such as asthma. Nevertheless, our study suggests that cytokine alteration of structural cell populations could play a role in asthma. Such a mechanism could account for the marked persistence of asthma despite anti-inflammatory therapy and long term “quisescence”. We also believe our system has the potential to explore the effect of external factors on the cell development and differentiation.
We differentiated the cells from EBs, not ESCs directly. EBs, which form spontaneously from ESCs under appropriate culture conditions have been used as precursor/stem cell sources for the differentiation of many cell types. By definition, ESCs are not present in the lung. However, there are clearly pluripotent precursors as we and others have shown 55–58. A hierarchy of epithelial stem/precursor/progeny cells has been proposed 59 and a similar hierarchy for mesenchymal cells seems likely. Whether the IL-4-induced differentiated cells characterized in the present report resemble a process that occurs in the lung or one that could affect a circulating source of mesenchymal cells such as fibrocytes remains to be determined.
In summary, the current study demonstrates that IL-4 induces differentiation of fibroblast-like cells that demonstrate a more “fibrogenic” phenotype, which was due to, in part, reduced expression of miR-155 compared with control cells. This demonstrates that distinct populations of fibroblast-like cells can originate if differentiation occurs in the presence of a cytokine and may represent a mechanism of disease development and maintenance.
Supplementary Material
Acknowledgments
The authors wish to thank Lillian Richards for excellent secretarial support.
Funding: This study was supported by the NHLBI RO1-064088, by the Pulmonary Section, Department of Internal Medicine, the Chancellor’s office and the Larson Endowment of UNMC (to S.I. Rennard); the Uehara Memorial Foundation and the Japanese Respiratory Research Foundation, Pfizer Fellowship (to T. Sato). The UNMC Microarray Core receives partial support from NIH grant number P20 RR016469 from the INBRE Program of the National Center for Research Resources.
Abbreviations
- IL-4
interleukin-4
- hESCs
human embryonic stem cells
- EBs
embryoid bodies
- miRNA
microRNA
- RTTC
rat tail tendon collagen
- NIH
National Institutes of Health
- UNMC
University of Nebraska Medical Center
- ESCRO
Embryonic Stem Cell Research Oversight
- IRB
Institutional Review Board
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
fetal bovine serum
- bFGF
basic fibroblast growth factor
- TGF
transforming growth factor
- α-SMA
alpha smooth muscle actin
- PG
prostaglandin
- FITC
fluorescein isothiocyanate stain-green immunofluoresence
- ELISA
enzyme-linked immunosorbent assay
- EIA
enzyme immunoassay
- rRNA
ribosomal RNA
- SD
standard deviation
- UTR
untranslated region
- AT1R
angiotensin II type 1 receptor
- HPF
high-power fields
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohnishi K, Takagi M, Yoshimochi K, Satomi S, Konttinen YT. Matrix metalloproteinase mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest. 1998;78:1077–87. [PubMed] [Google Scholar]
- 2.Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994;8:823–31. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- 3.Rennard SI, Hunninghake GW, Bitterman P, Crystal RG. Production of fibronectin by the human alveolar macrophage: Mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci. 1981;78:7147–51. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–21. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 5.Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–9. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 6.Kahari VM. Activation of dermal connective tissue in scleroderma. Ann Med. 1993;25:511–8. [PubMed] [Google Scholar]
- 7.Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, et al. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994;72:283–92. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- 8.Lewis CC, Chu HW, Westcott JY, Tucker A, Langmack EL, Sutherland ER, et al. Airway fibroblasts exhibit a synthetic phenotype in severe asthma. J Allergy Clin Immunol. 2005;115:534–40. doi: 10.1016/j.jaci.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 9.Kotaru C, Schoonover KJ, Trudeau JB, Huynh ML, Zhou X, Hu H, et al. Regional fibroblast heterogeneity in the lung: implications for remodeling. Am J Respir Crit Care Med. 2006;173:1208–15. doi: 10.1164/rccm.200508-1218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugiura H, Liu X, Duan F, Kawasaki S, Togo S, Kamio K, et al. Cultured lung fibroblasts from ovalbumin-challenged “asthmatic” mice differ functionally from normal. Am J Respir Cell Mol Biol. 2007;37:424–30. doi: 10.1165/rcmb.2007-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holz O, Zuhlke I, Jaksztat E, Muller KC, Welker L, Nakashima M, et al. Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J. 2004;24:575–9. doi: 10.1183/09031936.04.00143703. [DOI] [PubMed] [Google Scholar]
- 12.Muller KC, Welker L, Paasch K, Feindt B, Erpenbeck VJ, Hohlfeld JM, et al. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res. 2006;7:32. doi: 10.1186/1465-9921-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Togo S, Holz O, Liu X, Sugiura H, Kamio K, Wang X, et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am J Respir Crit Care Med. 2008;178:248–60. doi: 10.1164/rccm.200706-929OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato T, Liu X, Nelson A, Nakanishi M, Kanaji N, Wang X, et al. Reduced MiR-146a Increases Prostaglandin E2 in Chronic Obstructive Pulmonary Disease Fibroblasts. Am J Respir Crit Care Med. 2010;182:1020–29. doi: 10.1164/rccm.201001-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–37. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 16.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–20. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 18.Lama VN, Phan SH. The extrapulmonary origin of fibroblasts: stem/progenitor cells and beyond. Proc Am Thorac Soc. 2006;3:373–6. doi: 10.1513/pats.200512-133TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–37. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2000;97:11307–12. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three dimensional collagen gel matrix. In Vitro Cell Dev Biol. 1996;32:427–33. doi: 10.1007/BF02723005. [DOI] [PubMed] [Google Scholar]
