Abstract
In the present study, the authors compared the long-term risk of nasopharyngeal carcinoma (NPC) of male participants in an NPC multiplex family cohort with that of controls in a community cohort in Taiwan after adjustment for anti-Epstein-Barr virus (EBV) seromarkers and cigarette smoking. A total of 43 incident NPC cases were identified from the 1,019 males in the NPC multiplex family cohort and the 9,622 males in the community cohort, for a total of 8,061 person-years and 185,587 person-years, respectively. The adjusted hazard ratio was 6.8 (95% confidence interval (CI): 2.3, 20.1) for the multiplex family cohort compared with the community cohort. In the evaluation of anti-EBV viral capsid antigen immunoglobulin A and anti-EBV deoxyribonuclease, the adjusted hazard ratios were 2.8 (95% CI: 1.3, 6.0) and 15.1 (95% CI: 4.2, 54.1) for those positive for 1 EBV seromarker and positive for both seromarkers, respectively, compared with those negative for both EBV seromarkers. The adjusted hazard ratio was 31.0 (95% CI: 9.7, 98.7) for participants who reported a family history of NPC and who were anti-EBV-seropositive compared with individuals without such a history who were anti-EBV-seronegative. The findings suggest that both family history of NPC and anti-EBV seropositivity are important determinants of subsequent NPC risk and that the effect of family history on NPC risk cannot be fully explained by mediation through EBV serologic responses.
Keywords: carcinoma; cohort studies; herpesvirus 4, human
Nasopharyngeal carcinoma (NPC) has a striking geographic and ethnic distribution. In most parts of the world, the incidence of NPC is less than 1 per 100,000 person-years; however, it occurs at relatively high rates in southern China and southeastern Asia (1). Epstein-Barr virus (EBV) is a well-documented etiologic agent for the development of NPC. EBV is known to infect the vast majority of adults worldwide (≈95%), usually with lifelong persistence. However, only a small fraction of EBV-infected individuals develop NPC in their lifetime (2). Various cofactors have been postulated to be important determinants of NPC, including age, salted fish consumption early in life, occupational exposure to wood dust, long-term cigarette smoking, and a host of genetic factors (3).
Studies of immigrant populations have shown that NPC incidence among the Chinese is higher than among African Americans and Caucasians in the United States. NPC risk is lower in second- and third-generation Chinese immigrants to the United States than in first-generation immigrants, but the rates are still higher than those of other ethnic groups (4, 5). These immigrant studies suggest that both genetic factors and cultural lifestyle play a role in the etiology of NPC.
Familial aggregation of NPC has been widely documented in both Chinese and low-risk populations (6–13). Odds ratios for NPC ranging from 4 to 20 have been reported in case-control studies of individuals who reported a first-degree family history of NPC compared with those with no such history (14–22). In a complex segregation analysis in China, Jia et al. (23) also reported that multiple genetic and environmental factors likely contributed to the development of NPC. In a population-based cohort study in Greenland and Denmark, the relative risk of NPC was 8.0 (95% confidence interval (CI): 1.1, 3.9) among 766 first-degree relatives (24). Familial tendency for NPC may result from genetic and/or environmental risk factors shared by family members.
In our previous studies, both EBV seropositivity and long-term cigarette smoking were found to be associated with an increased NPC risk (25–27). EBV seropositivity was found to be more prevalent in family members of NPC cases (28). Seromarker measurements may reflect EBV infection itself, but they may also reflect an individual's genetic ability to generate high levels of antibodies against EBV or his/her susceptibility to EBV reactivation/replication. Smoking behavior of an individual may be influenced by parents and siblings (29). Although the results of many studies have shown a tendency for familial aggregation of NPC, the authors of these studies did not examine EBV status and cigarette smoking simultaneously. Our aim was to evaluate the effect of family history on NPC risk and determine how it might be mediated through other genetically driven or shared environmental risk factors, such as EBV serology or cigarette smoking. To explore this issue, we compared the relative risk of NPC among those with ≥2 affected family members with that of individuals lacking such a family history, after accounting for serologic EBV patterns and cigarette smoking.
MATERIALS AND METHODS
Cohort recruitment
Community cohort.
Details of the study have been published previously (26). Briefly, study subjects were recruited between 1984 and 1986 from 6 townships in Taiwan. A total of 9,699 males who were ≥30 years of age participated in our study. Seven were diagnosed with NPC before enrollment in the study, and an additional 67 reported a family history of NPC. These 74 individuals were excluded from this analysis. Each study participant provided written informed consent for an interview, collection of a blood specimen, and an otorhinolaryngologic examination and medical consultation with physicians at the National Taiwan University Hospital. The institutional review board at National Taiwan University approved the study protocol, and informed consent was obtained.
All participants were personally interviewed at local research centers by well-trained research assistants who used a structured questionnaire. The information obtained from the questionnaire interview included sociodemographic characteristics and information on cigarette smoking, alcohol drinking, diet, personal history of sinusitis or other nasal disease, and family history of NPC. Blood samples were collected at the time of enrollment, immediately stored at −30°C, and later tested for antibody titers to 2 EBV antigens associated with NPC: anti-EBV viral capsid antigen (VCA) immunoglobulin A (IgA) and anti-EBV deoxyribonuclease (DNase). We tested for anti-EBV VCA IgA through the use of indirect immunofluorescence assay (30), and those participants with titers ≥1:10 were categorized as positive. Anti-EBV DNase antibody levels were evaluated using an enzyme neutralization assay, and those with a result of ≥2 units of DNase activity were deemed positive. One unit of DNase activity is the amount of enzyme required to convert 1 μg of double-strand DNA into acid-soluble material in 10 minutes at 37°C (31).
