Abstract
Decision making is commonly conceived to reflect the interplay of mutually antagonistic systems: executive processes must inhibit affective information to make adaptive choices. Consistent with this interpretation, prior studies have shown that the dorsolateral prefrontal cortex (dlPFC) is activated by executive processing and deactivated during emotional processing, with the reverse pattern found within the ventrolateral prefrontal cortex (vlPFC). To evaluate whether this pattern generalizes to other affective stimuli – here, monetary rewards – we modified the emotional oddball task to use behaviorally-irrelevant reward stimuli, while matching analysis methods and task parameters to those of previous research. Contrary to the double-dissociation model advanced for emotional stimuli, we found that monetary stimuli produced activations within both the dlPFC and the vlPFC. This suggests that monetary stimuli are treated like affective stimuli by vlPFC but like task-relevant target stimuli by dlPFC. Our results suggest differential functional roles in affective and executive processing for these brain regions: the dlPFC supports contingency processing, while the vlPFC evaluates affective or conceptual information.
Keywords: decision making, executive function, emotion, value, striatum, fMRI
1. Introduction
Decision making has been often portrayed as a competition between two systems, with clear-headed judgments following from cognitive suppression of emotional responses and hot-headed choices arising from emotional interference with cognition (Bernheim and Rangel, 2004; Kahneman and Frederick, 2002; Lowenstein, 1996; Mayberg, 1997). This common theoretical conception has led to neuroscience studies that have looked for the physical basis of this competitive relationship within the brain (Drevets and Raichle, 1992; McClure et al., 2004; Yamasaki et al., 2002), often postulated to reflect interactions between a dorsal executive network (Fuster, 2000; Goldman-Rakic, 1996) and a ventral affective network (Adolphs, 2002).
To dissociate between cognitive and affective processing within the prefrontal cortex (PFC), Yamasaki and colleagues (2002) created an “emotional oddball task”. In the traditional oddball task (Herrmann and Knight, 2001; Picton, 1992), participants view a series of standard stimuli, most of which require the same behavioral responses; e.g., squares that require a right-button press. When an infrequent target (or “oddball”) stimulus appears – such as a circle that requires a left-button response – the participant must inhibit the prepotent behavioral response and engage an alternative response. Coincident with these stimuli are well-characterized neural changes: the target stimuli evoke increased fast electrophysiological responses that have prefrontal and parietal sources (Picton, 1992; Sutton et al., 1965) and functional magnetic resonance imaging (fMRI) activation in dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (PPC) (Casey et al., 2001; McCarthy et al., 1997; Strange et al., 2000). These effects have been shown to reflect the executive demands specific to the stimulus-behavior contingencies evoked by the targets; e.g., similar patterns of activation can be evoked by task variants that control for perceptual and motor demands of the targets (Huettel and McCarthy, 2004). Conversely, equally infrequent novel stimuli that do not require a change in behavior (e.g., emotionally neutral photographs of humans) do not evoke dlPFC activation (Yamasaki et al., 2002).
In their emotional oddball task, Yamasaki and colleagues (2002) introduced additional infrequent and behaviorally irrelevant novel stimuli: emotionally valent photographs. This allowed them to directly compare the executive processing related to the standard oddball target stimuli with the affective processing produced by the task-irrelevant emotional stimuli. Replicating previous studies, the oddball target stimuli produced activations within the dorsolateral prefrontal cortex (dlPFC), commonly associated with the dorsal executive network (Casey et al., 2001; McCarthy et al., 1997; Strange et al., 2000; Wang et al., 2009). The new emotional stimuli resulted in activations in the ventrolateral prefrontal cortex (vlPFC), an area commonly associated with responses to affective stimuli (Mayberg, 1997). Moreover, there was a double dissociation within these regions: target stimuli produced deactivations within the vlPFC and emotional stimuli deactivated the dlPFC. This pattern concurred with the theoretical model of competition between the executive and affective networks.
It remains unclear whether these effects of task-irrelevant emotional novels – i.e., enhanced activation in vlPFC and suppressed activation in dlPFC – generalize to other forms of affective stimuli, like motivational rewards. Emotional images and motivational rewards are processed, at least in part, through different pathways; notably, evaluation of rewards relies heavily on dopaminergic midbrain neurons and their projection targets (for reviews, see Dayan and Balleine, 2002; Haber and Knutson, 2010). Yet, substantial similarities also exist. Reactions to emotional images and learning about rewards rely on overlapping neural circuitry that includes the striatum, the amygdala, and the ventromedial prefrontal cortex (vmPFC) (for reviews, see Balleine et al., 2007; LeDoux, 2007; Murray et al., 2007, respectively). Moreover, the emotional and valuative responses to stimuli can interact. In the phenomenon of selective satiety, the perceived pleasantness and reward value of a specific food decrease in tandem with consumption (for review, see Rolls, 2007). Given these similarities, the presentation of rewards without behavioral change should result in diminished activation (or deactivations) of dlPFC, consistent with models of affect-cognition interactions (Kahneman and Frederick, 2002; Lowenstein, 1996; Mayberg, 1997).