- 22.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J Exp Med. 1962;115:453–66. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennard SI, Church RL, Rohrbach DH, Shupp DE, Abe S, Hewitt AT, et al. Localization of the human fibronectin (FN) gene on chromosome 8 by a specific enzyme immunoassay. Biochemical Genetics. 1981;19:551–66. doi: 10.1007/BF00484626. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura H, Ichikawa T, Liu X, Kobayashi T, Wang XQ, Kawasaki S, et al. N-acetyl-L-cysteine inhibits TGF-beta1-induced profibrotic responses in fibroblasts. Pulm Pharmacol Ther. 2009;22:487–91. doi: 10.1016/j.pupt.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Li YJ, Wang XQ, Sato T, Kanaji N, Nakanishi M, Kim M, et al. Prostaglandin E2 Inhibits Human Lung Fibroblast Chemotaxis through Disparate Actions on Different E-Prostanoid Receptors. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2009-0163OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–25. [PMC free article] [PubMed] [Google Scholar]
- 27.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–7. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christen T, Verin V, Bochaton-Piallat M, Popowski Y, Ramaekers F, Debruyne P, et al. Mechanisms of neointima formation and remodeling in the porcine coronary artery. Circulation. 2001;103:882–8. doi: 10.1161/01.cir.103.6.882. [DOI] [PubMed] [Google Scholar]
- 29.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. Faseb J. 2006;20:1045–54. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 30.Hagood JS, Prabhakaran P, Kumbla P, Salazar L, MacEwen MW, Barker TH, et al. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol. 2005;167:365–79. doi: 10.1016/S0002-9440(10)62982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 32.Postlethwaite AE, Holness MA, Katai H, Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest. 1992;90:1479–85. doi: 10.1172/JCI116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doucet C, Brouty BD, Pottin CC, Jasmin C, Canonica GW, Azzarone B. IL-4 and IL-13 specifically increase adhesion molecular and inflammatory cytokine expression in human lung fibroblasts. Int Immunol. 1998;10:1421–33. doi: 10.1093/intimm/10.10.1421. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J Allergy Clin Immunol. 2001;107:1001–8. doi: 10.1067/mai.2001.114702. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Kohyama T, Wang H, Zhu YK, Wen FQ, Kim HJ, et al. Th2 cytokine regulation of type I collagen gel contraction mediated by human lung mesenchymal cells. Am J Physiol. 2002;282:L1049–56. doi: 10.1152/ajplung.00321.2001. [DOI] [PubMed] [Google Scholar]
- 36.Nana-Sinkam SP, Hunter MG, Nuovo GJ, Schmittgen TD, Gelinas R, Galas D, et al. Integrating the MicroRNome into the study of lung disease. Am J Respir Crit Care Med. 2009;179:4–10. doi: 10.1164/rccm.200807-1042PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104:15805–10. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE, Callegari E, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–8. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta. 2009;1792:497–505. doi: 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 41.Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, et al. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58:1001–9. doi: 10.1002/art.23386. [DOI] [PubMed] [Google Scholar]
- 42.Martin MM, Buckenberger JA, Jiang J, Malana GE, Nuovo GJ, Chotani M, et al. The human angiotensin II type 1 receptor +1166 A/C polymorphism attenuates microrna-155 binding. J Biol Chem. 2007;282:24262–9. doi: 10.1074/jbc.M701050200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Sethupathy P, Borel C, Gagnebin M, Grant GR, Deutsch S, Elton TS, et al. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3′ untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am J Hum Genet. 2007;81:405–13. doi: 10.1086/519979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi JT, et al. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450:1096–9. doi: 10.1038/nature05992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, et al. Kaposi’s sarcoma-associated herpesvirus encodes an ortholog of miR-155. J Virol. 2007;81:12836–45. doi: 10.1128/JVI.01804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin Q, McBride J, Fewell C, Lacey M, Wang X, Lin Z, et al. MicroRNA-155 is an Epstein-Barr virus-induced gene that modulates Epstein-Barr virus-regulated gene expression pathways. J Virol. 2008;82:5295–306. doi: 10.1128/JVI.02380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuhn DE, Martin MM, Feldman DS, Terry AV, Jr, Nuovo GJ, Elton TS. Experimental validation of miRNA targets. Methods. 2008;44:47–54. doi: 10.1016/j.ymeth.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pottier N, Maurin T, Chevalier B, Puissegur MP, Lebrigand K, Robbe-Sermesant K, et al. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: implication in epithelial-mesenchymal interactions. PLoS One. 2009;4:e6718. doi: 10.1371/journal.pone.0006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, et al. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773–84. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desmouliere A, Gabbiani G. Modulation of fibroblastic cytoskeletal features during pathological situations: the role of extracellular matrix and cytokines. Cell Motil Cytoskeleton. 1994;29:195–203. doi: 10.1002/cm.970290302. [DOI] [PubMed] [Google Scholar]
- 52.Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Lab Investigation. 1993;68:696–707. [PubMed] [Google Scholar]
- 53.Montesano R, Orci L. Transforming growth factor-β stimulates collagen-matrix contraction by fibroblasts: Implication for wound healing. Proc Natl Acad Sci. 1988;85:4894–7. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987;165:251–6. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, et al. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med. 2004;170:1158–63. doi: 10.1164/rccm.200307-908OC. [DOI] [PubMed] [Google Scholar]
- 56.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–70. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 57.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 58.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest. 2005;85:962–71. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 59.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107:1414–9. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.