Abstract
Mast cells are located in the central nervous system (CNS) of many mammals and stress induces their degranulation. We postulated that mast cells are associated with wakefulness and stimulatory tone in the CNS, as reflected by spontaneous motor activity. Because stress also precipitates drug-seeking behavior in cocaine addicts, we also postulated that cocaine manifests its effects through this relationship. We investigated the influence of single and repeated injections of cocaine on circulating corticosterone, motor activity and degranulation of mast cells in both the thalamus and meninges of mice. Mice were subjected to 5 consecutive days of cocaine or saline followed by a single injection of cocaine or saline 11 days later. Spontaneous locomotor activity was measure for one hour after the final injection before death. Neither a single injection nor prior treatment with cocaine increased motor activity compared to saline-injected controls, however, repeated administration of cocaine induced a significant sensitization to its behavioral effect when delivered 11 days later. In mice that received only saline, motor activity correlated positively with mast cell degranulation in the meninges but not in the thalamus. Cocaine, regardless of the treatment schedule, disrupted this correlation. The concentration of corticosterone did not differ amongst groups and did not correlate with either behavior or mast cell parameters in any group. The correlation between behavioral activity and the mast cell degranulation in the meninges suggests that these parameters are linked. The disruptive effect of cocaine on this relationship indicates a role downstream from mast cells in the regulation of motor activity.
Keywords: Cocaine, mast cell, meninges, mouse, spontaneous locomotor activity, thalamus
1. Introduction
Mast cells are found not only in the periphery, but also in the central nervous system (CNS). Their number and distribution depend on the species and even on the individual animal (Silverman et al., 1994). Intracranial mast cells, derived from multipotent stem cells in bone marrow, enter the nervous system during development (Lambracht-Hall et al., 1990) and appear to continue populating the brain of adults by entry from the vasculature (Silverman et al., 1994), albeit in lower numbers. In the rodent brain, mast cells are almost exclusively located in the thalamus (Johnson and Krenger, 1992) where they are abundant in the ventral complex, medial dorsal, lateral and paraventricular nuclei (Asarian et al., 2002; Goldschmidt et al., 1984). Outside the parenchyma, mast cells are also common in the meninges.
Mast cells are highly sensitive to stress, as illustrated in rats (Esposito et al., 2001) and mice (Cirulli et al., 1998) where acute immobilization or even simple handling causes a significant portion of their mast cells to visibly degranulate in the thalamus as well as throughout the body (Persinger, 1980; Theoharides et al., 1995). Various chemicals, including corticotropin-releasing factor (CRF) (Crompton et al., 2003), a potent stress hormone that initiates hypothalamic-pituitary-adrenal (HPA) activity, induce mast cells to degranulate and release a host of compounds.
One of several compounds released during the degranulation of mast cells is histamine. This compound can act as a neurotransmitter in the brain via several different receptors. Relevant to locomotor activity, the H1 receptor is involved in movement, arousal and energy metabolism (Ohshima et al., 2007). Activity at this receptor causes a period of behavioral hyperactivity, as illustrated when histamine is injected intracerebroventricularly (icv). In contrast, the H3 receptor causes a brief period of hypoactivity (Chiavegatto et al., 1998).
Cocaine also has a potent effect on behavioral activity. Acutely administered, cocaine is a potent locomotor stimulant (Carey et al., 2001), producing this effect by inhibiting the reuptake of serotonin, norepinephrine and dopamine and thereby increasing the extracellular concentrations of these neurotransmitters in the CNS. When administered to mice that were previously injected with cocaine for several consecutive days, cocaine causes even greater behavioral activity than when administered to naïve mice (de Jong et al., 2009). This sensitization to the stimulant effect of cocaine can persist even after several days of drug withdrawal. Cocaine-induced hyperactivity can also be potentiated by thioperamide, an inverse agonist at the H3 receptor whose activity promotes histamine release (Brabant et al., 2009). This interaction indicates that the locomotor effect of cocaine is sensitive to histamine.
Stress-induced increases in CRF may be involved in the development, maintenance and reemergence of addiction (Goeders, 2002). For example, psychologically or physically stressful experiences can sensitize individuals to the effects of drugs of abuse and encourage drug-seeking behavior (Piazza and Le Moal, 1998). If mast cells are also involved in the stimulant or addictive effects of cocaine, it is possibly linked to their common sensitivity to stress and CRF. The present studies were designed to determine whether mast cells are linked to behavioral locomotor activity, to the ability of cocaine to increase behavioral activity, or to cocaine’s ability to sensitize mice to subsequent exposure to cocaine.
