Abstract
The developmentally arrested infective larva of hookworms encounters a host-specific signal during invasion that initiates the resumption of suspended developmental pathways. The resumption of development during infection is analogous to recovery from the facultative arrested dauer stage in the free-living nematode Caenorhabditis elegans. Infective larvae of the canine hookworm Ancylostoma caninum resume feeding and secrete molecules important for infection when exposed to a host mimicking signal in vitro. This activation process is a model for the initial steps of the infective process. Dauer recovery requires protein synthesis, but not RNA synthesis in C. elegans. To determine the role of RNA and protein synthesis in hookworm infection, inhibitors of RNA and protein synthesis were tested for their effect on feeding and secretion by A. caninum infective larvae. The RNA synthesis inhibitors α-amanitin and actinomycin D inhibit feeding dose-dependently, with IC50 values of 30 and 8 µM, respectively. The protein synthesis inhibitors puromycin (IC50 =110 µM), cycloheximide (IC50 =50 µM), and anisomycin (IC50 =200 µM) also displayed dose-dependent inhibition of larval feeding. Significant inhibition of feeding by α-amanitin and anisomycin occurred when the inhibitors were added before 12 h of the activation process, but not if the inhibitors were added after 12 h. None of the RNA or protein synthesis inhibitors prevented secretion of the activation-associated protein ASP-1, despite nearly complete inhibition of feeding. The results indicate that unlike dauer recovery in C. elegans, de novo gene expression is required for hookworm larval activation, and the critical genes are expressed within 12 h of exposure to activating stimuli. However, secretion of infection-associated proteins is independent of gene expression, indicating that the proteins are pre-synthesized and stored for rapid release during the initial stages of infection. The genes that are inhibited represent a subset of those required for the transition to parasitism, and therefore represent interesting targets for further investigation. Furthermore, while dauer recovery provides a useful model for hookworm infection, the differences identified here highlight the importance of exercising caution before making generalizations about parasitic nematodes based on C. elegans biology.
Keywords: hookworm, Ancylostoma caninum, activation, RNA synthesis, protein synthesis, dauer larva, insulin signaling
1. Introduction
During invasion of its definitive host, the developmentally arrested third-stage infective larva (L3) of parasitic nematodes encounters a host-specific signal that re-initiates suspended developmental pathways. This “activation” process results in the expression of a parasitic gene set (Petronijevic and Rogers, 1983; Rogers and Petronijevic, 1982) encoding proteins necessary for parasitism, and culminates with the development of the mature adult reproductive stages. This process is analogous to the recovery from the developmentally arrested dauer state of Caenorhabditis elegans and other free-living nematodes (Hawdon and Schad, 1991a; Hotez et al., 1993; Rogers and Sommerville, 1963) Following exposure to permissive conditions, dauer larvae resume feeding and pharyngeal pumping, molt to the L4 stage, and resume longitudinal growth (Cassada and Russell, 1975). These morphological changes are accompanied by changes in gene expression (Halaschek-Wiener et al., 2005; Jones et al., 2001; Wang and Kim, 2003). In the hookworm Ancylostoma caninum, L3 exposed to host-like signals undergo partial activation, as indicated by the resumption of feeding, the release of infection associated molecules (Hawdon et al., 1995; Hawdon et al., 1999; Hawdon and Schad, 1990, , 1992; Zhan et al., 1999), and changes in gene expression (Datu et al., 2009). Hookworm L3 activation, as well as dauer recovery in C. elegans, is mediated by cGMP and insulin-like signaling (Brand and Hawdon, 2004; Gao et al., 2009; Hawdon and Datu, 2003; Kiss et al., 2009), indicating a conservation of this activation pathway between the species, and suggests that dauer recovery may be a useful model for hookworm activation (Hawdon and Hotez, 1996; Hawdon and Schad, 1991a).
Dauer recovery requires protein synthesis, as incubation of dauer larvae with cyclohexamide prevents recovery in response to permissive conditions (Reape and Burnell, 1991b). However, incubation of dauer larvae with mRNA synthesis inhibitors had no effect on pharyngeal pumping or growth during early recovery, suggesting neither mRNA synthesis nor new gene expression is required for the initial stages exit from the dauer stage (Reape and Burnell, 1991a). Because of the parallels between dauer recovery and hookworm L3 activation, we hypothesized that protein synthesis, but not mRNA synthesis, would be required for hookworm L3 activation. Surprisingly we found that both mRNA and proteins synthesis are required for activation of hookworm L3. However, neither inhibitor affected secretion of an activation-associated protein. Our data indicate that some molecular processes involved in resumption of development during hookworm infection differ significantly from C. elegans dauer recovery, and that feeding and secretion during activation are independent and separable processes.
2. Materials and Methods
2.1. Parasites
A Baltimore strain of A. caninum (US National Parasite Collection No. 100655.00) was maintained in previously hookworm free beagles as described (Schad, 1982). Dogs were housed and treated according to a protocol approved by the George Washington University Institutional Care and Use Committee. Infective L3 were recovered from charcoal coproculture by a modified Baermann technique and stored for periods up to 3 weeks in buffer BU (50 mM Na2HPO4/22 mM KH2PO4/70 mM NaCl, pH 6.8 (Hawdon and Schad, 1991b) at 22°C until used for activation studies.
