Abstract
Dietary factors are believed to modulate arsenic toxicity, potentially influencing risk of arsenical skin lesions. The authors evaluated associations among dietary patterns, arsenic exposure, and skin lesion risk using baseline food frequency questionnaire data collected in the Health Effects of Arsenic Longitudinal Study (HEALS) in Araihazar, Bangladesh (2000–2009). They identified dietary patterns and estimated dietary pattern scores using factor analysis. Scores were tested for association with incident skin lesion risk and interaction with water arsenic exposure by using ∼6 years of follow-up data (814 events among 9,677 individuals) and discrete time hazards models (adjusting for key covariates). The authors identified 3 clear dietary patterns: the “gourd and root,” “vegetable,” and “animal protein” patterns. The gourd and root pattern score was inversely associated with skin lesion risk (Ptrend = 0.001), with hazard ratios of 0.86, 0.73, and 0.69 for the second, third, and fourth highest quartiles. Furthermore, the association between water arsenic and skin lesion incidence was stronger among participants with low gourd and root scores (multiplicative Pinteraction < 0.001; additive Pinteraction = 0.05). The vegetable pattern and animal protein pattern showed similar but weaker associations and interactions. Eating a diet rich in gourds and root vegetables and increasing dietary diversity may reduce arsenical skin lesion risk in Bangladesh.
Keywords: arsenic; Bangladesh; diet; drinking; factor analysis, statistical; malnutrition; skin; water
It has been estimated that 28%–62% of the ∼140 million people in Bangladesh drink groundwater that is naturally contaminated with arsenic (1). Chronic arsenic exposure through drinking water has been associated with increased risk for a wide range of diseases, including cancers of the lung (2), bladder (3), liver (4), skin (5), and kidney (6, 7), as well as neurologic (8, 9) and cardiovascular (10) disease. Skin lesions are a classic sign of arsenic toxicity, an indicator of susceptibility to arsenic-related diseases, and a precursor to arsenic-induced skin cancers (5).
Dietary factors are believed to play a critical role in modulating arsenic toxicity. Several lines of experimental and epidemiologic evidence suggest that micronutrients, such as selenium, vitamin A, iron, folate, and zinc, are involved in host defense against arsenic (11). However, in observational studies conducted using food frequency questionnaires (FFQs), isolating the effects of individual nutrients is difficult, as human diets are complex, consisting of correlated intakes of many foods and nutrients that potentially interact to influence health outcomes (12). Measuring dietary patterns, rather than specific nutrients, is a promising method for comprehensively characterizing dietary habits (13, 14) and estimating diet-related disease risks, in part, because broad dietary patterns can account for interactions among nutrients (12). Such patterns may predict disease more accurately than individual foods or nutrients, eventually contributing to the development of practical dietary guidelines (15).
The association between dietary patterns and arsenic-related toxicity has not been examined in Bangladesh (or elsewhere), a developing country where malnutrition (16) may contribute to increased arsenic toxicity (17). In this study, we prospectively examine the associations among FFQ-derived dietary patterns, arsenic exposure through drinking water, and arsenical skin lesion incidence in a large prospective cohort.
MATERIALS AND METHODS
Study area and study population
The Health Effects of Arsenic Longitudinal Study (HEALS) described by Ahsan et al. (18) is a prospective investigation of health outcomes associated with arsenic exposure through drinking water in a cohort of adults in Araihazar, Bangladesh, a rural area east of the capital city, Dhaka, with relatively homogeneous sociocultural characteristics. Between October 2000 and May 2002, we recruited married individuals (aged 18–75 years) who were residents of the study area for at least 5 years and primarily consumed drinking water from a local well. Using a precohort survey, we enumerated 65,876 individuals residing in Araihazar, from which we identified a sampling frame of 14,828 eligible residents. Of these 14,828 individuals, 2,778 were not at home during any of the 3 attempted recruiting visits. Of the 12,050 remaining eligible residents, 11,746 (97.5% response rate) men and women (4,801 married couples and 2,144 married individuals whose spouses did not participate) were enrolled. All 5,966 wells in the study area were tested for arsenic. At baseline, trained study physicians, blinded to the arsenic measurements, conducted in-person interviews and clinical evaluations and collected spot urine and blood samples from participants in their homes using structured protocols. Similar in-person follow-up interviews were conducted biennially for the entire cohort during the following periods: follow-up 1 during September 2002–May 2004, follow-up 2 during June 2004–August 2006, and follow-up 3 during January 2007–February 2009. The study protocol was approved by the institutional review boards of the University of Chicago, Columbia University, and the Bangladesh Medical Research Council. Informed consent was obtained from all participants.
Skin lesion status
At baseline and each follow-up interview, a structured protocol was used to ascertain skin lesions by the study physicians, who had undergone training for the detection and diagnosis of skin lesions (19). Through a whole-body examination, the study physician recorded the presence or absence of melanosis (hyperpigmentation of the skin surface), leukomelanosis (hypopigmentation of the skin surface), and keratosis (thickening of the skin typically on the palms and soles), as well as their location, size, and shape (19). During the entire follow-up period, 183 incident cases of keratosis and 631 incident cases of melanosis/leukomelanosis were observed. For the present analysis, skin lesion incidence was defined as a new occurrence of skin lesions of any type (as determined at one of the follow-up interviews) among individuals who previously had no manifestation of any lesion (as determined at baseline and previous follow-up interviews).
Arsenic exposure assessment
Well water arsenic concentrations of all 5,966 wells in the study area were measured by graphite furnace atomic absorption spectrometry, with a detection limit of 5 μg/L. Samples below the limit were reanalyzed by inductively coupled plasma-mass spectrometry, with a detection limit of 0.1 μgL (20). At baseline, participants identified their primary drinking well, and the arsenic concentration of this well was assigned as their baseline exposure. We were unable to ascertain the well water arsenic concentration for 1 well, resulting in missing data for 3 participants.