2. Results
2.1. Behavioral activity
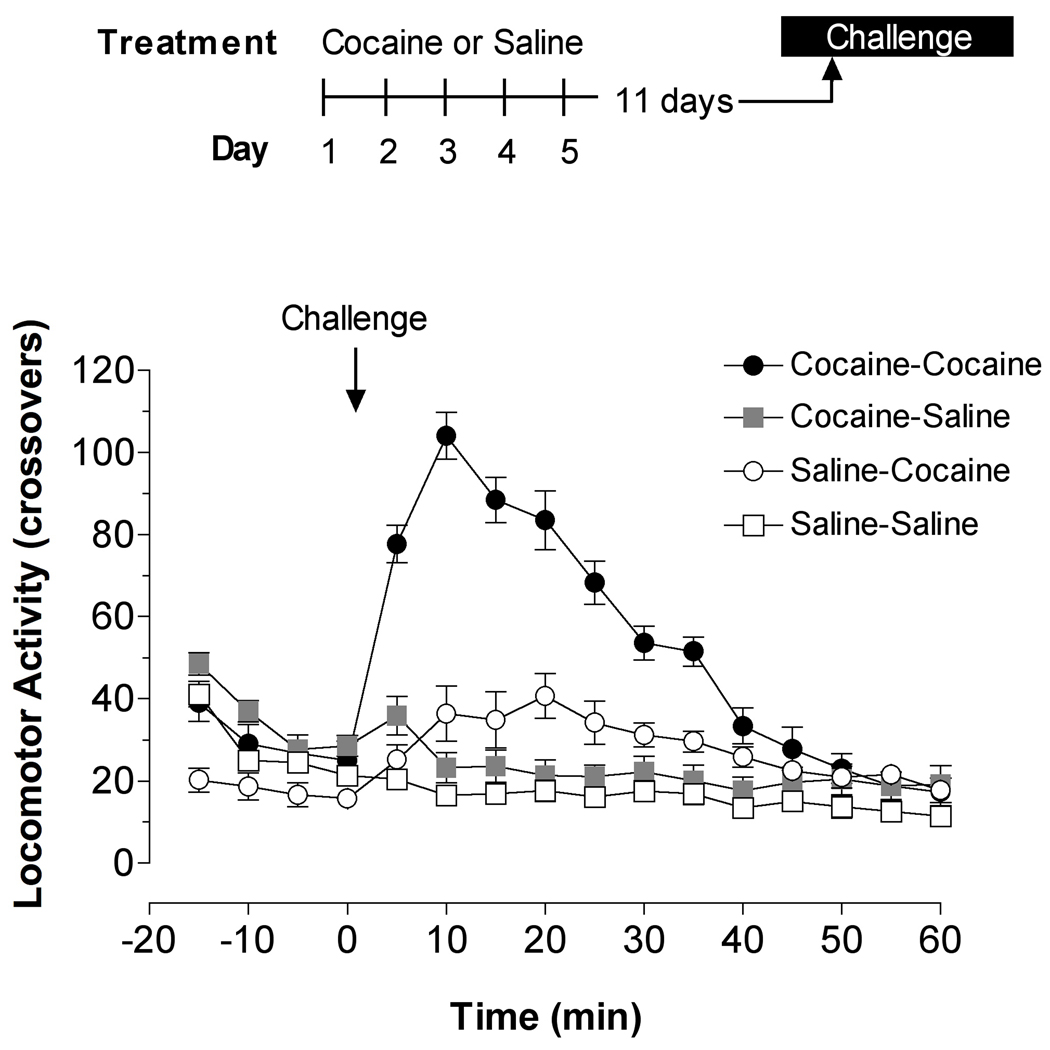
At the 15 mg/kg dose administered, the behavioral activity of mice was increased by cocaine but only when this drug was injected daily for 5 days in addition to the final challenge (Figure 1). This treatment schedule resulted in the sensitization of mice to the behavioral effect produced by a final injection of cocaine delivered 1 hr prior to their death (Table 1). The activity of the cocaine-cocaine group was significantly greater than that of any of the other groups tested when assessed by either the change induced during the first 15 min after injection of the challenge drug, by the average activity of mice during the 60 min interval, or by the total behavioral activity assessed during the span of the 60-min recording period.
Figure 1.
Behavioral sensitization of mice to the stimulant effect of cocaine on spontaneous locomotor activity. The time-line on the top indicates the treatment schedule for four groups of mice. Mice were injected with either 15 mg/kg of cocaine or saline intraperitoneally for 5 consecutive days and then 11 days later injected again with the same dose of cocaine or saline as a challenge dose. Locomotor activity, in terms of crossovers, was monitored at 5-min intervals for 60 min after the last injection, at which point the mice were killed. The values plotted illustrate the dramatic increase in locomotor activity in mice injected with cocaine for 5 days in addition to during the challenge (cocaine-cocaine) compared to any of the other treatment groups.
Table 1.
Effect of Cocaine on Behavioral Activity and Corticosterone
| Parameter ±SEM |
Sal-Sal | Sal-Coc | Coc-Sal | Coc-Coc |
|---|---|---|---|---|
| Sum in first 15 Minutes | 17.93 ±1.40 | 32.16 ±5.08 | 25.89 ±3.62 | 88.62 ±4.91* |
| Average Number of Behaviors | 17.89 ±1.69 | 24.62 ±2.27 | 22.87 ±2.22 | 45.76 ±3.55* |
| Sum of Behaviors | 304.1 ±28.76 | 418.5 ±38.54 | 388.67 ±37.70 | 777.78 ±60.38* |
| Circulating Corticosterone (ng/ml) | 139.6 ±26.29 | 111.52 ±11.83 | 114.92 ±24.38 | 115.5 ±19.51 |
indicates that the value is significantly different from each of the other categories based on ANOVA followed by Fisher’s posthoc analysis at P< 0.05. Each group is designated by the two treatments, saline (Sal) or cocaine (Coc). The first listed is the daily injection delivered for 5 days prior to the day of testing. The second drug listed is the final challenge delivered 60 min prior to death.
