Abstract
Rationale
Airway thiol redox disturbances, including depletion of the antioxidant, glutathione (GSH), are differentiating features of severe asthma in children.
Objectives
Given the role of the transcription factor, Nrf2, in maintaining GSH homeostasis and antioxidant defense, we quantified expression and activity of Nrf2 and its downstream targets in the airways and systemic circulation of asthmatic children. We hypothesized that Nrf2 activation and function would be impaired in severe asthma, resulting in depletion of thiol pools and insufficient GSH synthesis and conjugation.
Methods
Peripheral blood mononuclear cells (PBMCs) and airway lavage cells were collected from children 6–17 years with severe (n=51) and mild-to-moderate asthma (n=38). The thiols GSH and cysteine (CyS) were quantified and expression and activity of Nrf2 and its downstream targets were assessed.
Results
Children with severe asthma had greater oxidation and lower concentrations of GSH and Cys in the plasma and airway lavage. Although Nrf2 mRNA and protein increased in severe asthma as a function of increased thiol oxidation, the Nrf2 expressed was highly dysfunctional. Nrf2 activation and downstream targets of Nrf2 binding, including GSH-dependent enzymes, were not different between groups. The duration of asthma was a key factor associated with Nrf2 dysfunction in severe asthma.
Conclusions
Children with severe asthma have a global disruption of thiol redox signaling and control in both the airways and systemic circulation that is associated with post-translational modification of Nrf2. We conclude that the Nrf2 pathway is disrupted in severe asthma as a function of chronic oxidative stress, which ultimately inhibits GSH synthesis and antioxidant defense.
Keywords: Severe asthma, Oxidative stress, Inflammation, Glutathione, Cysteine, Nuclear factor (erythroid-derived 2)-like 2 (Nrf2)
INTRODUCTION
Severe, therapy-resistant asthma in children is a challenging disorder that is extremely difficult to treat.1 Whereas children with mild-to-moderate asthma achieve good symptom control with low doses of inhaled corticosteroids (ICS), children with severe asthma have ongoing symptoms despite therapy with high doses of ICS and even oral corticosteroids.2 While the underlying mechanisms of severe asthma in children are unclear, excessive free radical formation is a key feature of the disorder.3–5 This free radical burden eventually overwhelms endogenous oxidation-reduction (“redox”) reactions, leading to “oxidative stress” with a global disruption of redox signaling and control.5 Because redox signaling is essential for cellular homeostasis6, this oxidative stress may ultimately account for the persistent airway inflammation and irreversible airway injury that accompany severe asthma in children. 7,8
The thiols cysteine (Cys) and glutathione (GSH) are products of sulfur amino acid metabolism that are central to redox signaling. In their reduced forms, Cys and GSH both contain functional sulfhydryl groups (-SH) which are readily converted to disulfides (-SS) in the presence of oxidizing reactions, forming cystine (CySS) and glutathione disulfide (GSSG), respectively. While these reactions are initially protective, excessive oxidation can result in Cys and GSH depletion and increased susceptibility to oxidative-induced cellular dysfunction and tissue damage.6 Indeed, we have previously shown that children with severe asthma, compared to children with mild-to-moderate asthma, have significant depletion of GSH in the epithelial lining fluid with increased GSSG formation and a shift in the GSH/GSSG redox couple to the oxidized state.5 This finding was also noted in airway cells and was associated with increased markers of lipid peroxidation and DNA nucleoside oxidation and altered innate immune function.4,5 While the mechanisms linking altered GSH homeostasis to severe asthma are unclear, they may be related to GSH synthesis. However, whereas previous studies have shown associations between glutathione-S-transferase (GST) isoenzymes and asthma inflammation and severity,9,10 these studies were focused only on genotypes and no functional assays were performed. Thus the role of GSTs and other GSH-related factors in asthma pathogenesis is not entirely understood.11
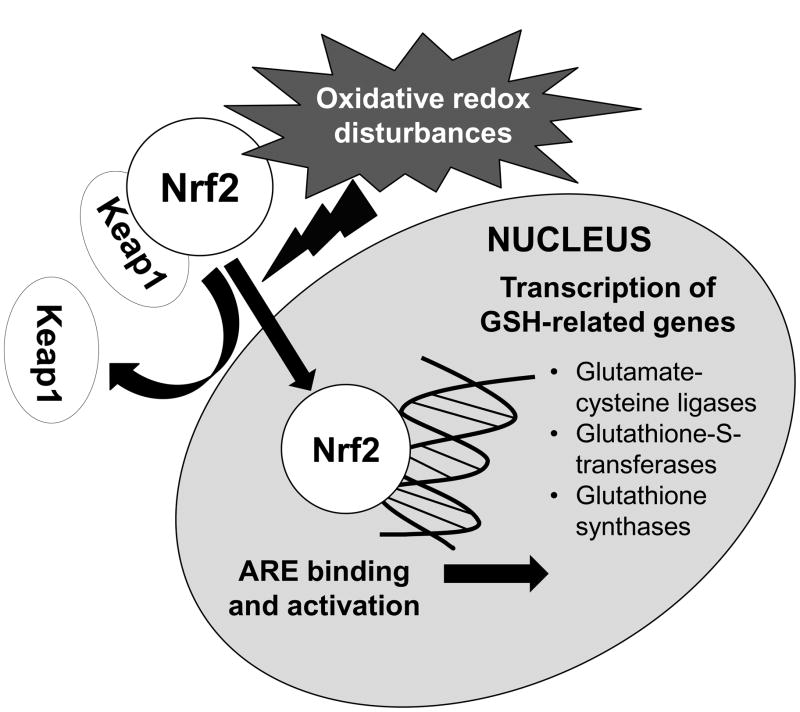
Nuclear factor erythroid 2-related factor (Nrf2) is a transcription factor with a basic leucine zipper motif that plays a key role in redox regulation. Nrf2 is expressed by nearly all cell types, where it is sequestered in the cytosol by an inhibitory protein, Kelch-like ECH-associated protein (Keap1). With oxidative redox disturbances, Nrf2 is activated and translocates to the nucleus, where it binds and activates the antioxidant response element (ARE) and upregulates several genes associated with GSH synthesis and antioxidant defense (Figure 1).12 To date, Nrf2 expression and activity have not been studied in asthmatic subjects. Given the magnitude of the airway redox disturbances we have previously observed in children with severe asthma,3–5 the purpose of this study was to quantify homeostasis of the Cys/CySS and GSH/GSSG couples in the airways and systemic circulation of children with severe versus mild-to-moderate asthma and to determine the expression and activity of Nrf2 and its downstream targets of activation. We hypothesized that severe asthma would be associated with impairment of Nrf2 activation and function from chronic and sustained oxidative redox disturbances, resulting in insufficient GSH synthesis and conjugation and depletion of the Cys and GSH thiol pools.
