Abstract
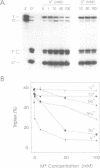
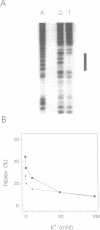
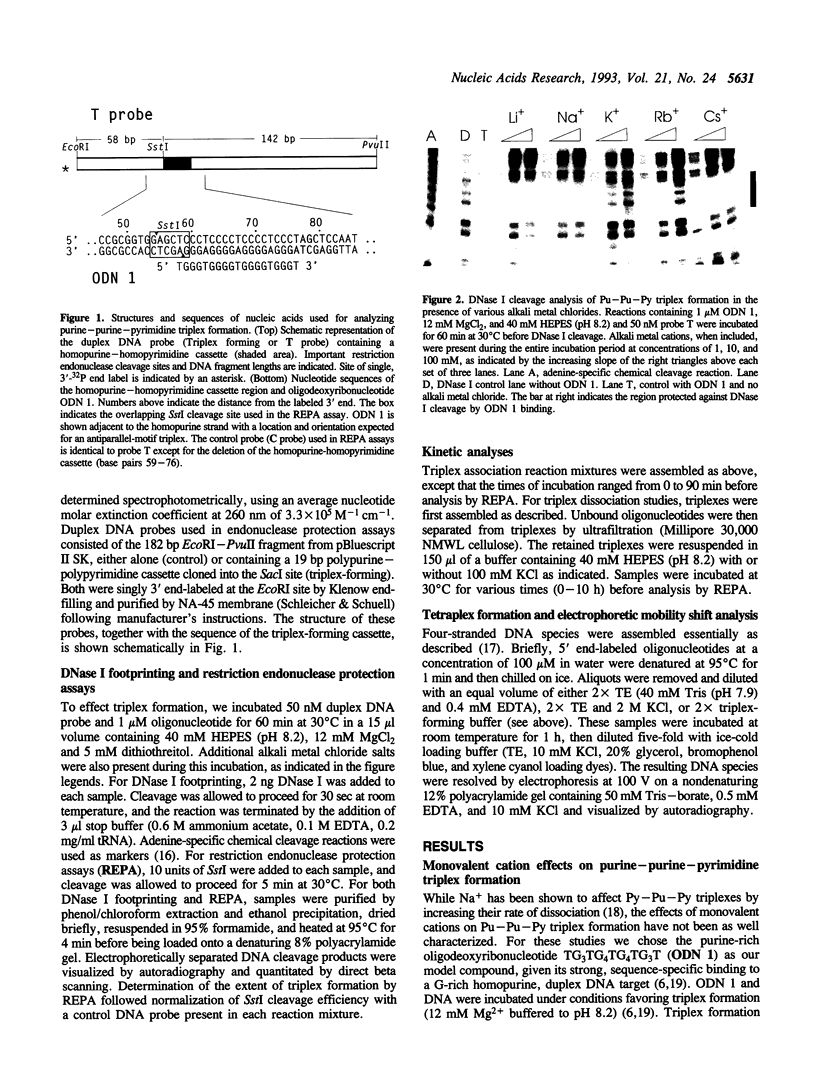
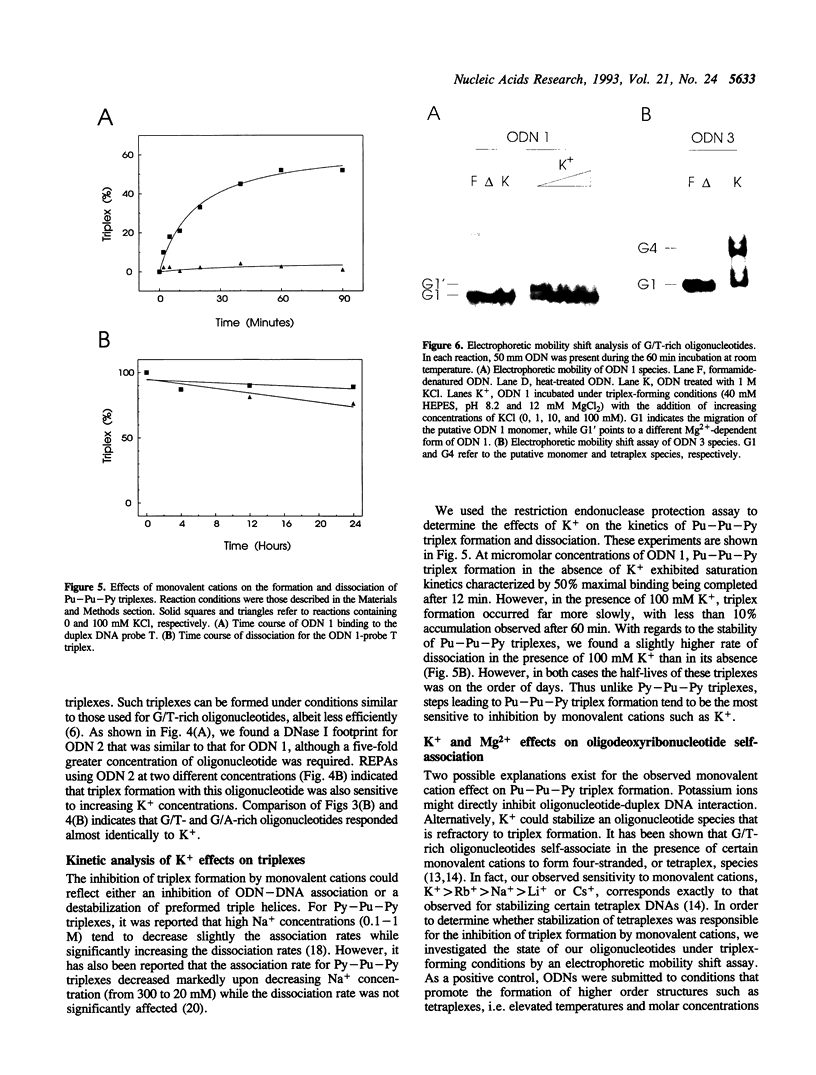
The binding of a 19-mer guanosine-rich oligodeoxyribonucleotide, TG3TG4TG4TG3T (ODN 1), to a complementary polypurine DNA target was investigated by DNase I footprinting and restriction endonuclease protection assays. Monovalent cations inhibited intermolecular purine-purine-pyrimidine triple-helical DNA formation, with K+ and Rb+ being most effective, followed by NH4+ and Na+. Li+ and Cs+ had little to no effect. Similar results were observed with the G/A-rich oligonucleotide AG3AG4AG4AG3AGCT. Kinetic studies indicated that monovalent cations interfered with oligonucleotide-duplex DNA association but did not significantly promote triplex dissociation. The observed order of monovalent cation inhibition of triplex formation is reminiscent of their effect on tetraplex formation with G/T-rich oligonucleotides. However, using electrophoretic mobility shift assays we found that the oligonucleotide ODN 1 did not appear to form a four-stranded species under conditions promoting tetraplex formation. Taken together, our data suggest that processes other than the self-association of oligonucleotides into tetraplexes might be involved in the inhibitory effect of monovalent cations on purine-pyrimidine-purine triplex formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal P. A., Dervan P. B. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science. 1991 Mar 15;251(4999):1360–1363. doi: 10.1126/science.2003222. [DOI] [PubMed] [Google Scholar]
- Beal P. A., Dervan P. B. The influence of single base triplet changes on the stability of a pur.pur.pyr triple helix determined by affinity cleaving. Nucleic Acids Res. 1992 Jun 11;20(11):2773–2776. doi: 10.1093/nar/20.11.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergemann A. D., Johnson E. M. The HeLa Pur factor binds single-stranded DNA at a specific element conserved in gene flanking regions and origins of DNA replication. Mol Cell Biol. 1992 Mar;12(3):1257–1265. doi: 10.1128/mcb.12.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergemann A. D., Ma Z. W., Johnson E. M. Sequence of cDNA comprising the human pur gene and sequence-specific single-stranded-DNA-binding properties of the encoded protein. Mol Cell Biol. 1992 Dec;12(12):5673–5682. doi: 10.1128/mcb.12.12.