Multiplex family cohort.
As described previously, 475 multiplex families were identified from 20,450 NPC cases diagnosed between 1980 and 2003 (28, 32). A multiplex family was defined as one that had ≥2 NPC cases among first-, second-, or third-degree adult relatives. Of these, a total of 358 multiplex families, including 2,240 unaffected first-degree relatives (1,104 males and 1,136 females), were enrolled in the study between 1996 and 2004. There were 154 first-degree relatives (84 males and 70 females) for whom incomplete national identification numbers prohibited linkage to national registries and who were therefore excluded from this analysis. In total, 75.9% of the diagnoses of NPC in the 358 multiplex families were confirmed by cross-referencing with the National Cancer Registry, the Death Certificate Registry, and the Chronic Major Disease Registry and/or abstraction of medical charts. For NPC cases diagnosed after the implementation of the National Cancer Registry system in 1979, the confirmation rate was as high as 87.7%. Because we only recruited male subjects in the community cohort, we excluded females from the multiplex cohort, giving us a total of 1,020 male unaffected first-degree relatives. Informed consent was obtained from study subjects. Institutional review boards at both National Taiwan University and the National Institutes of Health approved the study protocol and the informed consent procedure.
All subjects were given a self-administered questionnaire that assessed various risk factors, including socioeconomic status, dietary intake, smoking exposure, occupational history, and alcohol consumption. Biologic samples, including saliva, buccal (mouth) cells, and 35 mL of blood, were obtained for genotyping and EBV antibody testing. Samples were processed within 24 hours and then frozen at −80°C. Serum samples obtained from the blood draw at the time of enrollment in our family study were tested for levels of antibodies to VCA IgA and anti-DNase. EBV testing was performed by the same testing laboratory using the same methods, with cutoff values identical to those used in the above-mentioned community cohort study. For each batch of tests, 1 anti-EBV seronegative serum sample and 1 anti-EBV seropositive serum sample were used for quality control.
Ascertainment of incident developed nasopharyngeal carcinoma
Newly diagnosed cases of NPC were ascertained using the national identification number by computerized linkage with the National Cancer Registry. Linkage was performed for the period between the recruitment date and December 31, 2008. We identified a total of 36 and 11 newly diagnosed NPC cases from the community cohort and multiplex family cohort, respectively. Those subjects with NPC diagnosed within a year of study enrollment were excluded (community cohort: n = 3; multiplex family cohort: n = 1) because those cases were much more likely to be preexisting NPC rather than incident cases.
Statistical analysis
We used the Nelson-Aalen method to estimate the cumulative incidence of NPC among subjects with a family history of NPC (multiplex family cohort members) and those without such a family history (community cohort members) (33). The Mantel-Haenzel log-rank test was used to compare cumulative incidence curves. The number of person-years of follow-up for each subject was calculated as the time between the date of enrollment and the date of diagnosis of NPC, the date of death, or the date of last link to data from the National Cancer Registry (December 31, 2008), whichever came first. Incidence rates were calculated by dividing the number of incident cases of NPC by the number of person-years of follow-up. Cox's proportional hazards regression analyses were used to assess the univariate hazard ratio of developing NPC with a 95% confidence interval. Adjusted hazard ratio estimates were also calculated after adjustment for various risk factors, including age, ethnicity, years of schooling, anti-EBV seromarkers, cigarette smoking, and family history. The statistical significance for an interaction was tested through the comparison of the likelihood of models with and without the inclusion of an interaction term. The results of anti-EBV VCA IgA and anti-EBV DNase were missing for 53 and 54 individuals, respectively, including 1 NPC case from the community cohort. The results of tests for anti-EBV VCA IgA levels determined by indirect immunofluorescent assay were missing for 263 multiplex family cohort members (including 3 NPC cases) (25.8%) because this particular test was discontinued during the study and replaced by an enzyme-linked immunosorbent assay-based anti-EBV VCA IgA test. The results of anti-EBV DNase tests were missing for 16 individuals (1.6%) from the multiplex family cohort. Cumulative exposure to cigarette smoke was defined in pack-years as the number of packs of cigarettes smoked per day multiplied by the number of years of cigarette smoking. Data on cumulative cigarette smoking were not available for 1 subject from the family cohort and 349 subjects from the community cohort, including 1 NPC case from the community cohort. All statistical tests were 2-tailed.
RESULTS
A total of 1,019 multiplex family cohort members and 9,622 community cohort members were included in this analysis. Table 1 shows the distribution of baseline characteristics among participants in the 2 cohort studies according to age, ethnicity, years of schooling, cigarette smoking status, and anti-EBV seromarkers. Individuals from the multiplex family cohort were younger and more likely to be Fukkienese than were those from the community cohort. Although most of the multiplex family cohort members had ≥7 years of schooling, nearly 70% of the community cohort members had only 1–6 years of schooling. The prevalence of cigarette smoking was 59.5% in multiplex cohort family members and 64.3% in community cohort members. Seropositivity to anti-EBV VCA IgA was observed among 24.3% of multiplex family cohort members, compared with 1.1% of the community cohort members. The numbers of individuals who were seropositive for anti-EBV DNase was similar between the 2 cohorts.
Table 1.
