Abstract
The objective of this US study was to assess the association of toenail nicotine level as a novel biomarker with lung cancer risk independent of reported smoking history. A nested case-control study of 210 male lung cancer cases and 630 matched controls aged 40–75 years participating in the Health Professionals Follow-up Study was conducted. Toenail samples collected in 1987 were analyzed for nicotine levels, and incident lung cancer cases were diagnosed between 1988 and 2000. Mean toenail nicotine level among cases was 0.95 ng/mg compared with 0.25 ng/mg among controls (P < 0.0001). In univariate analyses, the relative risk of lung cancer for the highest versus lowest quintiles of toenail nicotine level was 10.50 (95% confidence interval: 5.61, 19.64; P for trend < 0.0001). When the authors adjusted for pack-years from reported smoking history in multivariate analyses, the relative risk for toenail nicotine levels in the highest quintile was still significant in predicting lung cancer risk: 3.57 (95% confidence interval: 1.73, 7.37; P for trend < 0.0001). In conclusion, the toenail nicotine biomarker was found to be a strong predictor of lung cancer independent of smoking history, suggesting that the adverse effects of cigarette smoke may be underestimated in studies based on smoking history only.
Keywords: cohort studies, lung neoplasms, nails, nicotine, tobacco
Lung cancer is a well-established consequence of smoking tobacco, and about 90% of lung cancer cases are attributed to smoking (1–3). The risk has been estimated by comparing active smokers with never smokers. Exposure to secondhand tobacco smoke, a well-established lung cancer risk factor, among never smokers is often neglected when never smokers are used as the comparison group for active smokers. This factor, and the imperfect measurement of the actual amount of tobacco smoke exposure among active smokers (including error in reporting amounts and difficulty in accounting for variation in depth of inhalation (4, 5)), lead to attenuation of risk (6). These factors that lead to misclassification and attenuation are difficult to avoid when using questionnaires as the method of tobacco exposure assessment. The information on exposure to tobacco smoke collected by questionnaires nevertheless has advantages in studies of risk of chronic diseases because of feasibility and the ability to capture information on past exposure.
Biomarkers are being developed for tobacco exposure assessment because they can potentially reduce measurement error and biases in reporting. However, most available tobacco biomarkers, such as urine and salivary cotinine (7–9), are short-lived. One of the longer-term biomarkers for tobacco exposure is 4-aminobiphenyl adducts to hemoglobin (10, 11), but it has limited value in epidemiologic studies because of lack of specificity, cost, and poor prediction of lung cancer risk (12, 13). Other tobacco-specific nitrosamines such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol have recently been used to determine lung cancer risk for heavy smokers (13, 14), but they are prone to significant metabolic variability because of age and other factors, a nonlinear association with exposure, and the need for sophisticated laboratory analyses and caution in handling these carcinogenic chemicals (15, 16). Furthermore, they require collection and special storage of urine or blood samples.
More recently, 2 tobacco biomarkers have been developed that show promise for wider use in research. The hair nicotine biomarker initially used for forensic or experimental purposes has become more widely adopted because of more cost-effective analyses, ease of collection, no need for special storage and handling procedures, and the ability to reflect long-term exposure (17–20). In addition, the toenail nicotine biomarker has some additional advantages because it has a relatively long half-life because of the slow growth rate of toenails (about 1 cm per year) and the ability to be collected easily by study participants (21, 22).
In this study, toenail samples were collected from men participating in the Health Professionals Follow-up Study at the beginning of follow-up. Using a nested case-control design, we examined the relation of toenail nicotine levels to incidence of lung cancer during 12 years of follow-up. We hypothesized that toenail nicotine levels will strongly predict risk of lung cancer.
MATERIALS AND METHODS
The Health Professionals Follow-up Study is a prospective cohort of men initiated in 1986, when 51,529 predominantly white men aged 40–75 years of age answered a detailed questionnaire by mail on diet and medical history (23). This cohort consists of dentists, veterinarians, pharmacists, optometrists, osteopathic physicians, and podiatrists. All 50 US states were represented, and no exclusions were made by race. Every 2 years, follow-up questionnaires were mailed to all surviving cohort members to collect data on lifestyle and anthropometric measures. In 1987, a majority of the participants (n = 33,737) also provided samples of toenail clippings.