Well water arsenic exposure was categorized into quintiles; however, because the first and second quintiles roughly corresponded to the World Health Organization's guideline for arsenic in drinking water (10 μg/L) and the national standard for drinking water in Bangladesh (50 μg/L), respectively, we adjusted the cutpoints to correspond to these regulatory levels.
Dietary pattern assessment
Dietary intakes were assessed by trained interviewers at baseline using a validated semiquantitative 39-item FFQ that was designed for HEALS participants. Focus groups were used to determine the food items to include, and validity was assessed by 7-day food diary data on 189 randomly selected participants (21). Correlations between food group intakes from FFQs and 7-day food diaries ranged from 0.19 to 0.78 (21). Total energy, macronutrient, and micronutrient intakes were estimated by using the US Department of Agriculture Nutrient Database for Standard Reference (22). All nutrients intakes were adjusted for total energy intake by using the residual method (23).
For each of the 39 food items (measured in g/day), we added 1 g/day (to avoid values of zero) and then log transformed all 39 items to obtain approximate normal distributions. We then applied maximum likelihood factor analysis to all 39 items. Factor analysis is a statistical method that uses many correlated variables to derive a smaller number of unobserved variables or factors that explain a substantial amount of variation in the correlated variables (12, 13, 15). A varimax rotation was used to obtain normally distributed, uncorrelated factors. Estimated factor scores were computed for each individual as a linear combination of the standardized intake values multiplied by their respective factor loadings (no loadings were set to zero). Factors with an eigenvalue of >1.0 and above the elbow of the scree plot (12) were retained and included in our skin lesion analysis. Dietary pattern analysis was carried out with the FACTOR procedure in SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina).
Exclusions and eligibility
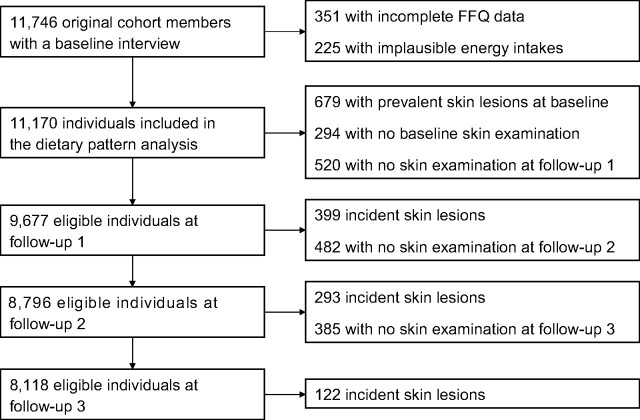
The participants included in this analysis are described in Figure 1. For the dietary pattern analysis, we excluded individuals with incomplete FFQ data (n = 351) or implausible total energy intake values (for females, <500 (n = 7) or >3,500 (n = 82) kcal/day; for males, <800 (n = 16) or >4,000 (n = 120) kcal/day) (23, 24), resulting in 11,170 eligible individuals. For association analyses, we also excluded individuals who had prevalent skin lesions at baseline or who did not receive skin examinations at baseline or follow-up 1, resulting in an analysis cohort of 9,677 individuals. At each follow-up wave, individuals with incident skin lesions detected at a prior follow-up examination were excluded from the analysis cohort, and individuals who did not receive skin examinations were censored after their prior follow-up examination (which occurred at the prior follow-up interview).
Figure 1.
Definition of the participants in the Health Effects of Arsenic Longitudinal Study (HEALS) included in this analysis, Araihazar, Bangladesh, 2000–2009. FFQ, food frequency questionnaire.
Covariates
All covariate data were derived from the baseline interview. Sociodemographic factors included sex, age (years), formal education (years), land ownership (yes/no), television ownership (yes/no), and smoking (never, former, or current). Trained study physicians measured height and weight 3 times using a locally manufactured tape measure and a Misaki (Japan) scale (calibrated weekly). Body mass index (average weight (kg)/average height (m)2) was calculated. For eligible individuals, there were very few missing data on these covariates (>99.9% complete).
Statistical analysis
Discrete-time hazard models were used to estimate hazard ratios and their 95% confidence intervals for skin lesion incidence (using skin lesion data from all 3 follow-up examinations). These models are based on the probability (i.e., the discrete-time hazard) of skin lesion incidence at each study interval conditional on being skin lesion-free at the previous study interval (25). The conditional probability was estimated by using a log-linear model with a different intercept for each study interval, but with common regression coefficients across all intervals. The regression coefficients were interpreted as log discrete-time hazard ratios, analogous to log hazard ratios that arise in the traditional continuous-time proportional hazards model (26). Because the enrollment of participants into the cohort was clustered on household (i.e., married couples) and households were clustered on primary well, robust standard errors (based on well clusters) were used to account for correlation between observations from the same well by estimating models using generalized estimating equations (27).
Associations for dietary pattern quartiles were adjusted for sex and categorical age groups. Multivariate models included further adjustment for several variables that were selected a priori on the basis of hypothesized associations with dietary factors and/or skin lesion risk: categorical body mass index, smoking status, years of formal education (categorical), land ownership, television ownership, study interval, and total energy intake (categorical quartiles). Tests for trend were obtained by including an ordinal exposure variable in the model.
We also examined how dietary patterns modify the association between arsenic exposure and skin lesion risk. Associations between arsenic quintiles and skin lesion incidence were examined in each of the quartiles of each dietary pattern score. We tested for multiplicative interaction by including the product term of the ordinal arsenic variable and ordinal dietary pattern variable in the discrete time hazard model. Additive interaction was assessed by using the relative excess risk due to interaction (RERI) measure (28). P values and 95% confidence intervals (bias corrected and accelerated) were determined by using 5,000 bootstrap resamples (29).