Characteristics of Participants in the Multiplex Family Cohort and Community Cohort Studies, Taiwan, 1984–2008
| Multiplex Family Cohort |
Community Cohort |
P Value | |||
| No. | % | No. | % | ||
| Age at enrollment, years | <0.001 | ||||
| <45 | 612 | 60.1 | 2,962 | 30.8 | |
| 45–54 | 185 | 18.1 | 2,639 | 27.4 | |
| ≥55 | 222 | 21.8 | 4,021 | 41.8 | |
| Ethnicitya | <0.001 | ||||
| Fukkinese | 697 | 68.4 | 3,226 | 33.5 | |
| Hakka | 249 | 24.4 | 6,395 | 66.5 | |
| Other | 73 | 7.2 | 0 | 0.0 | |
| Years of schoolingb | <0.001 | ||||
| 0–6 | 188 | 18.4 | 6,383 | 67.8 | |
| ≥7 | 831 | 81.6 | 3,036 | 32.2 | |
| Cumulative cigarette smoking, pack-yearsc | <0.001 | ||||
| 0 | 412 | 40.5 | 3,316 | 35.7 | |
| <30 | 472 | 46.4 | 3,883 | 41.9 | |
| ≥30 | 134 | 13.1 | 2,074 | 22.4 | |
| Anti-Epstein-Barr virus viral capsid antigen immunoglobulin Ad | <0.001 | ||||
| Negative | 572 | 75.7 | 9,460 | 98.9 | |
| Positive | 184 | 24.3 | 109 | 1.1 | |
| Anti-Epstein-Barr virus deoxyribonucleasee | 0.018 | ||||
| Negative | 908 | 90.5 | 8,420 | 88.0 | |
| Positive | 95 | 9.5 | 1,148 | 12.0 | |
| Total | 1,019 | 9,622 | |||
Data for ethnicity were not available for 1 participant in the community cohort.
Data for years of schooling were not available for 203 participants in the community cohort.
Data for cumulative cigarette smoking were not available for 1 participant in the multiplex family cohort and 349 participants in the community cohort.
Data for anti-Epstein-Barr virus viral capsid antigen immunoglobulin A tested by using immunofluorescence assay were not available for 263 participants in the multiplex family cohort and 53 participants in the community cohort.
Data for anti-Epstein-Barr virus deoxyribonuclease were not available for 16 participants in the multiplex family cohort and 54 participants in the community cohort.
The cumulative incidence of NPC is shown in Figure 1 by study cohort (A), anti-EBV seromarkers (B), and cumulative cigarette smoking (C). Members of the multiplex family cohort had a higher cumulative incidence of NPC than did those in the community cohort. Individuals with anti-EBV seropositivity had a higher cumulative incidence of NPC than did those with anti-EBV seronegativity. Smokers with a cumulative cigarette-smoking exposure ≥30 pack-years had a higher cumulative incidence of NPC than did those with cigarette-smoking exposures <30 pack-years and never smokers. The longer the follow-up duration, the greater were the differences in cumulative NPC incidence.
Figure 1.
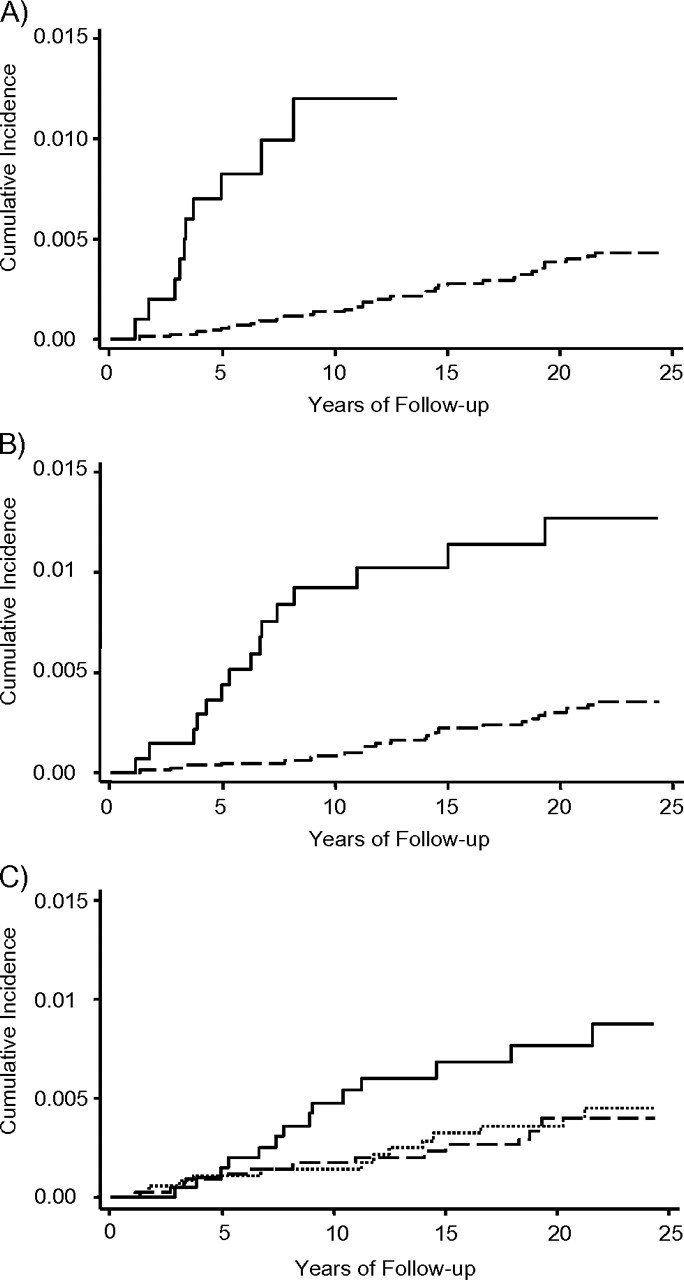
Cumulative incidence of nasopharyngeal carcinoma during follow-up among 10,641 males in Taiwan by A) study cohort (solid line: multiplex family cohort; dashed line: community cohort), B) anti-Epstein-Barr virus seromarkers (solid line: seropositive for anti-Epstein-Barr virus viral capsid antigen immunoglobulin A, anti–Epstein-Barr virus deoxyribonuclease, or both; dashed line: seronegative for both seromarkers), and C) cumulative pack-years of cigarette smoking (solid line: cumulative cigarette smoking ≥30 pack-years; dashed line: cumulative cigarette smoking <30 pack-years; dotted line: never smokers). Log-rank test statistics were 37.80 (P < 0.001), 23.49 (P < 0.001), and 5.71 (P = 0.0576) for A, B, and C, respectively.