Study design
To assess the association between toenail nicotine levels and lung cancer risk, only those men participating in the Health Professionals Follow-up Study who provided toenail samples in 1987 were included. We utilized a nested case-control design by identifying lung cancer cases diagnosed between 1988 and 2000, after the toenail samples were collected, and matching them to 3 controls randomly selected from the remaining cohort who did not develop lung cancer. Both cases and controls had to have toenail samples available for the study, and we excluded men with any history of cancer before the date of return of the nail specimens. Controls were matched on age and month of nail return. Most individuals with lung cancer are active smokers; therefore, 3 controls were used to be able to include 1 of 3 as a smoker and therefore an approximately comparable number of active smokers as among the cases.
A total of 221 lung cancer cases were matched to 666 controls (including extra controls) within the Health Professionals Follow-up Study population, for a total sample size of 887. Among those cases, 3 did not have enough toenail tissue for analyses and were excluded along with their matched controls. Another 35 samples were excluded because data on smoking status for these men were not available. The final analyses included 210 cases and 630 controls for a total sample size of 840. The institutional review boards for both Harvard University and University of California, San Diego approved this study.
Case ascertainment
On each biennial questionnaire, participants were asked whether they had been diagnosed with lung cancer during the previous 2 years. The follow-up rate with respect to the incidence of cancer was 96% of the total possible person-years. After permission from identified cases (or next of kin for decedents) was received, hospital records and pathology reports were obtained and reviewed by a physician for histologic confirmation. Information on lung cancer cell type was available for approximately 80%. Deaths in the cohort were ascertained through family members and the National Death Index (24).
Smoking and covariates
Participants reported their smoking status in 1986 as never, past, or current. A past smoker was defined as someone who had smoked more than 20 packs of cigarettes and had quit at the time of the survey. For current smokers, they selected the category that represented their daily number of cigarettes smoked (1–4, 5–14, 15–24, 25–34, 35–44, or ≥45). We collapsed the categories for number of cigarettes into 1–24 and 25 or more cigarettes per day because of small numbers. Cigarette consumption for the pack-years calculation was based on determining how many cigarettes per day smokers on average smoked during each decade of their life (by selecting the midpoint of smoking category) and multiplying that value by the total number of years of cigarette consumption and then dividing by 20.
Current weight was requested on questionnaires in 1986, and body mass index (weight (kg)/height (m2)) was calculated with the use of the height reported at baseline. Physical activity was calculated as metabolic equivalent task-hours per week (1 metabolic equivalent task-hour is equivalent to 1 hour of resting) from time spent in various leisure-time activities (e.g., walking, running, biking, or playing tennis) as reported on the 1986 questionnaire.
Laboratory analyses
Toenail samples were analyzed at the Wellington Hospital (New Zealand) biochemistry laboratory using the high-performance liquid chromatography with electrochemical detection method (25). The within-batch coefficient of variation percentage in this study was less than 10.4% throughout the analyses. Laboratory staffs were blinded to case-control and smoking status. Cases and controls were submitted to the laboratory as matched sets. To reduce random error, all samples were run in duplicate when there were enough toenail samples (87%).
Statistical analyses
The toenail nicotine levels were not normally distributed and we therefore used the nonparametric Wilcoxon rank sum test to assess the difference in nicotine levels between cases and their controls. The chi-square test was used to assess categorical variables in relation to lung cancer. We used the PHREG procedure in SAS software (SAS Institute, Inc., Cary, North Carolina) for conditional logistic regression analyses for matched analyses to model the independent predictors of lung cancer.
Toenail nicotine levels were initially analyzed as quintiles using indicator variables, with the lowest quintile as the referent group. We also modeled nicotine level as a continuous log-transformed variable for our analyses. The models assessed the smoking variables in relation to lung cancer, which included smoking status (never, past, current), pack-years (0, 1–9, 10–19, 20–29, 30–39, ≥40), and smoking status with number of cigarettes smoked included (never, past, current 1–24 cigarettes/day, current ≥25 cigarettes/day). In the multivariate analyses, these variables were included one at a time with quintiles of toenail nicotine levels and a continuous log-transformed nicotine variable. Physical activity was also included in the model as a covariate because of its association with toenail nicotine levels and lung cancer risk in our study.