Here, we adapted the emotional oddball task into a monetary oddball task that used real rewards, including both monetary gains and losses, which were delivered infrequently and without requiring a change in behavior. These stimuli allowed us to separate processes engaged due to alteration of behavior from those engaged by behaviorally irrelevant monetary stimuli. We conducted two independent sets of analyses on fMRI data: whole-brain voxelwise analyses, and region-of-interest (ROI) analyses using the approach of Yamasaki and colleagues (2002). The natural hypothesis is that monetary stimuli should produce the same double-dissociation within PFC as found for emotional stimuli. However, our analyses reveal that monetary stimuli produced activations within both the dlPFC and vlPFC, inconsistent with this theoretical competitive relationship.
2. Results
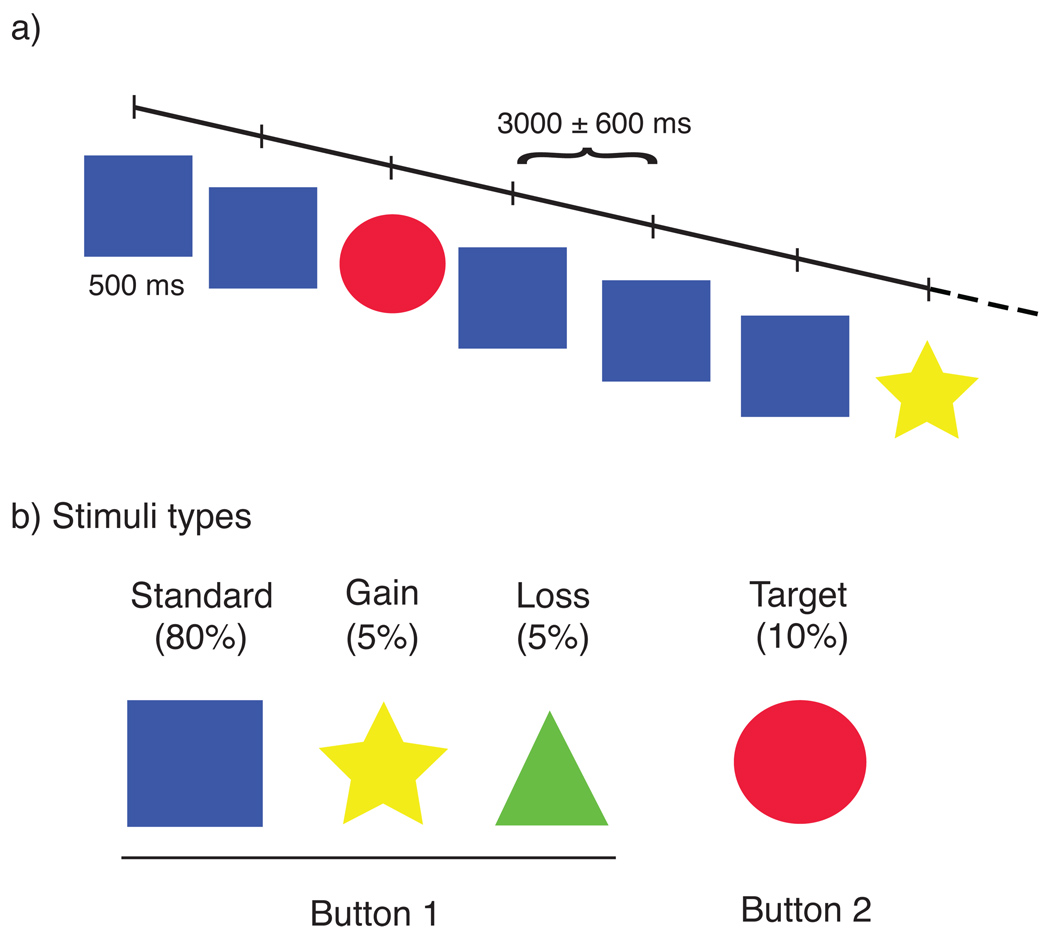
We examined the fMRI data from twenty subjects participating in a monetary oddball task (Figure 1), using both whole-brain regression and time-course analyses (see Methods).
Figure 1. Monetary Oddball Task.
(a) On each trial, participants were presented with a stimulus for 500ms and responded with a button press. (b) On 80% of trials, participants were presented with the standard image (blue square), and an accurate response was to press the 1st button. On 10% of trials, participants were presented with a target stimulus (red circle) and needed to alter their behavioral response (push the 2nd button). The remaining 10% were divided between monetary gains (5%, yellow stars worth +$2) and monetary losses (5%, green trials worth −$1), on which the participant should continue pressing the 1st button.