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney M., Czernuszewicz G., Postel E. H., Flint S. J., Hogan M. E. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science. 1988 Jul 22;241(4864):456–459. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durland R. H., Kessler D. J., Gunnell S., Duvic M., Pettitt B. M., Hogan M. E. Binding of triple helix forming oligonucleotides to sites in gene promoters. Biochemistry. 1991 Sep 24;30(38):9246–9255. doi: 10.1021/bi00102a017. [DOI] [PubMed] [Google Scholar]
- Giovannangéli C., Rougée M., Garestier T., Thuong N. T., Hélène C. Triple-helix formation by oligonucleotides containing the three bases thymine, cytosine, and guanine. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8631–8635. doi: 10.1073/pnas.89.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C. The anti-gene strategy: control of gene expression by triplex-forming-oligonucleotides. Anticancer Drug Des. 1991 Dec;6(6):569–584. [PubMed] [Google Scholar]
- Ing N. H., Beekman J. M., Kessler D. J., Murphy M., Jayaraman K., Zendegui J. G., Hogan M. E., O'Malley B. W., Tsai M. J. In vivo transcription of a progesterone-responsive gene is specifically inhibited by a triplex-forming oligonucleotide. Nucleic Acids Res. 1993 Jun 25;21(12):2789–2796. doi: 10.1093/nar/21.12.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson B. L., Dervan P. B. Adenine specific DNA chemical sequencing reaction. Nucleic Acids Res. 1987 Oct 12;15(19):7823–7830. doi: 10.1093/nar/15.19.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Praseuth D., Habhoub N., Decout J. L., Thuong N. T., Lhomme J., Hélène C. Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-[alpha]-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res. 1987 Oct 12;15(19):7749–7760. doi: 10.1093/nar/15.19.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd DNA triple-helix formation: an approach to artificial gene repressors? Bioessays. 1992 Dec;14(12):807–815. doi: 10.1002/bies.950141204. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dervan P. B., Wold B. J. Kinetic analysis of oligodeoxyribonucleotide-directed triple-helix formation on DNA. Biochemistry. 1990 Sep 18;29(37):8820–8826. doi: 10.1021/bi00489a045. [DOI] [PubMed] [Google Scholar]
