To the Editor:
Chronic idiopathic urticaria (CIU) is defined clinically as the presence of hives for at least 6 weeks with no identifiable cause. An autoimmune mechanism is thought to be involved in up to 50% of CIU patients with the presence of IgG antibodies to FcεRI, FcεRII, or IgE.1,2 The Chronic Urticaria (CU) Index (IBT Labs; Lenexa, KS) is a commercially-available in vitro basophil histamine release assay in which patient serum is mixed with donor basophils and the released histamine levels are measured through a quantitative enzyme immunoassay. A CU Index value greater than or equal to 10 indicates that the patient has either an autoimmune basis for their urticaria (antibodies for either IgE, FcεRI, or anti-FcεRII) or an alternate histamine releasing factor.3,4 H1-antihistamines have been the mainstay for management of chronic urticaria, with H2-antihistamines and leukotriene receptor antagonists (LTRAs) available as adjunct treatment. These three classes of medications represent reasonably well tolerated medications. Current guidelines recommend first-line treatment of non-sedating H1-antihistamines that can be increased up to four times the manufacturer's recommended dosing, and if there is no response a change in antihistamine or addition of LTRA, and finally the addition of cyclosporin A, H2-antihistamine, dapsone, or omalizumab.5
Previously, Eckman et al. found significantly higher CU Index values for CIU patients in comparison to non-CIU subjects, but the CU Index did not correlate with the presence of FcεRI or IgE antibodies,6 which may be due to the insensitivity of the ELISA binding assay and the presence of non-functional antibodies.7 Sabroe et al. demonstrated the clinical severity of CIU only corresponded to the broad presence or absence of serum histamine releasing factors,8 which may also represent predominantly autoantibodies.7 Najib et al. evaluated basophil activation by examining surface CD203 expression (BAT-CD203) induced by serum from CIU patients.2 They found that there was no relationship between the basophil activation and the maximum number of medications used, which was their measure of clinical severity. While the CU