2.1 Behavioral data
Average response times and accuracy rates are shown in Table 1. Target trials resulted in increased response times and decreased accuracy, as compared to standard trials (p<0.05, within-participants t-tests). Consistent with our description of the monetary stimuli as behaviorally irrelevant, gains and losses resulted in no change in accuracy, although an increase in response time was found for gain trials.
Table 1. Behavioral response times and accuracy.
Average response times and accuracy rates for each of the stimuli types.
| Stimulus type |
Response time mean (sd) msec |
Accuracy |
|---|---|---|
| Standard | 380 (71) | 99.3% |
| Targets | 482 (91) * | 87.2% * |
| Gains | 524 (112) * | 97.5% |
| Losses | 465 (120) | 98.5% |
Asterisks (*) indicate significant difference (paired t-test, p<.05) compared to the Standard stimulus.
2.2 Whole-Brain Regression Analyses
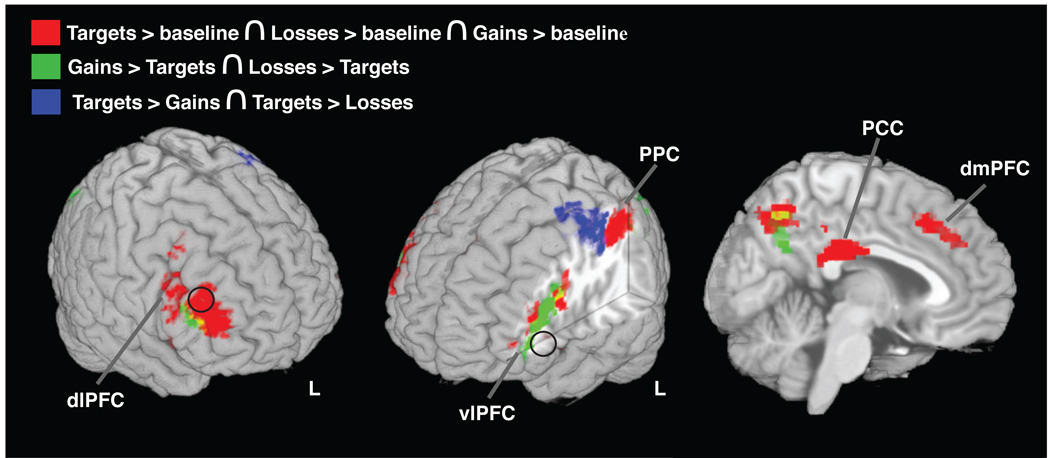
We found significant activation to targets (targets > baseline) within regions broadly constituting the dorsal executive network - the dorsolateral and dorsomedial prefrontal cortex (dlPFC and dmPFC, respectively), posterior parietal cortex (PPC), and posterior cingulate cortex (PCC) - in addition to bilateral anterior insula (aINS) and a small dorsal aspect of right ventrolateral prefrontal cortex (vlPFC). This pattern of target-related activation matches that from prior studies using variants of the oddball task (e.g., Fichtenholtz et al., 2004; Huettel et al., 2002; Yamasaki et al., 2002). Within all of these regions, we also found activations to both monetary gains and losses compared to baseline (Figure 2, and Table 2), suggesting that unexpected monetary gains and losses evoke executive processes overlapping with the executive processes associated with task-relevant targets.
Figure 2. Dissociating activations to targets, gains, and losses.
Shown are conjunctions and contrasts of neural activations to targets and monetary trials. In red are neural regions activated by targets, gains, and losses (conjunction of activations to targets, gains, and losses). In green are regions where monetary trials produced greater activation than targets (intersect of gains > targets and losses > targets). In blue are regions where targets produced greater activations than monetary trials (intersect of targets > losses and targets > gains). Black circles designate the locations of the ROIs derived from Yamasaki and colleagues (see text for details).
Table 2. Activation table for regions that presented increased activation to the presentation of targets, gains, and losses.
The coordinates of centroids of overlap activations are presented, with included neural structures within each cluster, identified using the probabilistic Harvard-Oxford atlases within FSLview. Included are all overlap clusters with over 10 voxels.