Figure 1.
Overview of Nrf2 activation and transcription of selected genes related to glutathione synthesis and conjugation.
METHODS
Sample
This study was approved by the Emory University Institutional Review Board. Informed consent and assent were obtained from all subjects. A convenience sample of children 6–17 years of age with persistent asthma treated with a daily asthma controller medication was obtained from a pediatric subspecialty clinic. All children were stable at the time of assessment with pulmonary function values within 10% of baseline and no signs of acute respiratory illnesses. Children with acute illnesses or worsening of asthma control were treated accordingly and reassessed at a later date. Severe asthma was diagnosed according to American Thoracic Society Workshop criteria13 after possible co-morbid conditions such as sinusitis and gastroesophageal reflux were addressed. Adherence to ICS for at least six months was demonstrated for all children with persistent asthma by an analysis of prescription refills.2 Thresholds for high-dose ICS were adjusted for children and were defined as ≥ 440 mcg of fluticasone equivalent per day for children < 12 years and ≥ 880 mcg for children 12–17 years of age.2,14
Procedures
Participants underwent phenotypic characterization consisting of questionnaires and allergy evaluation as previously described.2 Exhaled nitric oxide was determined online (NIOX Mino®, Aerocrine, Solna, Sweden) according to published recommendations.15 Spirometry was performed with a portable spirometer (KoKo® PDS, Ferraris, Louisville, CO)16 and the best of three forced vital capacity (FVC) maneuvers was interpreted with population reference equations.17 Whole blood (25 mL) was collected into heparinized tubes containing a density gradient (Vacutainer® CPT™, Becton, Dickinson and Company, Franklin Lakes, NJ). A subset of subjects further underwent bronchoscopy with bronchoalveolar lavage (BAL) for clinical indications, namely persistent asthma symptoms despite ICS treatment.18 Bronchoscopy was performed under general anesthesia using a laryngeal mask airway and flexible bronchoscope (Olympus BF-3C160, Olympus America Inc., Melville, NY). BAL fluid was collected from the right middle lobe with three 1 mL/kg (50 mL maximum) saline lavages. The BAL return volume was divided between the research and clinical laboratories. The samples submitted to the clinical laboratories were subjected to standard culture and sensitivity testing, viral respiratory panels, and cytopathological stains for bacteria and fungi.
Sample preparation
Blood samples were centrifuged at 1800 RCF for 20 minutes at 25°C and BAL samples were centrifuged 1200 rpm for 7 minutes at 25°C to separate the peripheral blood mononuclear cells (PBMCs) and airway lavage cells, respectively. To prevent auto-oxidation prior to thiol analysis, 250 μL of plasma and BAL supernatant were added to an equal volume of a 5% perchloric acid solution containing iodoacetic acid (6.7 μmol/L) and boric acid (0.1 mol/L) with 5 μmol/L γ-glutamyl-glutamate.19 PBMCs and airway lavage cells were added to 5 volumes of RNAlater® prior to RNA extraction (Applied Biosciences, Carlsbad, CA). Remaining samples not dedicated to thiol or RNA analysis were frozen at −80°C with protease inhibitors (Complete Mini EDTA-free, Roche Applied Science, Mannheim, Germany).
Thiol determination
Thiols (Cys, GSH) and thiol disulfides (CySS, GSSG) were measured in the plasma and BAL supernatant as previously described5 by high-performance liquid chromatography after derivatization of the samples with dansyl chloride.19 Derivatives were separated on a 10 μm Ultrasil amino-column with fluorescence detection at 365 nm (Waters Alliance 2690, Waters Corporation, Milford, MA). Cys, GSH, CySS and GSSG were quantified relative to γ-glutamyl-glutamate by integration.20 The redox potentials (Eh) of the CyS/CySS and GSH/GSSG thiol pairs were calculated with the Nernst equation, E h = Eo + RT/nF ln [disulfide]/([thiol1][thiol2]), where Eo is the standard potential for the redox couple, R is the gas constant, T is the absolute temperature, n is the number of electrons transferred, and F is Faraday’s constant. A more positive number indicates greater oxidation of the thiol/disulfide pair.21
Quantitative mRNA analysis
RNA was isolated from PBMCs and airway lavage cells using a commercial kit (RNeasy Mini, Qiagen, Valencia, CA) according to the manufacturer’s instructions. 10 ng of total RNA per sample was reverse transcribed using MultiScribe® Reverse Transcriptase (62.U/50 μL reaction), RNase Inhibitor, oligoT primers and MgCl2 at a concentration of 5.5 mM (Applied Biosystems, Carlsbad, CA). cDNA aliquots were used to quantitate relative levels of Nrf2 (Hs00232352_m1), Keap1 (Hs00202227_m1), the GST isoenzymes mu (GSTM1, Hs01683722_m1), pi (GSTP1, Hs00168310_m1) and theta (GSTT1, Hs00184475), glutathione synthetase (GSS, Hs00609286), and glutamate-cysteine ligase (catalytic subunit, GCLC, Hs00155249_m1; modifier subunit, GCLM, Hs00157694_m1) in a 96-well assay system (StepOnePlus real-time PCR assay; Applied Biosystems, Carlsbad, CA) with TaqMan primer pairs and probes (Applied Biosystems, Carlsbad, CA). Data were normalized to five housekeeping genes: β2 microglobulin (B2M, 4333766F), glyceraldehydes 3-phosphate dehydrogenase (GAPDH, 4333764F), β-glucuronidase (GUSB, 4333767F), phosphoglycerate kinase 1 (PGK1, 4333765F), and peptidylprolyl isomerase A (PPIA, 43337663F) whose expression did not differ significantly between the severe and mild-to-moderate asthmatic groups. Net cycle threshold (CT) values for each gene or interest were used to calculate ΔCT values for each subject, using the average CT of the five housekeeping genes as a reference.