Statistical analyses were performed by using SAS, version 9.2, including the GENMOD procedure, and STATA, version 11, including the bootstrap command (StataCorp LP, College Station, Texas).
RESULTS
Baseline characteristics of the HEALS cohort, according to incident skin lesion status, are presented in Table 1. Skin lesion risk was higher in males (compared with females) and smokers (compared with nonsmokers). Skin lesion risk also appeared to increase with increasing age, decreasing body mass index, decreasing years of formal education, and increasing well water arsenic concentration.
Table 1.
Baseline Characteristics of Eligible HEALS Participants, According to Incident Skin Lesion Status (n = 9,549), Araihazar, Bangladesh, 2000–2009
| Characteristics of Participants | Incident Skin Lesions, no. | Total Cohort, no. | Age- and Sex-adjusted Model |
|
| HR | 95% CI | |||
| Total | 814 | 9,677 | ||
| Sex | ||||
| Male | 571 | 3,753 | 1.00 | |
| Female | 243 | 5,924 | 0.37 | 0.32, 0.43 |
| Age, years | ||||
| 18–30 | 72 | 3,174 | 1.00 | |
| 31–40 | 219 | 3,503 | 2.24 | 1.71, 2.93 |
| 41–50 | 303 | 2,160 | 4.80 | 3.69, 6.24 |
| 51–75 | 220 | 840 | 7.68 | 5.79, 10.19 |
| Smoking | ||||
| Never | 313 | 6,588 | 1.00 | |
| Former | 125 | 564 | 2.19 | 1.67, 2.86 |
| Current | 376 | 2,523 | 4.52 | 3.47, 5.89 |
| Body mass index, kg/m2 | ||||
| <17.0 | 151 | 1,631 | 1.00 | |
| 17.0–18.4 | 205 | 2,112 | 1.10 | 0.90, 1.35 |
| 18.5–22.9 | 368 | 4,434 | 1.10 | 0.91, 1.32 |
| ≥23 | 83 | 1,439 | 0.74 | 0.58, 0.96 |
| Formal education, years | ||||
| 0 | 400 | 4,280 | 1.00 | |
| 1–4 | 138 | 1,382 | 1.05 | 0.88, 1.26 |
| 5–7 | 149 | 2,149 | 0.81 | 0.68, 0.98 |
| 8–16 | 126 | 1,861 | 0.61 | 0.50, 0.75 |
| Land ownership | ||||
| No | 377 | 4,688 | 1.00 | |
| Yes | 437 | 4,988 | 0.94 | 0.81, 1.09 |
| Television ownership | ||||
| No | 563 | 6,290 | 1.00 | |
| Yes | 251 | 3,387 | 0.74 | 0.64, 0.87 |
| Total energy intake, kcal/day | ||||
| <1,865 | 207 | 2,419 | 1.00 | |
| 1,865–2,189 | 181 | 2,420 | 0.98 | 0.80, 1.19 |
| 2,190–2,260 | 211 | 2,418 | 1.01 | 0.84, 1.23 |
| ≥2,261 | 215 | 2,420 | 0.94 | 0.78, 1.14 |
| Well water arsenic, μg/L | ||||
| 0.1–10 | 131 | 2,358 | 1.00 | |
| 10.1–50 | 125 | 2,118 | 1.17 | 0.92, 1.50 |
| 50.1–100 | 140 | 1,726 | 1.70 | 1.34, 2.16 |
| 100.1–200 | 318 | 2,855 | 2.22 | 1.81, 2.73 |
| ≥200.1 | 100 | 617 | 3.76 | 2.85, 4.95 |
| Follow-up wave (biennial) | ||||
| First wave | 399 | 9,677 | 1.00 | |
| Second wave | 293 | 8,796 | 0.84 | 0.73, 0.98 |
| Third wave | 122 | 8,118 | 0.39 | 0.32, 0.47 |
Abbreviations: CI, confidence interval; HEALS, Health Effects of Arsenic Longitudinal Study; HR, hazard ratio.
Factor analysis of the 39 FFQ-derived food items resulted in 3 factors (i.e., patterns) with eigenvalues greater than 1. These patterns were assigned names based on foods that loaded heavily on the factor: the “gourd and root,” “vegetable,” and “animal protein” patterns. These factors had eigenvalues of 5.54, 1.96, and 1.16, respectively. The next 2 largest eigenvalues were 0.61 and 0.59, indicating that the animal protein pattern was just above the elbow of the scree plot (12), further justifying the use of only the top 3 factors. The variance explained by each factor (after rotation) was 3.18, 3.04, and 2.44, respectively. Factor loadings are shown in Table 2, representing the correlation between the standardized food items and the unobserved factor. Each factor score had a mean of zero, a standard deviation of ∼0.82, and an approximate normal distribution.
Table 2.