| Cluster Centroid Coordinates (MNI, mm) |
||||
|---|---|---|---|---|
| Cluster (# voxels) |
Included Brain Regions | X | Y | Z |
| 23 | L Middle Frontal gyrus | −40 | 48 | 8 |
| 175 | L Middle Frontal gyrus | −40 | 32 | 20 |
| 2326 | R Frontal Pole | 46 | 24 | 16 |
| R Insula | ||||
| R Middle Frontal gyrus | ||||
| R Inferior Frontal gyrus | ||||
| R Precentral gyrus | ||||
| 297 | R Anterior Cingulate cortex | 6 | 24 | 42 |
| R Superior Frontal gyrus | ||||
| 299 | L Insula | −32 | 20 | −2 |
| 266 | L Middle Frontal gyrus | −46 | 10 | 26 |
| 123 | R Middle Frontal gyrus | 38 | 6 | 50 |
| 579 | Posterior Cingulate Cortex | 2 | −28 | 28 |
| 1635 | L Superior parietal lobule | −34 | −50 | 44 |
| L Lateral Occipital cortex | ||||
| L Supramarginal gyrus | ||||
| L Postcentral gyrus | ||||
| 349 | R Precuneus | 10 | −64 | 46 |
By contrasting between the different classes of infrequent (oddball) stimuli, we examined the specific activations produced by behavioral-relevance (for targets) from those due to behaviorally irrelevant valuative processing (for gains and losses). Targets produced greater activation compared to monetary stimuli (i.e., the intersection of targets > losses and targets > gains contrasts) in the precentral and postcentral gyri, consistent with the specific motor preparatory demands of the target trials (Figure 2 and Table 3). Monetary trials produced significantly greater activation relative to targets (i.e., the intersection of gains > targets and losses > targets contrasts), within the lateral occipital cortex (LOC), precuneus, and along the border between dlPFC and vlPFC (Figure 2 and Table 3). Notably, no voxels within the amygdala exhibited significant activation to reward novels (main effects of gains or losses, or for their conjunction), whereas Yamasaki and colleagues (2002) found a significant amygdala response to their emotional novels.
Table 3. Activation table for the regions that presented significant differences in the contrasts of targets to monetary stimuli (gains and losses) and the reverse.
Conventions similar to Table 2.
| Cluster Centroid Coordinates (MNI, mm) |
||||
|---|---|---|---|---|
| Cluster (# voxels) |
Included Brain Regions | X | Y | Z |
| Targets > Gains ∩ Targets > Losses | ||||
| 938 | L Precentral gyrus | −38 | 20 | 52 |
| L Postcentral gyrus | ||||
| Gains > Targets ∩ Losses > Targets | ||||
| 141 | L Inferior Frontal gyrus | −52 | 30 | 18 |
| 511 | L Inferior Frontal gyrus | −42 | 22 | 20 |
| L Middle Frontal gyrus | ||||
| L Frontal Pole | ||||
| 498 | L Lateral Occipital cortex | −32 | −66 | 44 |
| L Angular gyrus | ||||
| L Superior Parietal lobule | ||||
| 221 | Precuneous | 2 | −62 | 40 |
| 283 | R Lateral Occipital cortex | 34 | −70 | 40 |
Significant deactivations to targets relative to the standard baseline were found in the left frontal pole, superior frontal gyrus (SFG), dlPFC, vlPFC, precuneus, and right precentral gyrus. Significant deactivations to both gains and losses (conjunction of gains > baseline and losses > baseline) were only found in the bilateral occipital pole. In contrast to the results of Yamasaki and colleagues (2002), no deactivated voxels were found within the dlPFC for the main effects of either gains or losses, or for their conjunction.
2.3 Time-Course Analyses
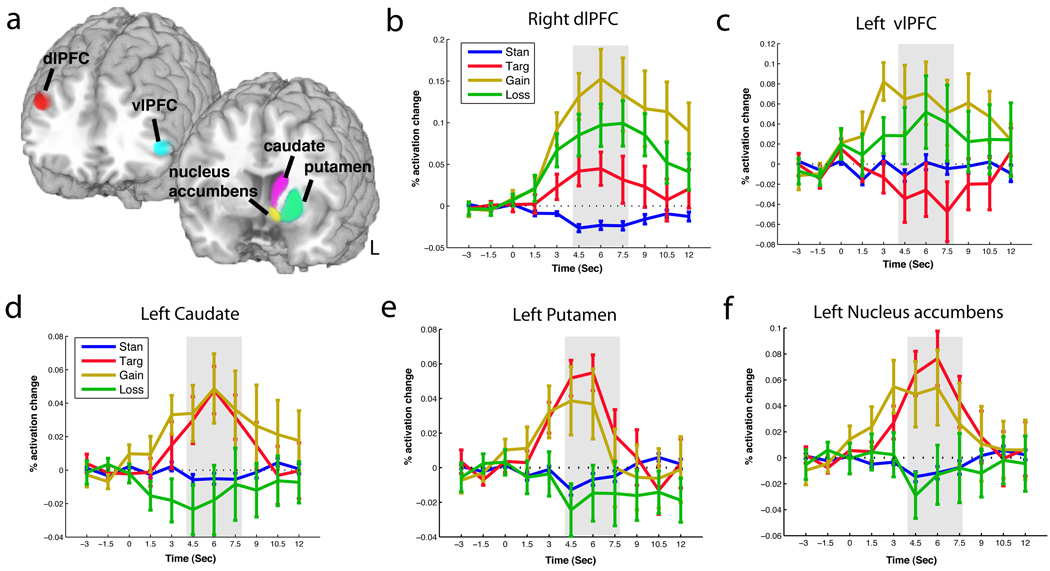
As a stronger comparison of our results to previous findings, we replicated the analysis methods of Yamasaki and colleagues (2002). We used right dlPFC and left vlPFC ROIs, each an 8-mm sphere centered on the activation centroid reported by Yamasaki and colleagues (dlPFC: MNI coordinate: x42, y30, z30, and vlPFC: MNI coordinate: x-51, y33, z6 [converted from Talairach with Pickatlas, Wake Forest University]). This ROI-based analysis (Figures 2 and 3a) revealed dlPFC activations to targets, gains, and losses (Figure 3b). However, within the vlPFC, we found the same dissociation between executive and valuative stimuli as Yamasaki and colleagues found between executive (deactivations) and emotional stimuli (activations), with significant activations to gains and losses with deactivations for targets (t-tests, p < .05; Figure 3c). A Region*Condition interaction test (Poldrack et al., 2008) allowed us to statistically verify these apparent dissociations – returning significant main effects for ROI and condition as well as their interaction (2-way repeated measures ANOVA of average response [window from 4.5 to 7.5s] by ROI and condition, ps<.05). These results showed that task-irrelevant monetary rewards produce activations similar to those produced by targets in the dlPFC, but activations similar to emotional novels within the vlPFC.
