Abstract
Objective
The goal of this study was to confirm a microarray study that suggested that Kindlin-2 might play a role in the development and progression of bladder cancer. There has been no previous examination of Kindlin-2 expression in human bladder cancer.
Methods
A combination of real time PCR, western analysis and immunohistochemistry was used to characterize Kindlin-2 expression in arsenite (As+3) and cadmium (Cd+2) transformed human cell lines, their tumor transplants in immune-compromised mice, and in archival specimens of human bladder and bladder cancer.
Results
The results show that the Kindlin-2 expression patterns in the cell lines were not duplicated in the tumor tissues. However, it was shown that Kindlin-2 was expressed in the stromal element of all the transplanted tumors and archival specimens of human bladder cancer. It was also shown that a small number of high grade invasive urothelial cancers have focal expression of Kindlin-2 in the tumor cells.
Conclusion
Kindlin-2 is expressed in the stromal component of most, if not all, human bladder cancers. Kindlin-2 is not expressed in normal urothelium. Kindlin-2 is expressed in a small subset of high grade invasive bladder cancers and may have potential as a prognostic marker for tumor progression.
Keywords: Urothelial Cancer, Kindlin-2, Stroma, Bladder, Urothelium, Biomarker
INTRODUCTION
Bladder cancer has a strong association with environmental exposures1. This association is particularly strong for arsenic and correlates to the same endemic areas where populations were identified with arsenic-induced skin cancer2–7. Several studies have also implicated Cd+2 in the development of bladder cancer8,9,10; however, the high level of Cd+2 accumulation in individuals who smoke cigarettes, along with the strong association of bladder cancer and smoking, is the major factor indirectly implicating Cd+2 in the development of urothelial cancer11,12. This laboratory employs the UROtsa cell line to model urothelial cell transformation as a consequence of exposure to As+3 and Cd+2. UROtsa is an immortalized, but not tumorigenic, cell line that displays features of transitional urothelium when propagated on a serum-free growth medium13. This cell line has been used to show that both Cd+2 and As+3 can cause the malignant transformation of human urothelial cells14, 15, 16. In total, 6 As+3 and 7 Cd+2 transformed cell lines have been isolated from the parental cells and all were shown to form tumor transplants that displayed histologic features of human urothelial carcinoma. An interesting finding in these studies was that, while all 13 As+3 and Cd+2 transformed cell lines could form subcutaneous tumors, only 2 of the As+3 and 1 of the Cd+2 transformed cell lines were able to effectively colonize the organs within the peritoneal cavity when injected directly into this anatomical site. This finding is potentially important since it is known from patients with bladder cancer, that tumors tend to spread locally, requiring the ability of tumor cells to colonize local tissues and organ sites following escape from the bladder. The present study is the initial attempt to begin to define differences in gene expression between the As+3 and Cd+2 transformed cell lines that may distinguish those that are capable and those that are not capable of establishing peritoneal growth. The strategy employed was to perform a microarray analysis of mRNA expression in each cell line, identify the major changes in expression between the two classes of cell lines regarding peritoneal growth, verify the microarray data for the gene indicated to be the most consistently repressed between the isolate classes, and to determine the expression of this gene in archival specimens of human bladder cancer.
MATERIALS AND METHODS
Cell Culture
The procedures for the culture of the parental UROtsa cell line and the As+3 and Cd+2 induced malignant transformants have been described previously14, 15, 16.
Purification of RNA and Array Analysis
RNA was purified from triplicate cultures of each UROtsa cell line by the RNeasy Mini kit (Qiagen, Valencia, CA). RNA was checked for the integrity of the ribosomal bands using agarose gel electrophoresis. For each of the transformed cell lines, aliquots of the three parallel samples of RNA were mixed in equal amounts (1:1:1) before submission for array analysis and they represented one sample for array hybridization. Global gene expression analysis was performed by Genome Explorations (Memphis, TN) using the GeneChip Human Genome U133 Plus 2.0 arrays (Affymetrix, Santa Clara, CA). For analysis, each probe set was filtered for MAS5 Detection on each array using p-values equal to or less than 0.05. Induced and repressed genes were identified that were differentially expressed between the cell lines based on their ability to form peritoneal tumors, by using a t-test with p-values equal to or less than 0.05 and an absolute fold change of at least 2.0. The Microarray Study has been deposited in the NIH GEO database.