2.2. In vitro activation of L3
Ancylostoma caninum L3 were activated by incubation under host-like conditions as described previously (Hawdon et al., 1999). Briefly, L3 collected from coprocultures were decontaminated with 1% HCl in BU buffer for 30 min at 22 C. Approximately 250 L3 were incubated at 37 C, 5% CO2 for 24 h in 0.1 ml RPMI1640 tissue culture medium supplemented with 25 mM HEPES pH 7.0, and antibiotics (RPMI-c) (Hawdon and Schad, 1990) in individual wells of 96-well microtiter plates. L3 were stimulated by inclusion of 15% (v/v) of a <10 kD ultrafiltrate of canine serum and 25 mM S-methyl-glutathione (GSM; Sigma, St. Louis, MO) dissolved in RPMI-c (Hawdon et al., 1995). Non-activated L3 were incubated in RPMI without the stimuli. Protein and mRNA synthesis inhibitors were obtained from Sigma and dissolved in RPMI-c (puromycin, α-amanitin), ethanol (cycloheximide) or methanol (anisomycin, actinomycin-D) to make stock solutions. Stocks were diluted with RPMI-c to the indicated concentrations in the incubations. Control incubations included ethanol or methanol at the highest concentration used in the experiment, and never exceeded 1.0%. Treatments were done in triplicate, and the percentage of feeding L3 was determined as described (Hawdon et al., 1996). The half maximal inhibitory concentration (IC50) for each inhibitor was calculated using GraphPad PRISM (ver 4.01) to fit dose-response data using either the sigmoidal dose-response or sigmoidal dose response with variable slope model.
2.3. ES collection and Western blotting
To determine if the protein and RNA synthesis inhibitors blocked secretion, excretory/secretory (ES) products from non-activated, activated, and inhibited L3 incubations were assayed by Western blot for the presence of the secreted protein Ac-ASP-1 (Hawdon et al., 1996). Approximately 5000 L3 were incubated in individual wells of a 24 well tissue culture plate containing 500 µL of RPMI-c alone (non-activated), 15% canine serum filtrate plus 25 mM GSM (activated), or filtrate, GSM and inhibitor (inhibited). Following incubation for 24 h, medium containing the L3 was transferred to separate microcentrifuge tubes and centrifuged for 5 min at 14,000 rpm. The supernatants were filtered through a 0.45 µm syringe filter to remove any L3 and cast cuticles, and stored at −20 C. Prior to electrophoresis, the ES products from approximately 5000 L3 (larval equivalents, LE) were concentrated by ultrafiltration using Centricon 10 cartridges (Amicon, Beverley, MA), washed with 1 ml of BU, concentrated again by ultrafiltration, and separated in a 11% SDS-polyacrylamide gel. Separated proteins were transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA) by electroblotting at 16V for 16 h (Towbin et al., 1979). The membrane was blocked with 5% non-fat dry milk in wash buffer (PBS, pH 7.4, 0.1% Tween 20) for 4 h at 4°C. The blocked membrane was incubated for 1 h at 22°C with a 1:5000 dilution of rabbit antiserum against recombinant Ancylostoma secreted protein 1 (rASP-1)(Hawdon et al., 1996). The membrane was washed 3 times with wash buffer for 10 min at 22°C, followed by incubation with a 1:5000 dilution of horseradish peroxidase-conjugated goat anti-rabbit Ig (Boehringer Mannheim, Indianapolis, IN) for 1 h at 22°C. Following washing, the bands were visualized using chemiluminescent detecting reagents according to the manufacturer’s instructions (ECL+, Amersham Pharmacia Biotech, Piscataway, NJ).
To determine whether ASP-1 was synthesized prior to activation, a soluble lysate of untreated, ensheathed L3 was prepared as described (Kiss et al., 2009). Following acetone precipitation, the proteins were resuspended in PBS and the protein concentration determined by the bicinchoninic acid method (Micro BCA, Thermo Fisher) according to the manufacturer’s instructions. Lysate (0.1 – 4 µg) and recombinant ASP-1 were separated on a 4–20% Tris-glycine SDS-polyacrylamide gel (Invitrogen) and blotted to nitrocellulose membrane using an iBlot gel transfer apparatus (Invitrogen). Following transfer, the membrane was blocked and washed as above, and incubated with ASP-1 antiserum (1:1000) followed by horseradish peroxidase-conjugated goat anti-rabbit Ig. The chemiluminescent signal was detected as described above.