Rotated Factor Pattern Loadingsa for the 39 Food Items Measured at the HEALS Baseline Interview (n = 9,549b), Araihazar, Bangladesh, 2000–2009
| Food Item | Gourd and Root Pattern | Vegetable Pattern | Animal Protein Pattern |
| Ridge gourdc | 0.52 | ||
| Snake gourdc | 0.51 | ||
| Ghosalac | 0.49 | ||
| Spinach stalks | 0.45 | ||
| Radish | 0.41 | ||
| Parwarc | 0.39 | ||
| Pumpkin | 0.37 | ||
| Green papaya | 0.32 | ||
| Sweet potato | 0.31 | ||
| Guava | |||
| Yam | |||
| Eggplant | |||
| Dried fish | |||
| Puffed rice | |||
| Salted fish | |||
| Cauliflower | 0.55 | ||
| Tomato | 0.54 | ||
| Beansd | 0.47 | ||
| Bitter gourdc | 0.40 | ||
| Cabbage | 0.38 | 0.40 | |
| Bottle gourdc | 0.39 | ||
| Okra | 0.37 | ||
| Jack fruit | 0.32 | ||
| Mango | 0.30 | ||
| Spinach | 0.30 | ||
| Small fishe | |||
| Potato | |||
| Milk | 0.44 | ||
| Poultryf | 0.44 | ||
| Eggs (hen) | 0.43 | ||
| Banana | 0.43 | ||
| Tea | 0.40 | ||
| Bread (wheat) | 0.39 | ||
| Beef/mutton | 0.38 | ||
| Watermelon | 0.38 | ||
| Big fishe | 0.33 | ||
| Lentil | |||
| Steamed rice | |||
| Water rice |
Abbreviation: HEALS, Health Effects of Arsenic Longitudinal Study.
Factor loadings of <0.30 are not shown.
Individuals missing data for 1 or more food items (n = 351) or reporting implausible total energy intake values (n = 225) were excluded from the analysis.
A kind of squash.
“Scarlet runner.”
Fresh water fish.
Duck or fowl.
The distribution of the baseline characteristics according to dietary pattern scores is shown in Table 3. Higher animal protein scores were strongly associated with indicators of higher socioeconomic status, including body mass index, education, land ownership, and television ownership. Increasing gourd and root scores were associated with female sex and land ownership, while increasing vegetable scores were strongly associated with older age and more years of education.
Table 3.
Distribution of Baseline Characteristics of HEALS Participants According to Dietary Pattern Factor Score Quartiles, Araihazar, Bangladesh, 2000–2009a
| Characteristics of Participants | Gourd and Root Pattern |
Vegetable Pattern |
Animal Protein Pattern |
||||||||||||
| Quartiles of Factor Scores |
Ptrend | Quartiles of Factor Scores |
Ptrend | Quartiles of Factor Scores |
Ptrend | ||||||||||
| Q1 |
Q4 |
Q1 |
Q4 |
Q1 |
Q4 |
||||||||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | ||||
| Sex, female | 59.2 | 67.1 | <0.0001 | 66.7 | 56.8 | 0.04 | 79.3 | 59.7 | <0.0001 | ||||||
| Age, years | 36.6 (10.0) | 35.7 (9.7) | 0.83 | 36.5 (10.1) | 37.0 (9.9) | <0.0001 | 35.4 (9.4) | 38.1 (9.9) | 0.72 | ||||||
| Smoking, ever | 34.3 | 26.7 | 0.31 | 30.8 | 34.4 | 0.14 | 21.5 | 44.9 | <0.0001 | ||||||
| Body mass index, kg/m2 | 19.7 (3.2) | 19.9 (3.1) | 0.39 | 19.5 (3.2) | 20.0 (3.2) | 0.03 | 19.1 (2.7) | 20.8 (3.6) | <0.0001 | ||||||
| Education, years | 3.4 (3.8) | 3.5 (3.7) | 0.16 | 2.9 (3.7) | 3.9 (3.8) | <0.0001 | 2.0 (2.9) | 5.5 (4.2) | <0.0001 | ||||||
| Owns land | 47.7 | 55.5 | <0.0001 | 46.6 | 56.3 | 0.01 | 42.2 | 62.8 | <0.0001 | ||||||
| Owns television | 35.3 | 34.2 | 0.12 | 31.8 | 39.0 | 0.14 | 18.0 | 56.1 | <0.0001 | ||||||
| Energy intake, kcal/day | 2,209 (578) | 2,326 (542) | <0.0001 | 1,996 (516) | 2,518 (533) | <0.0001 | 2,188 (528) | 2,327 (570) | <0.0001 | ||||||
| Water arsenic, μg/L | 98.8 (111) | 98.6 (112) | 0.43 | 101 (106) | 103 (119) | 0.81 | 101 (109) | 89.7 (102) | 0.06 | ||||||
Abbreviations: HEALS, Health Effects of Arsenic Longitudinal Study; Q1, quartile 1; Q4, quartile 4; SD, standard deviation.
a For each dietary pattern, P values were generated by using a single linear regression model that included all listed characteristics of participants as covariates.
Factor scores for the gourd and root pattern showed a strong inverse association with skin lesion risk in both the age- and sex-adjusted model and the multivariate-adjusted model (Ptrend < 0.001) (Table 4). There was a suggestive inverse association with skin lesion risk for the vegetable factor score, but the overall trend was not strong (multivariate-adjusted Ptrend = 0.22). The number of observed skin lesions increased with increasing animal protein factor scores; however, we observed an inverse association with skin lesion risk in the age- and sex-adjusted model (Ptrend = 0.002) because the animal protein scores are higher in males, who have higher overall skin lesion risk compared with females. The inverse association was attenuated in the multivariate model (Ptrend = 0.23). Adjustment for water arsenic exposure did not impact any of the observed associations.
Table 4.