GST activity
Total GST activity (cytosolic and microsomal) in the plasma and BAL supernatant was assessed using an assay kit (Cayman Chemical, Ann Arbor, MI). GST activity was determined after conjugation of 1-chloro-2,4-dinitrobenzene with GSH. GST activity was analyzed over five minutes at 340 nm with a 1-chloro-2,4-dinitrobenzene extinction coefficient of 0.0096 μM−1 cm−1.
Nrf2 activation
Nrf2 ARE binding was assessed in PBMC lysates using an ELISA-based Nrf2 TransAM™ transactivation kit (Active Motif, Carlsbad, CA). Briefly, cell lysates (minimum 5 μg) were loaded in a 96-well plate with immobilized oligonucleotides containing the consensus ARE to which the Nrf2 antibody binds. This signal is detected at an optical density of 450 nm after the addition of a horseradish peroxidase-conjugated secondary antibody and corresponds to the amount of Nrf2 activation in the sample.
Immunoblotting
PBMC samples (5–20 μg of protein per sample) were separated on SDS-PAGE using a 4–20% gradient ReadyGel (BioRad, Hercules, CA) and transferred to nitrocellulose. After transfer, membranes were rinsed with PBS and blocked with Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE). After blocking, membranes were washed in PBS containing 0.1% Tween-2 (PBS-T) and antibodies applied at a 1:300 ratio using the SnapID system (Millipore, Billerica, MA) per the manufactures instructions. Dual staining was performed using anti-Nrf2 or anti-Keap1 (AbCam, Cambridge, MA) followed by Goat anti-Rabbit IgG (H+L) conjugated to IRDye 680LT (LiCor Biosciences, Lincoln, NE) and β-actin Mouse mAB (Li-Cor Biosciences, Lincoln, NE) followed by Goat anti-Mouse IgG (H+L) conjugated to IRDye 800CW (Li-Cor Biosciences, Lincoln, NE). Membranes were washed 3 times with PBS-T after the application of each antibody and analyzed with an Odyssey® infrared imaging system (Li-Cor Biosciences, Lincoln, NE) at 700 and 800 nm. Equal loading of the samples was determined using beta-actin as a reference.
Data analysis
Data analysis was performed with PASW Statistics 18 (SPSS Inc., Chicago, IL). Cys, GSH, GSH and GSSG were logarithmically transformed prior to statistical analyses due to a non-normal distribution. mRNA gene expression was analyzed relative to the mild-to-moderate group and expressed as 2−ΔΔCT values. Nrf2 activation was normalized to protein (50 μg) and expressed as the optical density at 450 nm. T-tests were performed for between group comparisons and Pearson correlation coefficients and linear regression were used to determine variable associations. Two-tailed tests of significance were used with α < 0.05 as the threshold of significance.
RESULTS
Eighty-nine children with persistent asthma treated with daily asthma controller therapy were enrolled, including 38 children with mild-to-moderate asthma and 51 children with severe asthma. All children had historical evidence of bronchial hyperreactivity or reversibility of at least 12% in the forced expiratory volume in one second (FEV1) after bronchodilator administration. Features of the groups appear in Table 1. Consistent with the definition, children with severe asthma were highly symptomatic and were treated with more asthma controller therapies. Airflow limitation was also more pronounced in children with severe asthma and was further associated with increased markers of allergic sensitization, including increased exhaled nitric oxide and serum immunoglobulin E concentrations. Compared to children with mild-to-moderate asthma, children with severe asthma were also more likely to be black and have greater duration of asthma (Table I).
Table 1.
Features of the subjects. Data are shown as mean ± SD or frequency (%).
| Feature | Mild-to-moderate asthma (n = 38) | Severe asthma (n = 51) | p-value |
|---|---|---|---|
| Age (years) | 11 ± 4 | 12 ± 4 | 0.273 |
|
| |||
| Males | 18 (47) | 34 (67) | 0.084 |
|
| |||
| White | 22 (58) | 7 (14) | < 0.001 |
| Black | 15 (40) | 41 (80) | |
|
| |||
| Asthma duration (years) | 9 ± 4 | 11 ± 4 | 0.028 |
|
| |||
| Daily Medications | |||
| Inhaled corticosteroids (ICS) | 35 (92) | 51 (100) | 0.041 |
| Long-acting beta agonists | 12 (32) | 43 (84) | < 0.001 |
| Montelukast | 24 (63) | 46 (90) | 0.002 |
| Oral corticosteroids | 0 | 17 (33) | < 0.001 |
|
| |||
| ICS dose (mcg fluticasone/day) | 383 ± 287 | 801 ± 250 | < 0.001 |
|
| |||
| Healthcare Utilization | |||
| Emergency room visit (previous year) | 15 (40) | 41 (80) | < 0.001 |
| Hospitalization (previous year) | 7 (18) | 28 (55) | < 0.001 |
| Intensive care admission (ever) | 5 (13) | 33 (65) | < 0.001 |
| Intubation (ever) | 5 (13) | 27 (53) | < 0.001 |
|
| |||
| Pulmonary function | |||
| FVC (% predicted) | 104 ± 16 | 94 ± 20 | 0.016 |
| FEV1 (% predicted) | 98 ± 13 | 79 ± 20 | < 0.001 |
| FEV1/FVC | 0.83 ± 0.08 | 0.73 ± 0.12 | < 0.001 |
| FEV1/FVC (% predicted) | 93 ± 8 | 85 ± 12 | 0.008 |
| FEF25–75 (% predicted) | 83 ± 19 | 57 ± 26 | < 0.001 |
|
| |||
| Daily asthma symptoms (cough or wheeze) | 1 (3) | 24 (47) | 0.002 |
|
| |||
| Exhaled nitric oxide (online, ppb)1 | 38 ± 45 | 49 ± 43 | 0.047 |
|
| |||
| Serum immunoglobulin E (kU/L)1 | 118 ± 251 | 593 ± 966 | 0.001 |
|
| |||
| Blood eosinophils (%)1 | 4 ± 3 | 6 ± 4 | 0.278 |
p-value obtained after logarithmic transformation.