Table 2 shows the incidence rates and adjusted hazard ratios for developing NPC by family history, cumulative pack-years of cigarette smoking, and anti-EBV seromarkers. There were 33 incident NPC cases over a total of 185,587.3 person-years of follow-up in the community cohort and 10 incident NPC cases over a total of 8,060.8 person-years of follow-up in the multiplex family cohort. The mean durations of follow-up were 19.3 and 7.9 years for the community cohort and the multiplex family cohort, respectively. The incidence rates for NPC were 17.8 and 124.1 per 100,000 person-years in the community cohort and the multiplex family cohort, respectively. The NPC incidence in individuals who were seropositive for anti-EBV VCA IgA and/or anti-EBV DNase was higher than that observed in individuals seronegative for both anti-EBV VCA IgA and anti-EBV DNase. Participants with ≥30 pack-years of cigarette smoking had a higher NPC incidence than did those who smoked for <30 pack-years. Compared with participants in the community cohort, participants in the multiplex family cohort had an adjusted hazard ratio of developing NPC of 11.7 (95% CI: 4.6, 29.9) after adjustment for age, ethnicity, and years of schooling. After further adjustment for anti-EBV seromarkers and cumulative pack-years of cigarette smoking, the NPC risk remained significantly higher for members of the multiplex family cohort than for community cohort members (adjusted hazard ratio = 6.8, 95% CI: 2.3, 20.1). Compared with those individuals who were seronegative for anti-EBV VCA IgA, those who were seropositive for anti-EBV VCA IgA had a hazard ratio for 11.6 (95% CI: 4.7, 28.6) after adjustment for age, ethnicity, and years of schooling. Compared with those who were seronegative for anti-EBV DNase, those who were seropositive had a hazard ratio of 3.4 (95% CI: 1.7, 6.6) after adjustment of age, ethnicity, and years of schooling. After further adjustment for family history and cumulative cigarette smoking, the adjusted hazard ratio for developing NPC was 2.8 (95% CI: 1.3, 6.0) for participants who were seropositive for only 1 anti-EBV seromarker and 15.1 (95% CI: 4.2, 54.1) for those who were seropositive for both anti-EBV seromarkers compared with participants who were seronegative for both anti-EBV seromarkers. Although a residual increase in risk was observed for cumulative pack-years of cigarette smoking after adjustment for family history and anti-EBV seromarkers, the effect was not statistically significant (P = 0.16).
Table 2.
Incidence of and Adjusted Hazard Ratios, for Nasopharyngeal Carcinoma, by Family History, Anti-Epstein-Barr Virus Seromarker Status, and Cigarette Smoking at Study Entry, Taiwan, 1984–2008
| Variable | No. of Participants | Person-Years | No. of Nasopharyngeal Carcinoma Cases | Cumulative Incidencea | Adjusted Hazard Ratiob | 95% Confidence Interval | Adjusted Hazard Ratioc | 95% Confidence Interval |
| Family history | ||||||||
| Community cohort | 9,622 | 1,85,587.3 | 33 | 17.8 | 1.0 | Referent | 1.0 | Referent |
| Multiplex family cohort | 1,019 | 8,060.8 | 10 | 124.1 | 11.7 | 4.6, 29.9 | 6.8 | 2.3, 20.1 |
| Anti-EBV VCA IgAd | ||||||||
| Negative | 10,032 | 1,87,921.4 | 32 | 17.0 | 1.0 | Referent | ||
| Positive | 293 | 3,395.2 | 7 | 206.2 | 11.6 | 4.7, 28.6 | ||
| Anti-EBV deoxyribonucleasee | ||||||||
| Negative | 9,328 | 1,70,610.8 | 30 | 17.6 | 1.0 | Referent | ||
| Positive | 1,243 | 21,836.7 | 12 | 55.0 | 3.4 | 1.7, 6.6 | ||
| Combination of VCA IgA and deoxyribonucleasef | ||||||||
| Both negative | 8,880 | 1,67,090.5 | 24 | 14.4 | 1.0 | Referent | 1.0 | Referent |
| Either one positive | 1,373 | 23,248.6 | 12 | 51.6 | 3.5 | 1.7, 7.1 | 2.8 | 1.3, 6.0 |
| Both positive | 70 | 938.6 | 3 | 319.6 | 23.4 | 6.9, 79.1 | 15.1 | 4.2, 54.1 |
| Cumulative cigarette smoking, pack-yearsg | ||||||||
| 0 | 3,728 | 69,212.0 | 13 | 18.8 | 1.0 | Referent | 1.0 | Referent |
| <30 | 4,355 | 82,033.8 | 14 | 17.1 | ||||
| ≥30 | 2,208 | 36,085.3 | 14 | 38.8 | 2.3 | 1.1, 5.0 | 1.8 | 0.8, 4.1 |
Abbreviations: EBV, Epstein-Barr virus; IgA, immunoglobulin A; VCA, viral capsid antigen.