Associations Between Dietary Factor Scores and Incident Skin Lesion Risk Among HEALS Participants, Araihazar, Bangladesh, 2000–2009
| Dietary Pattern Factor Score Quartile | Incident Skin Lesions, no. | Total Cohort, no. | Age- and Sex-adjusted Model |
Multivariate-adjusted Modela |
||
| HR | 95% CI | HR | 95% CI | |||
| Gourd and root | ||||||
| Quartile 1 | 248 | 2,420 | 1.00 | 1.00 | ||
| Quartile 2 | 221 | 2,419 | 0.87 | 0.73, 1.04 | 0.86 | 0.72, 1.03 |
| Quartile 3 | 189 | 2,419 | 0.75 | 0.62, 0.91 | 0.73 | 0.61, 0.89 |
| Quartile 4 | 156 | 2,419 | 0.71 | 0.58, 0.89 | 0.69 | 0.57, 0.85 |
| Ptrend | 0.0004 | 0.0001 | ||||
| Vegetable | ||||||
| Quartile 1 | 205 | 2,420 | 1.00 | 1.00 | ||
| Quartile 2 | 212 | 2,419 | 1.02 | 0.84, 1.24 | 1.08 | 0.90, 1.30 |
| Quartile 3 | 194 | 2,418 | 0.87 | 0.71, 1.06 | 0.92 | 0.76, 1.12 |
| Quartile 4 | 203 | 2,420 | 0.89 | 0.73, 1.08 | 0.92 | 0.75, 1.13 |
| Ptrend | 0.10 | 0.22 | ||||
| Animal protein | ||||||
| Quartile 1 | 192 | 2,420 | 1.00 | 1.00 | ||
| Quartile 2 | 187 | 2,419 | 0.82 | 0.67, 1.01 | 0.85 | 0.70, 1.04 |
| Quartile 3 | 202 | 2,419 | 0.76 | 0.63, 0.93 | 0.83 | 0.69, 1.02 |
| Quartile 4 | 233 | 2,419 | 0.72 | 0.59, 0.88 | 0.87 | 0.70, 1.08 |
| Ptrend | 0.002 | 0.23 | ||||
Abbreviations: CI, confidence interval; HEALS, Health Effects of Arsenic Longitudinal Study; HR, hazard ratio.
Multivariate-adjusted model includes age, sex, water arsenic exposure, body mass index, education, television ownership, land ownership, smoking, and follow-up wave.
Associations between arsenic exposure and skin lesion risk are presented by dietary pattern score quartiles in Table 5. The association is weaker in the higher quartiles of the gourd and root pattern factor score (e.g., these associations are much stronger in the first compared with the fourth quartile), and statistical interaction is suggested by the P values for both the multiplicative (P = 0.05) and additive (P = 0.001) interaction estimates (multiplicative interaction hazard ratio (HR) = 0.95, 95% confidence interval (CI): 0.90, 1.00; RERI = −0.06, 95% confidence interval: −0.10, −0.03). A RERI of −0.06 is interpreted as follows: For a 1-unit increase in water arsenic exposure (i.e., a “unit” being 1 of the 5 categories) and a 1-unit increase in the gourd and root pattern scores (i.e., a “unit” being a quartile), the hazard ratio for skin lesions is 0.06 less than if there were no interaction. For the vegetable pattern, the multiplicative interaction P value was 0.04 (interaction HR = 0.95, 95% confidence interval: 0.90, 1.00), but the additive interaction P value was 0.55 (RERI = −0.01, 95% CI: −0.05, 0.03). The strength of the arsenic association to skin lesion risk also appears to decrease as the animal protein pattern scores increase, but this interaction was modest (additive P = 0.28; multiplicative P = 0.20).
Table 5.
Hazard Ratios and 95% Confidence Intervalsa for the Association Between Water Arsenic Exposure and Skin Lesion Risk Among HEALS Participants, Shown by Baseline Dietary Pattern Factor Score Quartile, Araihazar, Bangladesh, 2000–2009
| Dietary Pattern Factor Score Quartile | Water Arsenic Exposure Category, μg/L |
|||||||||
| 0.1–10 |
10.1–50 |
50.1–100 |
100.1–200 |
>200 |
||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Gourd and root | ||||||||||
| Quartile 1 | 1.00 | Referent | 1.44 | 0.89, 2.34 | 2.26 | 1.42, 3.60 | 3.50 | 2.34, 5.25 | 5.30 | 3.19, 8.81 |
| Quartile 2 | 1.00 | Referent | 1.14 | 0.75, 1.75 | 1.65 | 1.07, 2.57 | 1.80 | 1.23, 2.65 | 3.19 | 2.00, 5.09 |
| Quartile 3 | 1.00 | Referent | 1.22 | 0.45, 1.99 | 1.58 | 0.97, 2.59 | 2.17 | 1.45, 3.29 | 2.80 | 1.61, 4.89 |
| Quartile 4 | 1.00 | Referent | 0.76 | 0.52, 1.40 | 1.21 | 0.74, 1.98 | 1.43 | 0.93, 2.20 | 3.30 | 1.92, 5.67 |
| Multiplicative Pinteractionb = 0.05 | ||||||||||
| Additive Pinteractionc = 0.001 | ||||||||||
| Vegetable | ||||||||||
| Quartile 1 | 1.00 | Referent | 0.96 | 0.55, 1.68 | 1.48 | 0.88, 2.48 | 2.63 | 1.69, 4.12 | 5.68 | 3.39, 9.52 |
| Quartile 2 | 1.00 | Referent | 1.36 | 0.89, 2.09 | 1.75 | 1.14, 2.70 | 1.89 | 1.26, 2.83 | 3.72 | 2.23, 6.21 |
| Quartile 3 | 1.00 | Referent | 1.39 | 0.87, 2.24 | 1.66 | 1.02, 2.71 | 2.28 | 1.53, 3.40 | 2.90 | 1.69, 5.00 |
| Quartile 4 | 1.00 | Referent | 0.82 | 0.49, 1.36 | 1.78 | 1.13, 2.79 | 1.96 | 1.35, 2.85 | 2.39 | 1.43, 3.97 |
| Multiplicative Pinteractionb = 0.04 | ||||||||||
| Additive Pinteractionc = 0.55 | ||||||||||
| Animal protein | ||||||||||
| Quartile 1 | 1.00 | Referent | 1.83 | 1.05, 3.19 | 2.27 | 1.26, 4.12 | 2.93 | 1.77, 4.84 | 5.28 | 2.96, 9.43 |
| Quartile 2 | 1.00 | Referent | 1.03 | 0.62, 1.71 | 1.33 | 0.80, 2.21 | 2.29 | 1.51, 3.47 | 3.43 | 1.99, 5.89 |
| Quartile 3 | 1.00 | Referent | 0.97 | 0.61, 1.54 | 1.65 | 1.05, 2.59 | 1.69 | 1.15, 2.47 | 3.56 | 2.20, 5.75 |
| Quartile 4 | 1.00 | Referent | 1.02 | 0.65, 1.60 | 1.63 | 1.01, 2.40 | 2.16 | 1.52, 3.05 | 2.74 | 1.65, 4.54 |
| Multiplicative Pinteractionb = 0.20 | ||||||||||
| Additive Pinteractionc = 0.28 | ||||||||||
Abbreviations: CI, confidence interval; HEALS, Health Effects of Arsenic Longitudinal Study; HR, hazard ratio.