The aim of including the reported smoking variables with toenail nicotine levels in the same model was to assess the association of toenail nicotine levels with lung cancer independent of the risk predicted by reported smoking. The different reported smoking variables were not included in the same model because of colinearity.
Potential interaction by smoking status category (never, past, and current separately) and toenail nicotine levels was assessed by using a cross-product term in multivariate models so that the effect of log-nicotine in each smoking stratum was represented by these interaction terms and also by including smoking status as indicator variables in the model. We also carried out stratified analyses for the association between toenail nicotine levels and lung cancer according to smoking status (never smokers, past smokers, current smokers) using the LOGISTICS procedure in SAS software (SAS Institute, Inc.). Tests of linear trends for increasing quintiles of nicotine levels were conducted by assigning the median value for categories and treating them as a single continuous variable. Reported P values are based on 2-sided tests.
RESULTS
The distribution of potential lung cancer risk factors in the study population according to quintile of toenail nicotine is shown in Table 1. Men in the highest quintile of toenail nicotine were slightly younger than men in the lower toenail nicotine quintiles. Men in the highest quintiles of nicotine, who were more likely to be smokers, were less likely to be physically active. There was no difference in body mass index between quintiles. The percentage of pack-years distribution in the study population was as follows: 0 pack-years, 36.3%; 1–9 pack-years, 9.9%; 10–19 pack-years, 10.8%; 20–29 pack-years, 13.5%; 30–39 pack-years, 9.8; and ≥40 pack-years, 19.8%. Across quintiles of nicotine, mean pack-years increased with higher quintiles. Never and past smokers were less likely to have nicotine levels in the highest quintile, whereas the majority of smokers had nicotine levels in the highest quintile (Table 1).
Table 1.
Age-adjusted Characteristics of US Men in 1986 Participating in the Health Professionals Follow-up Study According to Quintile of Toenail Nicotine Level
| Toenail Nicotine Quintile, ng/mg |
|||||
| 1 | 2 | 3 | 4 | 5 | |
| No. of men | 179 | 164 | 163 | 166 | 168 |
| Median toenail nicotine, ng/mg | 0.04 | 0.07 | 0.10 | 0.20 | 1.28 |
| Age in years, mean (SD) | 62.8 (8.8) | 62.7 (8.0) | 63.8 (7.5) | 63.8 (7.1) | 60.7 (8.0) |
| Physical activity, METs | 19.2 | 21.0 | 18.8 | 19.6 | 15.0 |
| Body mass index, kg/m2 | 25.0 | 25.0 | 25.2 | 25.5 | 24.7 |
| Pack-years of smoking, no. | 11.2 | 12.7 | 15.8 | 19.3 | 37.3 |
| Smoking status, % | |||||
| Never smoker | 29.2 | 24.0 | 23.6 | 17.5 | 5.7 |
| Past smoker | 21.6 | 21.3 | 21.5 | 23.0 | 12.6 |
| Current smoker | 0.0 | 1.2 | 1.1 | 12.6 | 85.2 |
Abbreviations: MET, metabolic equivalent task-hour; SD, standard deviation.
As expected, age was not significantly different between cases and controls because it was a matching variable (Table 2). Body mass index was also not different between cases and controls, but controls were significantly more physically active than cases (P = 0.009). Toenail nicotine levels were significantly higher among cases compared with controls. The mean toenail nicotine levels ratio for cases and controls was 3.6:1; for pack-years, the ratio of the means was 3:1. The distribution of never, past, and current smokers varied greatly according to cases and controls. Only 9.1% of cases were never smokers, and only 6.2% of controls were current smokers. The percentage of cases who were past smokers was only slightly higher (54.1%) than that among controls (48.4%).
Table 2.
