Abstract
Many studies have chronicled the “epidemiologic synergy” between human immunodeficiency virus (HIV) and herpes simplex virus type 2 (HSV-2). HIV adversely affects the natural history of HSV-2 and results in more frequent and severe HSV-2 reactivation. Few longitudinal studies, however, have examined whether HSV-2 is associated with increased HIV plasma viral loads or decreased CD4 counts. The authors estimated the effect of HSV-2 seropositivity on HIV RNA viral load and on CD4 count over time among 777 HIV-seropositive US women not receiving suppressive HSV-2 therapy in the HIV Epidemiology Research Study (1993–2000). Linear mixed models were used to assess the effect of HSV-2 on log HIV viral load and CD4 count/mm3 prior to widespread initiation of highly active antiretroviral therapy. Coinfection with HSV-2 was not associated with HIV RNA plasma viral loads during study follow-up. There was a statistically significant association between HSV-2 seropositivity and CD4 count over time, but this difference was small and counterintuitive at an increase of 8 cells/mm3 (95% confidence interval: 2, 14) per year among HSV-2-seropositive women compared with HSV-2-seronegative women. These data do not support a clinically meaningful effect of baseline HSV-2 seropositivity on the trajectories of HIV plasma viral loads or CD4 counts.
Keywords: CD4 lymphocyte count; herpes simplex; herpesvirus 2, human; HIV; viral load
Many studies have chronicled the “epidemiologic synergy” between human immunodeficiency virus (HIV) and herpes simplex virus type 2 (HSV-2) infections (1–3). Chronic perianal ulceration due to HSV-2 infection was one of the first opportunistic infections identified among acquired immunodeficiency syndrome (AIDS) patients (4). HSV-2 is one of the most common coinfections in HIV-positive individuals, and more serious clinical manifestations of HSV-2 occur in those coinfected with HIV (5–7). Reactivation of HSV-2 genital lesions is also more frequent among women with HSV-2/HIV coinfections (8).
Few studies have examined the effect of HSV-2 on the progression of HIV infection (8). A cross-sectional study of men and women in Rakai, Uganda, found that HSV-2 seropositivity was associated with increased HIV viral load in those with early HIV infection, but not in those with prevalent HIV infection (9). A second observational study in Uganda found that individuals with prevalent HIV infection coinfected with HSV-2 had viral loads 0.3-log-copies/mL higher than those without HSV-2 infection (10). This finding led to speculation that HSV-2 infection may affect the set point of HIV viral load, leading to accelerated disease progression following initial HIV acquisition (8). Other coinfections in HIV-positive populations, such as tuberculosis and hepatitis, have been associated with a more rapid decline in CD4+ T cells and accelerated time to AIDS (11, 12). In addition, other herpesviruses have been associated with AIDS-defining illnesses. Human herpesvirus 8, the etiologic agent of Kaposi’s sarcoma, stimulates HIV replication in acutely infected cells as well as reactivation in chronically infected cells (13). If HSV-2 is shown to be associated with higher HIV viral loads and a more rapid decline in CD4 counts in HIV-positive individuals, screening HIV-positive individuals for HSV-2 seropositivity and treating HSV/HIV-coinfected individuals with daily HSV-2 suppressive therapy may be warranted in addition to highly active antiretroviral therapy (HAART) to slow HIV progression.
Following the initial hypothesis that HSV-2 may affect HIV progression, 2 subsequent longitudinal studies among US men with early HIV infection found no association between HSV-2 seropositivity and HIV viral load (14, 15). Data on the effect of HSV-2 seropositivity on HIV viral load in HIV-infected individuals are therefore inconclusive and are limited in women. Few data exist on the effect of HSV-2 seropositivity on CD4 count. In this study, we investigated the associations of HSV-2 seropositivity with HIV plasma viral load and CD4+ T-lymphocyte count over time among US women enrolled in the HIV Epidemiology Research Study (HERS).
MATERIALS AND METHODS
Study design
The HERS study followed a cohort of women over a period of 7 years (1993–2000) to examine the biologic, psychological, and social effects of HIV infection on women's health. The study enrolled 871 HIV-infected and 439 HIV-uninfected women aged 16–55 years from 4 cities (Baltimore, Maryland; Bronx, New York; Detroit, Michigan; and Providence, Rhode Island). The study rationale, organization, and methods have been described in detail elsewhere (16).