Multivariate-adjusted model includes age, sex, water arsenic, body mass index, education, television ownership, land ownership, smoking, and follow-up wave.
Multiplicative Pinteraction term generated by multiplying well water arsenic exposure score (e.g., 1–5) by factor scores quartiles (e.g., 1–4).
Additive P value for the relative excess risk due to interaction (refer to Materials and Methods).
For the nutrients that have been previously suggested to play a role in arsenic metabolism/toxicity (riboflavin, pyridoxine, folate, calcium, protein, fiber, cysteine, methionine, cobalamin, iron, zinc, niacin, and vitamins A, C, and E; refer to the Discussion), we tested correlations between the log-transformed energy-adjusted estimates of nutrient intakes and dietary factor scores. Most nutrients showed significant correlations with all 3 scores; the nutrients most correlated with the gourd and vegetable pattern were vitamin C (r = 0.43), vitamin A (r = 0.37), vitamin E (r = 0.34), folate (r = 0.27), calcium (r = 0.25), and fiber (r = 0.25). However, adjusting for these nutrients did not affect our estimates of association or interaction. The associations between micronutrients and skin lesion risk likely will be formally addressed in a future analysis.
DISCUSSION
This is the first study to assess associations among dietary patterns, arsenic exposure, and skin lesion risk. We used factor analysis to derive 3 dietary patterns from FFQ data. An increasing gourd and root factor score showed a clear association with decreasing skin lesion risk. This score also modified the association between water arsenic exposure and skin lesions risk, with decreased arsenic-related risk for individuals with high gourd and root scores. Similar associations and interactions were observed for the vegetable pattern score and the animal protein pattern score, but these associations were not statistically significant.
The motivation for dietary pattern analysis is based on the inherent difficulty of accurately measuring specific nutrients using FFQs and isolating the effects of individual nutrients in the context of a complex diet of correlated foods and nutrients (12, 13, 15). Using the dietary pattern approach, we did not measure specific nutrients, but rather focused on broad patterns derived from the correlation structure of the FFQ data.
In Bangladesh, the derived dietary pattern scores are likely related to the concepts of “nutrient intake inadequacy” (30) and “dietary diversity” (31), with low pattern scores reflecting a general micronutrient deficiency. In other words, individuals consuming diets that are primarily rice (i.e., lacking nutrient diversity) will have low factor scores, as steamed rice, the primary source of calories in this population, does not have a strong positive loading on any factor. In contrast, individuals consuming diets rich in many nonrice items will have higher factor scores. The few studies that have examined overall undernutrition and skin lesion risk have used body mass index as a surrogate of undernutrition (rather than measuring the diet itself) and have focused on prevalent rather than incident skin lesions (32, 33).
To our knowledge, there are no prior studies of dietary patterns, arsenic, and skin lesion risk; however, the roles of specific nutrients have been explored. In a cross-sectional analysis of baseline HEALS data, FFQ-derived intakes of several B vitamins (riboflavin, pyridoxine, and folic acid) and antioxidants (vitamins A, C, and E) showed inverse associations with prevalent skin lesions and interactions with arsenic exposure (34). A case-control study in West Bengal, India, assessed diet by using 24-hour and 1-week recalls and concluded that low intakes of calcium, animal protein, folate, and fiber were associated with increased skin lesion risk (35). Serum folate has been shown to have an inverse association with skin lesion risk (36).
Several authors have explored the role of dietary factors in arsenic metabolism (i.e., the conversion of inorganic arsenic to monomethylaronic acid and dimethylarsinic acid), as variation in metabolism may affect arsenic-related health risks. Using HEALS FFQ data, Heck et al. (24, 37) found that high intakes of protein, methionine, and cysteine were associated with increased arsenic excretion (i.e., increased total arsenic in urine) and that high intakes of cysteine, methionine, calcium, protein, and cobalamin were associated with increased urinary concentrations of arsenic metabolites. Data from the United States indicate that individuals with low protein, iron, zinc, and niacin have a higher percentage of urinary monomethylaronic acid (and a lower percentage of dimethylarsinic acid) (38). Plasma folate was positively associated with the percentage of urinary dimethylarsinic acid and negatively associated with the percentages of urinary monomethylaronic acid and inorganic arsenic in HEALS (39), suggesting that folate and other factors involved in 1-carbon metabolism influence arsenic metabolism. Accordingly, folate supplementation appears to influence arsenic methylation (40) and lower plasma arsenic concentrations (41) in folate-deficient individuals.
The research described above provides evidence that a wide array of dietary factors may influence arsenic metabolism and/or skin lesion risk. The gourd and root pattern score was correlated with intakes of some of these (refer to Results); however, adjusting for any of these nutrients did not alter our results, suggesting that no single nutrient accounts for the observed association and interaction.