Figure 3. Time course comparison of target, gain, and loss activations in the vlPFC, dlPFC, and striatal nuclei.
(a) Shown are the ROIs used to examine the left vlPFC (cyan), right dlPFC (red), the left caudate (purple), left putamen (light green), and nucleus accumbens (yellow). (b) Time courses of modulation of left vlPFC by standard, target, gain, and loss conditions. Error bars indicate ±1 standard error of the mean. The time window of statistical analyses is shown in grey. (c) Time courses of modulation in dlPFC to standard, target, gain, and loss conditions. (d) Time courses of modulations of left caudate by standard, target, gain, and loss conditions. (e) Time courses of modulations of left putamen by standard, target, gain, and loss conditions. (f) Time courses of modulations of left nucleus accumbens by standard, target, gain, and loss conditions.
Using additional focused-region-of-interest analyses based upon Tricomi and colleagues (2004), we examined executive and reward processing within the striatum. We extracted time courses from the caudate in each hemisphere, to dissociate between our behaviorally-relevant target trials and our reward-relevant but behaviorally-irrelevant monetary stimuli. In both left and right caudate, we found activations for targets and gains (t-tests, p<.05), with weak, non-significant deactivations to losses (Figure 3d). We extended these analyses by also examining the putamen and nucleus accumbens (in each hemisphere), and found the same pattern of significant activations across all three striatal nuclei, bilaterally(Figure 3e–f). This suggests these dorsal and ventral striatal nuclei contain signals of both the behavioral relevance and the behaviorally irrelevant reward value of stimuli, rather than only the contingent signals suggested by Tricomi and colleagues (2004).
3. Discussion
We examined how executive and valuative processes are dissociated within the prefrontal cortex and striatum through the use of a monetary oddball task. Our initial analyses replicated the activations to target stimuli produced in other instances of the oddball task, with activations throughout the dorsal executive network - bilaterally through the dlPFC, dmPFC, PPC, PCC - as well as bilateral aINS and a small dorsal aspect of right vlPFC (Casey et al., 2001; McCarthy et al., 1997; Strange et al., 2000; Tricomi et al., 2004; Wang et al., 2009). And, similarly to prior work, we found that behaviorally irrelevant monetary stimuli (gains and losses) engage the vlPFC in a manner similar to that reported to behaviorally irrelevant emotional stimuli. However, we also found that these monetary stimuli engage the dlPFC similarly to behaviorally relevant targets, even though the monetary stimuli are behaviorally irrelevant; i.e., they do not require changes in behavioral response or result in diminished behavioral accuracy.
3.1 dlPFC: Contingency detection and recall
Our results suggest that infrequent monetary rewards engage contingency processing within dlPFC. The dlPFC has long been implicated in the control processing necessary for learning environmental contingencies and producing appropriate behavioral responses to unexpected stimuli (Botvinick et al., 2001; Duncan and Owen, 2000; Goldman-Rakic, 1996; Hon et al., 2006; Huettel and McCarthy, 2004; Mansouri et al., 2009; Miller and Cohen, 2001; Mullette-Gillman and Huettel, 2009; Ridderinkhof et al., 2004; Robbins, 2007; Walton et al., 2004; Wise et al., 1996). Behaviorally irrelevant and novel emotional stimuli, as used by Yamasaki and colleagues, evoke no changes from the current response contingency and no dlPFC activation (Yamasaki et al., 2002). Conversely, when emotional stimuli have been presented as behaviorally relevant targets, they generate dlPFC activation (Fichtenholtz, et al. 2004).