Only women who were HIV-positive at baseline (n = 871), had baseline HSV-2 results (25 women excluded), and had at least 2 study visits (69 women excluded) were included in the analyses, leaving a total of 777 women. To address confounding by indication for HAART use, data were right censored at January 1, 1998, before HAART use was widespread in the HERS study (n = 2,125 visits excluded). Visits where women reported current use of acyclovir or valacyclovir (n = 51 visits) were also excluded from the analysis. These exclusions left 5,346 visits (71.1% of the total visits) for analysis, with up to 10 possible study visits per woman (each at approximately 6-month intervals).
Laboratory methods
Antibodies to HIV were identified by enzyme-linked immunosorbent assay and were confirmed by Western blot using procedures standardized across study sites (16). Heparinized whole blood was stained with monoclonal antibodies using a modified whole blood method, and percentages of CD4 T cells and CD4 counts were determined by flow cytometry and were calculated from complete blood counts with differential, respectively (17). Plasma HIV viral load was measured by the branched DNA (bDNA) technique with lower limits of detection of 500 and 50 copies/mL (Chiron Corporation, Emeryville, California). Date of immunologic AIDS diagnosis was defined as the visit date on which a participant first had a CD4 count less than 200 or a CD4 percentage less than 14. Date of clinical AIDS diagnosis was defined as the date on which one or more of the AIDS-defining conditions included in the 1987 Centers for Disease Control and Prevention definition were present on a participant's medical record abstraction form (18).
Sera obtained at baseline were tested for HSV-2 type-specific antibodies using the reference standard Western blot at the University of Washington. Laboratory measures for the detection of syphilis, gonorrhea, Chlamydia trachomatis, Trichomonas vaginalis, and Candida are described in detail elsewhere (16).
Baseline statistical analyses
Associations between HSV-2 serostatus and baseline characteristics were calculated using the Wilcoxon rank sum test and the χ2 statistic. For categorical variables with any expected cell size less than 10, Fisher's exact test was used.
HIV RNA viral load was log10-transformed and measured in log10 copies per milliliter, whereas CD4 T cells were quantified as the number of cells per cubic millimeter of blood divided by 100. HIV viral load values below the detection limits of 500 or 50 copies/mL were assigned the midpoint value of 250 or 25 copies/mL, respectively.
Mixed models
Separate linear mixed models were used to evaluate associations between the main exposure, HSV-2 serostatus at baseline, and 1) HIV RNA viral load and 2) CD4 count over time. Covariates considered for inclusion in both models were time since baseline, the interaction between time and HSV-2 serostatus, presence of other genital infections at baseline, race, and age at baseline. Including an interaction term between HSV-2 and time allowed estimation of the effect of HSV-2 at baseline on change in viral load or CD4. This was the main variable of interest. Presence of other genital infections was coded as a dichotomous variable and was used as a surrogate for genital immune reactivity; women with T. vaginalis, Candida, chlamydia, syphilis, or gonorrhea in the 6 months prior to the baseline visit received a value of 1, whereas women without any of these genital infections received a 0. Race was coded as a dichotomous variable indicating whether or not a woman was black or nonblack.
For the model with viral load as the outcome, CD4 count at baseline was included as a covariate; similarly, for the model with CD4 count as the outcome, viral load at baseline was included as a covariate. All continuous variables (baseline age, baseline CD4 count, and baseline viral load) were centered at the median. To allow nonlinear relations with the outcome, cubic splines with 4 knots at the 5th, 35th, 65th, and 95th percentiles were fit for the continuous variables age, CD4 count, and viral load. The splines were restricted to be linear beyond the outer knots, and nonlinear terms were normalized by dividing by the square of the difference in the outer knots (19). Nonlinear relations were explored by including quadratic terms for time and the interaction between time and HSV-2. Interaction terms were retained in the model if the likelihood ratio test comparing the model without the interaction term with the full model was statistically significant at an alpha level of 0.05. Potential confounders were retained in the model if removing them changed the HSV-2 coefficient estimate by 10% or more (20).