2.2. Histological analysis: mast cells in the thalamus and meninges
Histological analysis of the brains of mice injected either acutely or chronically with cocaine suggest that neither the number of mast cells in the thalamus nor the incidence of their degranulation, as indicated by the percent of those found in a visible state of degranulation (illustrated in Figure 2), was influenced by treatment with cocaine (Table 2). Also quantified was the incidence of mast cells in the meninges (at the thalamic level) that were degranulated, and this too was not influenced by pretreatment with cocaine, whether acutely or chronically delivered.
Figure 2.
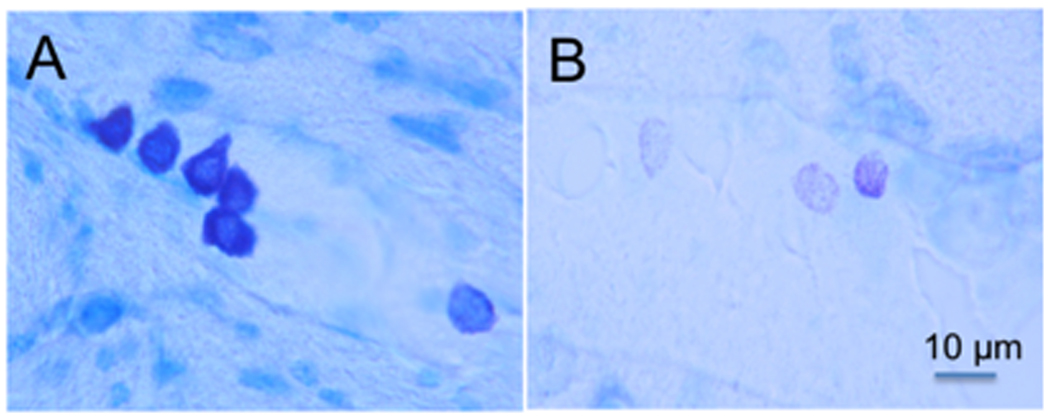
Mast cells in the CNS. (A) Cluster of darkly pigmented mast cells stained with toluidine blue (purplish black) in the thalamus of a mouse. The 5 mast cells on the left are typical of the fully granulated mast cells in the mouse thalamus. The lighter cell on the right in panel A depicts a mast cell that is partially degranulated containing only scattered granules. (B) Three partially degranulated mast cells appear as stippled (pinkish-violet) cells on a background (blue) of pale parenchymal cells. Individual granules that do not touch each other are visible in these cells at higher magnification. Magnification of both photographs is indicated by the bar in panel B. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Effect of cocaine (Coc) and saline (Sal) on thalamic and meningeal mast cells
| Parameter ±SEM |
Sal-Sal | Sal-Coc | Coc-Sal | Coc-Coc |
|---|---|---|---|---|
| Number of Mast Cells in the Thalamus | 23.3 ±5.97 | 25.2 ±6.41 | 21.78 ±7.82 | 22.4 ±9.61 |
| Percent of Mast Cells Degranulated In the Thalamus | 35.06 ±7.21 | 32.18 ±5.11 | 20.29 ±4.82 | 25.72 ±6.03 |
| Percent of Mast Cells Degranulated In the Meninges | 34.42 ±6.03 | 35.15 ±5.64 | 28.7 ±4.71 | 36.92 ±5.09 |
indicates that the value is significantly different from each of the other categories based on ANOVA followed by Fisher’s posthoc analysis at P< 0.05.
2.3. Correlation between behavioral activity and mast cell data in the thalamus
By exploiting the high variability in the behaviors of mice, in the number of mast cells, and in the incidence of mast cell degranulation, we were able to analyze the relationships between these parameters within each treatment group using correlational analyses. The strength of these relationships were indicated by the correlation coefficient (R). Within the thalamic population of mast cells, neither their number nor their incidence of degranulation correlated significantly with the behavioral parameters measured in any of the treatment groups (Table 3).
Table 3.