An alternative explanation could be that the dlPFC is simply engaged by the presence of an unexpected or novel event. Such an explanation is initially attractive, as it would explain the activations to all infrequent stimuli found both within this study and within that of Fichtenholtz and colleagues (Fichtenholtz et al., 2004), and potentially explain why we found twice as much activation for gains and losses as we found for targets (the gains and losses were each half as frequent and therefore twice as unexpected). However, this explanation cannot account for the deactivations found by Yamasaki and colleagues to unexpected behaviorally irrelevant and novel emotional stimuli (Yamasaki et al., 2002). Of future interest will be determining under what conditions, if any, emotional stimuli also produce dlPFC activations, and whether monetary rewards can generate the dlPFC deactivations of Yamasaki and colleagues. Alternatively, the nature of rewarding stimuli might result in contingency processing regardless of their behavioral relevance.
Interestingly, multiple studies have suggested that the dlPFC is involved in value processing – with activations modulated by the presence or level of rewards (Plassmann et al., 2010; Savine and Braver, 2010). Our activations to monetary gains concur with this view. However, our found activations to monetary losses suggests that the level of dlPFC activation may reflect the motivational salience of stimuli, with motivation to achieve gains or avoid losses, rather than a monotonic value function across losses and gains (salience and valence). Such salience-modulation of dlPFC is further supported by the activations we find for target trials, which are certainly salient although they present no external rewards or losses.
3.2 vlPFC: Conceptual processing, including affective concepts
Within the vlPFC, we found that monetary gains and losses produced activations similarly to the emotional novels presented by Yamasaki and colleagues (2002), whereas targets produced deactivation. These results indicate that this anterior vlPFC activation is produced by both monetary and emotional affective stimuli. Previous studies have suggested that this region is modulated by the value of available options during goal-directed choice (Hare et al., 2008), and that deactivations of this region reflect self-control processing to inhibit value signals of undesired actions (Camus et al., 2009; Hare et al., 2009). We find activations to both positive and negative rewards, suggesting this region may encode the affective magnitude, an affective signal that does not differentiate between positive and negative stimuli. Our deactivations during target trials are compatible with the idea that this region receives inhibitory signals from the executive system (Camus et al., 2009; Hare et al., 2009). Yet, our results argue against any simple opposition between dorsal and ventral PFC regions, given that monetary gains and losses activated both aspects.
An alternative explanation of vlPFC function arises from studies of conceptual mnemonic retrieval, which suggest that the vlPFC is engaged by higher order contingency processing of semantic information (Badre and Wagner, 2007; Buckner and Koutstaal, 1998; Dobbins and Wagner, 2005; McDermott et al., 2000) In non-affective memory tasks, activations of the vlPFC occur during episodic retrieval of conceptual information, with deactivations during simple perceptual or novelty-detection tasks (for example, see Dobbins and Wagner, 2005). These results suggest that the vlPFC is not processing affective information, but rather co-occurring semantic information. Their results concur with our vlPFC activations for monetary stimuli (which would engage conceptual processing) and deactivations for target stimuli (similarly to that found for novelty-detection). This interpretation also concurs with recent suggestions of level-of-processing differences between the dlPFC and vlPFC (for reviews, see Badre and Wagner, 2007; Badre and D'Esposito, 2009), with the dlPFC engaged by simple contingency processes to identify stimuli and determine the behavioral response, while the vlPFC is engaged during abstract conceptual processing. However, such a depth of processing account does not suggest an explanation for the deactivations found in the vlPFC during target presentation.
3.3 Striatum: Integration of value and action
Tricomi and colleagues investigated caudate function using variants of the oddball task that incorporated behaviorally relevant monetary rewards (Tricomi et al., 2004). They found that caudate activation was evoked by stimuli in which there was a contingency between action and reward. Vitally, these experiments did not dissociate between reward evaluation and behavioral response processing, so their data cannot predict caudate responses to behaviorally irrelevant rewards or behavioral processing in the absence of a reward.
We found that striatal regions were engaged both by targets (i.e., behavioral change without a reward) and monetary gains (i.e., rewards without behavioral change). In concurrence with this, Lau and Glimcher (2007 and 2008) examined ventral striatum neurons while non-human primates performed a reward foraging task (Lau and Glimcher, 2007; Lau and Glimcher, 2008). Lau found that individual neurons exhibited tuning properties modulated by both the received reward and the action taken. Parallel striatal responsivity to rewards and behavioral changes concurs with previous accounts of involvement of the striatum in action-outcome learning (for reviews, see Balleine et al., 2007; Seger, 2008), and with previous studies showing alterations of striatal responsivity during learning (Delgado et al., 2005; Tricomi et al., 2009). Note that the potential dissociation within the striatum – increased activation to gains, but neutral or decreased activation to losses – is consistent with prior studies (Delgado, Nystrom et al. 2000; Breiter, Aharon et al. 2001; Delgado, Locke et al. 2003; Tom, Fox et al. 2007).