A mixed model was first fit with random intercepts for each subject, and then models were fit by adding random effects for slopes. The random effect for slopes did not improve the fit of the model, and only random intercepts were used in subsequent models. Model fit was evaluated using likelihood ratio test statistics and by examining the percentage of variation not explained by the random effects included in the model. The effect of HSV-2 was evaluated by examining the coefficients for HSV-2 and interaction terms with time and the polynomial term for time.
Sensitivity analyses
To examine the effect of antiretroviral use that remained after censoring the data at January 1, 1998, we performed a sensitivity analysis in which we restricted the mixed models to those person-visits where participants reported no current use of antiretrovirals. We also performed a sensitivity analysis in which we removed all visits where women reported current use of the antiviral medications Cytovene (Roche Pharmaceuticals, Nutley, New Jersey), Foscavir (Astra Merck, Inc., Wayne, Pennsylvania), or amantadine because these medications have suppressive effects on HSV-2.
Proportional hazards model
The association between HSV-2 and time to clinical or immunologic AIDS diagnosis was evaluated among HIV-positive women at baseline using Cox proportional hazards models. The models were adjusted for CD4 count at baseline, presence of other genital infections in the 6 months prior to baseline, race, and age at baseline centered at the median of 35 years. Again, the data were censored at January 1, 1998, to examine the study period before HAART use was widespread. The proportional hazards assumption was evaluated by including interactions with categorical and continuous time variables.
The analyses reported in this paper were generated using SAS software, version 9.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
The median age of the 777 HIV-positive eligible women was 35 years. Black, non-Hispanic was the largest racial group (61.5%), followed by white, non-Hispanic (20.2%) and Hispanic/Latino (18.3%). Most women were single (43.0%), with a median reported age at first intercourse of 15 years. Among those women sexually active at baseline, almost half (46.6%) reported that they did not always use a condom in the 6 months prior to the baseline visit. HSV-2-positive women were more likely to be black, non-Hispanic (P = 0.0003) and were more likely to be older (P = 0.0009), although the difference in median age between HSV-2-positive and HSV-2-negative women was small (Table 1).
Table 1.
Baseline Characteristics of 777 HIV-Positive US Women From the HIV Epidemiology Research Study (1993–2000), Stratified by HSV-2 Serostatus
| Characteristic | HSV-2 Seropositive (N = 527)a |
HSV-2 Seronegative (N = 250)a |
P Valueb | ||||||
| No. | % | Median | Range | No. | % | Median | Range | ||
| Baseline viral load, log copies/mL | 3.1 | 1.4–5.7 | 3.2 | 1.4–5.3 | 0.44 | ||||
| Baseline CD4 count, cells/100 | 362 | 6–1,684 | 412 | 4–1,577 | 0.06 | ||||
| Age, years | 36 | 19–55 | 34 | 19–53 | 0.0009 | ||||
| Age at first intercourse, years | 15 | 8–28 | 15 | 8–27 | 0.65 | ||||
| Race | 0.0003 | ||||||||
| Black | 347 | 65.8 | 131 | 52.4 | |||||
| Nonblack | 180 | 34.2 | 119 | 47.6 | |||||
| Marital status | 0.92 | ||||||||
| Married/cohabiting | 139 | 26.7 | 72 | 28.9 | |||||
| Separated/divorced | 108 | 20.8 | 50 | 20.1 | |||||
| Single | 224 | 43.1 | 106 | 42.6 | |||||
| Widowed | 48 | 9.2 | 21 | 8.4 | |||||
| Current smoker | 397 | 75.3 | 189 | 75.6 | 0.94 | ||||
| Condom use in the last 6 months | 0.55 | ||||||||
| No vaginal sex in the last 6 months | 136 | 26.1 | 58 | 23.3 | |||||
| Always used a condom | 202 | 38.7 | 106 | 42.6 | |||||
| Did not always use a condom | 184 | 35.2 | 85 | 34.1 | |||||
| No. of male sexual partners in the last 6 months | 1 | 1–99 | 1 | 1–25 | 0.22 | ||||
| Sexually transmitted infections | |||||||||
| HSV-1 seropositive | 375 | 71.2 | 201 | 80.4 | 0.006 | ||||
| Chlamydia culture positive | 3 | 0.9 | 3 | 1.46 | 0.68c | ||||
| Gonorrhea culture positive | 2 | 0.4 | 3 | 1.3 | 0.34c | ||||
| Syphilis seropositive | 53 | 10.2 | 12 | 4.9 | 0.015 | ||||
| Trichomonas culture or wet mount positive | 79 | 15.1 | 26 | 10.4 | 0.081 | ||||
| Candida culture positive | 205 | 39.6 | 86 | 34.7 | 0.19 | ||||
Abbreviations: HIV, human immunodeficiency virus; HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2.