- McShan W. M., Rossen R. D., Laughter A. H., Trial J., Kessler D. J., Zendegui J. G., Hogan M. E., Orson F. M. Inhibition of transcription of HIV-1 in infected human cells by oligodeoxynucleotides designed to form DNA triple helices. J Biol Chem. 1992 Mar 15;267(8):5712–5721. [PubMed] [Google Scholar]
- Milligan J. F., Krawczyk S. H., Wadwani S., Matteucci M. D. An anti-parallel triple helix motif with oligodeoxynucleotides containing 2'-deoxyguanosine and 7-deaza-2'-deoxyxanthosine. Nucleic Acids Res. 1993 Jan 25;21(2):327–333. doi: 10.1093/nar/21.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Orson F. M., Thomas D. W., McShan W. M., Kessler D. J., Hogan M. E. Oligonucleotide inhibition of IL2R alpha mRNA transcription by promoter region collinear triplex formation in lymphocytes. Nucleic Acids Res. 1991 Jun 25;19(12):3435–3441. doi: 10.1093/nar/19.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel E. H., Flint S. J., Kessler D. J., Hogan M. E. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8227–8231. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougée M., Faucon B., Mergny J. L., Barcelo F., Giovannangeli C., Garestier T., Hélène C. Kinetics and thermodynamics of triple-helix formation: effects of ionic strength and mismatches. Biochemistry. 1992 Sep 29;31(38):9269–9278. doi: 10.1021/bi00153a021. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Van Dyke M. W. A rapid method for the purification of deprotected oligodeoxynucleotides. Nucleic Acids Res. 1991 Feb 11;19(3):674–674. doi: 10.1093/nar/19.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D., Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature. 1990 Mar 29;344(6265):410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Novel DNA superstructures formed by telomere-like oligomers. Biochemistry. 1992 Jan 14;31(1):65–70. doi: 10.1021/bi00116a011. [DOI] [PubMed] [Google Scholar]
- Venczel E. A., Sen D. Parallel and antiparallel G-DNA structures from a complex telomeric sequence. Biochemistry. 1993 Jun 22;32(24):6220–6228. doi: 10.1021/bi00075a015. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Raghuraman M. K., Cech T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989 Dec 1;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]