We used 1-df tests for interaction between the ordinal arsenic and dietary pattern variables. For the gourd and root pattern and the animal protein pattern, we observed additive and multiplicative interaction estimates that lead us to similar conclusions regarding “effect modification” (i.e., individuals with high gourd and root scores had lower arsenic-related risk, while animal protein scores did not alter risk substantially). In contrast, for the vegetable pattern, we observe multiplicative, but not additive, interaction. However, it is well known that the presence or absence of interaction depends upon the scale used to measure it (42), so the interpretation of these results depends upon on an understanding of scale dependence for interactions.
In this work, we did not consider how drinking habits affect one's true arsenic exposure. However, in previous analyses (unpublished), we have shown that using exposure measures that incorporate information on daily water consumption (i.e., “daily arsenic dose”), duration of well use (i.e., “cumulative arsenic index”), and past water sources (“average daily arsenic dose”) results in associations with skin lesion risk that are nearly identical to those observed for the water arsenic concentration variable. Thus, we only examined well water arsenic in this analysis. This measure is likely to represent long-term chronic arsenic exposure, as all HEALS participants were required to have lived at their current residence for at least 5 years prior to recruitment and consumed water from their current well for at least 3 years (19).
Our reliance on FFQ data is a limitation, as the FFQ is not the ideal method to assess food intakes (43). However, the FFQ was designed and validated for the HEALS population (21), and their relatively simple diet resulted in a small number of food items, which did not have to be subjectively combined into fewer categories for analysis purposes. We chose a commonly used analysis method (i.e., maximum likelihood factor analysis with a varimax rotation), although using principal component analysis produced very similar results. The factors were selected according to established criteria (i.e., eigenvalues >1 and the scree plot) (12). The derived patterns were very similar to those identified in a previous HEALS analysis (44), despite the fact that the previous analysis had different exclusion criteria, did not log-transform food intakes, and used principal component factor analysis. This gives us confidence that the observed patterns are robust and can be derived using various analytical methods.
Because of the prospective nature of this study, our results are not likely to be affected by recall bias, selection bias, or reverse causality. We attempted to control for confounding by socioeconomic status using 3 related factors: education, land ownership, and television ownership. We were unable to control for physical activity, except through adjustment for body mass index, occupation, and total energy intake.
This study provides additional support for the hypothesis that diet plays a critical role in susceptibility to arsenic-related toxicity. Although reducing arsenic exposure is the ideal way to reduce skin lesion risk in Bangladesh (45), eating a diet rich in gourds and root vegetables and increasing dietary diversity may further reduce risk. This work suggests that the effects of diet on skin lesion susceptibility are likely to be complex and may be difficult to assess in observational research when examining one nutrient at a time.
Acknowledgments
Author affiliations: Department of Health Studies, University of Chicago, Chicago, Illinois (Brandon L. Pierce, Maria Argos, Stephanie Melkonian, Paul J. Rathouz, Habibul Ahsan); Department of Environmental Medicine, New York University, New York, New York (Yu Chen); Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, New York (Faruque Parvez, Habibul Ahsan); Columbia University and University of Chicago Research Office in Bangladesh, Mohakhali, Dhaka, Bangladesh (Tariqul Islam, Alauddin Ahmed, Rabiul Hasan); and Departments of Medicine and Human Genetics and Cancer Research Center, University of Chicago, Chicago, Illinois (Habibul Ahsan).
The work was supported by the National Institutes of Health (grants P42ES010349, R01CA102484, R01CA107431, CA014599).
The authors would like to thank all HEALS staff and fieldworkers for their ongoing commitment to the study. They would also like to thank Dr. Joseph Graziano for his comments on this manuscript prior to submission.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- FFQ
food frequency questionnaire
- HEALS
Health Effects of Arsenic Longitudinal Study
- HR
hazard ratio
- RERI
relative excess risk due to interaction
References
- 1.Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ. 2000;78(9):1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 2.Celik I, Gallicchio L, Boyd K, et al. Arsenic in drinking water and lung cancer: a systematic review. Environ Res. 2008;108(1):48–55. doi: 10.1016/j.envres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Mink PJ, Alexander DD, Barraj LM, et al. Low-level arsenic exposure in drinking water and bladder cancer: a review and meta-analysis. Regul Toxicol Pharmacol. 2008;52(3):299–310. doi: 10.1016/j.yrtph.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Waalkes MP. Liver is a target of arsenic carcinogenesis. Toxicol Sci. 2008;105(1):24–32. doi: 10.1093/toxsci/kfn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu HS, Liao WT, Chai CY. Arsenic carcinogenesis in the skin. J Biomed Sci. 2006;13(5):657–666. doi: 10.1007/s11373-006-9092-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen CJ, Chen CW, Wu MM, et al. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992;66(5):888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Y, Marshall G, Ferreccio C, et al. Kidney cancer mortality: fifty-year latency patterns related to arsenic exposure. Epidemiology. 2010;21(1):103–108. doi: 10.1097/EDE.0b013e3181c21e46. [DOI] [PubMed] [Google Scholar]
- 8.Brinkel J, Khan MH, Kraemer A. A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. Int J Environ Res Public Health. 2009;6(5):1609–1619. doi: 10.3390/ijerph6051609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vahidnia A, van der Voet GB, de Wolff FA. Arsenic neurotoxicity—a review. Hum Exp Toxicol. 2007;26(10):823–832. doi: 10.1177/0960327107084539. [DOI] [PubMed] [Google Scholar]