Frequencies do not always add to the total number because of missing values.
P values were calculated using the Wilcoxon rank sum test for continuous variables and the χ2 statistic for categorical variables, unless noted otherwise.
P values were calculated using Fisher's exact test because of small cell sizes.
Approximately two-thirds of the women (67.8%) were seropositive for HSV-2 at baseline. Almost three-quarters of the women (74.1%) were seropositive for HSV-1 at baseline, 38% of the women were culture-positive for Candida (38.0%), and almost 13% were positive for T. vaginalis. Prevalences of chlamydia (1.2%) and gonorrhea (0.5%) were relatively low at baseline. Prevalence of syphilis was higher, at 7.7%. HSV-1-seronegative women were more likely to be HSV-2 seropositive, as were women with syphilis. Among HSV-2-seronegative women, the prevalence of hepatitis C virus was 60.4%, while the prevalence of hepatitis C virus among HSV-2-seropositive women was 60.8%. Since hepatitis C virus positivity was not associated with HSV-2 serostatus, it was not considered a confounder in the mixed models.
The median baseline HIV viral load was similar between HSV-2-seropositive (3.1 log copies/mL, 95% confidence interval: 1.4, 5.7) and HSV-2-seronegative (3.2 log copies/mL, 95% confidence interval: 1.4, 5.3) women (P = 0.44) (Table 1). Baseline CD4 count was slightly higher (P = 0.06) for those who were HSV-2 seronegative at baseline (median CD4 count 412 cells/mm3 compared with 362 cells/mm3 for HSV-2-seropositive women) (Table 1).
After censoring, women completed between 2 and 10 study visits, with a median of 7. There were 278 visits (5.2%) where a woman was currently receiving HAART after censoring. Women were not using any antiretrovirals at 3,074 visits (57.5%) and were on sub-HAART regimens at 1,992 visits (37.3%). Data on HAART use were missing for 2 women at one visit. There were 193 visits (3.6%) for which a value for viral load was missing and 175 visits (3.3%) for which a CD4 count was missing.
The final HIV viral load model was adjusted for baseline CD4 count and its associated spline terms, presence of genital infections, and race (Table 2). Age was not retained in the final model since removing it changed the HSV-2 coefficient estimate by less than 10%. The HSV-2 coefficient and the coefficients representing the interaction between HSV-2 and time were nonsignificant in the viral load model. We also assessed whether the effect of HSV-2 on viral load over time was nonlinear by including an interaction term between HSV and the polynomial term for time. The likelihood ratio test comparing the model without the interaction term with the model with it was nonsignificant (P = 0.10), so the term was not retained in the final model.
Table 2.
Summary of Estimated Regression Coefficients From Final Mixed Models for Associations Between Baseline HSV-2 Seropositivity and 1) HIV Viral Load and 2) CD4 Count/100 Over Study Follow-up, HIV Epidemiology Research Study, United States, 1993–2000
| Variable | HIV Log Viral Load Model |
CD4 Count/100 Model |
||||
| a | SE | P Value | a | SE | P Value | |
| Intercept | 2.80 | 0.12 | <0.0001 | 3.95 | 0.47 | <0.0001 |
| HSV-2 | 0.02 | 0.06 | 0.75 | −0.18 | 0.17 | 0.28 |
| Time since baseline | 0.38 | 0.03 | <0.0001 | −0.68 | 0.05 | <0.0001 |
| HSV-2 × time since baseline | −0.02 | 0.02 | 0.23 | 0.08 | 0.03 | 0.006 |
| Time since baseline squared | −0.10 | 0.01 | <0.0001 | 0.09 | 0.01 | <0.0001 |
| Baseline CD4 count/100b | −0.34 | 0.05 | <0.0001 | |||
| Baseline viral load (log10)b | −1.91 | 0.39 | <0.0001 | |||
| Race | 0.18 | 0.06 | 0.002 | 0.31 | 0.15 | 0.04 |
| Presence of genital infections | 0.12 | 0.05 | 0.03 | |||
Abbreviations: HIV, human immunodeficiency virus; HSV-2, herpes simplex virus type 2; SE, estimated standard error.