Index qualitatively measures autoimmunity and potentially other serum histamine releasing factors, its significance as a quantitative measure for patients with CIU in their clinical management has not been established. Therefore, we sought to investigate the clinical utility of the CU Index, specifically with respect to disease severity and responsiveness to medications.
We performed an IRB-approved retrospective chart review of all patients seen in the outpatient University of Wisconsin Allergy clinic diagnosed with CIU (based on ICD-9 codes) over a recent two year period and correlated their measured CU index to disease severity. Patients 18 years and older with symptoms consistent with chronic idiopathic urticaria (presence of hives for more than 6 weeks with no identifiable cause) were included, while those with history or symptoms suggesting acute urticaria or a physical urticaria were excluded from analysis. The patients' entire medication course for management of their urticaria was also obtained and categorized. In order to assess the clinical status of the CIU, patients were considered responders (controlled patients) if they only required antihistamines with or without LTRAs in order to achieve subjective control without symptoms of urticaria. Those who remained symptomatic or continued to have physical evidence of urticaria (even if they reported their urticaria relatively controlled) despite the use of antihistamines with or without LTRAs were considered to be refractory.
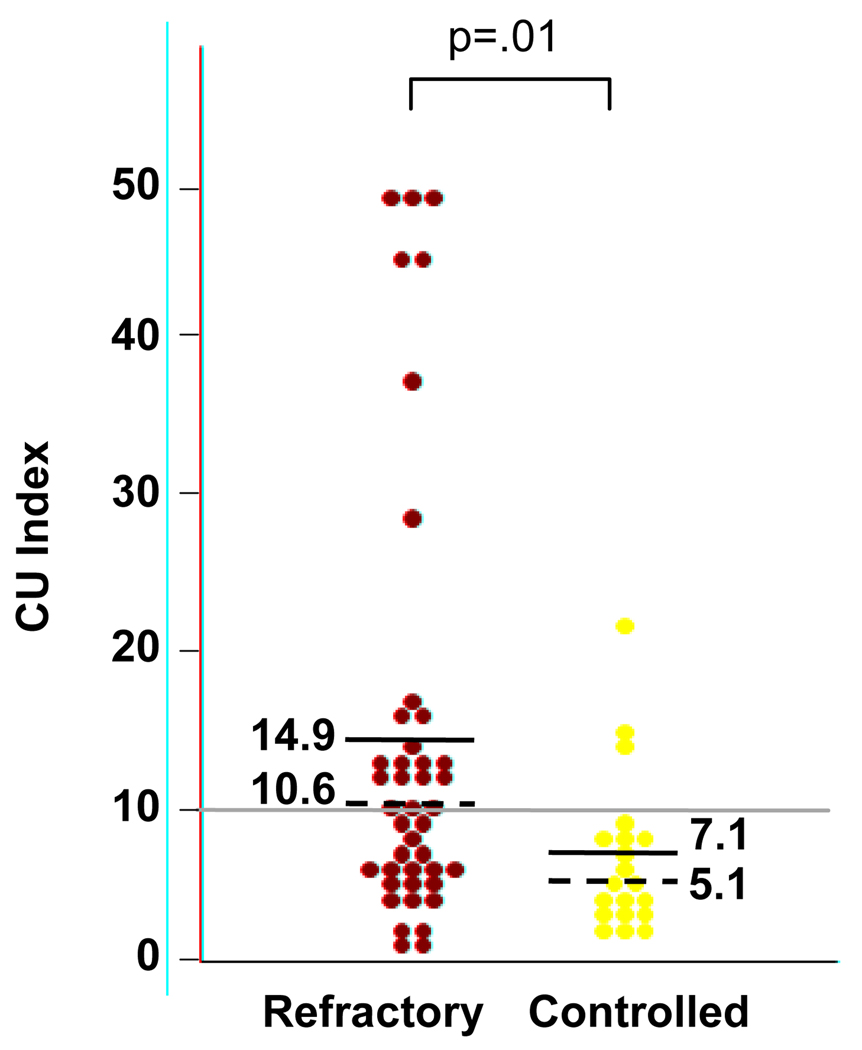
There were 171 patients with a physician-confirmed diagnosis of chronic idiopathic urticaria. The CU Index was ordered in 80 patients, of whom 80% of patients were female, mean age was 44 (range 19–88), and 90% were Caucasian, 4% were African American, and 6% were Asian. There was a selection bias with respect to ordering the CU Index in 17 of the patients. Therefore, the results from the remaining 63 CIU patients with a measured CU index were categorized: 20 as controlled and 43 as refractory. As shown in Figure 1, the median CU Index for refractory patients (10.6, interquartile range 6.1–14.9) was significantly higher (p=0.01, Mann