Abstract
It has been reported that intracerebroventricular injection of a μ receptor antagonist blocked 2 but not 100 Hz electroacupuncture (EA)-produced analgesia in an uninjured animal model. Because persistent pain changes neural response to external stimulation, we hypothesized that the mechanisms of EA anti-hyperalgesia may be different in persistent pain than in health. Hyperalgesia, decreased paw withdrawal latency (PWL) to a noxious thermal stimulus, was induced by subcutaneously injecting complete Freund’s adjuvant (CFA) into the hind paws of rats. Selective antagonists against μ (CTOP: D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-ThrNH2, 6.25 nmol) and κ (Nor-BIN: nor-binaltorphimine, 10 nmol) opioid receptors were infused into the rostral ventromedial medulla (RVM) 10 min before 30-min EA treatment at acupoint Huanti (GB30) 1 hr 30 min post-CFA. PWL was measured before and 2.5 post-CFA. Both 10 Hz and 100 Hz EA-produced anti-hyperalgesia were blocked by intra-RVM μ, but not κ, receptor antagonists. Double immunofluorescence staining demonstrated that μ receptor-containing neurons were GABAnergic and that GABAa receptor-containing neurons were serotonergic in the RVM. The results demonstrated an involvement of RVM μ, but not κ, receptors in EA-produced anti-hyperalgesia. In summary, EA may induce release of endogenous endomorphins that activate μ opioid receptors in GABAnergic neurons to suppress the release of GABA. This removes the tonic inhibition of GABA on serotonergic neurons in the RVM, and activation of these serotonergic neurons inhibits pain. EA may be used as complementary treatment for inflammatory pain.
Keywords: acupuncture, hyperalgesia, pain, opioid receptor, RVM
1. Introduction
Acupuncture analgesia is well documented in clinical trials on patients with chronic pain (Berman et al., 2004; Efthimiou and Kukar, 2010; Martin et al., 2006; Witt et al., 2005). However, its underlying mechanisms are not fully established.
The involvement of endogenous opioids in acupuncture analgesia has been studied in healthy volunteers and uninjured animal models in past decades. Studies in healthy humans demonstrate that naloxone, a specific opiate antagonist, reverses acupuncture analgesia (Jiang et al., 1978; Mayer et al., 1977) and that beta-endorphin increases in human cerebrospinal fluid after acupuncture treatment (Mayer, 2000). Animal studies show similar effects (Mayer, 2000). Further study showed that electroacupuncture- (EA) produced analgesia was blocked by microinjections of naloxone into the preoptic area, septal area, nucleus accumbens, amygdale, caudate nucleus, periaqueductal grey, and the nucleus raphe magnus (He, 1987). Moreover, in an uninjured animal model, 2 and 100 Hz EA analgesia is mediated, respectively, by μ and κ opioid receptors (Han, 2003).
While those studies greatly contribute to our understanding of the mechanisms of acupuncture analgesia, they have limited clinical relevance as they were carried out in healthy subjects. It has been reported that EA has different effects on healthy and pathological conditions. For example, EA significantly increases plasma adrenocorticotropic hormone (ACTH) and corticosterone levels in inflamed but not in naive rats (Li et al., 2008). Further, recent chronic pain acupuncture/EA studies, including our own (Lao et al., 2004), have shown that EA produces anti-hyperalgesia in inflammatory pain animal models (Yang et al., 2010; Zhang et al., 2002). It has been demonstrated that the spinal μ opioid receptor antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) blocks 10 and100 Hz EA-produced anti-hyperalgesia in a complete Freund’s adjuvant (CFA)-induced inflammatory pain rat model, while the κ receptor antagonist nor-binaltorphimine (Nor-BNI) does not (Zhang et al., 2004). In contrast, spinal endomorphin-1, an endogenous μ receptor agonist, mediates 2 but not 100 Hz EA analgesia in uninjured rat models (Han et al., 1999). These studies demonstrated that the spinal opioid receptors are differently involved in EA action in pathological conditions than in health. Thus it is important to investigate mechanisms of EA anti-hyperalgesia under pathological conditions.
At the supraspinal level, intracerebroventricular injection of CTOP, a μ receptor antagonist, blocked 2 but not 100 Hz EA-produced analgesia in an uninjured animal model (Huang et al., 2000). This study indicated that supraspinal opioids are implicated in EA analgesia in uninjured animals. Supraspinal opioid receptor involvement in EA anti-hyperalgesia in inflamed rats has not been studied.
The rostral ventromedial medulla (RVM) is critical for the modulation of dorsal horn nociceptive transmission. Research showed that EA treatment inhibits the nociceptive response of excitatory RVM neurons and that EA-produced inhibitory effects are blocked in uninjured rats by naloxone pretreatment (Ao et al., 1996), but the role of RVM μ and κ opioid receptors in EA-produced anti-hyperalgesia in an inflammatory pain rat model was not examined. However, intra-RVM infusion of either DAMGO, a μ opioid receptor agonist, or U69593, a κ opioid receptor agonist, increased paw withdrawal latency (PWL) in an inflammatory pain rat model (Schepers et al., 2008a). We hypothesized that μ and κ receptors in RVM are differently involved in EA action under pathological conditions.