Estimated regression coefficients from the final mixed model.
Variable is centered at the median.
The final CD4 model was adjusted for baseline viral load and race. Neither age, presence of genital infections, nor the spline terms for baseline viral load were retained in the final model since their removal changed the HSV-2 coefficient estimate by less than 10%. The interaction term between HSV-2 and time was statistically significant (P = 0.006), indicating that HSV-2-seropositive women, compared with HSV-2-seronegative women, had an increase of 8 cells/mm3 per year over the study period. This result, while statistically significant, was in the opposite direction from that expected and is likely not clinically meaningful because of the small change in CD4 count over time. As in the viral load model, we assessed whether the effect of HSV-2 on CD4 over time was nonlinear by including an interaction term between HSV and the polynomial term for time. The likelihood ratio test comparing the model without the interaction term with the model with it was nonsignificant (P = 0.32), so the term was not retained in the final model.
HSV-2 therefore did not notably influence the trajectories of HIV viral load and CD4 counts over time. The mean predicted HIV plasma viral load values and CD4 counts from the mixed models are shown graphically in Figure 1A and 1B, respectively, stratified by HSV-2 serostatus. The graphs presented are the predicted lines for those individuals in the referent group of each category (median baseline viral load, median CD4 count, absence of genital infections, and nonblack race). Plots for other groups looked similar but are not shown.
Figure 1.
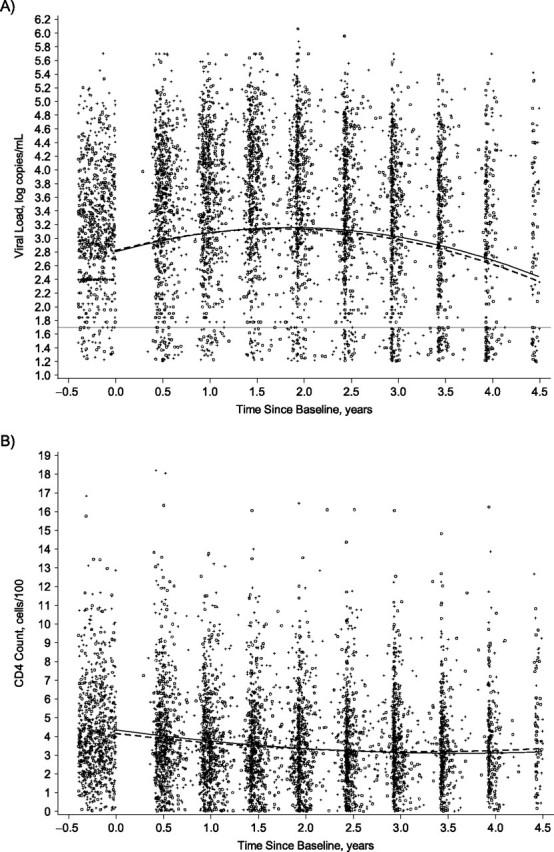
Mean predicted values from the mixed model by herpes simplex virus type 2 (HSV-2) status with individual visit data for A) human immunodeficiency virus (HIV) viral load (log copies/mL) and B) CD4 counts (cells/100), United States, 1993–2000. Solid line: HSV-2 seronegative at baseline; dashed line: HSV-2 seropositive at baseline; open circle: CD4 or HIV viral load for a woman HSV-2 seronegative at baseline; +: CD4 or HIV viral load for a woman HSV-2 seropositive at baseline. Horizontal line at viral load = 1.7 log copies/mL indicates the limit of detection. Refer to the Materials and Methods section of the text for more information about the HIV viral load and CD4 models. The graphs are the predicted lines for those women in the referent group of each category (median baseline viral load or median baseline CD4 count, absence of genital infections, and nonblack race). Data were jittered before time 0 in both graphs and around the viral load limit of detection in the viral load graph.