Whitney U test) than controlled patients (5.1, 3.6–8.4) with a quantified CU Index (one patient had the non-numeric result of “negative”). The overall prevalence for a positive CU Index was 40%. However, the prevalence of a positive CU Index was significantly greater in refractory patients than in controlled patients (52% vs. 15%; p=0.01, Fisher’s exact test).
Figure 1.
CU Index values for CIU patients that were refractory or controlled with the use of antihistamines with or without LTRAs. Mean values are listed and represented by the solid lines. Median values are listed and represented by the dashed line. The gray line indicates the defined threshold of 10 for a positive CU Index value.
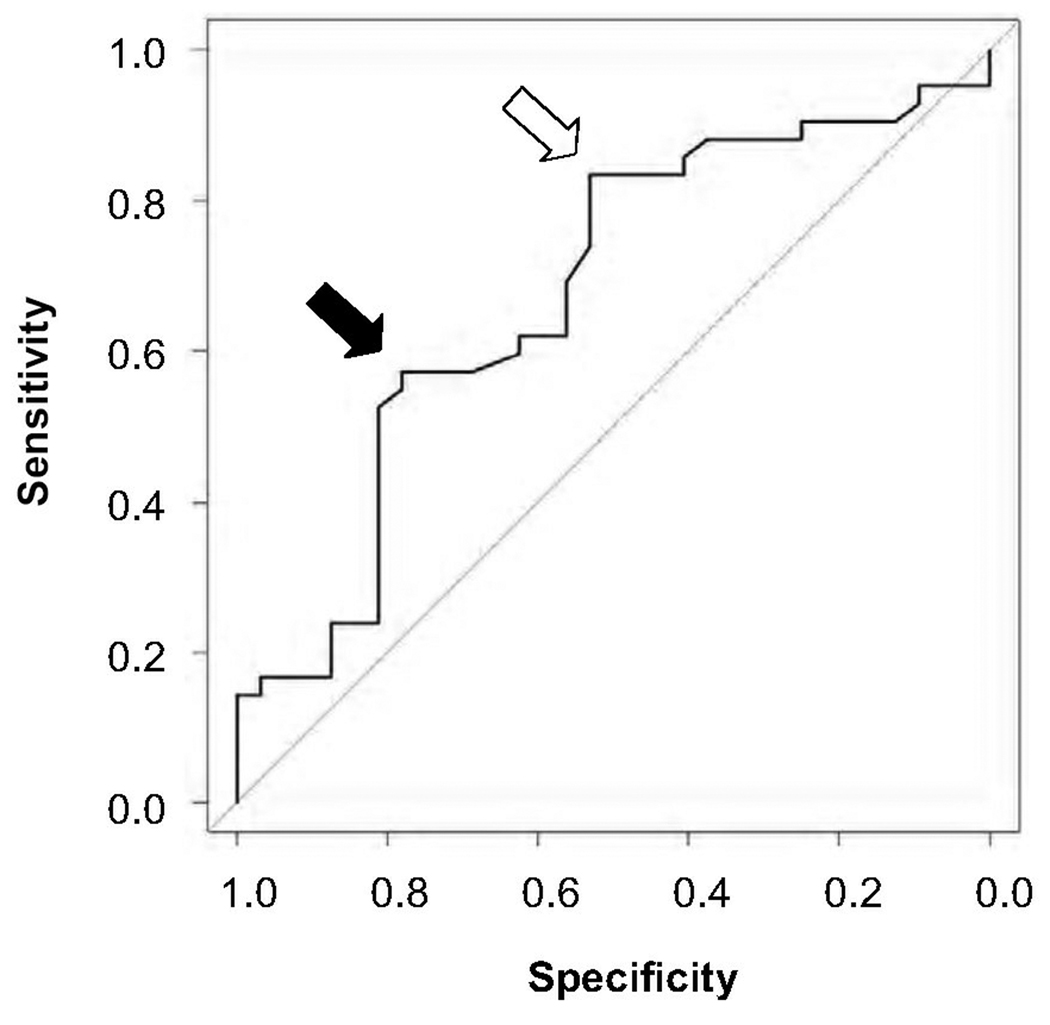
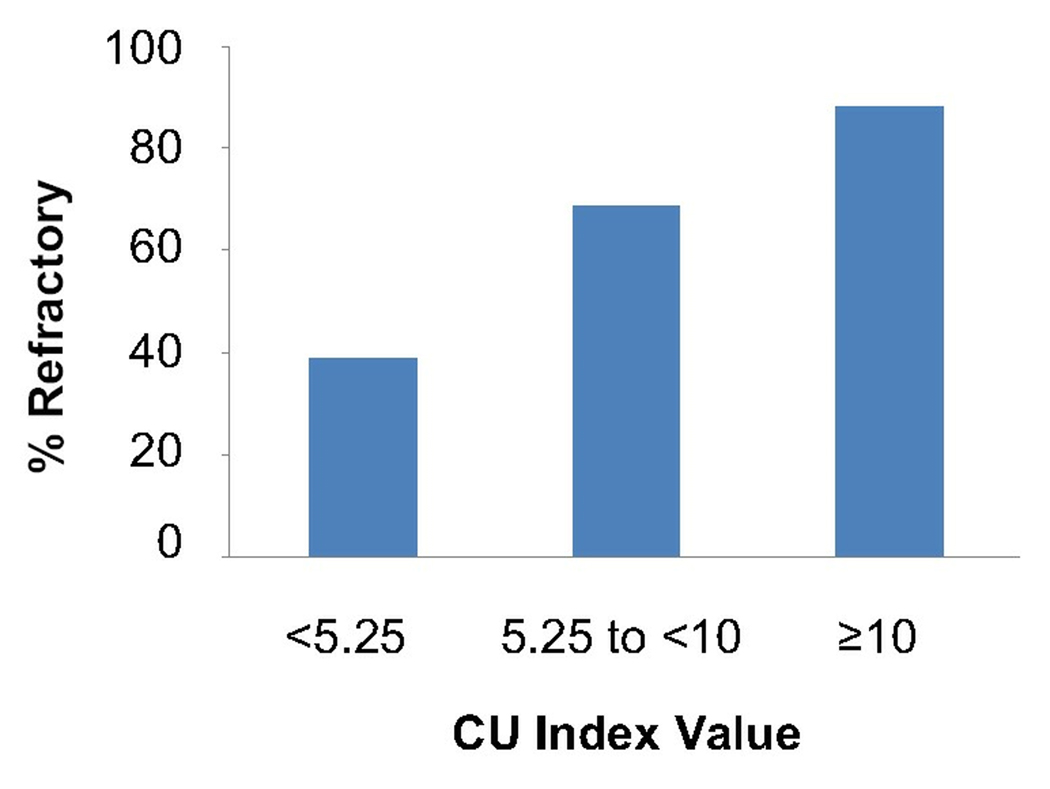
With the categorization of refractory and responsive patients and analysis of their respective CU indices, we find that a positive or elevated CU Index value suggests an increased severity of chronic urticaria. To examine the predictive value of the CU Index for clinical severity, we analyzed 75 out of the 80 patients for whom we had a CU Index value and could be categorized as controlled or refractory. There were five patients whose responsiveness could not be determined due to loss to follow-up (4) or refusal to take daily medications (1). A receiver-operator curve indicates that a decision threshold of 5.25 on the CU index provides 81% sensitivity and 53% specificity for identifying CIU patients with disease refractory to the use of antihistamines with or without LTRAs (Figure 2). However, if the decision threshold of 10.0 is used, sensitivity decreases to 51%, but specificity increases to 81%. Of patients with CU Index values less than 5.25, 39% were refractory; for CU Index values between 5.25 and less than 10, 68% were refractory; and for CU index values greater than or equal to 10, 88% were refractory (Figure 3). This suggests that almost 9 out of 10 patients with a CU Index greater than or equal to 10 required more than antihistamine and LTRA therapy. Thus, the CU Index could help guide whether a more aggressive level of medication will be required to achieve symptom control.