3. Results
3.1. Effect of RNA and protein synthesis inhibitors on L3 activation
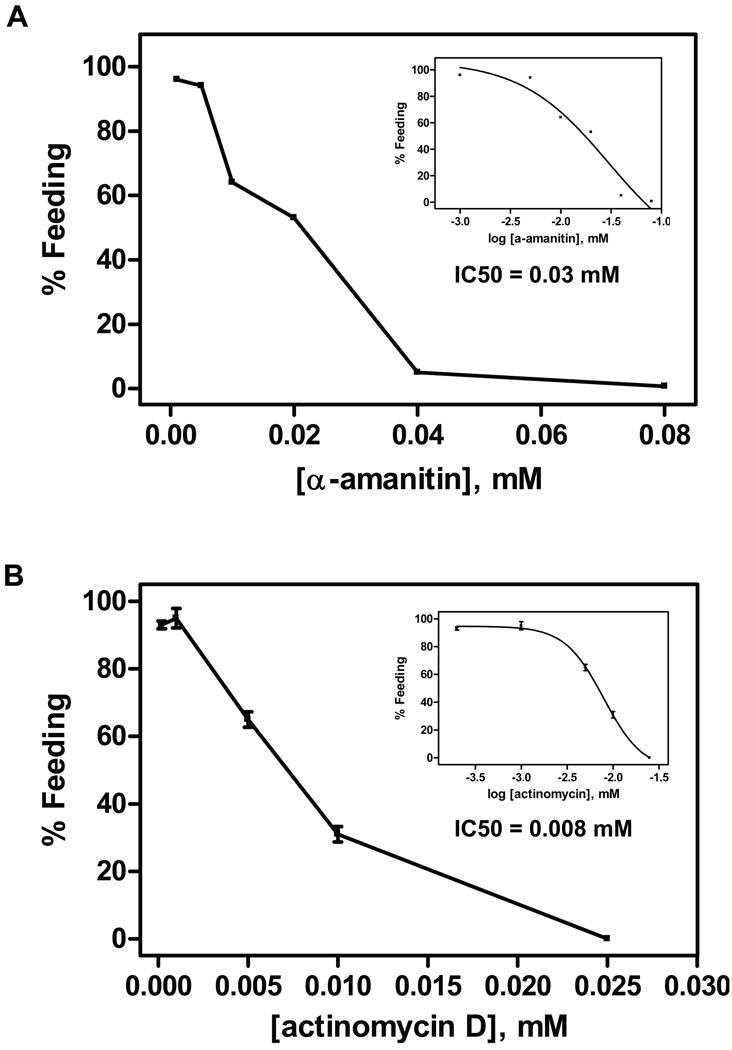
To determine whether mRNA synthesis was required for activation, A. caninum L3 were co-incubated with either actinomycin D or α-amanitin and the activating stimulus containing a <10 kD ultrafiltrate of canine serum and S-methylglutathione (fil+GSM). As shown in Figure 1, inhibition of activation by both actinomycin D and α-amanitin is dose-dependent, with IC50 of 7.9 µM and 30 µM, respectively. Feeding was completely inhibited by 40 µM α-amanitin and 25 µM actinomycin D. The inhibitors had no effects on L3 motility. These compounds inhibit RNA synthesis by different mechanisms. Actinomycin D binds to DNA and prevents transcription (Ernst and Oleinick, 1977), whereas α-amanitin is a specific inhibitor of RNA polymerase II (Lindell et al., 1970). The data suggest that mRNA synthesis is required for activation. This contrasts with C. elegans, which were able to initiate recovery from dauer when mRNA synthesis was inhibited (Reape and Burnell, 1991a).
Figure 1.
Effect of RNA synthesis inhibitors on Ancylostoma caninum L3 activation in vitro. Increasing concentrations of mRNA synthesis inhibitors were co-incubated with canine serum filtrate and S-methyl-glutathione (GSM) in triplicate samples. The inset represents curve fitting to dose response models using GraphPad PRISM (ver 4.01) for the calculation of IC50 values. A) Effect of α-amanitin on L3 feeding. The sigmoidal dose response model was used for IC50 calculation. B) Effect of actinomycin D on L3 feeding. The sigmoidal dose response model with variable slope was used for IC50 calculation. Each experiment was repeated at least once.
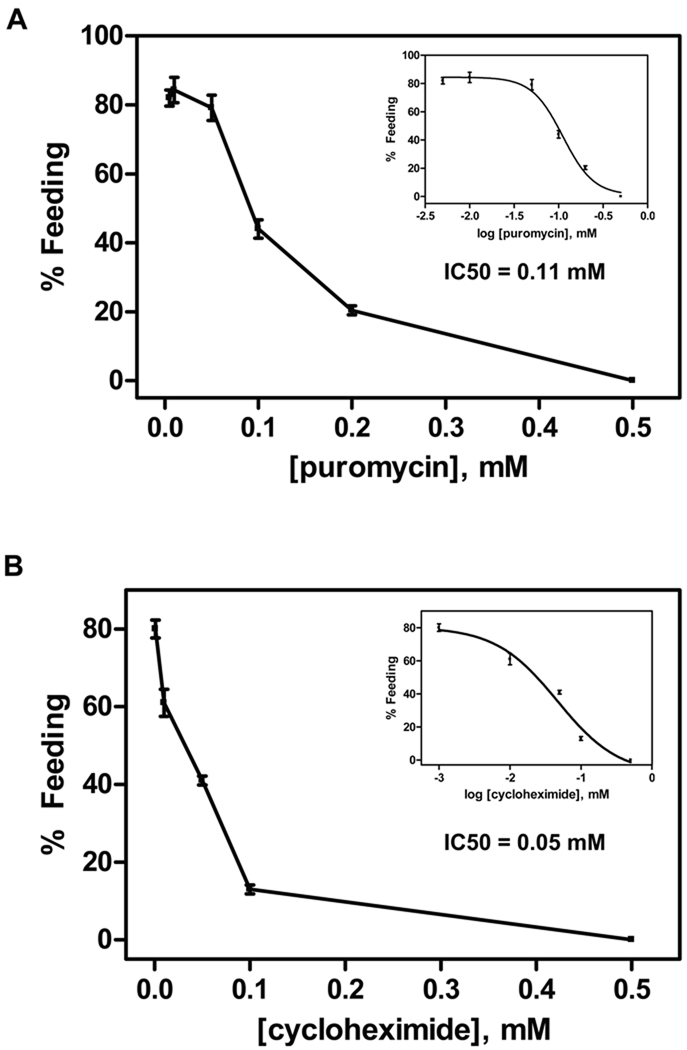
Next, L3 were co-incubated with stimulus and protein synthesis inhibitors to determine if protein synthesis was required for activation. Both puromycin (IC50 = 111 µM) and cyclohexamide (IC50= 50 µM) exhibited dose-dependent inhibition of activation, and completely inhibited activation at 500 µM (Figure 2). Similar dose-dependent inhibition was seen with anisomycin, with an IC50 of 200 µM and complete inhibition at 500 µM (not shown). None of the inhibitors affected L3 motility at the concentrations tested. Cycloheximide and anisomycin block the translocation step and the peptidyl transferase reaction, respectively, in the 80S ribosome during protein synthesis, whereas puromycin causes the premature release of nascent polypeptide chains by its addition to growing chain end (Alberts et al., 1994). Inhibition of activation by these inhibitors indicates that protein synthesis is also required for hookworm L3 activation and feeding, as in C. elegans.