Per 100,000 person-years.
Adjusted for age (continuous), ethnicity (Fukkinese or non-Fukkinese), and years of schooling (0–6 years or ≥7 years).
Model included age (continuous), ethnicity (Fukkinese or non-Fukkinese), years of schooling (0–6 years or ≥7 years), family history, cumulative cigarette smoking, and combination of anti-EBV VCA IgA tested by immunofluorescence assay and anti-EBV deoxyribonuclease.
Data for anti-EBV VCA IgA tests were not available for 316 participants, including 4 nasopharyngeal carcinoma cases.
Data for anti-EBV deoxyribonuclease tests were not available for 70 participants, including 1 nasopharyngeal carcinoma case.
Data for the combination of VCA IgA and deoxyribonuclease were not available for 318 participants, including 4 nasopharyngeal carcinoma cases.
Data for cumulative cigarette smoking were not available for 350 participants, including 2 nasopharyngeal carcinoma cases.
The association with NPC for the combination of family history, anti-EBV seromarkers, and cumulative pack-years of cigarette smoking after adjustment for age, ethnicity, and years of schooling was further evaluated. As shown in Table 3, no evidence for departure from a multiplicative model was observed for any of the joint effects evaluated. Of interest is the fact that individuals with a family history of NPC who were also seropositive for anti-EBV seromarkers had an adjusted hazard ratio of 31.0 (95% CI: 9.7, 98.7) compared with individuals with no family history of NPC who were anti-EBV seronegative.
Table 3.
Incidence of and Adjusted Hazard Ratios for Nasopharyngeal Carcinoma, by Combination of Anti-Epstein-Barr Virus Seromarkers, Family History, and Cumulative Cigarette Smoking at Study Entry, Taiwan, 1984–2008
| Variable |
No. of Participants | Person-Years | No. of Cases | Cumulative Incidencea | Hazard Ratiob | 95% Confidence Interval | P for Interaction | |
| Combination of VCA IgA and deoxyribonuclease | Family historyc | 0.533 | ||||||
| Both negative | No | 8,357 | 1,62,372.5 | 22 | 13.5 | 1.0 | Referent | |
| Both negative | Yes | 523 | 4,718.0 | 2 | 42.4 | 5.5 | 1.1, 26.8 | |
| Any positive | No | 1,210 | 22,104.7 | 10 | 45.2 | 3.1 | 1.4, 6.9 | |
| Any positive | Yes | 233 | 2,082.5 | 5 | 240.1 | 31.0 | 9.7, 98.7 | |
| Cumulative cigarette smoking, pack-years | Family historyd | 0.310 | ||||||
| <30 | No | 7,199 | 1,44,173.4 | 18 | 12.5 | 1.0 | Referent | |
| <30 | Yes | 884 | 7,072.4 | 9 | 127.3 | 14.5 | 5.1, 41.2 | |
| ≥30 | No | 2,074 | 35,101.5 | 13 | 37.0 | 2.7 | 1.2, 6.0 | |
| ≥30 | Yes | 134 | 983.8 | 1 | 101.6 | 12.4 | 1.5, 102.8 | |
| Combination of VCA IgA and deoxyribonuclease | Cumulative cigarette smoking, pack-yearse | 0.862 | ||||||
| Both negative | <30 | 6,777 | 1,31,514.3 | 16 | 12.2 | 1.0 | Referent | |
| Both negative | ≥30 | 1,810 | 30,268.1 | 7 | 23.1 | 1.9 | 0.7, 5.1 | |
| Any positive | <30 | 1,038 | 17,771.0 | 9 | 50.6 | 4.2 | 1.9, 9.6 | |
| Any positive | ≥30 | 352 | 5,474.3 | 5 | 91.3 | 6.9 | 2.1, 21.9 | |
Abbreviations: IgA, immunoglobulin A; VCA, viral capsid antigen.
Per 100,000 person-years.
Adjusted for age (continuous), ethnicity (Fukkinese or non-Fukkinese), and years of schooling (0–6 years or ≥7 years).
Data for the combination of anti-Epstein-Barr virus seromarkers and family history were not available for 318 participants, including 4 nasopharyngeal carcinoma cases.
Data for the combination of cumulative cigarette smoking and family history were not available for 350 participants, including 2 nasopharyngeal carcinoma cases.
Data for the combination of anti-Epstein-Barr virus seromarkers and cumulative cigarette smoking were not available for 664 participants, including 6 nasopharyngeal carcinoma case.
DISCUSSION
To our knowledge, this is the first prospective study to evaluate the association between family history and NPC risk after accounting for anti-EBV seromarkers and cigarette smoking. The study utilized data from 2 cohort studies comprising over 10,000 individuals. The longitudinal design minimized recall bias concerns related to the ascertainment of NPC family history and exposure to environmental risk factors. We also avoided issue of causal temporality by assessing EBV serostatus at study entry, which meant that measurements occurred on average 10.2 years before diagnosis in cases. Subjects were identified via linkage to a long-standing population-based national tumor registry.