Expression of Kindlin-2 mRNA and Protein in UROtsa Cell Lines and Tumor Heterotransplants
The preparation of total RNA and protein from the parental UROtsa cells and the As+3 and Cd+2 transformed cells and their subcutaneous heterotransplants have been described previously14, 15, 16. Pre-existing samples from these studies were used to determine the expression of Kindlin-2 mRNA and protein in this study. The expression of Kindlin-2 mRNA was determined using real time RT-PCR with Kindlin-2 specific primers obtained from Qiagen (Valencia, CA). The level of Kindlin-2 mRNA was determined relative to the UROtsa cells grown in serum-containing medium using serial dilutions of this sample as the standard curve. The resulting relative levels were then normalized to the fold change in β-actin expression. The expression of Kindlin-2 protein was determined by Western blotting using 20 μg of total cellular protein on a 12.5% SDS-polyacrylamide gel and transferred to a hybond-P polyvinylidine difluoride membrane (Amersham Biosciences, Piscataway, NJ). Membranes were blocked in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBS-T) and 5% (w/v) nonfat dry milk for 1 h at room temperature. After blocking, the membranes were probed with a 1:1500 dilution of the Kindlin-2 primary antibody (Proteintech, Chicago, IL) in blocking buffer for 1 h. After washing three times in TBS-T, membranes were incubated with the anti-rabbit secondary antibody (1:2000) in antibody dilution buffer for 1 h. The blots were visualized using the Phototope-HRP Western blot detection system (Cell Signaling Technology, Beverly, MA).
Immunohistochemical Localization of Kindlin-2 in Tumor Heterotransplants and Archival Specimens of Human Bladder Cancer
The production of nude mice heterotransplants from the As+3 and Cd+2 -transformed UROtsa cell lines has been previously described14, 15, 16. Tumor tissues taken from these studies were utilized in the present study to determine the localization and expression of Kindlin-2 in tumor heterotransplants. Tissue sections for the immunohistochemical analysis of Kindlin-2 expression in human bladder were obtained from archival paraffin blocks that originated from previously completed patient diagnostic procedures. These archival specimens contained no patient identifiers and their use was approved by the University of North Dakota Internal Review Board. The immunostaining procedures used by the laboratory have been described previously15, 16.
Statistics
Statistical analysis consisted of ANOVA with Tukey post-hoc testing performed by Graphpad PRISM 5. All statistical significance is denoted at p < 0.05.
RESULTS
Selection of Kindlin-2 for Analysis of Gene Expression in Urothelial Cancer
Each of the 13 independent isolates of As+3 and Cd+2 -transformed UROtsa cell lines were characterized for their pattern of gene expression against the UROtsa parental cell line using the Affymetrix Human Genome U133 Plus 2.0 array chip. The data was then analyzed for differences between the 3 transformed cell lines able to effectively generate peritoneal tumors (As#1, As#3 and Cd#1) with those that formed no peritoneal tumors (As#2, As#5 and As#6; Cd#2 – Cd#7). Those cell lines designated as effective for generating peritoneal tumors produced over 100 separate tumor nodules per mouse. One cell line (As#4) generated a low number of peritoneal tumors, between 3 and 20 among the six mice tested, and was not used in the analysis. A set of five genes were identified that were the most consistently repressed in the cell lines able to form peritoneal tumors when compared to those unable to form peritoneal tumors. Similarly, a set of five genes were identified that were the most induced in the cell lines able to form peritoneal tumors when compared to those unable to form peritoneal tumors. These 10 genes are identified in Table 1. The PLEKHC1 gene, also known as Kindlin-2, was chosen for further characterization since it was the gene most consistently differentially repressed between the two sets of cell lines.