2. Results
2.1 A μ, but not a κ, opioid receptor antagonist significantly blocked 10 Hz EA anti-hyperalgesia
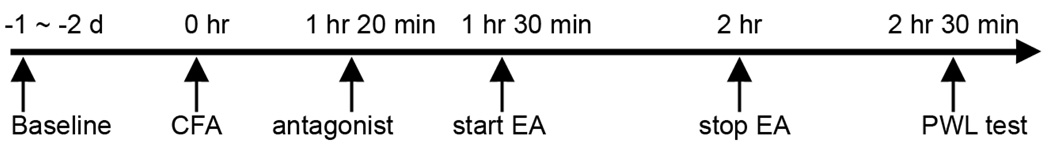
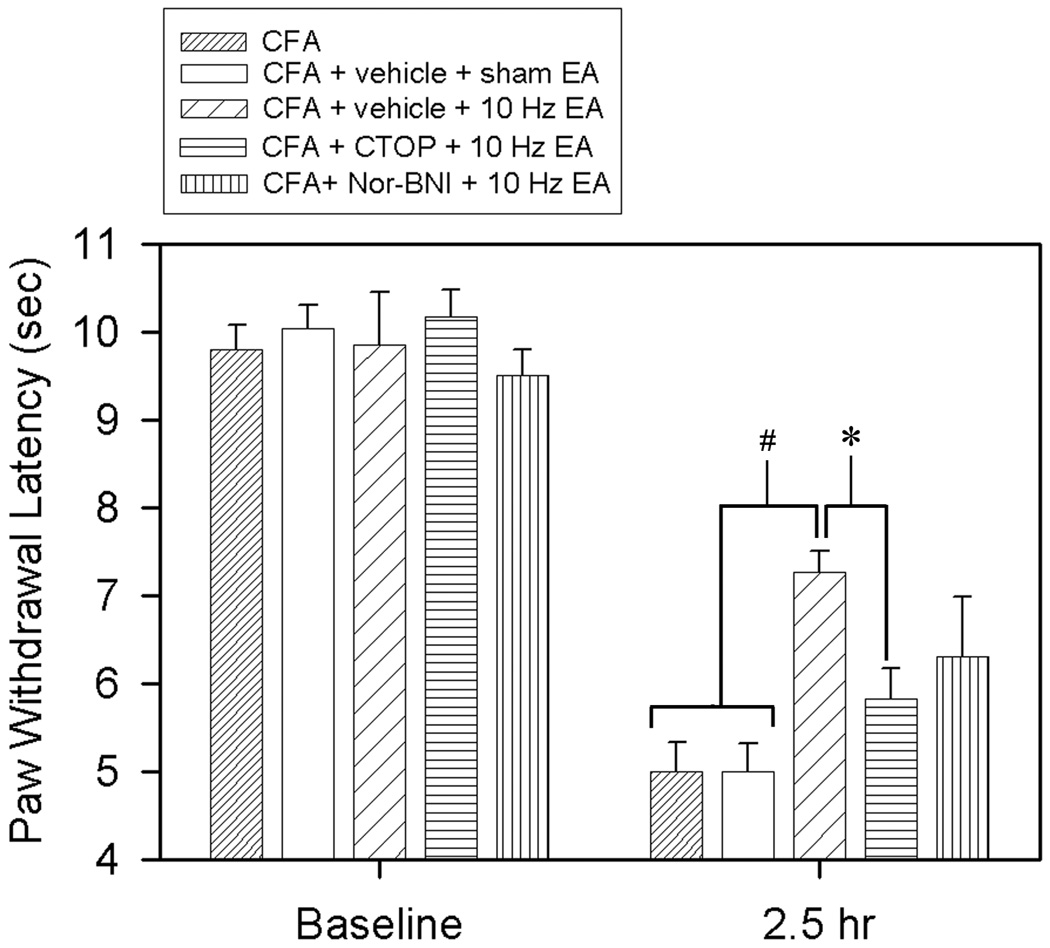
The experimental procedures are shown in Fig. 1. Before a CFA injection, overall mean baseline PWL to noxious heat stimuli was similar in all groups of rats, and there was no significant difference between left and right hind paw PWL. As shown in Fig. 2, an 0.08-ml injection of CFA into the left hind paw significantly (F(9,79) = 59.39, P<0.05) decreased latency while the contralateral hind paw remained at the pre-CFA level (data not shown). Both rats with intra-RVM vehicle and sham EA and those with a CFA injection alone showed the same PWL, indicating that sham EA did not affect PWL. EA at 10 Hz (vehicle + 10 Hz EA) significantly (P<0.05) increased PWL of the CFA-injected hind paw, an anti-hyperalgesic effect, 2.5 hours post-CFA injection compared to sham control (vehicle + sham EA). At 10 nmol, the κ opioid receptor antagonist Nor-BNI did not significantly impede 10 Hz EA-produced anti-hyperalgesia (Fig. 2). In contrast, a 6.25 nmol, CTOP, a μ opioid receptor antagonist, significantly blocked 10 Hz EA-produced anti-hyperalgesia. However, when CTOP was infused into a site 2 mm dorsal to the NRM, it did not block the EA-produced inhibition of thermal hyperalgesia (6.95 ± 0.71 sec). This indicates that a 0.5-µl CTOP infusion into the RVM is limited to the RVM
Fig. 1.
CFA injection, EA treatment and the behavioral test timeline.
Fig. 2.
Effects of saline, CTOP, and Nor-BNI in the RVM on 10 Hz EA-produced anti-hyperalgesia in inflamed rats. A CFA injection significantly decreased PWL of the CFA-injected hind paw (F(9,79) = 59.39, P<0.05). EA (vehicle + 10 Hz EA) significantly increased CFA-injected hind paw PWL compared to sham control (vehicle + sham EA). CTOP, but not Nor-BNI, significantly prevented a 10 Hz-produced PWL increase compared to saline. # and *, P<0.05 vs CFA alone/vehicle + sham EA and CTOP + 10 Hz EA respectively.