There are some considerations to our study. First, the 2 cohorts were recruited at different times (1980s versus the late 1990s). NPC diagnostic methods may have changed over time. However, the overall incidence of NPC in Taiwan was stable in the 1980s and 1990s (34). Second, individuals from the multiplex family cohort might have been more concerned with health than those from the community cohort. However, because persons with NPC are unlikely to remain symptom-free and because Taiwan has a national health care system with broad access, it is expected that nearly all NPC cases would ultimately be identified and reported to the National Cancer Registry. Third, there were some differences in the collection of data through structured questionnaires, the temperatures for biospecimen storage, and the response rates between 2 study cohorts. The questionnaire information was collected by personal interview for the community cohort and by self-administration for the multiplex family cohort. Although the collection methods differed, the questions about sociodemographic characteristics and cigarette smoking habits were simple and easy to answer. The storage temperatures for biopsecimens collected from the community and multiplex family cohorts were −30°C and −80°C, respectively. However, researchers tested for anti-EBV seromarkers within 3 months of recruitment, and therefore the difference in storage temperatures would not have affected the test results of anti-EBVs. Although the response rate in the multiplex family cohort was higher than in the community cohort, the cohorts were representative samples of multiplex families and community residents, respectively. Furthermore, the ascertainment of newly developed NPC through data linkage with the National Cancer Registry was exactly the same for both cohorts.
Limitations of our study included the small number of newly developed NPC cases resulting from the low incidence of NPC. However, despite this limitation, we were able to observe a statistically significant association between family history of NPC and NPC risk, which was consistent with findings from previous studies (14–22, 24). Although it was interesting to evaluate the joint effects of family history and anti-EBV seromarkers, it was difficult to draw any definitive conclusions because of the small numbers of NPC cases. Another potential limitation of our study is that anti-EBV VCA IgA results using the indirect immunofluorescent assay test were available for only 75% of individuals enrolled in the multiplex family cohort. It is reassuring to note, however, that participants with missing anti-EBV VCA IgA data were similar to those for whom data were not missing with respect to all exposure variables (age, ethnicity, educational level, smoking status, and anti-EBV DNase) evaluated in this analysis. Lastly, the anti-EBV seromarker tests were done in the same laboratory at different times.
The magnitude of risk associated with a family history of NPC in our study was higher than that reported in most previous studies (adjusted hazard ratio = 11.7) (14–22, 24). The most likely explanation for this difference is our requirement that members of our family cohort report at least 2 family members affected with NPC (i.e., multiplex families). In contrast, other studies considered individuals with ≥1 relatives affected with NPC. Our findings are therefore generalizable only to individuals who report at least 2 family members with NPC. In this group, our results suggest that unaffected individuals from NPC multiplex families with putatively higher genetic risk might be at greatly elevated risk of NPC.
In our study, the hazard ratio for family history declined from 11.7 to 6.8 after adjustment for EBV seromarkers and cigarette smoking. The hazard ratio for seropositivity to anti-EBV VCA IgA and anti-EBV DNase also declined from 23.4 to 15.1 after adjustment for family history and cigarette smoking. In both instances, however, significant residual effects were noted after adjustment. In contrast, the effect of cigarette smoking was no longer significant after we controlled for family history and EBV seropositivity. The effect of family history might theoretically be mediated through common genetic and/or environmental factors shared by family members, such as EBV seroreactivity and cigarette smoking. The fact that family history of NPC remained a significant risk factor after we controlled for these factors, however, suggests that familial aggregation in cigarette smoking behavior and in EBV exposure and/or antibody responses were unlikely to fully explain why individuals with a family history of NPC were at an increased risk of developing NPC themselves. In fact, based on our data, we estimated that approximately 28% of the risk of NPC associated with family history could be explained by EBV (35). It should be noted that in our study, we did not directly measure the level of EBV exposure. Rather, serologic responses to EBV, as determined by antibody levels detected in circulation, were used as a proxy for the degree of EBV exposure. To the extent that antibody production has an underlying host (e.g., genetic susceptibility) and exogenous determinants (e.g., degree of EBV exposure determined by frequency of reactivation of latent EBV infections), our study was not capable of differentiating between these different components.
To our knowledge, we are the first to evaluate the joint effect of family history of NPC, anti-EBV seromarkers, and cigarette smoking on NPC risk in a prospective manner. We found that both NPC family history and anti-EBV seropositivity were important determinants of subsequent NPC risk and that the effect of family history on NPC could not be fully explained by mediation through EBV serologic responses.
Acknowledgments
Author affiliations: Genomics Research Center, Academia Sinica, Taipei, Taiwan (Wan-Lun Hsu, Yin-Chu Chien, San-Lin You, Chien-Jen Chen); Graduate Institute of Epidemiology, College of Public Health, National Taiwan University, Taipei, Taiwan (Wan-Lun Hsu, Chun-Ju Chiang, Yu-Juen Cheng, Chien-Jen Chen); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Kelly J. Yu, Allan Hildesheim); National Institute of Cancer Research, National Health Research Institutes, Chunan, Taiwan (Jen-Yang Chen, Sheng-Ping Chou); Graduate Institute of Microbiology, College of Medicine, National Taiwan University, Taipei, Taiwan (Jen-Yang Chen, Czau-Siung Yang); Center of General Education, National Taipei College of Nursing, Taipei, Taiwan (Mei-Ying Liu); Department of Otolaryngology, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan (Mow-Ming Hsu, Pei-Jen Lou, Cheng-Ping Wang); Institute of Biomedical Engineering, College of Medicine and College of Engineering, National Taiwan University, Taipei, Taiwan (Cheng-Ping Wang); Department of Radiation Oncology, Chang Gung Memorial Hospital, Taoyuan, Taiwan (Ji-Hong Hong); Department of Otolaryngology, MacKay Memorial Hospital, Taipei, Taiwan (Yi-Shing Leu); Department of Otolaryngology, China Medical University Hospital, Taichung, Taiwan (Ming-Hsui Tsai); Department of Otorhinolaryngology-Head and Neck Surgery, Chung Shan Medical University Hospital, Taichung, Taiwan (Mao-Chang Su); Department of Speech Language Pathology and Audiology, Chung Shan Medical University Hospital, Taichung, Taiwan (Mao-Chang Su); School of Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan (Mao-Chang Su); Department of Otolaryngology, National Cheng Kung University Hospital, Tainan, Taiwan (Sen-Tien Tsai, Wen-Yuan Chao); Department of Education and Research, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan (Luo-Ping Ger); Department of Otolaryngology, Buddhist Tzu-Chi General Hospital, Hualien, Taiwan (Peir-Rong Chen); and Center for Pharmacogenomics and Complex Disease Research, New Jersey Dental, Newark, New Jersey (Scott R. Diehl).