- 10.States JC, Srivastava S, Chen Y, et al. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107(2):312–323. doi: 10.1093/toxsci/kfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anetor JI, Wanibuchi H, Fukushima S. Arsenic exposure and its health effects and risk of cancer in developing countries: micronutrients as host defence. Asian Pac J Cancer Prev. 2007;8(1):13–23. [PubMed] [Google Scholar]
- 12.Michels KB, Schulze MB. Can dietary patterns help us detect diet-disease associations? Nutr Res Rev. 2005;18(2):241–248. doi: 10.1079/NRR2005107. [DOI] [PubMed] [Google Scholar]
- 13.Kant AK. Dietary patterns and health outcomes. J Am Diet Assoc. 2004;104(4):615–635. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62(5):177–203. doi: 10.1301/nr.2004.may.177-203. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Pierce BL, Kalra T, Argos M, et al. A prospective study of body mass index and mortality in Bangladesh. Int J Epidemiol. 2010;39(4):1037–1045. doi: 10.1093/ije/dyp364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh N, Kumar D, Sahu AP. Arsenic in the environment: effects on human health and possible prevention. J Environ Biol. 2007;28(suppl 2):S359–S365. [PubMed] [Google Scholar]
- 18.Ahsan H, Chen Y, Parvez F, et al. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16(2):191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 19.Ahsan H, Chen Y, Parvez F, et al. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the Health Effects of Arsenic Longitudinal Study. Am J Epidemiol. 2006;163(12):1138–1148. doi: 10.1093/aje/kwj154. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Z, Zheng Y, Mortlock R, et al. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379(3):512–518. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Ahsan H, Parvez F, et al. Validity of a food-frequency questionnaire for a large prospective cohort study in Bangladesh. Br J Nutr. 2004;92(5):851–859. doi: 10.1079/bjn20041277. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Agriculture Agricultural Research Service. Nutrient Data Laboratory. USDA nutrient database for standard reference, release 15. Beltsville, MD: Beltsville Human Nutrition Research Center; 2002. ( http://www.ars.usda.gov/Services/docs.htm?docid=8964) [Google Scholar]
- 23.Willett W. Nutritional Epidemiology. New York, NY: Oxford University Press; 1990. [Google Scholar]
- 24.Heck JE, Nieves JW, Chen Y, et al. Dietary intake of methionine, cysteine, and protein and urinary arsenic excretion in Bangladesh. Environ Health Perspect. 2009;117(1):99–104. doi: 10.1289/ehp.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer JD, Willett JB. It's about time: using discrete-time survival analysis to study duration and the timing of events. J Educ Behav Stat. 1993;18(2):155–195. [Google Scholar]
- 26.Chizmar JF. A discrete-time hazard analysis of the role of gender in persistence in the economics major. J Econ Educ. 2000;31(2):107–118. [Google Scholar]
- 27.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Knol MJ, van der Tweel I, Grobbee DE, et al. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36(5):1111–1118. doi: 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- 30.Román-Viñas B, Ribas Barba L, Ngo J, et al. Validity of dietary patterns to assess nutrient intake adequacy. Br J Nutr. 2009;101(suppl 2):S12–S20. doi: 10.1017/S0007114509990547. [DOI] [PubMed] [Google Scholar]
- 31.Thorne-Lyman AL, Valpiani N, Sun K, et al. Household dietary diversity and food expenditures are closely linked in rural Bangladesh, increasing the risk of malnutrition due to the financial crisis. J Nutr. 2010;140(1):182S–188S. doi: 10.3945/jn.109.110809. [DOI] [PubMed] [Google Scholar]
- 32.Maharjan M, Watanabe C, Ahmad SA, et al. Mutual interaction between nutritional status and chronic arsenic toxicity due to groundwater contamination in an area of Terai, lowland Nepal. J Epidemiol Community Health. 2007;61(5):389–394. doi: 10.1136/jech.2005.045062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milton AH, Hasan Z, Shahidullah SM, et al. Association between nutritional status and arsenicosis due to chronic arsenic exposure in Bangladesh. Int J Environ Health Res. 2004;14(2):99–108. doi: 10.1080/0960312042000209516. [DOI] [PubMed] [Google Scholar]
- 34.Zablotska LB, Chen Y, Graziano JH, et al. Protective effects of B vitamins and antioxidants on the risk of arsenic-related skin lesions in Bangladesh. Environ Health Perspect. 2008;116(8):1056–1062. doi: 10.1289/ehp.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitra SR, Mazumder DN, Basu A, et al. Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environ Health Perspect. 2004;112(10):1104–1109. doi: 10.1289/ehp.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilsner JR, Liu X, Ahsan H, et al. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ Health Perspect. 2009;117(2):254–260. doi: 10.1289/ehp.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heck JE, Gamble MV, Chen Y, et al. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr. 2007;85(5):1367–1374. doi: 10.1093/ajcn/85.5.1367. [DOI] [PubMed] [Google Scholar]
- 38.Steinmaus C, Carrigan K, Kalman D, et al. Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect. 2005;113(9):1153–1159. doi: 10.1289/ehp.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamble MV, Liu X, Ahsan H, et al. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect. 2005;113(12):1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gamble MV, Liu X, Ahsan H, et al. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am J Clin Nutr. 2006;84(5):1093–1101. doi: 10.1093/ajcn/84.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gamble MV, Liu X, Slavkovich V, et al. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr. 2007;86(4):1202–1209. doi: 10.1093/ajcn/86.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 43.Kristal AR, Peters U, Potter JD. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol Biomarkers Prev. 2005;14(12):2826–2828. doi: 10.1158/1055-9965.EPI-12-ED1. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Factor-Litvak P, Howe GR, et al. Nutritional influence on risk of high blood pressure in Bangladesh: a population-based cross-sectional study. Am J Clin Nutr. 2006;84(5):1224–1232. doi: 10.1093/ajcn/84.5.1224. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, van Geen A, Graziano JH, et al. Reduction in urinary arsenic levels in response to arsenic mitigation efforts in Araihazar, Bangladesh. Environ Health Perspect. 2007;115(6):917–923. doi: 10.1289/ehp.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]