Forty-one children (mild-to-moderate asthma, n = 18; severe asthma, n = 23) underwent bronchoscopy with BAL. Features of the children who underwent bronchoscopy did not differ from those of the larger sample (mean age 11 ± 4 years, 56% male, 49% black). Whereas children with severe asthma underwent bronchoscopy as indicated for persistent asthma symptoms despite high-dose ICS treatment,18 children with mild-to-moderate asthma underwent bronchoscopy for recurrent pneumonia (n = 6), suspected foreign body aspiration (n = 2), suspected aspiration (n = 5), and upper airway evaluation (n = 5). All children included were stable at the time of bronchoscopy and were treated with a stable dose of ICS for at least 6 weeks (ICS dose: 782 ± 302 vs. 516 ± 242 μg/day fluticasone for severe vs. mild-to-moderate asthma, p = 0.004). All children who underwent bronchoscopy and were included in this study had no evidence of active airway infection, including negative viral respiratory panels and bacterial cultures. Bronchoscopy with BAL was well tolerated and no child required overnight hospitalization or prolonged observation.
Thiol measurement
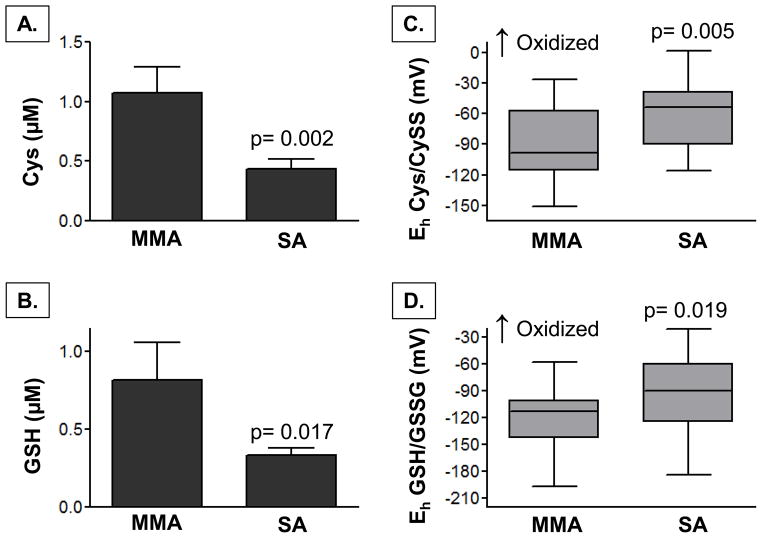
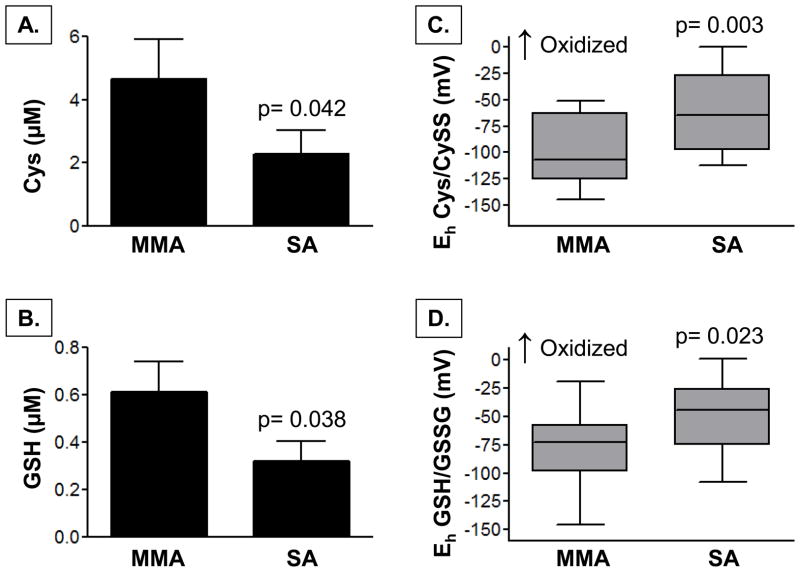
In the plasma, children with severe asthma had significantly lower concentrations of Cys and GSH (Figure 2), as well as lower total CyS and GSH thiol pools (CyS + CySS: 3.1 ± 2.1 vs. 4.5 ± 2.7 μM, p = 0.050; GSH + GSSG: 0.6 ± 0.5 vs. 1.3 ± 1.7 μM, p = 0.033). Whereas plasma CySS and GSSG concentrations were not different between groups (severe versus mild-to-moderate asthma, CySS: 2.8 ± 2.0 vs. 3.4 ± 2.1 μM; GSSG: 0.3 ± 0.4 vs. 0.5 ± 0.8 μM; p = ns), children with severe asthma had increased oxidation of the Cys/CySS and GSH/GSSG couples, reflected by a more positive redox potential (Eh) (Figure 2). Similar findings were noted in the BAL supernatant. Compared to children with mild-to-moderate asthma, children with severe asthma had lower concentrations of CyS and GSH (Figure 3) and lower total Cys and GSH thiol pools in the airway lavage (CyS + CySS: 2.9 ± 4.4 vs. 6.3 ± 6.5 μM, p = 0.043; GSH + GSSG: 0.7 ± 0.7 vs. 1.2 ± 0.9 μM, p = 0.031). Airway CySS and GSSG concentrations did not differ between groups (severe versus mild-to-moderate asthma, CySS: 1.5 ± 2.3 vs. 1.4 ± 2.2 μM; GSSG: 0.4 ± 0.4 vs. 0.6 ± 0.5 μM; p = ns). However, children with severe asthma again had greater oxidation of the CyS/CySS and GSH/GSSG couples to a similar magnitude of what was observed in the plasma (Figure 3).
Figure 2.
Plasma concentrations of (A) Cys, (B) GSH, and the redox potential (Eh) of (C) Cys/Cyss and (D) GSH/GSSG in children with mild-to-moderate asthma (MMA, n = 23) and severe asthma (SA, n = 36). Bar graphs depict the mean ± SEM. Box plots are shown with medians and the upper and lower quartiles in gray.
Figure 3.
BAL supernatant concentrations of (A) Cys, (B) GSH, and the redox potential (Eh) of (C) Cys/Cyss and (D) GSH/GSSG in children with mild-to-moderate asthma (MMA, n = 18) and severe asthma (SA, n = 23). Bar graphs depict the mean ± SEM. Box plots are shown with medians and the upper and lower quartiles in gray.