This study was supported by the Department of Health, Executive Yuan, Taipei, Taiwan (grants DOH 75-0203-18 and DOH 76-0203-17), the National Science Council, Taipei, Taiwan (grant NSC 95-2314-B-002-178), and intramural funds from the National Institutes of Health, United States.
These data were presented in part at the 81st Taiwan Otolaryngological Society Conference, Taipei, Taiwan, November 4–5, 2006.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- DNase
deoxyribonuclease
- EBV
Epstein-Barr virus
- IgA
immunoglobulin A
- NPC
nasopharyngeal carcinoma
- VCA
viral capsid antigen
References
- 1.Parkin DM, Whelan SL, Ferlay J, et al. Cancer Incidence in Five Continents. Vol VIII. (IARC scientific publication no 155) Lyon, France: IARC Press; 2002. [Google Scholar]
- 2.Epstein-Barr Virus and Kaposi's Sarcoma Herpesvirus/Human Herpesvirus 8. International Agency for Research on Cancer. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans vol 70). Lyon, France: IARC Press; 1997. [PMC free article] [PubMed] [Google Scholar]
- 3.Chien YC, Chen CJ. Epidemiology and etiology of nasopharyngeal carcinoma: gene-environment interaction. Cancer Rev Asia Pac. 2003;1(1):1–19. [Google Scholar]
- 4.Chen CJ, You SL, Lin LH, et al. Cancer epidemiology and control in Taiwan: a brief review. Jpn J Clin Oncol. 2002;32(suppl):S66–S81. doi: 10.1093/jjco/hye138. [DOI] [PubMed] [Google Scholar]
- 5.Sun LM, Epplein M, Li CI, et al. Trends in the incidence rates of nasopharyngeal carcinoma among Chinese Americans living in Los Angeles County and the San Francisco metropolitan area, 1992–2002. Am J Epidemiol. 2005;162(12):1174–1178. doi: 10.1093/aje/kwi345. [DOI] [PubMed] [Google Scholar]
- 6.Gajwani BW, Devereaux JM, Beg JA. Familial clustering of nasopharyngeal carcinoma. Cancer. 1980;46(10):2325–2327. doi: 10.1002/1097-0142(19801115)46:10<2325::aid-cncr2820461035>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Ko JY, Sheen TS, Hsu MM, et al. Familial clustering of nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 1998;118(5):736–737. doi: 10.1016/s0194-5998(98)70258-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Zhang J. Clinical hereditary characteristics in nasopharyngeal carcinoma through Ye-Liang's family cluster. Chin Med J (Engl) 1999;112(2):185–187. [PubMed] [Google Scholar]
- 9.Loh KS, Goh BC, Lu J, et al. Familial nasopharyngeal carcinoma in a cohort of 200 patients. Arch Otolaryngol Head Neck Surg. 2006;132(1):82–85. doi: 10.1001/archotol.132.1.82. [DOI] [PubMed] [Google Scholar]
- 10.Albeck H, Bentzen J, Ockelmann HH, et al. Familial clusters of nasopharyngeal carcinoma and salivary gland carcinomas in Greenland natives. Cancer. 1993;72(1):196–200. doi: 10.1002/1097-0142(19930701)72:1<196::aid-cncr2820720135>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Levine PH, Pocinki AG, Madigan P, et al. Familial nasopharyngeal carcinoma in patients who are not Chinese. Cancer. 1992;70(5):1024–1029. doi: 10.1002/1097-0142(19920901)70:5<1024::aid-cncr2820700503>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Coffin CM, Rich SS, Dehner LP. Familial aggregation of nasopharyngeal carcinoma and other malignancies. A clinicopathologic description. Cancer. 1991;68(6):1323–1328. doi: 10.1002/1097-0142(19910915)68:6<1323::aid-cncr2820680623>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Olajos J, Füle E, Erfán J, et al. Familial clustering of nasopharyngeal carcinoma in a non-endemic geographical region. Report of two Hungarian cases and a review of the literature. Acta Otolaryngol. 2005;125(9):1008–1013. doi: 10.1080/00016480510040155. [DOI] [PubMed] [Google Scholar]
- 14.Chen DL, Huang TB. A case-control study of risk factors of nasopharyngeal carcinoma. Cancer Lett. 1997;117(1):17–22. doi: 10.1016/s0304-3835(97)00182-1. [DOI] [PubMed] [Google Scholar]
- 15.Yu MC, Ho JH, Lai SH, et al. Cantonese-style salted fish as a cause of nasopharyngeal carcinoma: report of a case-control study in Hong Kong. Cancer Res. 1986;46(2):956–961. [PubMed] [Google Scholar]
- 16.Chen CJ, Liang KY, Chang YS, et al. Multiple risk factors of nasopharyngeal carcinoma: Epstein-Barr virus, malarial infection, cigarette smoking and familial tendency. Anticancer Res. 1990;10(2B):547–553. [PubMed] [Google Scholar]