TABLE 1.
Differentially Expressed Genes in Peritoneal-Forming, Metal-Transformed UROtsa Cells.
| Gene Symbol | Gene | Log 2 Fold Change* | Fold Change* |
|---|---|---|---|
| CALB1 | calbindin 1, 28kDa | 4.80 | 27.87 |
| SPRR3 | small proline-rich protein 3 | 3.32 | 10.05 |
| ADH7 | alcohol dehydrogenase 7 | 3.14 | 8.83 |
| ODZ2 | odz, odd Oz/ten-m homolog 2 | 2.98 | 7.94 |
| ZNF703 | zinc finger protein 703 | 2.65 | 6.32 |
| KRT7 | keratin 7 | −3.36 | 0.10 |
| PTN | pleiotrophin | −3.17 | 0.11 |
| CTHRC1 | collagen triple helix repeat containing 1 | −3.07 | 0.12 |
| LRRN1 | leucine rich repeat neuronal 1 | −2.84 | 0.14 |
| PLEKHC1 | pleckstrin homology domain containing, family C member 1 | −2.60 | 0.16 |
Average fold change in cell lines able to form peritoneal tumors compared with non-tumor forming lines.
Kindlin-2 mRNA and Protein Expression in Parental UROtsa Cells, the As+3 and Cd+2 Transformed Cell Lines and Their Subcutaneous Tumors
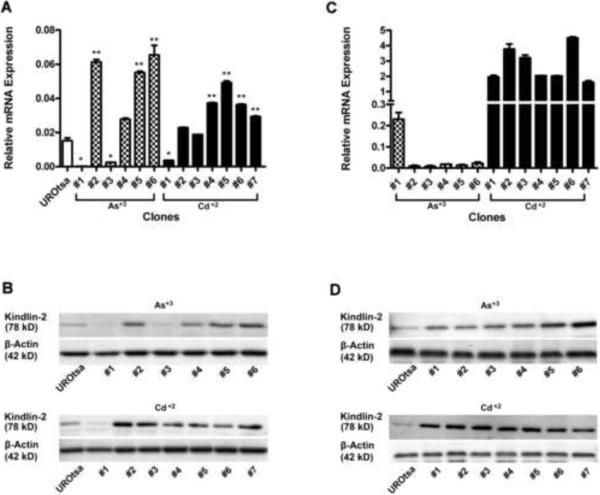
The expression of Kindlin-2 mRNA was determined on the parental UROtsa cells and the As+3 and Cd+2-transformed cell lines (Fig 1A). The results of this analysis demonstrated that Kindlin-2 mRNA was expressed in the parental UROtsa cell line. The 2 As+3-transformed cell lines (As#1, As#3) and the 1 Cd+2 (Cd#1) transformed cell line, that were able to effectively form peritoneal tumors, had reduced expression of Kindlin-2 mRNA when compared to the parental cell line and all the other As+3 and Cd+2 transformed cell lines (Fig 1A). The 3 As+3 -transformed and the 6 Cd+2 -transformed cell lines unable to form peritoneal tumors displayed Kindlin-2 mRNA levels similar to, or elevated above, those of the parental cell line. A corresponding analysis of Kindlin-2 protein expression by western blotting generally followed the above pattern of Kindlin-2 mRNA expression (Fig 1B). Western blotting showed that the parental cell line expressed Kindlin-2 protein. The relative amount of Kindlin-2 protein expression in the parental cells was below that of the 9 As+3 and Cd+2 -transformed cell lines unable to form peritoneal tumors. In contrast, the relative amount of Kindlin-2 protein expression in the parental cells was above that of the 2 As+3 -transformed cell lines (As#1, As#3) and the 1 Cd+2 (Cd#1) transformed cell line that were able to effectively form peritoneal tumors. Overall, the determination of Kindlin-2 mRNA and protein in each cell line confirmed the observation regarding Kindlin-2 expression in the initial microarray-based analysis. The expression of Kindlin-2 mRNA and protein was also determined for the subcutaneous tumors generated from the 6 As+3 and 7 Cd+2 -transformed cell lines (Figs. 1C & 1D). Kindlin-2 mRNA was expressed in all of the Cd+2 -transformed cell lines (Fig 1C). The expression of Kindlin-2 mRNA was similar in tumors generated from the Cd#1, Cd#4, Cd#5 and Cd#7 cell lines and, in comparison to these tumors, elevated in the tumors derived from the Cd#2, Cd#3 and Cd#6 cell lines. The corresponding analysis of Kindlin-2 protein in the Cd+2 -transformed cell lines by western blotting demonstrated that all the cell lines expressed Kindlin-2 protein, but that there was no correlation between the levels of Kindlin-2 mRNA expression and that of the corresponding protein (Fig 1D). Acute studies of Kindlin-2 expression were not performed on the parental UROtsa cell line.