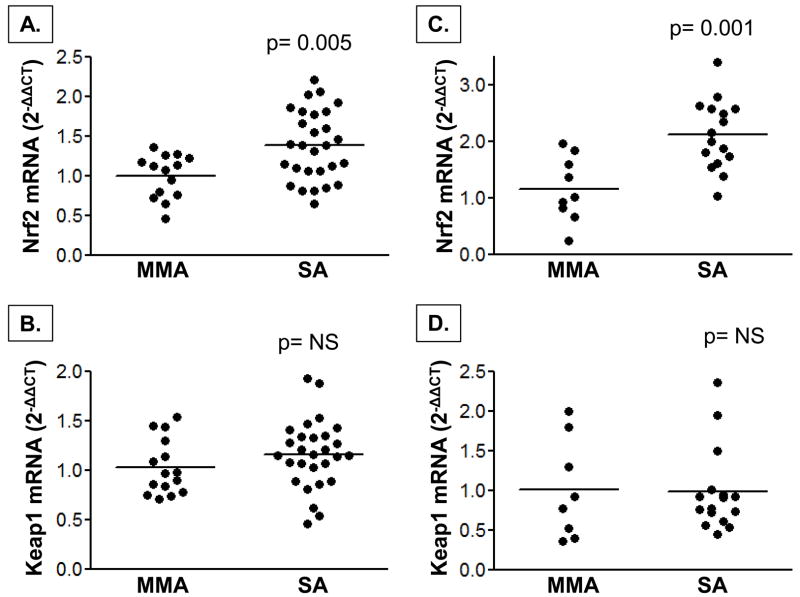
mRNA expression of Nrf2 and downstream genes
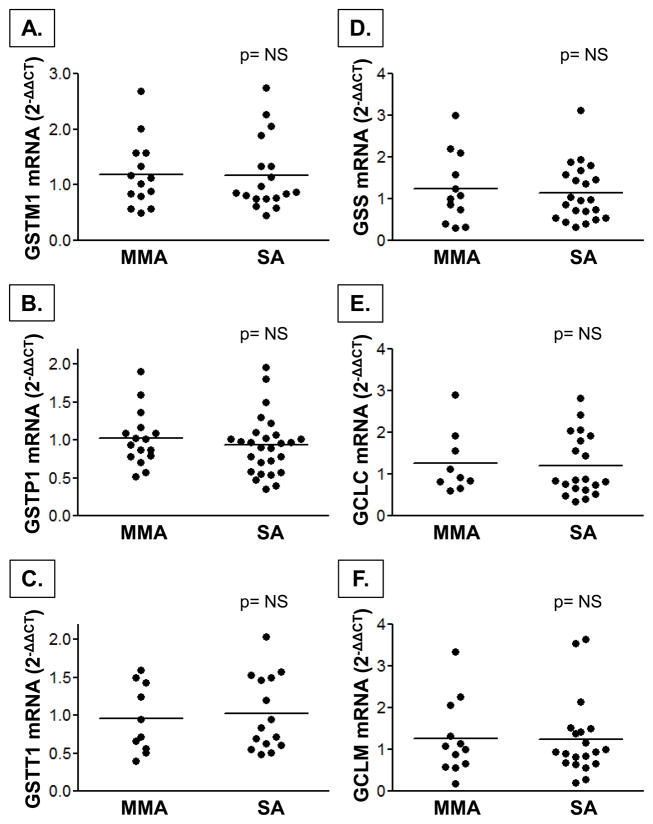
Nrf2 mRNA expression was higher in children with severe asthma in both the PBMCs and airway lavage cells (Figure 4). Keap1 mRNA expression was not different between groups. Despite increased Nrf2 expression in the airways and systemic circulation of children with severe asthma, mRNA expression of downstream targets of Nrf2 activation, including GSH synthesis genes (GSS, GCLC, GCLM) and GST genes (GSTP1, GSTT1, GSTM1) also did not differ between groups (Figure 5, airway lavage cell data not shown). Total GST activities in the plasma and BAL supernatant were also not different between children with severe asthma and mild-to-moderate asthma (severe vs. mild-to-moderate asthma, plasma: 2.8 ± 1.9 vs. 2.5 ± 1.9 nmol/min/mL, p = ns; BAL: 43 ± 27 vs. 49 ± 50 nmol/min/mL, p = ns).
Figure 4.
Nrf2 and Keap1 mRNA gene expression in PBMCs (A, B) and airway lavage cells (C, D) from children with mild-to-moderate (MMA) and severe asthma (SA). Data were normalized to 5 housekeeping genes and are shown relative to MMA group as 2−ΔΔCT values. Horizontal lines represent the mean.
Figure 5.
mRNA gene expression of downstream targets of Nrf2 activation responsible for GSH synthesis, including the (A–C) GST isoenzymes mu, pi, and theta, (D) glutathione synthetase, and (E, F) glutamate-cysteine ligase (catalytic and modifier subunits) in PBMCs from children with mild-to-moderate (MMA) and severe asthma (SA). Data were normalized to 5 housekeeping genes and are shown relative to MMA group as 2−ΔΔCT values. Horizontal lines represent the mean.
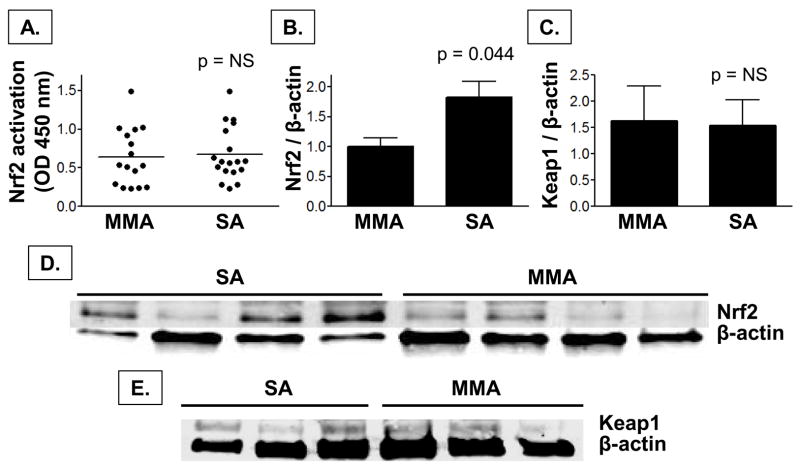
Nrf2 activation and protein expression
Because similar results for Nrf2 mRNA expression were observed in the both the systemic and airway cells, the remaining experiments were performed exclusively on PBMCs given the limited BAL cell pellet available for further analysis. Given the increased expression of Nrf2 mRNA we observed in children with severe asthma, we assessed whether Nrf2 activation and protein expression were also increased in this group. Compared to children with mild-to-moderate asthma, children with severe asthma did not have increased Nrf2 activation despite higher protein expression of Nrf2 (Figure 6). Consistent with our mRNA gene expression findings, Keap1 protein expression was also not different between groups (Figure 6).