When data were restricted to the 57.5% of study visits where women reported no current use of antiretrovirals, results from the viral load model were similar. In the CD4 model, the interaction between HSV and time was smaller (HSV-2-seropositive women, compared with HSV-2-seronegative women, had an increase in CD4 of 3 cells/mm3 per year over the study period) and was nonsignificant (P = 0.45). When study visits where women reported current use of the antivirals Cytovene (n = 12 visits), Foscavir (n = 328 visits), and amantadine (n = 2 visits) were excluded, results were similar for both the viral load and CD4 models.
These results were consistent with the Kaplan-Meier curves examining the time to AIDS diagnosis by baseline HSV-2 serostatus (data not shown). The hazard ratio comparing HSV-2-seropositive women with those who were HSV-2 seronegative at baseline was 0.99 (95% confidence interval: 0.77, 1.23).
DISCUSSION
These data from the HERS study do not support a clinically meaningful effect of baseline HSV-2 seropositivity on the trajectories of HIV plasma viral loads or CD4 counts among 777 HIV-seropositive women. By censoring the data at January 1, 1998, and removing most visits where women were receiving HAART, we examined the effect of HSV-2 seropositivity on the natural history of HIV.
Our results are fairly consistent with those of several smaller studies examining the effect of HSV-2 on viral load and CD4. For instance, in a longitudinal cohort study in San Diego, California (15), and a cross-sectional study in Rakai, Uganda (9), no differences in HIV plasma viral load were observed between HSV-2-seropositive and HSV-2-seronegative men and women with prevalent HIV infection. In the San Diego study, the median HIV viral load was 5.04 log10 copies/mL (range: 1.70–7.24) among 172 HSV-2-seronegative individuals compared with a median HIV viral load of 4.87 log10 copies/mL (range: 1.70–7.76) among 122 HSV-2-seropositive individuals (15). Among 115 HSV-2-seronegative individuals with HIV infection in the Rakai study, the mean HIV plasma RNA viral load was 4.18 log10 copies/mL (standard deviation, 0.83) compared with a mean HIV plasma RNA viral load of 4.09 log10 copies/mL (standard deviation, 0.84) among 230 HSV-2-seropositive individuals (9).
In contrast to our results, a 2005 observational study of 339 individuals with prevalent HIV infection not receiving acyclovir or HAART in Uganda found that HSV-2 coinfection was associated with a 0.3-log10-copies/mL higher mean HIV viral load (4.6 (standard deviation, 0.94) vs. 4.3 (standard deviation, 0.91) log copies/mL, P = 0.014) (10). While the effect of HSV-2 on CD4 count over time was statistically significant in our study, it is likely not clinically meaningful. The lack of a strong association between HSV-2 seropositivity and CD4 counts is supported by the Ugandan study, where no change in CD4 count over time was observed between HSV-2-seropositive and HSV-2-seronegative individuals.
It is plausible that HSV-2 infection is associated with increased HIV RNA plasma viral load, given data from longitudinal studies of HSV-2 suppression that demonstrate an effect of acyclovir on reducing HIV RNA plasma viral load (21). Most of our study population was acyclovir-naïve; therefore, we could not examine this association directly. One possibility for the lack of an association between HSV-2 seropositivity and HIV viral load in our study and the presence of an association in HSV-2 suppression studies is that acyclovir acts directly on HIV RNA rather than through HSV-2 suppression. A recent study found that acyclovir suppresses HIV in human herpesvirus-coinfected human tissues. Once acyclovir is phosphorylated by human herpesvirus kinases, it can directly inhibit HIV reverse transcriptase (22). These data suggest that acyclovir's anti-HIV activity in the presence of HSV may contribute to the response of acyclovir in HIV/HSV–coinfected patients.
In terms of study strengths, HERS is one of the largest longitudinal studies of women with or at risk of HIV infection. With its 7 years of follow-up and high retention rate, it represents one of the largest studies to date to analyze the effect of HSV-2 serostatus on HIV plasma viral load and CD4 counts. One study limitation is that HSV-2 serostatus was measured at baseline only. If women contracted HSV-2 over the 7 years of follow-up, they would subsequently be misclassified as HSV-2 seronegative in analyses. This nondifferential misclassification of the exposure could result in biasing the effect of HSV-2 on HIV plasma viral load or CD4 counts over time toward the null in expectation under a monotonicity assumption. A previous study of HSV-2 seroconversion among 850 HIV-infected individuals in London, United Kingdom, demonstrated an HSV-2 seroincidence of 1.8 per 100 person-years. If seroconversion was similarly low in this study, our results were likely not greatly biased by this limitation (23).