Correlations between the effects of cocaine on behavioral activity and mast cells in the thalamus and meninges
| Sal-Sal | Sal-Coc | Coc-Sal | Coc-Coc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Histology | Behavior | R | P | R | P | R | P | R | P |
| Percent of Mast Cells Degranulated In Meninges | 15 Min | 0.65 | 0.043* | 0.31 | 0.38 | 0.32 | 0.40 | 0.376 | 0.319 |
| Average | 0.78 | 0.008* | 0.51 | 0.129 | 0.36 | 0.34 | 0.44 | 0.238 | |
| Sum | 0.78 | 0.008* | 0.51 | 0.129 | 0.36 | 0.338 | 0.44 | 0.238 | |
| Percent of Mast Cells Degranulated In Thalamus | 15 Min | 0.273 | 0.51 | 0.072 | 0.844 | 0.281 | 0.464 | 0.211 | 0.616 |
| Average | 0.478 | 0.231 | 0.136 | 0.708 | 0.024 | 0.952 | 0.264 | 0.527 | |
| Sum | 0.476 | 0.233 | 0.136 | 0.708 | 0.024 | 0.952 | 0.264 | 0.528 | |
| Number of Mast Cells in Thalamus | 15 min | 0.313 | 0.379 | 0.119 | 0.744 | 0.061 | 0.876 | 0.101 | 0.796 |
| Average | 0.36 | 0.307 | 0.085 | 0.816 | 0.429 | 0.249 | 0.11 | 0.779 | |
| Sum | 0.361 | 0.305 | 0.087 | 0.812 | 0.431 | 0.247 | 0.11 | 0.779 | |
indicates that the value is significantly different based on ANOVA followed by Fisher’s posthoc analysis at P< 0.05
2.4. Correlation between behavioral activity and mast cell data in the meninges
In contrast to the thalamic mast cell population, the activity of mast cells in the meninges was found to co-vary with the behavioral activity of mice when all four groups were pooled (r=0.35, P=0.029, n=38). However, when the sensitized cocaine-cocaine group was eliminated from this analysis, the correlation between behavioral activity and meningeal mast cell degranulation was even more significant (r=0.48, P=0.009, n=29) in spite of the decreased number. Systematic analysis after omission of any of the remaining three groups resulted lower P values than that after omission of the cocaine-cocaine group, indicating the disruptive effect of sensitization to cocaine on this relationship.
Analysis of the correlations within each treatment group also supports a general correspondence between meningeal mast cell degranulation and behavioral activity that is especially strong in mice that are not sensitized to the effect of cocaine. In the control group, the activity of mast cells in the meninges was found to co-vary with the behavioral activity of mice suggesting a relationship between these parameters (Table 3). This positive correlation was evident when behavioral activity when expressed as the initial 15 min of behaviors, the average behavior, or the sum over 60 min (Figure 3). However, in mice that had been exposed to cocaine even once, the correlations between the number of behaviors and either mast cell parameter remained positive but was not significant. Regardless of when cocaine was administered, whether administered acutely 1 hour prior to death or for 5 days as a sensitizing regimen, or both, cocaine disrupted the normal correlation between mast cell degranulation and behavior.
Figure 3.
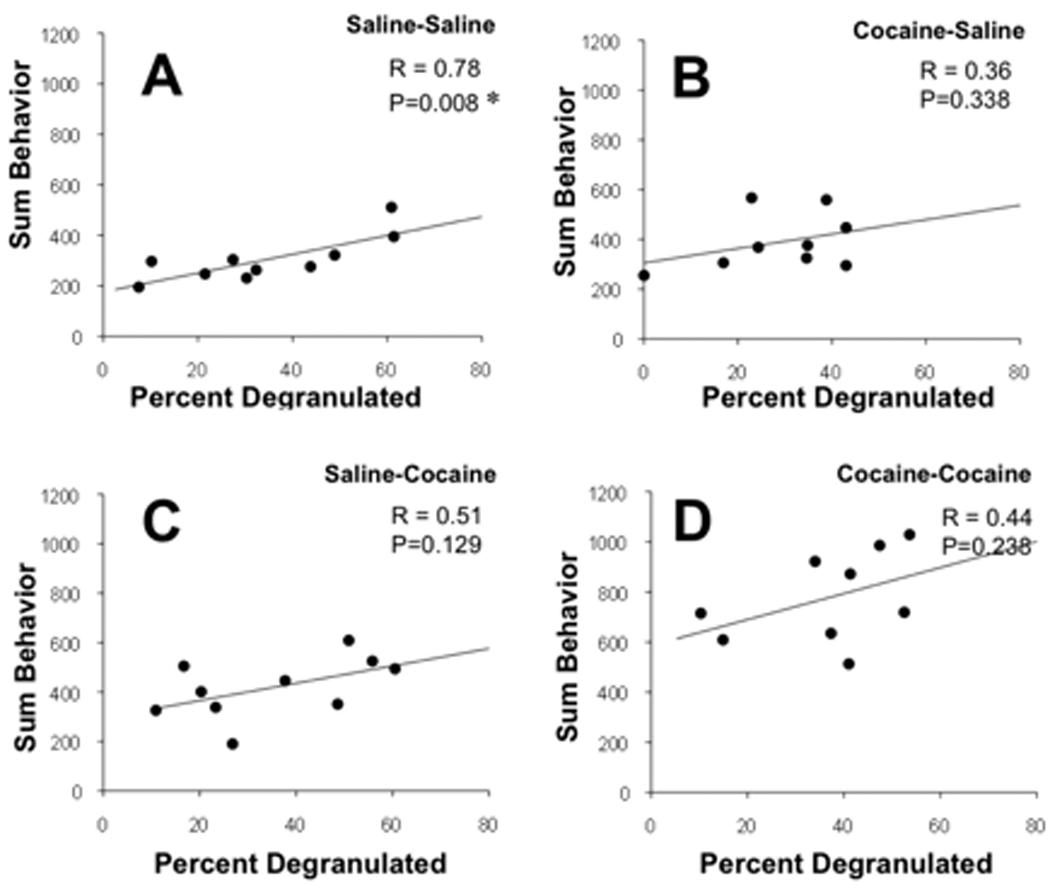
Correlations between the percent of meningeal mast cells that are degranulated and locomotor activity of mice after saline or cocaine treatment. The four treatment groups are designated at the top of each panel where the first treatment listed reflects the chronic daily injection schedule (5 consecutive days with 15 mg/kg of cocaine or saline) and the second reflects the acute challenge delivered 11 days later and just 60 min before death. Behavior was recorded for 60 min after the injections. Activity is shown as the average sum of behaviors during the entire 60 min episode after injections. The correlation coefficient (R) for each set of data and the probability that the correlation is significant (P) are indicated on each panel. P < 0.05 is taken as statistically significant and indicated by an asterisk.