Figure 6.
Nrf2 (A) activation and (B) protein expression and (C) Keap1 protein expression in PBMCs from children with mild-to-moderate asthma (MMA) and severe asthma (SA). Nrf2 and Keap1 expression were normalized to β-actin and are expressed relative to the MMA group as mean ± SEM values (Nrf2: MMA, n = 8; SA, n = 16; Keap1: MMA, n = 5; SA, n = 5). Representative images of Nrf2 and Keap1 protein expression are shown in panels D and E, respectively.
Clinical correlates of increased Nrf2 expression
To determine whether increased Nrf2 mRNA expression in this sample was associated with thiol redox status among individual study participants, correlations between PBMC Nrf2 mRNA expression (relative to the housekeeping ge.nes) and plasma GSH, GSSG, Cys, and CySS were assessed. Although no significant correlations between Nrf2 mRNA expression and Cys or CySS were noted, Nrf2 mRNA expression was significantly higher in children with lower plasma GSH concentrations and a more oxidized redox potential (Eh) for the GSH/GSSG couple (Figure 7).
Figure 7.
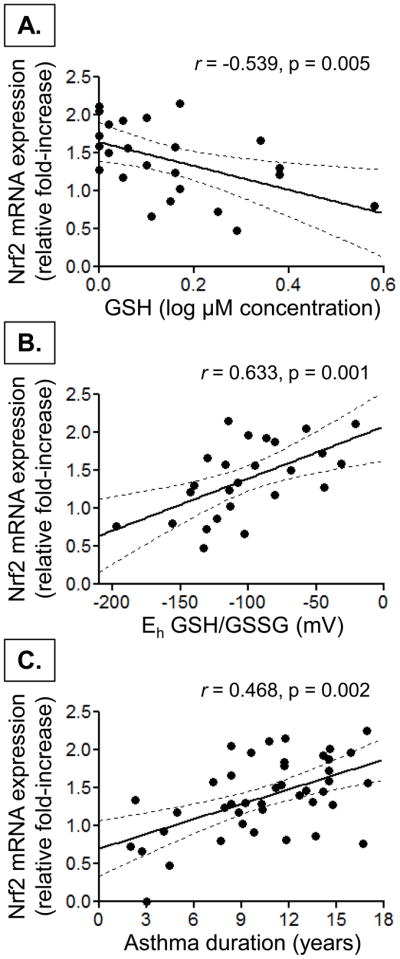
Scatterplot depicting the relationship between Nrf2 mRNA expression in PBMCs and (A) plasma GSH, (B) the redox potential (Eh) of the GSH:GSSG couple, and (C) asthma duration. Nrf2 mRNA expression is shown as the fold-increase in expression relative to the housekeeping genes. The dashed lines depict the 95% confidence interval.
To further determine clinical factors that might affect Nrf2 expression in this sample, univariate linear regression was performed using the increase in PBMC Nrf2 mRNA expression relative to the housekeeping genes as the dependent variable and sex, race, ICS dose, duration of asthma, the frequency of current asthma symptoms, hospitalization for asthma within the previous year, history of intubation, exhaled nitric oxide concentration and baseline lung function (FEV1 and FEV1/FVC) as predictors. There were no associations between Nrf2 mRNA expression and sex, race, hospitalization, exhaled nitric oxide, or lung function. However, with univariate analyses, Nrf2 mRNA expression was significantly associated with the duration of asthma (R2 = 0.219, p = 0.002), the frequency of current asthma symptoms (R2 = 0.102, p = 0.037), and history of intubation (R2 = 0.126, p = 0.020), as well as ICS dose (R2 = 0.120, p = 0.023). When multivariate analyses were performed with these three variables controlling for ICS dose, only asthma duration (p = 0.001) was significantly associated with Nrf2 mRNA expression (model R2 = 0.446, p < 0.001). A scatterplot depicting the relationship between Nrf2 mRNA expression and asthma duration is shown in Figure 7.
DISCUSSION
To our knowledge, this is the first study to describe post-translational modification of Nrf2 in subjects with asthma. Moreover, this is the first study to reveal disruption of the Nrf2 pathway as a mechanism underlying thiol redox disturbances in asthmatic children. Compared to children with mild-to-moderate asthma, children with severe asthma had lower concentrations of the thiols Cys and GSH in the plasma and airway lavage associated with a shift in the redox potential (Eh) of the Cys/CySS and GSH/GSSG redox couples to the more oxidized state. Remarkably, the magnitude of the Cys/CySS and GSH/GSSG oxidation was similar between the airway and systemic compartments, suggesting a global disruption of redox signaling and control that is not alleviated by endogenous airway defenses. While these thiol redox disturbances were accompanied by an expected upregulation of Nrf2 mRNA and protein, this did not translate to an increase in downstream targets of Nrf2 ARE binding and activation, including key enzymes involved in GSH synthesis and conjugation. Indeed, Nrf2 activation was not different between mild-to-moderate and severe asthmatic children. Given that Nrf2 expression was strongly associated with GSH redox disturbances and the duration of asthma, we conclude that the Nrf2 pathway is disrupted as a function of chronic oxidative stress. Thus in the case of severe asthma, the high expression of Nrf2 at baseline is not beneficial but rather reflects the inability of this pathway to increase GSH synthesis in response to GSH depletion. This Nrf2 “burnout” (i.e., the inability by Nrf2 to counteract even minor, acute redox disturbances) might further explain the increased risk and severity of exacerbations in children with severe asthma,2 particularly in the presence of secondary insults such as respiratory infection.22,23
While our study provides the first evidence of altered Nrf2 expression and activity in subjects with asthma, Nrf2 alterations have been previously described in smokers and adults with chronic obstructive pulmonary disease (COPD). In healthy smokers, Nrf2 is activated in the airway epithelium and increases expression of downstream genes containing functional AREs in the promoter region. 24,25 However, in smokers with mild COPD (stage 1–2), GSH-related gene expression is not increased despite airway GSH depletion and oxidative stress.26 As the duration of smoking and severity of COPD increases, airway Nrf2 protein is further depleted in these subjects.26–28 Thus the chronic and sustained oxidative stress conferred by cigarette smoking likely overwhelms the capacity of the Nrf2 pathway to provide antioxidant defense. Because our study was limited to children, it may be that Nrf2 expression increases initially and then progressively declines as severe asthma evolves and worsens from childhood to adulthood. Longitudinal studies of chronic changes in Nrf2 expression in children with severe asthma are needed.