Figure 2.
Effect of protein synthesis inhibitors on Ancylostoma caninum L3 activation in vitro. Increasing concentrations of protein synthesis inhibitors were co-incubated with canine serum filtrate and GSM in triplicate samples. The inset represents curve fitting to dose response models using GraphPad PRISM (ver 4.01) for the calculation of IC50 values. A) Effect of puromycin on L3 feeding. The sigmoidal dose response model with variable slope was used for IC50 calculation. B) Effect of cycloheximide on L3 feeding. The sigmoidal dose response model was used for IC50 calculation. Each experiment was repeated at least once.
3.2. Critical time for inhibitor addition
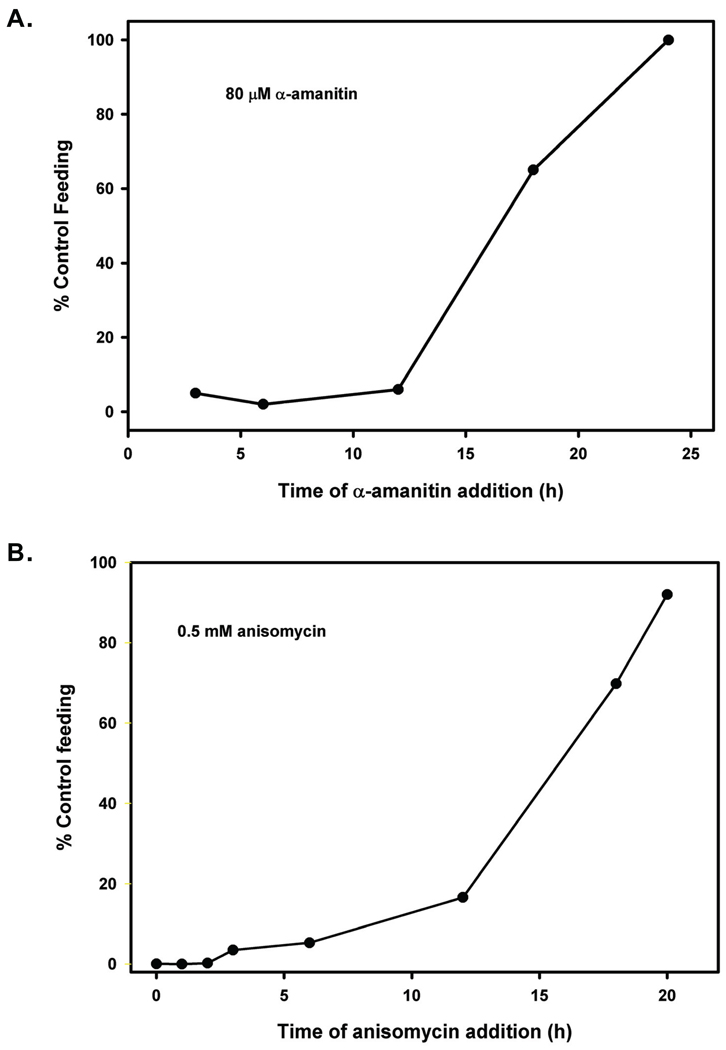
Activation of A. caninum L3 in vitro proceeds with well-defined and repeatable kinetics (Hawdon and Schad, 1993). The first L3 resume feeding after exposure to the stimulus for approximately 6 h. The number of feeding L3 increases linearly until 12 h, when it reaches a maximum level of approximately 90% of the population feeding. To determine when during this process RNA and protein synthesis was required, the inhibitors were added at various times during the incubation, and the worms assayed for feeding at 24 h. When either the RNA synthesis inhibitor α-amanitin (80 µM) or the protein inhibitor anisomycin (500 µM) was added before 12 h, activation at 24 h was significantly inhibited (Figure 3). However, when the inhibitors were added at 18 h or later, L3 activated to near maximal levels. This suggests that critical protein synthesis required for the activation process occurs before 12 h.
Figure 3.
Effect of time of inhibitor addition on activation of Ancylostoma caninum L3. Inhibitors were added to L3 incubated with filtrate and GSM at the times shown, and the percentage feeding determined at 24 h. Each time point was done in triplicate, and the experiment repeated twice. A) Effect of time of addition of the RNA synthesis inhibitor α-amanitin on activation. B) Effect of time of addition of the RNA synthesis inhibitor actinomycin D on activation.