2.5. Concentration of circulating corticosterone
In spite of the dramatic behavioral sensitization observed after chronic (5 day) and acute treatment with cocaine, the concentration of corticosterone measured in the trunk blood of mice at death did not differ between groups. In addition, when the concentration of circulating corticosterone was compared by correlation coefficient (R) to the mast cell parameters assessed in each mouse, no significant correlations were found within any treatment group (data not shown).
3. Discussion
3.1. Association between meningeal mast cell activity and behavioral activity is disrupted by cocaine
Mast cells are normally associated with allergies and inflammation in the periphery. Their presence in the CNS is not widely recognized and their contribution to CNS function is unclear. Histamine and other compounds released from mast cells have been postulated to affect neuronal activity and blood flow in the brain. Approximately half of the histamine in the brain and 90% in the thalamus is thought to be derived from mast cells (Goldschmidt et al., 1985). A relatively high proportion of the mast cell population in the brain is in some stage of degranulation even at rest (Florenzano and Bentivoglio, 2000; Wilhelm et al., 2000). Our studies reveal that the intensity of spontaneous locomotor activity in saline-injected control mice correlates with the incidence of degranulation of mast cells located in the meninges but not those in the thalamus of the same mice. Stabilization of mast cells using central injection of cromolyn sodium inhibits open field exploratory activity (Nautiyal et al., 2008), consistent with the modulation of behavioral activity by degranulation of mast cells in the CNS. Our data demonstrating a correlation between the activity of a select intracranial mast cell population and spontaneous locomotor activity suggest that it is the activity of meningeal mast cells that influence resting exploratory behavior. The association between mast cell activity in the meninges and motor activity was disrupted in mice given multiple or even one single dose of cocaine. This suggests that the regulation of normal activity is disrupted by these interventions and that the regulation of mast cell degranulation is superceded by cocaine-induced events.
3.2. Persistant disruptive effect of cocaine
In agreement with previous studies showing that cocaine leads to increased motor activity in rats (Carey et al., 2001; Steketee, 2005), our results confirm that mice who were presensitized to cocaine respond to challenges of the drug with increased behavioral activity. We did not see significantly increased behavior in the mice that were given cocaine for the first time as the dose of cocaine was selected to be sufficiently small to allow detection of a full range of sensitization without the limitation imposed by a ceiling effect. Disruption of the normal correlation between mast cell activity and spontaneous locomotor activity by such a small dose of cocaine indicates that this system is exquisitely sensitive to the effect of cocaine. The disruptive effect also persists for a long period of time as evidenced by the ability of the sensitizing regimen to interfere with the correlation 11 days later.
3.3. Mast cells’ histamine and cognitive functions, effect of cocaine
Because histamine is likely released by mast cells on degranulation, our study is consistent with the possibility that the release of histamine into the cerebrospinal fluid from the meninges influences behavioral activity. Histamine has been suggested to be a neurotransmitter in the CNS that helps regulate arousal and cognitive function (Reviewed by Passani et al., 2007). The origin of histamine may also be neuronal rather than exclusively from mast cell populations. Exogenously administered histamine can produce either hyperactivity or hypoactivity in rats depending on the dose and the time after an intracerebroventricular injection (Chiavegatto et al., 1998). All doses cause a transient hypoactivity (5–10 min), higher doses also cause hyperactivity (35–40 and 65–70 min), and even larger doses produce hypoactivity (35–40 min) that was not followed by hyperactivity. These effects likely depend on histamine’s action at 4 receptors designated H1, H2, H3 and H4. Antihistamines directed at the H1 receptor have long been known to inhibit wakefulness and activity. The H1 histamine receptor is also involved in circadian rhythms of movement, eating and drinking, arousal, thermoregulation and balance of energy metabolism (Ohshima et al., 2007) leading to behavioral hyperactivity.
