Figure 5.
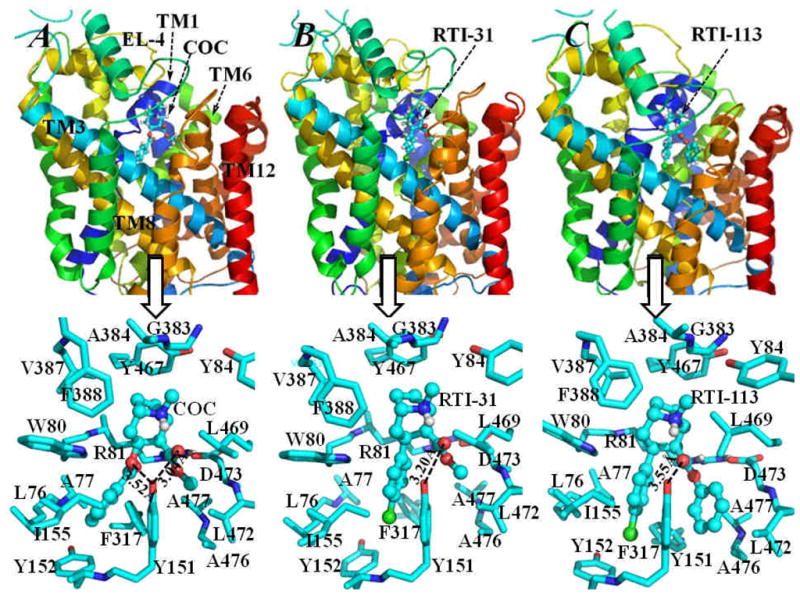
Energy-minimized structures of NET-NE/inhibitor complexes: (A) NET-NE/cocaine; (B) NET-NE/RTI-31; (C) NET-NE/RTI-113. The NET-NE protein is represented as colored ribbons and the inhibitors, i.e. cocaine (COC), RTI-31 and RTI-113, are shown in ball-and-stick style. The transmembrane helices 1, 3, 6, 8 and12, and extracellular loop 4 (EL-4) are also labeled. The low panel of this figure shows more details of inter-molecular interactions of NET-NE with each of the three inhibitors, residues of NET-NE within 5 Å around the inhibitor are shown as stick and colored by atom types. The distances between the hydroxyl oxygen on the side chain of Y151 of NET-NE and the carbonyl oxygen on the methyl ester group of both cocaine and RTI-31 are labeled. Also labeled is the distance between the hydroxyl oxygen of Y151 and the carbonyl oxygen on the benzoyl ester group of cocaine. For comparison, the distance between the hydroxyl oxygen of Y151 of NET-NE and the carbonyl oxygen on the 2β-carbophenoxy group of RTI-113 is also labeled.