Figure 1.
Expression of Kindlin-2 mRNA and protein in UROtsa parent, As (+3) & Cd (+2)-transformed isolates and subcutaneous tumor heterotransplants. Real-time PCR analysis of Kindlin-2 in UROtsa parent and transformed isolates (A) and the subcutaneous heterotransplants (C). Western analysis of Kindlin-2 in UROtsa and transformed isolates (B) and the subcutaneous heterotransplants (D). Statistical analysis consisted of ANOVA with Tukey post-hoc testing performed using GraphPad PRISM 5. All statistical significance is denoted at p<0.05. *Reduced compared to parental cells and other cell lines. ** Elevated compared to parental cells.
The expression of Kindlin-2 mRNA was at the level of detection in the tumor derived from the As#1 cell line and low, but clearly detectable in the tumors derived from the other 5 As+3 transformed cell lines (Fig 1C). Western analysis showed that all the tumors derived from the 6 As+3 transformed cell lines expressed Kindlin-2 protein (Fig 1F). Kindlin-2 protein expression was similar in tumors from 5 of the As+3 transformed cell lines with an elevated expression in tumor derived from the As#6 cell line. There was no correlation between the levels of Kindlin-2 mRNA expression and that of the corresponding protein. In marked contrast to the cell line results which confirmed the microarray analysis, there was no correlation between the expression of Kindlin-2 in the subcutaneous tumors and the ability of the Cd+2 and As+3 -transformed cell lines to generate peritoneal tumors.
Immunohistochemical Staining of Kindlin-2 in Tumor Heterotransplants
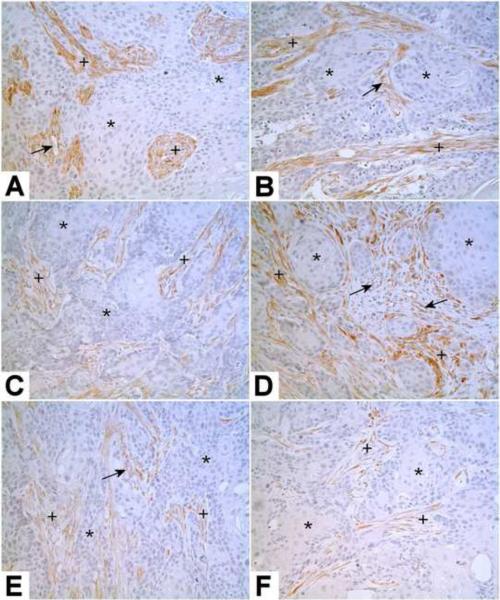
The immunohistochemical analysis of Kindlin-2 expression in the subcutaneous tumors showed no staining of Kindlin-2 in the urothelial tumor cells produced by any of the As+3 or Cd+2 transformed cell lines. In contrast, the stromal components of all the urothelial tumors were moderately to strongly positive for the expression of Kindlin-2 protein. The stromal components of the tumors are presumably of murine origin and recruited to the tumor site. There were also occasional blood vessels within the tumors which were weakly to moderately immunoreactive for Kindlin-2. An example of this immunostaining pattern of Kindlin-2 is illustrated for subcutaneous tumors generated from 2 As+3 and 2 Cd+2 -transformed cell lines (Fig 2 A–D). An identical result for Kindlin-2 staining was obtained for the intraperitoneal tumors produced from the Cd#1 and As#1 and As#3 cell lines with the urothelial cancer cells negative and the stromal cells positive for Kindlin-2 expression. An example is shown for the As#1 and Cd#1 cell lines (Fig 2 E&F).