The normal histaminergic tone promoting motor activity is supplemented by the stimulant effect of cocaine. Thioperamide, an inverse agonist for the H3 histamine receptor that encourages histamine release in the brain, potentiates the locomotor effects of cocaine (Brabant et al., 2009). Mice in which the histamine decarboxylase enzyme has been knocked out lack histamine and are less stimulated by cocaine than normal mice when given doses of cocaine parenterally (Brabant et al., 2007). Thioperamide, which enhances the release of histamine, is more effective at increasing the behavior effect of cocaine in histamine decarboxylase knockout mice than wild-type controls (Brabant et al., 2009), suggesting that histamine is not necessary for cocaine’s effects and may even inhibit its action. In addition to stimulation of locomotor activity, histamine may be antidepressant as several tricyclic antidepressants inhibit the uptake of histamine in addition to their actions on biogenic amines (Sakurai et al., 2006).
Mast cells in the hypothalamus are reported to modulate HPA activity in the dog (Matsumoto et al., 2001). They appear to enhance circulating corticosterone by their release of histamine and CRF. In spite of the increase in behavioral activity, no connection was found between either the behavioral response to cocaine and the circulating concentration of corticosterone. Behavioral sensitization to cocaine has been previously linked to adrenal hormones (de Jong et al., 2009) in a genotype-dependant way. Sensitization alters pre- and post-synaptic components of the midbrain dopamine system (De Jong et al., 2008). Adrenal hormones were found to be critical for the locomotor sensitization to cocaine in the DBA/2 strain but not in the C57BL/6 mice (de Jong et al., 2007). It is likely that our C57B1/6J mice have a genotype more similar to the C57BL/6 strain than the DBA/2 strain, explaining the lack of a correlation between corticosterone levels and behavior activity after cocaine.
3.4. Conclusions
In summary, spontaneous locomotor activity in mice correlates well with the incidence of mast cell degranulation in the meninges but not with those in the thalamus. This suggests that the granular contents from meningeal mast cells play a role in the regulation of arousal and the activating system in the brain. This relationship between a specific mast cell population and behavior was disrupted by cocaine delivered acutely, previously or in a sensitized paradigm, indicating that the relationship between mast cells and spontaneous behavior is very sensitive to the effect of cocaine.
4. Experimental procedures
4.1. Subjects
40 adult male C57B1/6J mice weighing 20–28 g were housed 4 per cage. Mice were allowed free access to food and water, and housed in a room with a constant temperature of 23°C on a 12 h light-dark cycle (light 6 am–6 pm). Each animal received 2 treatments of cocaine or saline. Cocaine HCl was used, which was obtained from Boynton Health Service at the University of Minnesota. All procedures were performed according to the guidelines of the International Association for the Study of Pain, the University of Minnesota Animal Care and Use Committee, and the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (DHEW Publication NIH 78-23, revised 1995).
4.2. Treatments
All mice were handled for two days prior to beginning the experiment to habituate them to the handling involved in injections. For five consecutive days the mice received intraperitoneal injections of either saline (Sal, 0.85% sodium chloride) or cocaine (Coc) at a dose of 15 mg/kg, based on the ability of this dose to cause sensitization in mice (Shuster et al., 1977). On each day, mice were placed in activity chambers for 20 minutes of habituation prior to injection. After injection, they were returned and their behavior was recorded for 45 minutes. 11 days were allowed to elapse before challenge injections. On the challenge days (20 mice each on each of 2 days), mice were given an injection of either saline or cocaine after 22 minutes of habituation, and behavior was recorded for 60 minutes after the injection. Mice were then killed for the collection of trunk blood, brains and spinal cords.
4.3. Tissue collection
Mice were killed at the same time of day to avoid any influence of circadian rhythm. After rapid decapitation, trunk blood, brains and spinal cords were removed and fixed for 2 days by immersion in 4% formaldehyde (pH 7.0) in PBS. Tissues were then soaked at least 24 hr in PBS containing 30% sucrose at 4°C. The diencephalon from the anterior commissure to the rostral midbrain was cut at 40 μ in the coronal plane using a sliding microtome. Tissue slices were mounted on gelatin-coated slides and stained with 0.125% acidified (pH: 2–2.5) aqueous toluidine blue O solution (JT Baker Chemical, Phillipsburg, NJ) for 30 min. After staining, sections were dehydrated in increasing alcohol series, dipped in xylene, and coverslipped using DPX solidifying mountant (Sigma). Cells were mapped based on the Mouse Brain Atlas (Franklin and Paxinos, 1997).
4.4 Assessment of mast cell degranulation
Mast cells were analyzed in all sections containing thalamic nuclei from the rostral to the caudal extent of the thalamus. Mast cells were also noted in leptomeningeal tissue in the choroid fissure between the thalamus and hippocampus. Mast cells were identified under light microscopy by their metachromatic-purple and cytoplasmatic granules, in contrast to the neurons and surrounding tissue, which were stained light blue. Toluidine blue stains both fully granulated mast cells (dark, metachromatically stained granules packed densely in the cells) and partially degranulated cells (which are pinker and paler). Because the degree of degranulation is commonly used to reflect activation of mast cells, we differentiated between granulated and degranulated cells that are visualized using toluidine blue. The definitive criteria distinguishing granulated from degranulated mast cells was whether individual granules were distinctly separated within the cells and did not abut other granules (degranulated), or whether no individually isolated granules could be detected due to their dense packaging (granulated). We have found a high correspondence (>95%) between observers using this approach.