HSV-2 serostatus could also have been misclassified if HSV-2 seropositivity was the result of oral HSV-2 infection in some women. Although oral HSV-2 infection exposure is possible, HSV-2 seropositivity is generally considered indicative of past exposure to genital herpes, because most women with oral HSV-2 infection have also been exposed genitally (24). In addition, because HSV-2 was measured at baseline only, the mixed-model analysis conducted did not enable estimation of total causal effects. We also did not include HAART use as a potential confounder in the models because it was time varying and was measured after the baseline exposure. While censoring the data on January 1, 1998, removed a large number of study visits where women were receiving HAART, confounding due to use of sub-HAART therapies was likely still present. To address this problem, we conducted a sensitivity analysis in which we fit the models to only those person-visits where women reported no current use of antiretrovirals; results were similar, albeit less precise. Future longitudinal research should test for HSV-2 serostatus at each study visit and use marginal structural modeling to assess the total causal effect of HSV-2 on the trajectories of HIV viral load and CD4 counts, adjusting for HAART use.
Another limitation of the study is that data on HSV-2 shedding from the genital tract and frequency of outbreaks of genital lesions were not available. We were therefore unable to determine whether transient increases in plasma HIV viral load occurred during periods of HSV-2 shedding or outbreaks. Furthermore, data on recency of HIV infection were not available to examine whether HIV viral load differed by HSV-2 serostatus for women with recent HIV infections.
Although HSV-2 and HIV-1 infections tend to be coprevalent, these data suggest that HSV-2 serostatus does not affect the trajectories of HIV plasma viral loads or CD4 counts. Further prospective studies are needed to examine the effect of HSV-2 acquisition and reactivation on HIV dynamics to determine whether HSV-2 can potentially affect the viral set point of HIV.
Acknowledgments
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Brooke E. Hoots, Stephen R. Cole, Jennifer S. Smith); Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Michael G. Hudgens); Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia (Denise J. Jamieson); National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia (Caroline C. King); Division of Infectious Diseases, Mount Sinai School of Medicine, New York, New York (Robert S. Klein); Miriam Hospital and Department of Medicine, Brown University, Providence, Rhode Island (Kenneth H. Mayer); Division of Infectious Diseases, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland (Anne M. Rompalo); and Department of Infectious Diseases, Wayne State University School of Medicine, Detroit, Michigan (Jack D. Sobel).
Brooke Hoots’ work was supported by National Institute of Allergy and Infectious Diseases (NIAID) grant T32AI070114-01.
Pangaja Paramsothy assisted with data acquisition and helped clarify study questionnaires and variable definitions.