Figure 2.
Immunostaining of Kindlin-2 in Tumor Heterotransplants. Kindlin-2 is shown in representative mouse subcutaneous heterotransplants from As#2 (A), As#4 (B), Cd#2 (C), Cd#3 (D) cell lines, and intraperitoneal heterotransplants from As#1 (E), Cd#1 (F) cell lines. The tumorous epithelium is negative for Kindlin-2 (*), but the stroma is moderately to strongly positive for Kindlin-2 (+). Some small blood vessels in the tumor shown weak to moderate staining of Kindlin-2 (arrows). Original magnification: 200×.
Immunohistochemical Staining of Kindlin-2 in Benign Human Bladder and Urothelial Cancer
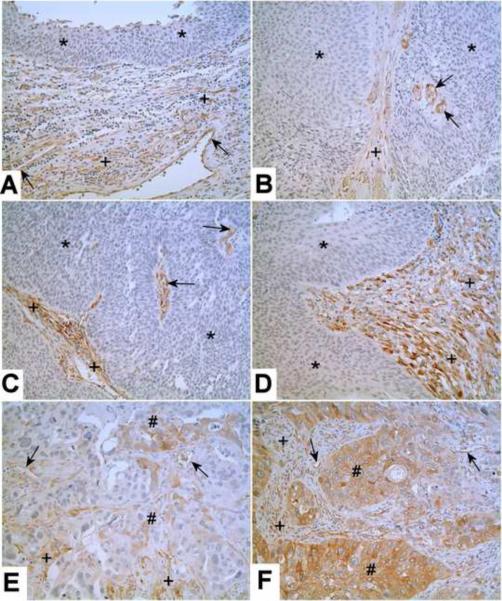
The immunohistochemical staining of Kindlin-2 was determined on 6 archival specimens of benign human bladder, 5 cases of low grade carcinoma, 6 cases of non-invasive high grade carcinoma and 16 cases of invasive high grade carcinoma. There was no staining of Kindlin-2 in the normal urothelial cells present in all six cases of benign human bladder (Fig 3A). In all six cases of benign human bladder there was Kindlin-2 staining of the stromal, endothelial and smooth muscle cells (Fig 3A). There was no staining of Kindlin-2 in the tumorous epithelium in all 5 cases of low grade carcinoma (Fig 3B). In all 5 cases, there were some stromal cells and small blood vessels in the papillary core that were moderately positive for Kindlin-2. The 6 cases of non-invasive high grade urothelial cancer showed no staining of Kindlin-2 in the tumorous epithelium, but did show moderate to strong staining for Kindlin-2 in the stroma and small blood vessels in the papillary core (Fig 3 C&D). In contrast to the noninvasive low and high grade cancers, 2 of the 16 cases of invasive, high grade urothelial cancer were found to have areas of Kindlin-2 expression in the tumorous epithelium. Kindlin-2 expression in the malignant cells was focal in expression and comprised approximately 30% of the tumor cells (Fig 3 E&F). In all the cases of high grade invasive urothelial cancer, the stromal cells and small blood vessels were stained for Kindlin-2.
Figure 3.
Immunostaining of Kindlin-2 in Benign Human Bladder and Urothelial Carcinoma. Kindlin-2 is shown in representative benign human bladder (A), low-grade urothelial carcinoma (B), high-grade noninvasive carcinoma (C, D), and high-grade invasive carcinoma (E, F). There is no staining of Kindlin-2 in benign urothelium, and the tumorous epithelium from low grade carcinoma and high grade noninvasive carcinoma (*, A–D), but in 2 cases of high grade invasive carcinoma, the tumorous epithelium shows focal positivity of Kindlin-2 (#, E and F). The stromal cells from benign bladder and urothelial carcinoma are positive for Kindlin-2 (+). Some small blood vessels from both benign bladder and urothelial carcinoma are also positive for Kindlin-2 (arrows). Original magnification: 200×.