Figure 2.
Receiver Operator Curve indicating the sensitivities and specificities for the optimized decision threshold value of 5.25 (open arrow) and the autoimmunity decision threshold of 10 (black arrow).
Figure 3.
Percent prevalence of patients refractory to antihistamines with or without LTRAs based on CU Index Value Ranges.
One limitation of this study is that it was retrospective and the patients were not assessed and managed according to a common protocol; patients were at most treated with twice the manufacturer's dosing of non-sedating H1-antihistamines, not four times as has been recently identified as effective.9 Another limitation is that the determination of response to medications was based on a subjective evaluation of the medical record (MJB and RKV) rather than a validated instrument such as the Urticaria Assessment Score.10 Despite these limitations, our results suggest that the CU Index has implications for predicting disease severity and responsiveness to medications. A prospective study of CIU patients is warranted to validate these findings and further evaluate the clinical utility of the CU Index.
Acknowledgments
This project was supported, in part, by NIH funding (AI007635).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaplan AP. Chronic Urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004 Sep;114(3):465–474. doi: 10.1016/j.jaci.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 2.Najib U, Bajwa ZH, Ostro MG, Sheikh J. A retrospective review of clinical presentation, thyroid autoimmunity, laboratory characteristics, and therapies used in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2009 Dec;103(6):496–501. doi: 10.1016/S1081-1206(10)60266-9. [DOI] [PubMed] [Google Scholar]
- 3.Altrich M. Laboratory Evaluation of Autoimmune Chronic Urticaria [White paper]. IBT Laboratories. 2009 June; Retrieved from http://www.ibtlabs.com/library/PDF/CU%20White%20Paper%20Final%20Online%20Version.pdf. [Google Scholar]
- 4.Altrich ML, Halsey JF, Altman LC. Comparison of the in vivo autologous skin test with in vitro diagnostic tests for diagnosis of chronic autoimmune urticaria. Allergy Asthma Proc. 2009 Jan–Feb;30(1):28–34. doi: 10.2500/aap.2009.30.3185. [DOI] [PubMed] [Google Scholar]
- 5.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM, Grattan CE, Kapp A, Maurer M, Merk HF, Rogala B, Saini S, Sánchez-Borges M, Schmid-Grendelmeier P, Schünemann H, Staubach P, Vena GA, Wedi B. Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009 Oct;64(10):1427–1443. doi: 10.1111/j.1398-9995.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- 6.Eckman JA, Hamilton RG, Saini SS. Independent evaluation of a commercial test for "autoimmune" urticaria in normal and chronic urticaria subjects. J Invest Dermatol. 2009 Jun;129(6):1584–1586. doi: 10.1038/jid.2008.416. [DOI] [PubMed] [Google Scholar]
- 7.Soundararajan S, Kikuchi Y, Joseph K, Kaplan AP. Functional assessment of pathogenic IgG subclasses in chronic autoimmune urticaria. J Allergy Clin Immunol. 2005 Apr;115(4):815–821. doi: 10.1016/j.jaci.2004.12.1120. [DOI] [PubMed] [Google Scholar]
- 8.Sabroe RA, Fiebiger E, Francis DM, Maurer D, Seed PT, Grattan CE, Black AK, Stingl G, Greaves MW, Barr RM. Classification of anti-FcepsilonRI and anti-IgE autoantibodies in chronic idiopathic urticaria and correlation with disease severity. J Allergy Clin Immunol. 2002 Sep;110(3):492–499. doi: 10.1067/mai.2002.126782. [DOI] [PubMed] [Google Scholar]
- 9.Staevska M, Popov TA, Kralimarkova T, Lazarova C, Kraeva S, Popova D, Church DS, Dimitrov V, Church MK. The effectiveness of levocetirizine and desloratadine in up to 4 times conventional doses in difficult-to-treat urticaria. J Allergy Clin Immunol. 2010 Mar;125(3):676–682. doi: 10.1016/j.jaci.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 10.Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008 Jun;63(6):777–780. doi: 10.1111/j.1398-9995.2008.01726.x. [DOI] [PubMed] [Google Scholar]