3.3. Effect of inhibitors on ASP-1 secretion
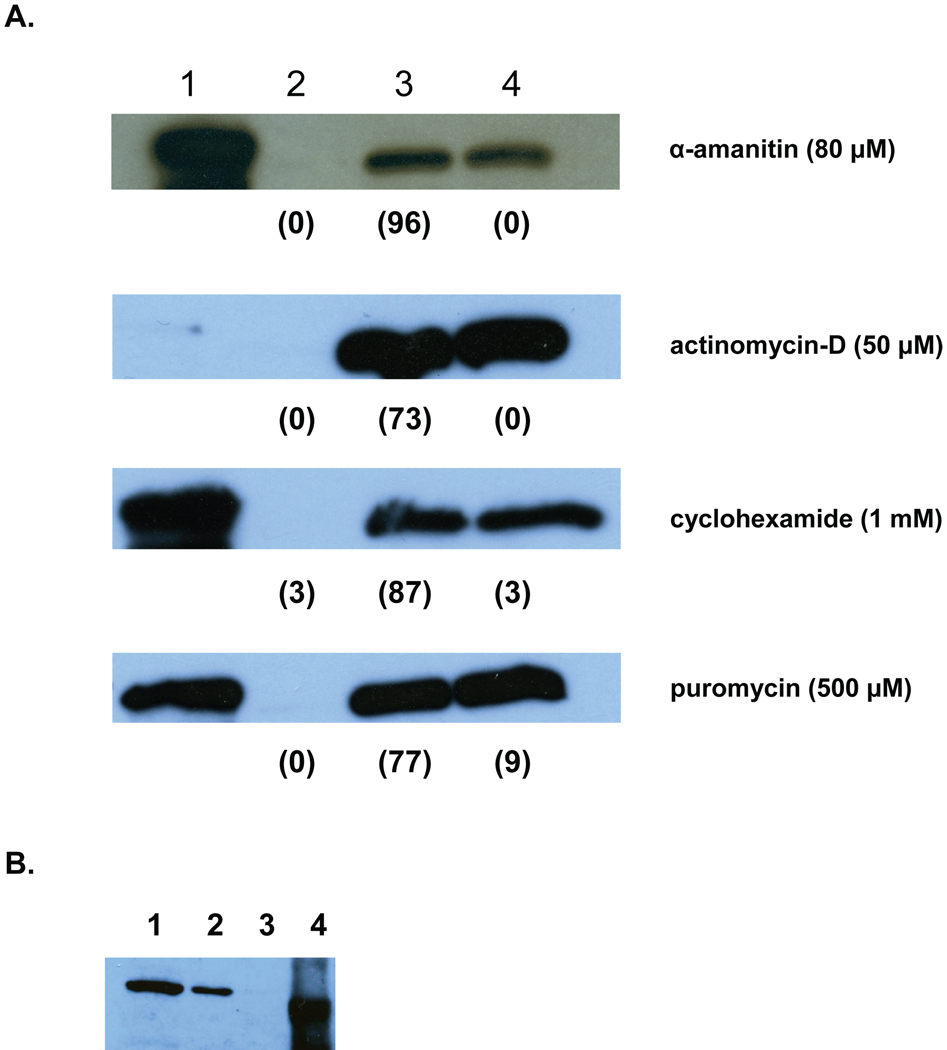
In addition to feeding, activation initiates the release of proteins by the L3. One such protein, the activation-associated secreted protein ASP-1, is released within 30 min exposure to the activating stimulus, with the majority secreted by 4 h (Hawdon et al., 1996). The rapid kinetics suggests that ASP-1 is pre-synthesized and released in response to the activation stimulus. To test this hypothesis, the effect of mRNA synthesis inhibitors (actinomycin D and α-amanitin) and protein synthesis inhibitors (puromycin and cycloheximide) on activation-associated secretion of ASP-1 was determined. As shown in Figure 4a, non-activated L3 released little or no ASP-1, whereas activated L3 released significant amounts of the protein. None of the inhibitors affected ASP-1 secretion in response to the activation stimulus. This indicates that de novo gene expression is not required for activation-associated secretion, and suggests that ASP-1 is released from stored supplies. To confirm that ASP-1 is synthesized and stored in the L3 stage prior to activation, a soluble lysate of untreated L3 was probed with the ASP-1 antiserum. As seen in Fig. 4b, ASP-1 is present in un-stimulated L3, indicating that it is synthesized prior to activation.
Figure 4.
Effect of RNA and protein synthesis inhibitors on secretion of ASP-1 during activation. A. Approximately 5000 A.caninum L3 were incubated without filtrate and GSM (non-activated), with filtrate and GSM (activated), or with filtrate and GSM and inhibitor at the indicated concentration (inhibited). After 24 h, the ES products were collected and separated by SDS-PAGE, blotted, and probed with ASP-1 antiserum. Lane 1, rASP-1; lane 2, non-activated L3 ES; lane 3, activated L3 ES; Lane 4, inhibited L3 ES. The numbers in parentheses indicate percentage feeding at 24 h. The lack of signal in the positive control for actinomycin D was due to inadvertent omission of the control sample from the gel. B. ASP-1 is synthesized prior to activation. Soluble extract of untreated A. caninum L3 were separated by SDS-PAGE, blotted, and probed with ASP-1 antiserum. Lane 1, 4 µg lysate; lane 2, 1 µg lysate; lane 3, 0.1 µg lysate; lane 4, 5 µg recombinant ASP-1.