4.5. Analysis of mast cell data
Mast cells are often found in clusters throughout the thalamus, typically in close proximity to the vasculature. Because of this, systematic sampling or random analysis of sections may yield artificially high or low numbers (Coggeshall and Lekan, 1996; Guillery and Herrup, 1997) depending on the location of selected sections relative to these pockets of mast cells. To accommodate the documented variability in mast cells (Persinger, 1979) and ensure accurate counts in small thalamic nuclei, we evaluated the number and distribution of all mast cells throughout the entire rostral to caudal extent of the thalamus (about 90 sections/animal). We noted in each case their state of granulation and nuclear location, as described above. Only mast cells with nuclei in the focal plane were counted. Prior to statistical analysis, the Abercrombie correction factor was applied to minimize the possibility of double counting of cells in consecutive tissue sections (Abercrombie, 1946). Data were analyzed and reported as mean values (mean ± S.E.M.). Significance was determined using ANOVA and Fisher Protected Least Squares Difference (PLSD) test. We compared behavioral activity and mast cell parameters of individual mice within groups to determine the degree of correlation (r) using Pearson’s correlation coefficient. Correlations between behavioral activity and either the number of mast cells or the incidence of their degranulation (percent degranulated) were deemed significant by a cutoff of significance at P < 0.05.
4.6. Corticosterone assay
Whole trunk blood was collected in plastic tubes containing EDTA and stored on ice. Blood samples were centrifuged (2500 g) for 30 min at 4°C, and the plasma was stored at −20°C until assayed (Jasper and Engeland, 1991; Thrivikraman and Plotsky, 1993). Corticosterone was assayed in duplicate samples of mouse plasma by the ImmuChemTM Double Antibody Corticosterone 1251 RIA kit from ICN Biochemicals Inc. (Costa Mesa, CA).
4.7. Statistics
The data involving comparisons between four groups were analyzed statistically using ANOVA followed by a post hoc analysis using the Fisher Protected Least Squares Difference (PLSD) test to determine differences between groups. A difference was considered significant if the probability that it occurred because of chance alone was less than 5% (P<0.05).
We compared the behavior and mast cell parameters of individual mice to determine the degree of correlation (R) within each group using Pearson’s correlation coefficient. Using this approach, correlations between the spontaneous motor activity and either the number of mast cells or the incidence of their degranulation (percent degranulated) were deemed significant by a cutoff of significance at P<0.05.
Acknowledgements
This work was supported by grant #05-24 from the University of Minnesota Academic Health Sciences, a grant from NIH from the National Institutes on Arthritis and Musculoskeletal and Skin Diseases [AR056092], and by a grant from the University of Minnesota Graduate School. The authors would like to thank Paul R. Larson for his excellent technical expertise and tireless assistance in the preparation of the many histological sections for these studies.
Abbreviations
- CNS
central nervous system
- CRF
corticotropin-releasing factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alice A. Larson, Email: larso011@umn.edu.
Mark J. Thomas, Email: tmhomas@umn.edu.
Katalin J. Kovács, Email: kovac001@umn.edu.
REFERENCES
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Asarian L, Yousefzadeh E, Silverman AJ, Silver R. Stimuli from conspecifics influence brain mast cell population in male rats. Horm. Behav. 2002;42:1–12. doi: 10.1006/hbeh.2002.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant C, Quertemont E, Anaclet C, Lin JS, Ohtsu H, Tirelli E. The psychostimulant and rewarding effects of cocaine in histidine decarboxylase knockout mice do not support the hypothesis of an inhibitory function of histamine on reward. Psychopharmacology (Berl) 2007;190:251–263. doi: 10.1007/s00213-006-0603-0. [DOI] [PubMed] [Google Scholar]
- Brabant C, Alleva L, Grisar T, Quertemont E, Lakaye B, Ohtsu H, Lin JS, Jatlow P, Picciotto MR, Tirelli E. Effects of the H3 receptor inverse agonist thioperamide on cocaine-induced locomotion in mice: role of the histaminergic system and potential pharmacokinetic interactions. Psychopharmacology (Berl) 2009;202:673–687. doi: 10.1007/s00213-008-1345-y. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E. Cocaine and serotonin: a role for the 5-HT(1A) receptor site in the mediation of cocaine stimulant effects. Behav Brain Res. 2001;126:127–133. doi: 10.1016/s0166-4328(01)00253-4. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Nasello AG, Bernardi MM. Histamine and spontaneous motor activity: biphasic changes, receptors involved and participation of the striatal dopamine system. Life Sci. 1998;62:1875–1888. doi: 10.1016/s0024-3205(98)00154-4. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Pistillo L, de Acetis L, Alleva E, Aloe L. Increased number of mast cells in the central nervous system of adult male mice following chronic subordination stress. Brain Behav. Immun. 1998;12:123–133. doi: 10.1006/brbi.1998.0505. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J. Comp. Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Crompton R, Clifton VL, Bisits AT, Read MA, Smith R, Wright IM. Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J. Clin. Endocrinol. Metab. 2003;88:5427–5432. doi: 10.1210/jc.2003-030377. [DOI] [PubMed] [Google Scholar]