2.2 A μ, but not a κ, opioid receptor antagonist significantly blocked 100 Hz EA anti-hyperalgesia
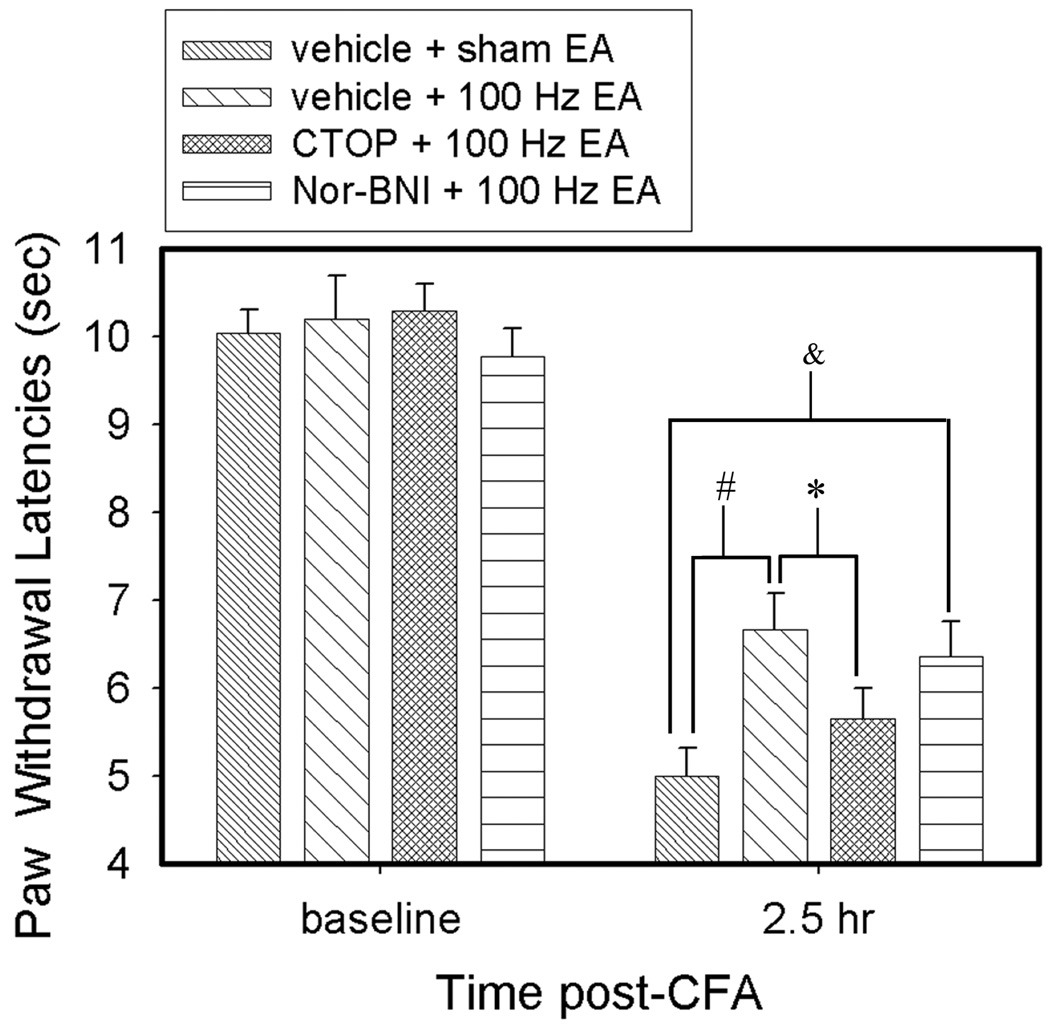
As shown in Fig. 3, CFA induced a significant (F(7,63) = 72.28, P<0.05) decrease of PWL. EA at 100 Hz (vehicle + 100 Hz EA) significantly (P<0.05) increased PWL of the CFA-injected hind paw, an anti-hyperalgesic effect, 2.5 hours post-CFA injection compared to sham control (vehicle + sham EA). The μ opioid receptor antagonist CTOP (6.25 nmol) significantly (P<0.05) blocked 100 Hz-produced anti-hyperalgesia. EA plus the κ opioid receptor antagonist Nor-BNI (10 nmol) significantly (P<0.05) increased PWL compared to vehicle + sham EA, indicating that Nor-BNI did not block 100 Hz-produced anti-hyperalgesia.
Fig. 3.
Effects of saline, CTOP, and Nor-BNI in the RVM on 100 Hz EA-produced anti-hyperalgesia in inflamed rats. CFA induced significant (F(7,63) = 72.28, P<0.05) decrease of PWL. EA (vehicle + 100 Hz EA) significantly increased PWL of the CFA-injected hind paw compared to sham control (vehicle + sham EA). CTOP, but not Nor-BNI, significantly prevented this 100 Hz-produced PWL increase compared to saline. # and *, P<0.05 vs vehicle + sham EA and CTOP + 100 Hz EA respectively; & P<0.05 between vehicle + sham EA and Nor-BNI +100 Hz EA.