The findings and conclusions of this study are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest: none declared.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- HAART
highly active antiretroviral therapy
- HERS
HIV Epidemiology Research Study
- HIV
human immunodeficiency virus
- HSV-2
herpes simplex virus type 2
References
- 1.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19(2):61–77. [PubMed] [Google Scholar]
- 2.Celum CL. The interaction between herpes simplex virus and human immunodeficiency virus. Herpes. 2004;11(suppl 1):36A–45A. [PubMed] [Google Scholar]
- 3.Wald A. Synergistic interactions between herpes simplex virus type-2 and human immunodeficiency virus epidemics. Herpes. 2004;11(3):70–76. [PubMed] [Google Scholar]
- 4.Siegal FP, Lopez C, Hammer GS, et al. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981;305(24):1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- 5.Sacks SL, Wanklin RJ, Reece DE, et al. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann Intern Med. 1989;111(11):893–899. doi: 10.7326/0003-4819-111-11-893. [DOI] [PubMed] [Google Scholar]
- 6.Marks GL, Nolan PE, Erlich KS, et al. Mucocutaneous dissemination of acyclovir-resistant herpes simplex virus in a patient with AIDS. Rev Infect Dis. 1989;11(3):474–476. doi: 10.1093/clinids/11.3.474. [DOI] [PubMed] [Google Scholar]
- 7.Gateley A, Gander RM, Johnson PC, et al. Herpes simplex virus type 2 meningoencephalitis resistant to acyclovir in a patient with AIDS. J Infect Dis. 1990;161(4):711–715. doi: 10.1093/infdis/161.4.711. [DOI] [PubMed] [Google Scholar]
- 8.Baeten JM, McClelland RS, Corey L, et al. Vitamin A supplementation and genital shedding of herpes simplex virus among HIV-1-infected women: a randomized clinical trial. J Infect Dis. 2004;189(8):1466–1471. doi: 10.1086/383049. [DOI] [PubMed] [Google Scholar]
- 9.Gray RH, Li X, Wawer MJ, et al. Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J Infect Dis. 2004;189(7):1209–1215. doi: 10.1086/382750. [DOI] [PubMed] [Google Scholar]
- 10.Duffus WA, Mermin J, Bunnell R, et al. Chronic herpes simplex virus type-2 infection and HIV viral load. Int J STD AIDS. 2005;16(11):733–735. doi: 10.1258/095646205774763298. [DOI] [PubMed] [Google Scholar]
- 11.Toossi Z, Johnson JL, Kanost RA, et al. Increased replication of HIV-1 at sites of Mycobacterium tuberculosis infection: potential mechanisms of viral activation. J Acquir Immune Defic Syndr. 2001;28(1):1–8. doi: 10.1097/00042560-200109010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Piroth L, Duong M, Quantin C, et al. Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS. 1998;12(4):381–388. doi: 10.1097/00002030-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Caselli E, Galvan M, Cassai E, et al. Human herpesvirus 8 enhances human immunodeficiency virus replication in acutely infected cells and induces reactivation in latently infected cells. Blood. 2005;106(8):2790–2797. doi: 10.1182/blood-2005-04-1390. [DOI] [PubMed] [Google Scholar]
- 14.Barbour JD, Sauer MM, Sharp ER, et al. HIV-1/HSV-2 co-infected adults in early HIV-1 infection have elevated CD4+ T cell counts. PLoS One. 2007;2(10):e1080. doi: 10.1371/journal.pone.0001080. (doi:10.1371/journal.pone.0001080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cachay ER, Frost SD, Richman DD, et al. Herpes simplex virus type 2 infection does not influence viral dynamics during early HIV-1 infection. J Infect Dis. 2007;195(9):1270–1277. doi: 10.1086/513568. [DOI] [PubMed] [Google Scholar]
- 16.Smith DK, Warren DL, Vlahov D, et al. Design and baseline participant characteristics of the Human Immunodeficiency Virus Epidemiology Research (HER) Study: a prospective cohort study of human immunodeficiency virus infection in US women. Am J Epidemiol. 1997;146(6):459–469. doi: 10.1093/oxfordjournals.aje.a009299. [DOI] [PubMed] [Google Scholar]
- 17.Rompalo AM, Shah N, Margolick JB, et al. Evaluation of possible effects of continued drug use on HIV progression among women. Int J STD AIDS. 2004;15(5):322–327. doi: 10.1177/095646240401500510. [DOI] [PubMed] [Google Scholar]
- 18.Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists; AIDS Program, Center for Infectious Diseases. MMWR Morb Mortal Wkly Rep. 1987;36(suppl 1):1S–15S. [PubMed] [Google Scholar]
- 19.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 20.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 21.Schacker T, Zeh J, Hu H, et al. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186(12):1718–1725. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 22.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4(3):260–270. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramaswamy M, Sabin C, McDonald C, et al. Herpes simplex virus type 2 (HSV-2) seroprevalence at the time of HIV-1 diagnosis and seroincidence after HIV-1 diagnosis in an ethnically diverse cohort of HIV-1-infected persons. Sex Transm Dis. 2006;33(2):96–101. doi: 10.1097/01.olq.0000187211.61052.c7. [DOI] [PubMed] [Google Scholar]
- 24.Looker KJ, Garnett GP. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex Transm Infect. 2005;81(2):103–107. doi: 10.1136/sti.2004.012039. [DOI] [PMC free article] [PubMed] [Google Scholar]