The post-translational modification of Nrf2 that we observed in children with severe asthma is intriguing and suggests that Nrf2, along with thiol redox status, may be a novel biomarker of disease status in asthma. Increased Nrf2 expression in severe asthma may further account for our previous observations of increased IL-8 expression 4,7 and decreased histone deacetylase (HDAC) activity4 in this group. The promoter region of the IL-8 gene contains an ARE element and in vitro studies have demonstrated increased IL-8 expression and enhanced IL-8 mRNA stability with Nrf2 activation.29 Another recent study noted decreased HDAC expression and activity in lung tissue from Nrf2−/− mice associated with increased inflammation and steroid resistance.30 While the clinical relevance of these previous studies is unclear, these observations highlight important relationships between Nrf2 and differentiating features of severe asthma in children that warrant further study.
This study does not inform the specific mechanism(s) underlying Nrf2 dysfunction in children with severe asthma. Nrf2 is expressed in nearly all tissues, including PBMCs and airway lavage cells, and is particularly abundant in systems such as the lung that are exposed to the external environment. 31 While the oxidative burden associated with severe asthma may be attributed to environmental exposures, dysfunction of epithelial cells and phagocytes, including granulocytic leukocytes and monocytes/macrophages, may also contribute.32,33 Indeed, we have previously reported persistent activation of airway macrophages in children with severe asthma associated with increased pro-inflammatory cytokine secretion,7 increased lipid and DNA oxidation,4 and impaired phagocytic function34 that may further increase free radical burden. Given the complexities of the innate antioxidant response, coupled with the clinical heterogeneity of severe asthma,35 it is possible that there are several mechanisms responsible for our observations. One such mechanism could be impaired liberation of Nrf2 from Keap1, which results in ubiquitination and proteosomal degradation of Nrf2.36,37 Other mechanisms include acetylation and phosphorylation of Nrf2,38,39 although the functional and clinical relevance of Nrf2 phosphorylation has been questioned.40 Alternatively, Nrf2 dysfunction and redox disturbances may result from single nucleotide polymorphisms in Nrf2, which have been associated with decreased lung function in the general population41 and increased risk of acute lung injury after significant trauma.42
This study has a number of limitations. First, because bronchoscopy can only be performed for clinical indications in children, the inclusion of a healthy, aged-matched control group was not possible. Regardless, the purpose of this study was to provide insight regarding a potential mechanism of severe versus mild-to-moderate asthma in children, and not a mechanism of asthma per se. Thus comparisons to children with mild-to-moderate asthma help eliminate potential confounding by asthma medication exposure and other key variables in the medical history. However, given the disproportionate representation of black males in our severe asthma group, it is possible that the Nrf2 dysregulation we observed could be due to underlying genetic differences. While enrollment of additional females and non-Hispanic whites would have led to more balanced groups, our group of children with severe asthma likely reflects important genetic differences in asthma severity. Indeed, other genetic-based studies have shown that blacks have the earliest age of asthma onset, the strongest family history of asthma, and the lowest lung function.43 Males with persistent wheezing also have lower lung function compared to females.44 While genetic differences underlying Nrf2 dysfunction in severe asthma were beyond the scope of our study, this is a potentially important finding that should be explored.
Because this study was focused on children, our findings may not be generalized to asthmatic adults. However, given previous observations of Nrf2 dysfunction in adults with COPD,26–28 the inclusion of children provides potentially useful information for the development of future disease-modifying interventions. Furthermore, although we did observe an overall trend of increased Nrf2 expression in children with severe asthma, there was heterogeneity within this group of subjects similar to what has been reported previously.35 Future studies are needed to tease out individualized patterns of Nrf2 responses, including the stability of Nrf2 expression and thiol redox disturbances over time, to understand the clinical relevance of our findings.
In summary, we have demonstrated for the first time significant thiol redox disturbances in the airways and systemic circulation of children with severe asthma associated with dysregulation of the transcription factor, Nrf2. Despite increased Nrf2 expression in children with severe versus mild-to-moderate asthma, Nrf2 activity and expression of downstream Nrf2 genes were not different between groups. Although additional studies are warranted, these findings highlight a potential mechanism for the persistent airway inflammation and oxidative stress that accompany severe asthma. Ultimately, interventions to overcome Nrf2 dysregulation and associated redox disturbances may be warranted in this population.
Acknowledgments
Funded by NIH RO1 NR012021 and supported in part by PHS Grant UL1RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources
The authors would like to acknowledge Karen DeMuth, Denise Whitlock, Shanae Wakefield, Christine Spainhour, Michelle Popler, and Emily Morrison for their assistance with subject recruitment and characterization.
ABBREVIATIONS
- ARE
Antioxidant Response Element
- BAL
Bronchoalveolar lavage
- COPD
Chronic obstructive pulmonary disease
- Cys
Cysteine (reduced form)
- CySS
Cystine
- CT
Cycle threshold
- FEF25–75
Mid-expiratory flow rate at 25–75% of vital capacity
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- GCLC
Glutamate-cysteine ligase, catalytic subunit
- GCLM
Glutamate-cysteine ligase, modifier subunit
- GSH
Glutathione (reduced form)
- GSS
Glutathione synthetase
- GSSG
Glutathione disulfide
- GST
Glutathione S-transferase
- GSTM1
Glutathione S-transferase mu isoform
- GSTP1
Glutathione S-transferase pi isoform
- GSTT1
Glutathione S-transferase tau isoform
- HDAC
Histone deacetylase
- ICS
Inhaled corticosteroid
- Keap1
Kelch-like ECH-associated protein 1
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- PBMC
Peripheral blood mononuclear cell
Footnotes
AMF is the Principal Investigator, oversaw all aspects of the research, and wrote the manuscript.