2.3 RVM μ opioid receptors are localized in GABAnergic neurons
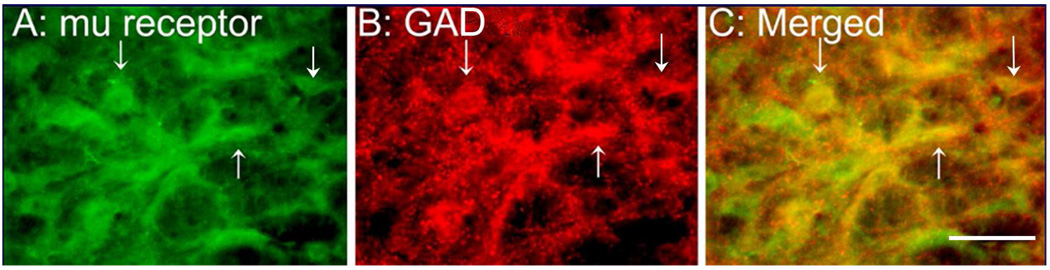
To investigate the mechanisms of RVM opioid receptors in EA action, we double labeled μ opioid receptors and glutamic acid decarboxylase (GAD), a marker of GABAnergic neurons. It was revealed that at least 30% of μ receptor-containing neurons were GABAnergic, and almost 70% of GABAnergic neurons contained μ opioid receptors. Control sections without primary antiserum showed no immunoreactive staining (Fig. 4).
Fig. 4.
Micrographs showing co-localization of μ and GAD in the RVM. (A–C) Sections were double labeled with anti-μ opioid receptor (green) and anti-GAD (red). (A) μ opioid receptor-immunoreactive neurons; (B) GAD-immunoreactive neurons; (C) merged graphs of panels A and B. Arrows indicate double-labeled μ opioid receptor /GAD neurons (yellow). Scale bar=50 µm.
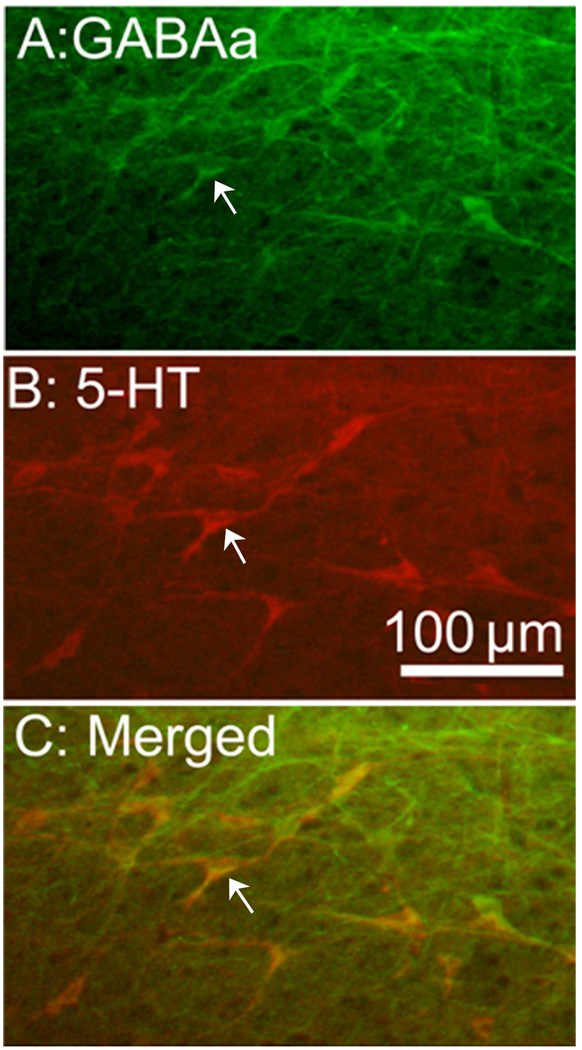
2.4 GABAa receptors are localized in serotonergic RVM neurons
Double labeling of GABAa receptor and serotonin demonstrated that 80% of GABAa receptor-containing neurons were serotonergic and vice versa. Control sections without primary antiserum showed no immunoreactive staining (Fig. 5).
Fig. 5.
Micrographs showing co-localization of GABAa receptors and serotonin in the RVM. Sections were double labeled with anti-GABAa receptor (green) and anti-serotonin (red). (A) GABAa receptor-immunoreactive neurons; (B) serotonin-immunoreactive neurons; (C) merged graphs of panels A and B. Arrows indicate double-labeled μ opioid receptor/GAD neurons (yellow). Scale bar=100 µm.
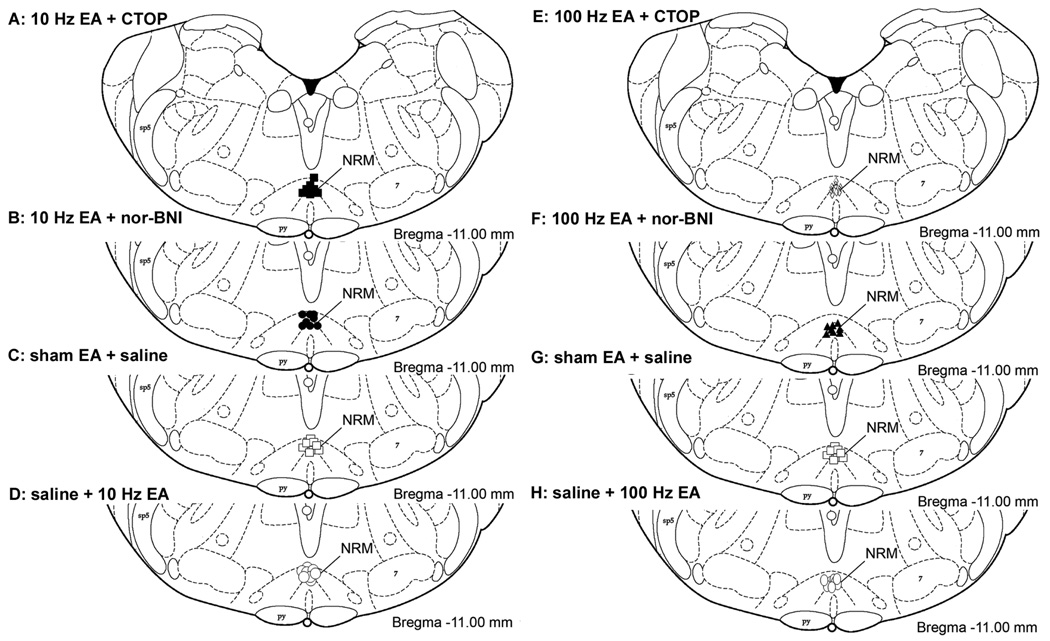
2.5 Placement of RVM injection sites
The injection sites were located within the RVM as shown in Fig. 6. The control site injection was 2 mm dorsal to the NRM (data not shown).