4. Discussion
Developmentally arrested infective L3 of hookworms encounter host-specific signals during invasion that re-activate suspended developmental pathways. Recovery from the dauer stage of the free-living nematode C. elegans is analogous to L3 resumption of development, and has been used as a model for investigations of hookworm L3 activation during infection (Hawdon and Schad, 1991a; Hotez et al., 1993). Dauer recovery and hookworm larval activation are complex, highly regulated processes that are initiated by exposure to specific environmental conditions. These environmental conditions initiate the expression of stage-specific genes that are required for development to the L4 and adult stages, and in the case of hookworm L3, for parasitism. These parasitism genes represent potential targets for intervention in the hookworm life cycle.
Here we demonstrate significant differences in the requirements for gene expression in hookworm L3 activation and C. elegans dauer recovery. When C. elegans dauer larvae are incubated with the mRNA synthesis inhibitors actinomycin-D and α-amanitin, they initiate recovery from dauer normally and resume pharyngeal pumping, but fail to molt to the L4 (Reape and Burnell, 1991a). This indicate that mRNA synthesis is not required for dauer exit or the initial pre-molt recovery period, and suggests that the mRNAs required for early dauer recovery are pre-synthesized and stored, whereas those required for the molt are synthesized post-recovery. However, the protein synthesis inhibitors puromycin, anisomycin, and cycloheximide prevented pharyngeal pumping and recovery, indicating that protein synthesis was required for dauer exit (Reape and Burnell, 1991b). These data suggested that mRNAs encoding the genes required for exit from the dauer stage are transcribed in the dauer larva, but not translated until after recovery has been initiated.
As in C. elegans, protein synthesis inhibitors block activation in A. caninum L3, indicating that new proteins are required for the activation process. Unlike C. elegans, however, hookworm L3 also require mRNA synthesis for activation. This indicates that de novo expression of one or more key proteins is required for larval activation, and that unlike C. elegans, the transcripts required for activation are not pre-synthesized. Critical gene expression occurs during the first 12 h following exposure to the stimulus, as addition of the inhibitors after this time has little effect on activation levels. Global analysis of dauer gene expression during recovery identified 1984 genes that showed significant expression changes (Wang and Kim, 2003). The genes were grouped temporally, with transient genes representing those that peak at approximately 2 h after exposure to food. This is prior to pharyngeal pumping and many of the morphological changes associated with dauer recovery (Cassada and Russell, 1975; Golden and Riddle, 1984). These transient genes may represent transcription factors that initiate expression of downstream genes required for activation.
Several other transient or early class proteins might represent key hookworm activation proteins. Datu et al (2008) found that expression of several genes increased within the first 6 h of activation. These included several members of the activation-associated secreted proteins/ pathogenesis related proteins (ASP/PRP) (Hawdon et al., 1996), proteases, and novel mRNAs. Also, several transcripts were down regulated, including small heat shock proteins, ASP/PRPs, proteases, and novel mRNA. While up regulated proteins represent likely candidates for critical activation genes, the inability to negatively regulate arrest-associated genes due to inhibited expression of a regulatory protein could also prevent activation. The functions of these differentially regulated proteins are mostly unknown, making confirmation of their role in activation problematic. Furthermore, additional genes that were not detected by the techniques used in these studies might also be required for activation. In any case, our results indicate that one or more members of the parasitic gene suite expressed early in activation are essential for activation to occur.
In addition to feeding, hookworm L3 activation is characterized by the secretion of proteins believed to be involved in infection. These include a metalloprotease, a hyaluronidase, and at least 2 ASP/PRP family members named ASP-1 and ASP-2 (Hawdon et al., 1995; Hawdon et al., 1996; Hawdon et al., 1999; Hotez et al., 1992; Zhan et al., 2002). Of these, the kinetics of ASP-1 secretion during activation has been best characterized. ASP-1 is released within 30 min exposure to the activation stimulus, with nearly 60% of the total ASP released in the 1st h and almost 90% released by 4 h (Hawdon et al., 1996). This is several hours before a significant number of L3 have begun feeding. As the buccal cavity of hookworm L3 remains sealed until feeding resumes (El Naggar, 1987), ASP-1 is secreted from a site other than the esophagus or intestine of the larva. The rapidity of release suggests that the protein is synthesized and stored for secretion during infection, although de novo gene expression and secretion could occur within 4 h. For example, infective dauer juveniles of the entomopathogenic nematode Heterorhabditis bacteriophora recover from the dauer arrest following exposure to its insect host (Dolan et al., 2002). During recovery, RNA synthesis in the esophageal gland cell increases within 3 h, indicating high levels of transcription. Feeding resumes by 5 h, and reaches a maximum at 36 h in this species. However, our inability to inhibit ASP-1 secretion with either protein or RNA synthesis inhibitors suggests that ASP-1 secretion does not require synthesis of new ASP-1 mRNA or protein. Furthermore, ASP-1 is secreted as early as 30 min after exposure to the stimulus, much earlier than gene expression occurs in H. bacteriophora. Finally, our results show that ASP-1 is present in unstimulated L3 before exposure to activation stimuli. This indicates that ASP-1, and perhaps other proteins secreted early in activation, are pre-synthesized and stored for quick release early in infection, and supports the hypothesis that they are important for establishment of parasitism (Hawdon et al., 1996).