DISCUSSION
The cell biology of the Kindlins has been the subject of a recent review17. The Kindlins represent a class of focal adhesion proteins implicated in integrin activation. The Kindlins have a bipartite FERM domain interrupted by a pleckstrin homology domain. They can bind directly to various classes of integrins as well as participate in inside-out integrin activation. Kindlin-2 is one of three family members that possess considerable sequence homology and its gene is located at chromosome 14q22.1. In addition to its name Kindlin-2, it is also reported in the literature as MIG2, KIND2, mig-2, UNC112, PLEKHC1, UNC112B, FLJ34213, FLJ44462, DKFZp686G11125 and FERMT2. Kindlin-2 deficient mice have been generated and the knockout animals died at or before embryonic age 7.5 days due to detachment of the epiblast and endoderm resulting in peri-implantation lethality. Heterozygote mice are viable and did not display any abnormalities. Using northern analysis, Kindlin-2 mRNA has been shown to be present in the heart, lung, skeletal muscle, kidney, bladder, and stomach18. Kindlin-2 has been shown to be present in fibroblasts, muscle, epithelial and endothelial cells. A review of the literature indicates that the Kindlin-2 protein has not been localized within the normal human bladder or characterized for its expression in urothelial cancer. The present study appears to be the first to report Kindlin-2 protein expression and localization in the normal human bladder and urothelial cancer.
The present study demonstrated that there was no expression of Kindlin-2 in the urothelium of the normal bladder, but there was modest expression in the non-epithelial elements of the bladder. This included fibroblasts, smooth muscle cells and some blood vessels. This finding indicates that the prior report18 of Kindlin-2 expression in the normal bladder was likely due to the presence of Kindlin-2 mRNA in the stroma and not epithelial elements of the bladder. An examination of archival specimens of low and high grade urothelial cancer demonstrated that all the stromal components were positive for the expression of the Kindlin-2 protein. This was especially pronounced for the high grade lesions. Blood vessels within the papillary core were also positive for Kindlin-2 expression. A potentially significant finding was that high grade invasive urothelial carcinomas had focal expression of Kindlin-2 in the tumor epithelium, while all other archival specimens examined had no staining of the malignant urothelium. This is potentially important since it indicates that Kindlin-2 expression could be a prognostic marker for a sub-set of urothelial cancers. The finding of increased expression in a small sub-set of high grade tumor cells of lesions undergoing invasion suggests that expression might be indicative of cancers destined to undergo progression. The low number of high grade invasive lesions that expressed Kindlin-2 indicates prognostic significance and will need to be determined by a research group that possesses a large human tissue bank.
The present study shows the value of translating microarray studies to human tissue resources even when the confirmation of the microarray results from the cell lines are not optimal. In the present study, the microarray screen showed that the 3 cell lines repressed in the expression of Kindlin-2 mRNA were those cell lines able to establish peritoneal tumors when injected into the peritoneal cavity. This mRNA expression profile was confirmed on extracts of the respective cell lines, showing that the level of Kindlin-2 protein expression also correlated with the ability of the 3 cell lines to form peritoneal tumors. However, this pattern of Kindlin-2 expression was not found in the subcutaneous and peritoneal tumor transplants generated from the cell lines. In contrast to the cell lines, it was shown that there was no expression of Kindlin-2 protein in the urothelial cells of any of the subcutaneous or peritoneal tumors. The finding might also suggest that Kindlin-2 expression is non-permissive for the establishment of tumor transplants from the urothelial cells. A positive finding from the study in the tumor transplants was the observation that Kindlin-2 was expressed in the stromal elements recruited to the tumor. The finding that Kindlin-2 was expressed in the stromal element of the tumor transplants motivated the examination of the human archival tissue and the initial documentation described above of Kindlin-2 expression in the normal human bladder and urothelial cancer.