3.4 Summary
We examined a reported dissociation between executive and emotional signals in PFC, during performance of an oddball task (Yamasaki and colleagues; 2002). We found that while responses in anterior vlPFC do generalize to monetary rewards, the responses in the dlPFC do not. Rather, monetary rewards evoke increased activation in the vlPFC, like emotional stimuli, but also increased activation in the dlPFC, like task-relevant targets. Combined, our results suggest differential functional roles for these brain regions in affective and executive processing: the dlPFC supports simple contingency processing (with salience-modulation), the vlPFC evaluates affective or conceptual information, and the striatum learns relationships between actions and their rewards.
4. Experimental procedures
4.1 Participants
Twenty-nine healthy, right-handed young adults participated in this experiment (age range: 18–36y; mean age: 24y; 16 female). Data from 9 participants were excluded prior to data analysis (scanner error, 2 participants; head movement of greater than one voxel, 3 participants; task accuracy below 60%, 4 participants), leaving data from 20 individuals in the reported sample. Participants were compensated based upon stimuli presentation during their fMRI session (as described below), and received an additional $5 for achieving 95% accuracy in their behavioral responses. Mean payment across participants was approximately $45. All participants provided informed consent under a protocol approved by the Institutional Review Board of Duke University Medical Center.
4.2 Task
In our monetary oddball task, participants viewed a rapidly presented series of colored shapes, each displayed for 500 ms with a stimulus-onset asynchrony of 3000 ± 600 ms (Figure 1a). The shape of the presented stimulus determined whether the participant should press Button-1 or Button-2 (index or middle finger on right hand; Figure 1b). Most trials were of standard stimuli (80%); i.e., squares that required a button-1 response. The remaining trials contained three types of infrequent stimuli. On target trials (10%), a circle appeared and required a Button-2 response. The final 10% of trials were divided between financial gains ($2, indicated by a star) and losses (−$1, indicated by a triangle). Importantly, the gain and loss trials required that the participant press Button-1, maintaining the behavioral response from the frequent standard trials. To equate their affective magnitudes, we used a 2:1 ratio between gains and losses as an approximation of the population median in loss aversion (Camerer, 1998; Kahneman and Tversky, 1979; Koszegi and Rabin, 2006).
Both standards and targets varied in color and size across stimuli to prevent visual habituation, while gain and loss stimuli remained constant to maximize their discriminability. Participants performed 5 or 6 runs (mean: 5.6) of 140 trials each. All stimuli were viewed through LCD goggles (Resonance Technologies, inc.), and all button presses were recorded using a custom fiber-optic response box.
4.3 FMRI data acquisition and analysis
FMRI data were collected with a gradientecho inverse-spiral pulse sequence (TR = 1500ms, TE = 31ms, 34 axial slices parallel to the AC-PC plane, 3.75*3.75*3.8mm) on a GE 4T scanner with an eight-channel phased-array head coil. High-resolution 3D full-brain SPGR images were acquired to aid in normalization and coregistration. Head motion was restricted using a vacuum cushion and tape.
We performed two types of analyses: regression using the general linear model and time-course evaluation. Our regression analyses used FEAT (FMRI Expert Analysis Tool) version 5.98, part of the FSL package (Smith et al., 2004; Woolrich et al., 2009). The following pre-processing steps were applied: motion correction using MCFLIRT, slice-timing correction, removal of non-brain voxels using BET (Smith, 2002), spatial smoothing with a Gaussian kernel of full-width-half-maximum of 6mm, and 50s high-pass temporal filtering. Registration to high resolution and standard images was carried out using FLIRT (Jenkinson and Smith, 2001).
Our first-level FEAT model contained 3 regressors, one for each of the rare stimuli types (e.g., targets, gains, and losses). To construct each regressor, we defined impulse functions of unit duration and unit weight at the onset of each stimulus, and convolved the resulting timecourse with a double-gamma hemodynamic response function. Of note, this model uses the standard trials as a task-related baseline to control for processing associated with visual perception and motor responses.
Second-level FEAT analyses combined across runs for each participant using a fixed-effects model. Third-level, across-participants analyses used a FLAME (stage 1) random-effects analysis, with automatic outlier de-weighting (Woolrich, 2008). All statistical inferences, including data visualization, are whole-brain corrected (cluster-significance threshold corrected to p < 0.05; voxel z > 2.3). Regions of interest (ROI) masks were created, and centroids of overlap activations were calculated using MRICRON (Rorden et al., 2007).
Our time-course analyses replicated the procedures of Yamasaki and colleagues (2002). The dlPFC ROI was an 8mm sphere around the activation centroid reported by Yamasaki and colleagues (MNI coordinate: x42, y30, z30). The vlPFC ROI was an 8mm sphere centered on the coordinate found by Yamasaki and colleagues (MNI coordinate: x-51, y33, z6, converted from Talariach with Pickatlas, Wake Forest University). Additional anatomical ROIs were defined in the caudate, putamen, and nucleus accumbens – based on prior literature indicating specific effects of behaviorally relevant rewards in those regions (Tricomi et al., 2004) – all derived from the probabilistic Harvard-Oxford atlas within FSLview. Each ROI was constructed by thresholding the probabilistic map for each structure at ≥25% probability (threshold selected to maximize the apparent spatial coverage while minimizing the overlap across regions). After the preprocessing steps above, we extracted the temporal waveforms within each designated ROI time-locked to the onset for each of the target, gain, and loss stimuli within each run. Each peri-stimulus epoch comprised 11 time points from 3sec before stimulus onset through 12sec after stimulus onset, using 1.5sec steps. To test for changes in activation, we used t-tests to contrast the average hemodynamic responses from 4.5 to 7sec after stimulus onset, combining these time points across participants.