Fig. 6.
Histological verification of cannula placements in the RVM. Distribution of microinjection sites in RVM for the drugs used in experiments with 10 Hz (A–D) or 100 Hz EA (E–H): CTOP (A, E), nor-BNI (B, F) and saline in sham EA-treated rats (C, G) and saline in EA treated rats (D, H). Rats in C and G are the same group of rats. The rostral-to-caudal coordinate is with respect to the bregma (Paxinos and Watson, 1998). Sp5, spinal trigeminal nucleus; 7, facial nucleus; Pyr, pyramidal tract.
3. Discussion
The present study demonstrates that μ opioid receptor antagonists block 10 and 100 Hz EA anti-hyperalgesia in a CFA-induced peripheral inflammatory pain rat model while κ opioid receptor antagonists do not. The data indicate that EA anti-hyperalgesia produced by both frequencies is mediated byμ, but not κ, receptors in the RVM. This suggests that the significant anti-hyperalgesia produced by high and low frequency EA during persistent pain is the result of the activation of μ receptors but not κ receptors. Previous studies demonstrated that an intra-RVM infusion of 30 nmol of U69593, a κ opioid receptor agonist, increased PWL in CFA-inflamed rats to the same extent as did 80 pmol intra-RVM infusion of DAMGO (Schepers et al., 2008a). The data suggest that a lower dose of μ opioid receptor agonist may efficiently activate μ opioid receptors to inhibit pain. In another word, μ opioid receptors in RVM are easily activated compared to κ opioid receptors in CFA-inflamed rats. This is consistent with our finding that EA more easily activates μ than κ receptors. This warrants further study.
Our previous study showed that blockage of spinal μ receptors prevents 10 and 100 Hz EA anti-hyperalgesia (Zhang et al., 2004). It seems that EA activates μ opioid receptors at both spinal and supraspinal levels. Other studies have shown synergistic anti-nociception between spinal and supraspinal opioids (Yeung et al., 1980), suggesting that EA activates both spinal and supraspinal opioid receptors to suppress pain.
Our study demonstrates that μ opioid receptors are localized in GABAnergic neurons in the RVM, which implies that endogenous endomorphin may modulate GABAnergic neuron activity. In a previous study, intra-RVM infusion of DAMGO significantly decreased extracellular GABA concentration and increased PWL (Schepers et al., 2008b). These data suggest that EA may induce the release of endogenous endomorphin to activate μ opioid receptors in GABAnergic neurons and to suppress the release of GABA.
We also showed that GABAa receptors are localized in serotonergic neurons in the RVM, suggesting that GABA may inhibit the activities of RVM serotonergic neurons. This is consistent with prior anatomical studies showing that GABA-immunoreactive terminals make symmetrical axodendritic synapses with RVM-spinal projection neurons (Cho et al., 1991). It has been reported that RVM GABAa receptor agonists produce hyperalgesia while antagonists produce anti-nociception (Heinricher and Kaplan, 1991). These data demonstrate that serotonergic RVM neurons receive tonic inhibitory input, mediated by GABAa receptors, from GABAnergic neurons. Accordingly, EA may decrease the release of GABA to disinhibit serotonergic neurons in the RVM.
It has been demonstrated that EA inhibits hyperalgesia by activating serotonergic RVM neurons that project to the spinal cord (Li et al., 2007). In another study, an intra-RVM infusion of DAMGO produced an increase of tail-flick latency that was blocked by intrathecal pretreatment with methysergide, a serotonin receptor antagonist (Hurley et al., 2003). These findings suggest that an EA-activated opioid system in the RVM may inhibit pain through a descending serotonergic system in the spinal cord.
Additionally, previous studies suggest that EA may activate the nucleus raphe obscurus and raphe pallidus (Guo et al., 2008), which are involved in regulation of presympathetic rostral ventrolateral medullary neurons (Moazzami et al., 2010). It has also been demonstrated that μ but not κ opioid receptors in the rostral ventrolateral medulla are involved in EA modulation of cardiovascular reflex responses (Li et al., 2001). These data and our own show that EA may modulate a variety of functions.
In summary, EA may induce release of endogenous endomorphins that activate μ opioid receptors in GABAnergic neurons to suppress the release of GABA. This removes the tonic inhibition of GABA on serotonergic neurons in the RVM. Activation of these serotonergic neurons inhibits pain.
4. Materials and methods
4.1 Animal preparation
Male Sprague Dawley rats (250–270 g body weight, Harlan) were kept under controlled conditions (22°C ± 0.5°C, relative humid ity 40–60%, twelve-hour (7:00am to 7:00pm) alternate light-dark cycles, and food and water ad libitum). The animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine. In two 30-minute periods two days before a baseline behavioral test, the rats were habituated to a plastic chamber used during the experiments.
4.2 Experimental procedures
Three sets of experiments were conducted: (1) effects of intra-RVM CTOP and nor-BNI on 10 Hz EA-produced anti-hyperalgesia; (2) effects of intra-RVM CTOP and nor-BNI on 100 Hz EA-produced anti-hyperalgesia; (3) immunohistochemical double labeling of μ opioid receptors and GAD and of GABAa receptors and serotonin in the RVM.