- de Jong IEM, Oitzl MS, de Kloet ER. Adrenalectomy prevents behavioural sensitisation of mice to cocaine in a genotype-dependent manner. Behavioural Brain Res. 2007;177:329–339. doi: 10.1016/j.bbr.2006.11.015. [DOI] [PubMed] [Google Scholar]
- De Jong IEM, Steenbergen PJ, De Kloet ER. Strain differences in the effects of adrenalectomy on the mibrain dopamine system: Implication for behavioral sensitization to cocaine. Neuroscience. 2008;153:594–604. doi: 10.1016/j.neuroscience.2008.03.004. [DOI] [PubMed] [Google Scholar]
- De Jong IEM, Steenbergen PJ, de Kloet ER. Behavioral sensitization to cocaine: cooperation between glucocorticoids and epinephrine. Psychopharmacology. 2009;204:693–703. doi: 10.1007/s00213-009-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, Jacobson S, Theoharides TC. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Florenzano F, Bentivoglio M. Degranulation, density, and distribution of mast cells in the rat thalamus: A light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J. Comp. Neurol. 2000;424:651–669. [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Vol. San Diego: Academic Press; 1997. [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J. Pharmacol. Exp. Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goldschmidt RC, Hough LB, Glick SD, Padawer J. Mast cells in rat thalamus: nuclear localization, sex difference and left-right asymmetry. Brain Res. 1984;323:209–217. doi: 10.1016/0006-8993(84)90291-9. [DOI] [PubMed] [Google Scholar]
- Goldschmidt RC, Hough LB, Glick SD. Rat brain mast cells: contribution to brain histamine levels. J. Neurochem. 1985;44:1943–1947. doi: 10.1111/j.1471-4159.1985.tb07191.x. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J. Comp. Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Jasper MS, Engeland WC. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am. J. Physiol. 1991;261:R1257–R1268. doi: 10.1152/ajpregu.1991.261.5.R1257. [DOI] [PubMed] [Google Scholar]
- Johnson D, Krenger W. Interactions of mast cells with the nervous system--recent advances. Neurochem. Res. 1992;17:939–951. doi: 10.1007/BF00993271. [DOI] [PubMed] [Google Scholar]
- Lambracht-Hall M, Dimitriadou V, Theoharides TC. Migration of mast cells in the developing rat brain. Brain Res. Dev. Brain Res. 1990;56:151–159. doi: 10.1016/0165-3806(90)90077-c. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Inoue Y, Shimada T, Aikawa T. Brain mast cells act as an immune gate to the hypothalamic-pituitary-adrenal axis in dogs. J. Exp. Med. 2001;194:71–78. doi: 10.1084/jem.194.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc. Nat.l Acad. Sci. U S A. 2008;105:18053–18057. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y, Iwase M, Izumizaki M, Ishiguro T, Kanamaru M, Nakayama H, Gejyo F, Homma I. Hypoxic ventilatory response during light and dark periods and the involvement of histamine H1 receptor in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1350–R1356. doi: 10.1152/ajpregu.00318.2007. [DOI] [PubMed] [Google Scholar]
- Passani MB, Giannoni P, Bucherelli C, Baldi E, Blandina P. Histamine in the brain: beyond sleep and memory. Biochem. Pharmacol. 2007;73:1113–1122. doi: 10.1016/j.bcp.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Persinger MA. Handling factors not body marking influence thalamic mast cell numbers in the preweaned albino rat. Behav Neural. Biol. 1980;30:448–459. doi: 10.1016/s0163-1047(80)91283-2. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol. Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Oreland L, Nishiyama S, Kato M, Watanabe T, Yanai K. Evidence for the presence of histamine uptake into the synaptosomes of rat brain. Pharmacology. 2006;78:72–80. doi: 10.1159/000095637. [DOI] [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacol. 1977;52:185–190. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Millar RP, King JA, Zhuang X, Silver R. Mast cells with gonadotropin-releasing hormone-like immunoreactivity in the brain of doves. Proc Natl Acad Sci U S A. 1994;91:3695–3699. doi: 10.1073/pnas.91.9.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD. Cortical mechanisms of cocaine sensitization. Crit. Rev. Neurobiol. 2005;17:69–86. doi: 10.1615/critrevneurobiol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, Rozniecki JJ, Webster E, Chrousos GP. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Plotsky PM. Absence of glucocorticoid negative feedback to moderate hemorrhage in conscious rats. Am. J. Physiol. 1993;264:E497–E503. doi: 10.1152/ajpendo.1993.264.4.E497. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, King B, Silverman AJ, Silver R. Gonadal steroids regulate the number and activational state of mast cells in the medial habenula. Endocrinology. 2000;141:1178–1186. doi: 10.1210/endo.141.3.7352. [DOI] [PubMed] [Google Scholar]