STS, GH, and LB performed data analysis and interpretation.
MP, DS, JH, DPJ, and LASB were involved in the conception, hypothesis delineation, and design of the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376:814–825. doi: 10.1016/S0140-6736(10)61054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–1225. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzpatrick AM, Brown LA, Holguin F, Teague WG. Levels of nitric oxide oxidation products are increased in the epithelial lining fluid of children with persistent asthma. J Allergy Clin Immunol. 2009;124:990–996. doi: 10.1016/j.jaci.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzpatrick AM, Teague WG, Burwell L, Brown MS, Brown LA. Glutathione oxidation is associated with airway macrophage functional impairment in children with severe asthma. Pediatr Res. 2011;69:154–159. doi: 10.1203/PDR.0b013e3182026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LA. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123:146–152. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick AM, Higgins M, Holguin F, Brown LA, Teague WG. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125:851–857. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzpatrick AM, Teague WG. Progressive airflow limitation is a feature of children with severe asthma. J Allergy Clin Immunol. 2011;127:282–284. doi: 10.1016/j.jaci.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexis NE, Zhou H, Lay JC, Harris B, Hernandez ML, Lu TS, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Peden DB. The glutathione-S-transferase Mu 1 null genotype modulates ozone-induced airway inflammation in human subjects. J Allergy Clin Immunol. 2009;124:1222–1228. doi: 10.1016/j.jaci.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ercan H, Birben E, Dizdar EA, Keskin O, Karaaslan C, Soyer OU, Dut R, Sackesen C, Besler T, Kalayci O. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J Allergy Clin Immunol. 2006;118:1097–1104. doi: 10.1016/j.jaci.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Minelli C, Granell R, Newson R, Rose-Zerilli MJ, Torrent M, Ring SM, Holloway JW, Shaheen SO, Henderson JA. Glutathione-S-transferase genes and asthma phenotypes: a Human Genome Epidemiology (HuGE) systematic review and meta-analysis including unpublished data. Int J Epidemiol. 2010;39:539–562. doi: 10.1093/ije/dyp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy NM, Kleeberger SR, Yamamoto M, Kensler TW, Scollick C, Biswal S, Reddy SP. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol Genomics. 2007;32:74–81. doi: 10.1152/physiolgenomics.00126.2007. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions; Am J Respir Crit Care Med; 2000. pp. 2341–2351. [DOI] [PubMed] [Google Scholar]
- 14.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 15.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 18.Payne D, McKenzie SA, Stacey S, Misra D, Haxby E, Bush A. Safety and ethics of bronchoscopy and endobronchial biopsy in difficult asthma. Arch Dis Child. 2001;84:423–426. doi: 10.1136/adc.84.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones DP, Carlson JL, Samiec PS, Sternberg P, Mody VC, Reed RL, Brown LA. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 20.Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 21.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 22.Reddy NM, Suryanarayana V, Kalvakolanu DV, Yamamoto M, Kensler TW, Hassoun PM, Kleeberger SR, Reddy SP. Innate immunity against bacterial infection following hyperoxia exposure is impaired in NRF2-deficient mice. J Immunol. 2009;183:4601–4608. doi: 10.4049/jimmunol.0901754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, Kleeberger SR. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med. 2009;179:138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubner RH, Schwartz JD, De Bishnu P, Ferris B, Omberg L, Mezey JG, Hackett NR, Crystal RG. Coordinate control of expression of Nrf2-modulated genes in the human small airway epithelium is highly responsive to cigarette smoking. Mol Med. 2009;15:203–219. doi: 10.2119/molmed.2008.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelsen SG, Duan X, Ji R, Perez O, Liu C, Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol. 2008;38:541–550. doi: 10.1165/rcmb.2007-0221OC. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2008;39:673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- 28.Goven D, Boutten A, Lecon-Malas V, Marchal-Sommé J, Amara N, Crestani B, Fournier M, Lesèche G, Soler P, Boczkowski J, Bonay M. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63:916–924. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Chen X, Song H, Chen HZ, Rovin BH. Activation of the Nrf2/antioxidant response pathway increases IL-8 expression. Eur J Immunol. 2005;35:3258–3267. doi: 10.1002/eji.200526116. [DOI] [PubMed] [Google Scholar]
- 30.Adenuga D, Caito S, Yao H, Sundar IK, Hwang JW, Chung S, Rahman I. Nrf2 deficiency influences susceptibility to steroid resistance via HDAC2 reduction. Biochem Biophys Res Commun. 2010;403:452–456. doi: 10.1016/j.bbrc.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 33.Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol. 2008;121:1372–1378. doi: 10.1016/j.jaci.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, Wenzel SE, Aujla S, Castro M, Bacharier LB, Gaston BM, Bleecker ER, Moore WC. Heterogeneity of severe asthma in childhood: Confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127:382–389. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 38.Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 39.Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res. 2010;44:1267–1288. doi: 10.3109/10715762.2010.507670. [DOI] [PubMed] [Google Scholar]
- 41.Siedlinski M, Postma DS, Boer JM, van der Steege G, Schouten JP, Smit HA, Boezen HM. Level and course of FEV1 in relation to polymorphisms in NFE2L2 and KEAP1 in the general population. Respir Res. 2009;10:73. doi: 10.1186/1465-9921-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, Kleeberger SR. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 43.Lester LA, Rich SS, Blumenthal MN, Togias A, Murphy S, Malveaux F, Miller ME, Dunston GM, Solway J, Wolf RL, Samet JM, Marsh DG, Meyers DA, Ober C, Bleecker ER. Ethnic differences in asthma and associated phenotypes: collaborative study on the genetics of asthma. J Allergy Clin Immunol. 2001;108:357–362. doi: 10.1067/mai.2001.117796. [DOI] [PubMed] [Google Scholar]
- 44.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]