In Experiment 1, rats were divided into the following five groups (n=8 per group): 1) CFA only; 2) CFA + vehicle + sham EA; 3) CFA + vehicle + 10 Hz EA; 4) CFA + CTOP (6.25 nmol in 0.5 ul) + 10 Hz EA; 5) CFA + nor-BNI (10 nmol in 0.5 ul) + 10 Hz EA. Another 6 rats were used for infusion site control; CTOP was injected into a site 2 mm dorsal to the NRM.
In Experiment 2, rats with CFA-induced inflammation were divided into the following four groups (n=8 per group): 1) vehicle + sham EA; 2) vehicle + 100 Hz EA; 3) CTOP (6.25 nmol in 0.5 ul) + 100 Hz EA; 4) nor-BNI (10 nmol in 0.5 ul) + 100 Hz EA. All antagonists were dissolved in saline and administered 10 minutes before EA treatment, which was administered 1 hour and 30 minutes after CFA injection. Antagonist dosage was based on a previous study (Zhang et al., 2004) and our pilot studies.
In Experiment 3, RVM sections from four rats were double labeled for μ opioid receptors and GAD and for GABAa receptors and serotonin in the RVM.
4.3 EA treatment
EA treatment was conducted according to procedures previously developed in our laboratory (Lao et al., 2004). EA at 10 or 100 Hz, 2 mA, 0.4 ms pulse width for 30 minutes, which produced significant anti-hyperalgesia in our previous study, was administered at acupoint GB30. In humans, GB30 is located at the junction of the lateral third and medial two-thirds of the distance between the greater trochanter and the sacral hiatus; underneath are the sciatic nerve, inferior gluteal nerve and gluteal muscles. Equivalent anatomical landmarks were used to locate these points in the rat. The transposition of an acupoint from the known human map to the anatomically comparable position in animals is widely used to determine points in animals (Lao et al., 2001; Lee and Beitz, 1993; Ma et al., 2005; Zhou et al., 2005) and has been demonstrated to be effective (Lao et al., 2001; Ulett et al., 1998). After cleaning the skin with alcohol swabs, one investigator swiftly inserted an acupuncture needle (gauge # 32, 0.5 inch in length) approximately one-half inch deep into each hind limb of the rat at GB30 while another gently held the animal. The two needles were stabilized with adhesive tape (Lao et al., 2004; Zhang et al., 2008). EA was delivered by a stimulator (Electrostimulator 8-C, Pantheon Research Inc) via electrodes that had been soldered to the needle handles in advance; the other end of each electrode was connected to an output channel of the stimulator. A symmetrical biphasic wave was delivered to each electrode so that it was alternately positive and negative to stimulate the bilateral needles alternately. To minimize discomfort, stimulation intensity was gradually increased over a period of two minutes to 2 mA, which we have found to be the maximum level that can be tolerated by unrestrained rats. During EA treatment, each rat was placed under an inverted clear plastic chamber (approximately 5”× 8”×11”) but was neither restrained nor given any anesthetic. Mild muscle twitching was observed. The animals remained awake and still during treatment and gave no observable signs of distress.
For sham control, acupuncture needles were inserted bilaterally into GB30 without electrical or manual needle manipulation. Sham EA showed little anti-hyperalgesia in our previous study (Lao et al., 2004), making it an appropriate control for non-specific needling effects. Sham- and EA-treated animals were handled identically. The investigators performing the behavioral tests were blind to treatment assignments.
4.4 Hyperalgesia testing
Inflammatory hyperalgesia was induced by injecting CFA subcutaneously into the plantar surface of one hind paw of the rat using a 25-gauge hypodermic needle (Lao et al., 2004). Hyperalgesia was determined by a decrease in PWL to a noxious thermal stimulus. PWL was tested with Hargreaves’s method (Hargreaves et al., 1988; Lao et al., 2004). Each rat was placed under an inverted clear plastic chamber on the glass surface of a Paw Thermal Stimulator System (UCSD, San Diego) and allowed to acclimatize for 30 min before the test. A radiant heat stimulus was applied to the plantar surface of each hind paw from underneath the glass floor with a projector lamp bulb (CXL/CXR, 8 V, 50 W). PWL to the nearest 0.1 sec was automatically recorded when the rat withdrew its paw from the stimulus. Stimulus intensity was adjusted to derive a baseline PWL of approximately 10.0 seconds in naive animals. Paws were alternated randomly to preclude order effects; a 20-second cut-off was used to prevent tissue damage. Four tests were conducted, with a 5-minute interval between each test. Mean PWL was established by averaging the tests.
4.5 Intra-RVM cannulation and drug infusion
Animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and held in a stereotaxic frame (Stoelting, Wood Dale IL). An incision was made on the midline of the head and a small hole was drilled. A 26-gauge stainless steel guide cannula (Plastic One, Roanoke, VA) was implanted 3 mm dorsal to the nucleus raphe magnus (NRM) of the RVM, 11 mm posterior to the bregma, and 6.5 mm ventral to the surface of the cerebellum according to Paxinos and Watson’s flat skull coordinate system. The guide cannula was secured with dental cement and two small screws. A dummy cannula, cut to extend 0.5 mm beyond the guide cannula, remained in the guide cannula except during drug infusion, and the cannula was covered with a dust cap. Following cannulation, animals were housed singly and allowed to recover for five days prior to the experiment.