Previously, we demonstrated that feeding and secretion were regulated by different pathways downstream of the initial activation signal. Inhibition of IIS using the phosphoinositide-3-OH-kinase (PI3-K) inhibitor LY294002 prevented feeding, but not secretion (Brand and Hawdon, 2004). Furthermore, the pathways appears to bifurcate at an upstream muscarinic receptor step, as the specific muscarinic receptor antagonist atropine completely inhibited feeding, but only partially inhibited secretion (Hawdon and Datu, 2003). Our ability to pharmacologically uncouple feeding from secretion with RNA and protein synthesis inhibitors in this study supports the idea that while feeding and secretion are initiated by the same activation stimulus, their down stream signaling pathways differ, and therefore represent separate targets for intervention.
In conclusion, we have shown that L3 activation is blocked by inhibitors of mRNA and protein synthesis when they are added during the first 12 h of the activation process. However, secretion of activation-associated protein ASP-1 is not blocked by the inhibitors. This is in contrast to C. elegans, in which protein synthesis, but not RNA synthesis, is required for the analogous process of dauer recovery. Taken together, these results indicate that activation of L3 depends on de novo transcription and translation within the first 12 h of exposure to stimulating conditions, but that secretion occurs without new gene expression. The genes that are expressed during activation are a subset of those required for the transition to parasitism, and as such represent interesting targets for further investigation. Furthermore, while dauer recovery provides a paradigm for hookworm larval activation during infection, there are significant differences between the nematodes, and caution should be used before making generalizations about the parasitic nematode infectious process based on analogous C. elegans biology.
Acknowledgments
We thank Geeta Varghese for parasite maintenance, Ben Datu for technical assistance, and Bin Zhan for providing recombinant ASP-1. The project was supported by Award Number R01AI069293 and 1R21AI062857 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors declare no conflicts of interest.
References
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. London: Garland Publishing; 1994. [Google Scholar]
- Brand A, Hawdon JM. Phosphoinositide-3-OH-kinase inhibitor LY294002 prevents activation of Ancylostoma caninum and Ancylostoma ceylanicum third-stage infective larvae. Int. J. Parasitol. 2004;34:909–914. doi: 10.1016/j.ijpara.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Cassada RC, Russell RL. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Datu BJ, Gasser RB, Nagaraj SH, Ong EK, O'Donoghue P, McInnes R, Ranganathan S, Loukas A. Transcriptional changes in the hookworm, Ancylostoma caninum, during the transition from a free-living to a parasitic larva. PLoS Negl. Trop. Dis. 2008;2:e130. doi: 10.1371/journal.pntd.0000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datu BJ, Loukas A, Cantacessi C, O'Donoghue P, Gasser RB. Investigation of the regulation of transcriptional changes in Ancylostoma caninum larvae following serum activation, with a focus on the insulin-like signalling pathway. Vet. Parasitol. 2009;159:139–148. doi: 10.1016/j.vetpar.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Dolan KM, Jones JT, Burnell AM. Detection of changes occurring during recovery from the dauer stage in Heterorhabditis bacteriophora. Parasitology. 2002;125:71–81. doi: 10.1017/s0031182002001762. [DOI] [PubMed] [Google Scholar]
- El Naggar HMS. Ultrastructural comparison of rhabditiform (developing) and filariform (resting) larvae of the human hookworm A. duodenale. Philadelphia: University of Pennsylvania; 1987. [Google Scholar]
- Ernst SG, Oleinick NL. Actinomycin D in Tetrahymena. 356 Non-specific inhibition of RNA synthesis and primary and secondary effects on protein synthesis. Exper Cell Res. 1977;110:363–373. doi: 10.1016/0014-4827(77)90303-2. [DOI] [PubMed] [Google Scholar]
- Gao X, Frank D, Hawdon JM. Molecular cloning and DNA binding characterization of DAF-16 orthologs from Ancylostoma hookworms. Int. J. Parasitol. 2009;39:407–415. doi: 10.1016/j.ijpara.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- Halaschek-Wiener J, Khattra JS, McKay S, Pouzyrev A, Stott JM, Yang GS, Holt RA, Jones SJM, Marra MA, Brooks-Wilson AR, Riddle DL. Analysis of long-lived C. elegans daf-2 mutants using serial analysis of gene expression. Genome Res. 2005;15:603–615. doi: 10.1101/gr.3274805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawdon JM, Datu B. The second messenger cyclic GMP mediates activation in Ancylostoma caninum infective larvae. Int. J. Parasitol. 2003;33:787–793. doi: 10.1016/s0020-7519(03)00088-2. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Hotez PJ. Hookworm: developmental biology of the infectious process. Curr. Opin. Gen. Dev. 1996;6:618–623. doi: 10.1016/s0959-437x(96)80092-x. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Jones BF, Hoffman DR, Hotez PJ. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J. Biol. Chem. 1996;271:6672–6678. doi: 10.1074/jbc.271.12.6672. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Jones BF, Perregaux MA, Hotez PJ. Ancylostoma caninum: metalloprotease release coincides with activation of infective larvae in vitro. Exp. Parasitol. 1995;80:205–211. doi: 10.1006/expr.1995.1025. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Narasimhan S, Hotez PJ. Ancylostoma secreted protein 2: cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum. Mol. Biochem. Parasitol. 1999;99:149–165. doi: 10.1016/s0166-6851(99)00011-0. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Schad GA. Serum stimulated feeding in vitro by third stage infective larvae of the canine hookworm Ancylostoma caninum. J. Parasitol. 1990;76:394–398. [PubMed] [Google Scholar]
- Hawdon JM, Schad GA. Developmental Adaptations in Nematodes. In: Toft CA, Aeschlimann A, Bolis L, editors. Parasitism: Coexistence or Conflict? Oxford: Oxford University Press; 1991a. pp. 274–298. [Google Scholar]
- Hawdon JM, Schad GA. Long-term storage of hookworm infective larvae in buffered saline solution maintains larval responsiveness to host signals. J. Helm. Soc. Wash. 1991b;58:140–142. [Google Scholar]
- Hawdon JM, Schad GA. Ancylostoma caninum: reduced glutathione stimulates feeding by third-stage infective larvae. Exp. Parasitol. 1992;75:40–46. doi: 10.1016/0014-4894(92)90120-y. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Schad GA. Ancylostoma caninum: glutathione stimulates feeding in third-stage larvae by a sulfhydryl-independent mechanism. Exp. Parasitol. 1993;77:489–491. doi: 10.1006/expr.1993.1110. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Hawdon JM, Schad GA. Hookworm larval infectivity, arrest, and amphiparatenesis: the Caenorhabditis elegans daf-c paradigm. Parasitol. Today. 1993;9:23–26. doi: 10.1016/0169-4758(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Narasimhan S, Haggerty J, Milstone L, Bhopale V, Schad GA, Richards FF. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect. Immun. 1992;60:1018–1023. doi: 10.1128/iai.60.3.1018-1023.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SJ, Riddle DL, Pouzyrev AT, Velculescu VE, Hillier L, Eddy SR, Stricklin SL, Baillie DL, Waterston R, Marra MA. Changes in gene expression associated with developmental arrest and longevity in Caenorhabditis elegans. Genome Res. 2001;11:1346–1352. doi: 10.1101/gr.184401. [DOI] [PubMed] [Google Scholar]
- Kiss J, Gao X, Krepp J, Hawdon J. Interaction of hookworm 14-3-3 with the forkhead transcription factor DAF-16 requires intact Akt phosphorylation sites. Parasit. Vectors. 2009;2:21. doi: 10.1186/1756-3305-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell TJ, Weinberg F, Morris PW, Roeder RG, Rutter WJ. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970;170:447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Petronijevic T, Rogers WP. Gene activity and the development of early parasitic stages of nematodes. Int. J. Parasitol. 1983;13:197–199. doi: 10.1016/0020-7519(83)90012-7. [DOI] [PubMed] [Google Scholar]
- Reape TJ, Burnell AM. Dauer larva recovery in the nematode Caenorhabditis elegans - I. The effect of mRNA synthesis inhibitors on recovery, growth and pharyngeal pumping. Comp. Biochem. Physiol. 1991a;98B:239–243. [Google Scholar]
- Reape TJ, Burnell AM. Dauer larva recovery in the nematode Caenorhabditis elegans - II. The effect of inhibitors of protein synthesis on recovery, growth and pharyngeal pumping. Comp. Biochem. Physiol. 1991b;98B:245–252. [Google Scholar]
- Rogers WP, Petronijevic T. The infective stage and the development of nematodes. In: Symons LEA, Donald AD, Dineen JK, editors. Biology and Control of Endoparasites. Australia: Academic Press; 1982. pp. 3–28. [Google Scholar]
- Rogers WP, Sommerville RI. The infective stage of nematode parasites and its significance in parasitism. Adv. Parasit. 1963;1:109–177. doi: 10.1016/s0065-308x(08)60503-5. [DOI] [PubMed] [Google Scholar]
- Schad GA. Arrested development of Ancylostoma caninum in dogs: influence of photoperiod and temperature on induction of a potential to arrest. In: Meerovitch E, editor. Aspects of Parasitology: a festschrift dedicated to the fiftieth anniversary of the Institute of Parasitology of McGill University. Montreal: McGill University; 1982. pp. 361–391. [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kim SK. Global analysis of dauer gene expression in Caenorhabditis elegans. Develop. 2003;130:1621–1634. doi: 10.1242/dev.00363. [DOI] [PubMed] [Google Scholar]
- Zhan B, Hawdon J, Shan Q, Ren H, Qiang H, Hu W, Xiao S-H, Li T, Xing G, Feng Z, Hotez P. Ancylostoma secreted protein 1 (ASP-1) homologues in human hookworms. Mol. Biochem. Parasitol. 1999;98:143–149. doi: 10.1016/s0166-6851(98)00157-1. [DOI] [PubMed] [Google Scholar]
- Zhan B, Hotez PJ, Wang Y, Hawdon JM. A developmentally regulated metalloprotease secreted by host-stimulated Ancylostoma caninum third-stage infective larvae is a member of the astacin family of proteases. Mol. Biochem. Parasitol. 2002;120:291–296. doi: 10.1016/s0166-6851(01)00453-4. [DOI] [PubMed] [Google Scholar]