Acknowledgments
We thank Gregory McCarthy, Lihong Wang, and Brandi Newell for comments on data analysis and the manuscript. This research was supported by NINDS-41328 and NIMH-70685 (SAH). SAH was supported by an Incubator Award from the Duke Institute for Brain Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest, financial or otherwise.
References
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim D, Rangel A. Addiction and Cue-Triggered Decision Processes. The American Economic Review. 2004;94:1558–1590. doi: 10.1257/0002828043052222. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proc Natl Acad Sci U S A. 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer C. Bounded Rationality in Individual Decision Making. Experimental Economics. 1998;1:163. 168. [Google Scholar]
- Camus M, Halelamien N, Plassmann H, Shimojo S, O'Doherty J, Camerer C, Rangel A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex decreases valuations during food choices. Eur J Neurosci. 2009;30:1980–1988. doi: 10.1111/j.1460-9568.2009.06991.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, Thomas KM, Noll DC. Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Hum Brain Mapp. 2001;13:26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol Bull. 1992;28:261–274. [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Brain Res Cogn Brain Res. 2004;20:67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Executive frontal functions. Exp Brain Res. 2000;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Knight RT. Mechanisms of human attention: event-related potentials and oscillations. Neurosci Biobehav Rev. 2001;25:465–476. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Hon N, Epstein RA, Owen AM, Duncan J. Frontoparietal activity with minimal decision and control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:9805–9809. doi: 10.1523/JNEUROSCI.3165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Mack PB, McCarthy G. Perceiving patterns in random series: dynamic processing of sequence in prefrontal cortex. Nat Neurosci. 2002;5:485–490. doi: 10.1038/nn841. [DOI] [PubMed] [Google Scholar]
- Huettel SA, McCarthy G. What is odd in the oddball task? Prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia. 2004;42:379–386. doi: 10.1016/j.neuropsychologia.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect Theory - Analysis of Decision under Risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Kahneman D, Frederick S. Representativeness revisited: Attribute substitution in intuitive judgment. In: Gilovich DGDKT, editor. Heuristics and Biases. Vol. New York: Cambridge University Press; 2002. pp. 49–81. ^eds. [Google Scholar]
- Koszegi B, Rabin M. A Model of Reference-Dependent Preferences. Quarterly Journal of Economics. 2006;121:1133–1165. [Google Scholar]
- Lau B, Glimcher PW. Action and outcome encoding in the primate caudate nucleus. J Neurosci. 2007;27:14502–14514. doi: 10.1523/JNEUROSCI.3060-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Value representations in the primate striatum during matching behavior. Neuron. 2008;58:451–463. doi: 10.1016/j.neuron.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lowenstein G. Out of Control: Visceral Influences on Behavior. Organizational Behavior and Human Decision Processes. 1996;65:272–292. [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. The Journal of neuropsychiatry and clinical neurosciences. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL., 3rd Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: an event-related fMRI study. J Cogn Neurosci. 2000;12:965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mullette-Gillman OA, Huettel SA. Neural substrates of contingency learning and executive control: dissociating physical, valuative, and behavioral changes. Front Hum Neurosci. 2009;3:23. doi: 10.3389/neuro.09.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE. Guidelines for reporting an fMRI study. Neuroimage. 2008;40:409–414. doi: 10.1016/j.neuroimage.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Sensory processing in the brain related to the control of food intake. Proc Nutr Soc. 2007;66:96–112. doi: 10.1017/S0029665107005332. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Savine AC, Braver TS. Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. J Neurosci. 2010;30:10294–10305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Seger CA. How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neurosci Biobehav Rev. 2008;32:265–278. doi: 10.1016/j.neubiorev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Strange BA, Henson RN, Friston KJ, Dolan RJ. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. Neuroimage. 2000;12:425–433. doi: 10.1006/nimg.2000.0637. [DOI] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O'Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Wang L, Mullette-Gillman OA, Gadde KM, Kuhn CM, McCarthy G, Huettel SA. The effect of acute tryptophan depletion on emotional distraction and subsequent memory. Soc Cogn Affect Neurosci. 2009;4:357–368. doi: 10.1093/scan/nsp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Murray EA, Gerfen CR. The frontal cortex-basal ganglia system in primates. Crit Rev Neurobiol. 1996;10:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]