For drug infusion, a 0.6 cm length of PE-50 tubing was connected to each end of a 15-cm length of PE-10 tubing. During infusion, the dummy cannula was replaced by an injector that was inserted 3 mm beyond the guide cannula to target the RVM. One end of the tubing was connected to the injector and the other to a 50-µl Hamilton syringe. The solution was infused at 0.1µl/min for a total of 0.5 µl with a pump (KD Scientific, Model 780210). After infusion, the injector was left in the cannula for another 2 minutes to allow the chemicals to spread at the injected area.
For infusion site control, CTOP was injected into a site 2 mm dorsal to the NRM in another 6 rats.
4.6 Immunofluorescence
Rats were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and perfused transcardially with 100 ml of saline followed by 500 ml of 4% paraformaldehyde in 0.1 mol/L phosphate buffer at pH 7.4. The brainstem containing the RVM was removed, immersed in the same fixative for 2 hours and transferred to a solution of 30% sucrose in a phosphate buffer for overnight cryoprotection. Forty micrometer-thick (40 µm) sections were cut with a cryostat at−20°C. Free-floating tissue sections were rinsed in phosphate-buffered saline (PBS). For double immunofluorescence labeling, RVM sections were blocked in PBS with 10% normal donkey serum for 60 minutes, incubated overnight at room temperature with a mixture of guinea pig polyclonal antibody against μ opioid receptors (1:3000, Chemicon) and mouse monoclonal antibodies against GAD (Sigma, 1:500), or with goat polyclonal antibody against 5-HT (ImmunoStar, 1:250) and rabbit polyclonal antibody against GABAa (Sigma, 1:500). After three 10-minute washings in PBS, sections were incubated in a mixture of CY2-conjugated donkey anti-guinea pig (1:100, Jackson ImmunoResearch Laboratories) and CY3-congugated donkey anti-mouse antibody sera (1:400) or CY3-congugated donkey anti-goat (1:500) and CY2-conjugated donkey anti-rabbit antibody sera (1:100) for 1 hour at room temperature. Control sections were similarly processed, except that the primary antisera were omitted. The stained sections were mounted on gelatin-coated slides, coverslipped with aqueous mounting medium (Biomeda Corp., CA) and examined under a Nikon fluorescence microscope. RVM immunoreactive neurons were counted in five sections from each rat.
4.7 Histology
After the experiment, the infusion site was verified by histology. The animals were perfused with saline and 10% formalin under analgesia with sodium pentobarbital. The brains were removed and immersed in 10% formalin for 2 hours and transferred to 30% sucrose. Tissue from the cannula site was cut into 40-µm thick coronal sections. Sections were stained and microscopically examined to determine the location of the cannula according to Paxinos and Watson’s atlas.
4.8 Statistical analysis
Data from the behavioral tests were presented as mean ± SE and analyzed using analysis of variance (ANOVA) followed by Bonferroni multiple comparisons (Graphpad Prism). P<0.05 was set as the level of statistical significance.
Acknowledgements
We would like to thank Dr. Lyn Lowry for her editorial support. This work was supported by NIH Grant R21AT004113 and P01 AT002605.
Abbreviation
- EA
electroacupuncture
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ao M, Wei J, Tan Z, Hu Q, Tang J. The influence of electroacupuncture with different frequencies on the discharges of neurons in rostral ventromedial medulla on rats. Acupuncture Res. 1996;21:41–45. [PubMed] [Google Scholar]
- Berman BM, Lao L, Langenberg P, Lee WL, Gilpin AM, Hochberg MC. Effectiveness of acupuncture as adjunctive therapy in osteoarthritis of the knee: a randomized, controlled trial. Ann. Intern. Med. 2004;141:901–910. doi: 10.7326/0003-4819-141-12-200412210-00006. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Basbaum AI, Cho HJ, Basbaum AI. GABAergic circuitry in the rostral ventral medulla of the rat and its relationship to descending antinociceptive controls. J. Comp. Neurol. 1991;303:316–328. doi: 10.1002/cne.903030212. [DOI] [PubMed] [Google Scholar]
- Efthimiou P, Kukar M. Complementary and alternative medicine use in rheumatoid arthritis: proposed mechanism of action and efficacy of commonly used modalities. Rheumatol. Int. 2010;30:571–586. doi: 10.1007/s00296-009-1206-y. [DOI] [PubMed] [Google Scholar]
- Guo Z-L, Moazzami AR, Tjen-A-Looi S, Longhurst JC. Responses of opioid and serotonin containing medullary raphe neurons to electroacupuncture. Brain Res. 2008;1229:125–136. doi: 10.1016/j.brainres.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J-S. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Han Z, Jiang Y-H, Wan Y, Wang Y, Chang J-K, Han J-S. Endomorphin-1 mediates 2 Hz but not 100 Hz electroacupuncture analgesia in the rat. Neurosci. Lett. 1999;274:75–78. doi: 10.1016/s0304-3940(99)00670-9. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- He LF. Involvement of endogenous opioid peptides in acupuncture analgesia. Pain. 1987;31:99–121. doi: 10.1016/0304-3959(87)90011-X. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Kaplan HJ. GABA-mediated inhibition in rostral ventromedial medulla: role in nociceptive modulation in the lightly anesthetized rat. Pain. 1991;47:105–113. doi: 10.1016/0304-3959(91)90017-R. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Chang J-K, Han J-S. Endomorphin and [mu]-opioid receptors in mouse brain mediate the analgesic effect induced by 2 Hz but not 100 Hz electroacupuncture stimulation. Neurosci. Lett. 2000;294:159–162. doi: 10.1016/s0304-3940(00)01572-x. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Banfor P, Hammond DL. Spinal pharmacology of antinociception produced by microinjection of [mu] or [delta] opioid receptor agonists in the ventromedial medulla of the rat. Neuroscience. 2003;118:789–796. doi: 10.1016/s0306-4522(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Ye Q, Shen YT, Zhu FX, Tang SQ, Liang NJ, Zeng XC. Effects of naloxone on experimental AA evaluated by sensory decision theory. Acta Zool. Sin. 1978;24:1–10. [Google Scholar]