Acknowledgements
This work was supported by grant number R01 ES015100 from the National Institute of Environmental Health Sciences, NIH. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bischoff CJ, Clark PE. Bladder Cancer. Curr Opin Oncol. 2009;21:272–277. doi: 10.1097/cco.0b013e328329f184. [DOI] [PubMed] [Google Scholar]
- 2.Cantor KP, Lubin JH. Arsenic, internal cancers, and issues in inference from studies at low-level exposures in human populations. Toxicol Appl Pharmacol. 2007;222:252–257. doi: 10.1016/j.taap.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiou HY, Hsueh Y, Liaw KF, et al. Incidence of internal cancers and ingested inorganic arsenic: a 7-year follow-up study in Taiwan. Cancer Res. 1995;55:1296–1300. [PubMed] [Google Scholar]
- 4.Luster MI, Simeonova PP. Arsenic and urinary bladder cell proliferation. Toxicol Appl Pharmacol. 2004;198:419–423. doi: 10.1016/j.taap.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Smith AH, Goycolea M, Haque R, et al. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147:660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- 6.Steinmaus C, Moore L, Hopenhayn-Rich C, et al. Arsenic in drinking water and bladder cancer. Cancer Invest. 2000;18:174–182. doi: 10.3109/07357900009038249. [DOI] [PubMed] [Google Scholar]
- 7.Tsuda T, Babazono A, Yamamoto E, et al. Ingested arsenic and internal cancer: a historical cohort study followed for 33 years. Am J Epidemiol. 1995;141:198–209. doi: 10.1093/oxfordjournals.aje.a117421. [DOI] [PubMed] [Google Scholar]
- 8.Kellen E, Zeegers M, Hond ED, et al. Blood cadmium may be associated with bladder carcinogenesis; the Belgian case control study on bladder cancer. Cancer Detect Prev. 2007;31:77–82. doi: 10.1016/j.cdp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Siemiatycki J, Dewar R, Nadon L, et al. Occupational risk factors for bladder cancer: Results from a case control study in Montreal, Quebec, Canada. Am J Epidemiol. 1994;140:1061–1080. doi: 10.1093/oxfordjournals.aje.a117207. [DOI] [PubMed] [Google Scholar]
- 10.Waalkes MP. Cadmium carcinogenesis in review. J Inorg Biochem. 2000;79:241–244. doi: 10.1016/s0162-0134(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 11.Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect. 2004;112:1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satarug S, Garrett SH, Sens MA, et al. Cadmium, environmental exposure and heath outcomes. Environ Health Perspect. 2010;118:182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi MR, Masters JRW, Park S, et al. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ Health Perspect. 2001;109:801–808. doi: 10.1289/ehp.01109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sens DA, Park S, Gurel V, et al. Inorganic cadmium- and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol Sci. 2004;79:56–63. doi: 10.1093/toxsci/kfh086. [DOI] [PubMed] [Google Scholar]
- 15.Cao L, Zhou XD, Sens MA, et al. Keratin 6 expression correlates to areas of squamous differentiation in multiple independent isolates of As+3- induced bladder cancer. J Appl Toxicol. 2010;30:416–430. doi: 10.1002/jat.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somji S, Zhou XD, Mehus A, et al. Variation of keratin 7 expression and other phenotypic characteristics of independent isolates of cadmium transformed human urothelial cells (UROtsa) Chem Res Toxicol. 2010;23:348–356. doi: 10.1021/tx900346q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai-Cheong JE, Parsons M, McGrath JA. The role of Kindlins in cell biology and relevance to human disease. Intern J Biochem Cell Biol. 2010;42:595–603. doi: 10.1016/j.biocel.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Ussar S, Wang HV, Linder S, et al. The Kindlins: Subcellular localization and expression during murine development. Exp Cell Res. 2006;312:3142–3151. doi: 10.1016/j.yexcr.2006.06.030. [DOI] [PubMed] [Google Scholar]