- 17.Sriamporn S, Vatanasapt V, Pisani P, et al. Environmental risk factors for nasopharyngeal carcinoma: a case-control study in northeastern Thailand. Cancer Epidemiol Biomarkers Prev. 1992;1(5):345–348. [PubMed] [Google Scholar]
- 18.Yu MC, Garabrant DH, Huang TB, et al. Occupational and other non-dietary risk factors for nasopharyngeal carcinoma in Guangzhou, China. Int J Cancer. 1990;45(6):1033–1039. doi: 10.1002/ijc.2910450609. [DOI] [PubMed] [Google Scholar]
- 19.Yuan JM, Wang XL, Xiang YB, et al. Non-dietary risk factors for nasopharyngeal carcinoma in Shanghai, China. Int J Cancer. 2000;85(3):364–369. [PubMed] [Google Scholar]
- 20.Henderson BE, Louie E, SooHoo Jing J, et al. Risk factors associated with nasopharyngeal carcinoma. N Engl J Med. 1976;295(20):1101–1106. doi: 10.1056/NEJM197611112952003. [DOI] [PubMed] [Google Scholar]
- 21.Yan L, Xi Z, Drettner B. Epidemiological studies of nasopharyngeal cancer in the Guangzhou area, China. Preliminary report. Acta Otolaryngol. 1989;107(5-6):424–427. doi: 10.3109/00016488909127534. [DOI] [PubMed] [Google Scholar]
- 22.Ung A, Chen CJ, Levine PH, et al. Familial and sporadic cases of nasopharyngeal carcinoma in Taiwan. Anticancer Res. 1999;19(1B):661–665. [PubMed] [Google Scholar]
- 23.Jia WH, Collins A, Zeng YX, et al. Complex segregation analysis of nasopharyngeal carcinoma in Guangdong, China: evidence for a multifactorial mode of inheritance (complex segregation analysis of NPC in China) Eur J Hum Genet. 2005;13(2):248–252. doi: 10.1038/sj.ejhg.5201305. [DOI] [PubMed] [Google Scholar]
- 24.Friborg J, Wohlfahrt J, Koch A, et al. Cancer susceptibility in nasopharyngeal carcinoma families—a population-based cohort study. Cancer Res. 2005;65(18):8567–8572. doi: 10.1158/0008-5472.CAN-04-4208. [DOI] [PubMed] [Google Scholar]
- 25.Cheng YJ, Hildesheim A, Hsu MM, et al. Cigarette smoking, alcohol consumption and risk of nasopharyngeal carcinoma in Taiwan. Cancer Causes Control. 1999;10(3):201–207. doi: 10.1023/a:1008893109257. [DOI] [PubMed] [Google Scholar]
- 26.Chien YC, Chen JY, Liu MY, et al. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345(26):1877–1882. doi: 10.1056/NEJMoa011610. [DOI] [PubMed] [Google Scholar]
- 27.Hsu WL, Chen JY, Chien YC, et al. Independent effect of EBV and cigarette smoking on nasopharyngeal carcinoma: a 20-year follow-up study on 9,622 males without family history in Taiwan. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1218–1226. doi: 10.1158/1055-9965.EPI-08-1175. [DOI] [PubMed] [Google Scholar]
- 28.Pickard A, Chen CJ, Diehl SR, et al. Epstein-Barr virus seroreactivity among unaffected individuals within high-risk nasopharyngeal carcinoma families in Taiwan. Int J Cancer. 2004;111(1):117–123. doi: 10.1002/ijc.20222. [DOI] [PubMed] [Google Scholar]
- 29.Avenevoli S, Merikangas KR. Familial influences on adolescent smoking. Addiction. 2003;98(suppl 1):1–20. doi: 10.1046/j.1360-0443.98.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 30.Henle G, Henle W. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer. 1976;17(1):1–7. doi: 10.1002/ijc.2910170102. [DOI] [PubMed] [Google Scholar]
- 31.Chen JY, Chen CJ, Liu MY, et al. Antibody to Epstein-Barr virus-specific DNase as a marker for field survey of patients with nasopharyngeal carcinoma in Taiwan. J Med Virol. 1989;27(4):269–273. doi: 10.1002/jmv.1890270403. [DOI] [PubMed] [Google Scholar]
- 32.Yang XR, Diehl S, Pfeiffer R, et al. Evaluation of risk factors for nasopharyngeal carcinoma in high-risk nasopharyngeal carcinoma families in Taiwan. Cancer Epidemiol Biomarkers Prev. 2005;14(4):900–905. doi: 10.1158/1055-9965.EPI-04-0680. [DOI] [PubMed] [Google Scholar]
- 33.Breslow N, Crowley J. A large sample study of the life table and product limit estimates under random censorship. Ann Stat. 1974;2(3):437–453. [Google Scholar]
- 34.Hsu C, Shen YC, Cheng CC, et al. Difference in the incidence trend of nasopharyngeal and oropharyngeal carcinomas in Taiwan: implication from age-period-cohort analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(5):856–861. doi: 10.1158/1055-9965.EPI-05-0821. [DOI] [PubMed] [Google Scholar]
- 35.Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med. 1992;11(2):167–178. doi: 10.1002/sim.4780110204. [DOI] [PubMed] [Google Scholar]