- Lao L, Zhang G, Wei F, Berman BM, Ren K. Electroacupuncture attenuates behavioral hyperalgesia and selectively reduces spinal Fos protein expression in rats with persistent inflammation. J. Pain. 2001;2:111–117. doi: 10.1054/jpai.2001.19575. [DOI] [PubMed] [Google Scholar]
- Lao L, Zhang R-X, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004;1020:18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- Lee J, Beitz A. The distribution of brain-stem and spinal cord nuclei associated with different frequencies of electroacupuncture analgesia. Pain. 1993;52:11–28. doi: 10.1016/0304-3959(93)90109-3. [DOI] [PubMed] [Google Scholar]
- Li A, Wang Y, Xin J, Lao L, Ren K, Berman BM, Zhang R-X. Electroacupuncture suppresses hyperalgesia and spinal Fos expression by activating the descending inhibitory system. Brain Res. 2007;1186:171–179. doi: 10.1016/j.brainres.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Lao L, Wang Y, Xin J, Ren K, Berman BM, Tan M, Zhang R. Electroacupuncture activates corticotrophin-releasing hormone-containing neurons in the paraventricular nucleus of the hypothalammus to alleviate edema in a rat model of inflammation. BMC Complement. Altern. Med. 2008;8:20. doi: 10.1186/1472-6882-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Tjen-A-Looi S, Longhurst JC. Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Auton Neurosci. 2001;89:38–47. doi: 10.1016/S1566-0702(01)00247-8. [DOI] [PubMed] [Google Scholar]
- Ma S-X, Ma J, Moise G, Li X-Y. Responses of neuronal nitric oxide synthase expression in the brainstem to electroacupuncture Zusanli (ST 36) in rats. Brain Res. 2005;1037:70–77. doi: 10.1016/j.brainres.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Martin DP, Sletten CD, Williams BA, Berger IH. Improvement in fibromyalgia symptoms with acupuncture: results of a randomized controlled trial. Mayo Clin. Proc. 2006;81:749–757. doi: 10.4065/81.6.749. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977;121:368–372. doi: 10.1016/0006-8993(77)90161-5. [DOI] [PubMed] [Google Scholar]
- Mayer DJ. Biological mechanisms of acupuncture. Prog. Brain Res. 2000;122:457–477. doi: 10.1016/s0079-6123(08)62157-3. [DOI] [PubMed] [Google Scholar]
- Moazzami A, Tjen-A-Looi SC, Guo ZL, Longhurst JC. Serotonergic projection from nucleus raphe pallidus to rostral ventrolateral medulla modulates cardiovascular reflex responses during acupuncture. J. Appl. Physiol. 2010;108:1336–1346. doi: 10.1152/japplphysiol.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers RJ-F, Mahoney JL, Shippenberg TS. Inflammation-induced changes in rostral ventromedial medulla mu and kappa opioid receptor mediated antinociception. Pain. 2008a;136:320–330. doi: 10.1016/j.pain.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Schepers RJ-F, Mahoney JL, Zapata A, Chefer V, Shippenberg TS. The effects of local perfusion of DAMGO on extracellular GABA and glutamate concentrations in the rostral ventromedial medulla. J. Neurochem. 2008b;104:806–817. doi: 10.1111/j.1471-4159.2007.05017.x. [DOI] [PubMed] [Google Scholar]
- Ulett GA, Han S, Han J-s. Electroacupuncture: mechanisms and clinical application. Biol. Psychiatry. 1998;44:129–138. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- Witt C, Brinkhaus B, Jena S, Linde K, Streng A, Wagenpfeil S, Hummelsberger J, Walther HU, Melchart D, Willich SN. Acupuncture in patients with osteoarthritis of the knee: a randomised trial. Lancet. 2005;366:136–143. doi: 10.1016/S0140-6736(05)66871-7. [DOI] [PubMed] [Google Scholar]
- Yang E, Koo S, Kim Y, Lee J, Hwang H, Lee M, Choi S. Contralateral electroacupuncture pretreatment suppresses carrageenan-induced inflammatory pain via the opioid-mu receptor. Rheumatol. Int. 2010 Feb 4; doi: 10.1007/s00296-010-1364-y. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Yeung JC, Rudy TA, Yeung JC, Rudy TA. Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of antinociceptive action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. J. Pharmacol. Exp. Ther. 1980;215:633–642. [PubMed] [Google Scholar]
- Zhang R-X, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020:12–17. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Xin J, Ren K, Qiao JT, Berman BM, Lao L. Electroacupuncture attenuates bone-cancer-induced hyperalgesia and inhibits spinal preprodynorphin expression in a rat model. Eur. J. Pain. 2008;12:870–878. doi: 10.1016/j.ejpain.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-Q, Ji G-C, Wu G-C, Zhao Z-Q. Excitatory amino acid receptor antagonists and electroacupuncture synergetically inhibit carrageenan-induced behavioral hyperalgesia and spinal fos expression in rats. Pain. 2002;99:525–535. doi: 10.1016/S0304-3959(02)00268-3. [DOI] [PubMed] [Google Scholar]
- Zhou W, Tjen-A-Looi SC, Longhurst JC. Brain Stem Mechanisms Underlying Acupuncture Modality-Related Modulation of Cardiovascular Responses in Rats. J. Appl. Physiol. 2005 doi: 10.1152/japplphysiol.01365.2004. 01365.2004. [DOI] [PubMed] [Google Scholar]