Age-adjusted Distribution of Lung Cancer Risk Factors at Baseline (1986) in the Health Professionals Follow-up Study for Cases Diagnosed Between 1988 and 2000 and Their Matched Controls
| Cases (n = 210) |
Controls (n = 630) |
P Valuea | |||
| Mean (SE) | % | Mean (SE) | % | ||
| Nicotine level, ng/mg | 0.95 (0.09) | 0.25 (0.02) | <0.0001 | ||
| Age, years | 62.5 (0.6) | 62.9 (0.3) | 0.52 | ||
| Physical activity, METs | 15.8 (1.2) | 19.6 (0.8) | 0.009 | ||
| Body mass index, kg/m2 | 25.1 (0.2) | 25.1 (0.1) | 0.9 | ||
| Pack-years of smoking, no. | 38.7 (1.6) | 12.9 (0.7) | <0.0001 | ||
| Smoking status | |||||
| Never smoker | 9.1 | 45.3 | |||
| Past smoker | 54.1 | 48.4 | |||
| Current smoker | 36.9 | 6.2 | <0.0001 | ||
Abbreviations: MET, metabolic equivalent task-hour; SE, standard error.
P value for continuous variables is from the Wilcoxon rank sum test and for categorical variables is from the chi-square test.
Table 3 includes the univariate models matched for age and date of toenail return and the multivariate models additionally adjusted for tobacco smoke variables to assess the association between reported smoking variables and measured nicotine levels in relation to risk of lung cancer. All these variables showed a highly significant dose-response association with lung cancer risk in both the univariate and multivariate analyses. The highest relative risks were for those who smoked for 40 or more pack-years (relative risk (RR) = 26.05, 95% confidence interval (CI): 13.90, 48.81; P for trend < 0.0001) and for current smokers smoking 25 or more cigarettes per day (RR = 50.70, 95% CI: 23.72, 108.36; P for trend < 0.0001). Regarding toenail nicotine levels, the men in the fifth quintile versus first quintile had a relative risk of 10.50 (95% CI: 5.61, 19.64; P for trend < 0.0001).
Table 3.
Relative Risks and 95% Confidence Intervals for the Tobacco Predictors of Lung Cancer Risk in Univariate and Multivariate Analyses for Men Participating in the Health Professionals Follow-up Study (Followed Between 1986 and 2000)
| Variable | Multivariate Analysis |
|||||
| Univariate Analysis |
Model 1a |
Model 2b |
||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Smoking status | ||||||
| Never smoker (19 casesc) | 1.00 | 1.00 | ||||
| Past smoker (113 cases) | 5.42 | 3.21, 9.15 | 4.99 | 2.91, 8.57 | ||
| Current smoker, 1–24 cigarettes/day (10 cases) | 11.61 | 4.28, 31.51 | 5.62 | 1.94, 16.34 | ||
| Current smoker, ≥25 cigarettes/day (67 cases) | 50.70 | 23.72, 108.36 | 20.59 | 8.52, 49.75 | ||
| P for trend | <0.0001 | <0.0001 | ||||
| Pack-years of smoking | ||||||
| 0 (19 cases) | 1.00 | 1.00 | ||||
| 1–9 (12 cases) | 2.18 | 0.98, 4.84 | 1.92 | 0.83, 4.44 | ||
| 10–19 (9 cases) | 1.46 | 0.62, 3.47 | 1.36 | 0.55, 3.34 | ||
| 20–29 (30 cases) | 5.44 | 2.82, 10.51 | 4.66 | 2.35, 9.24 | ||
| 30–39 (35 cases) | 10.39 | 5.28, 20.44 | 7.68 | 3.75, 15.73 | ||
| ≥40 (105 cases) | 26.05 | 13.90, 48.81 | 15.80 | 8.06, 30.97 | ||
| P for trend | <0.0001 | <0.0001 | ||||
| Nicotine quintile | ||||||
| 1 (26 cases) | 1.00 | 1.00 | 1.00 | |||
| 2 (21 cases) | 1.02 | 0.52, 1.99 | 0.82 | 0.40, 1.69 | 0.83 | 0.38, 1.82 |
| 3 (26 cases) | 1.43 | 0.74, 2.75 | 1.23 | 0.61, 2.47 | 1.09 | 0.51, 2.34 |
| 4 (40 cases) | 2.10 | 1.12, 3.93 | 1.34 | 0.68, 2.67 | 1.30 | 0.62, 2.73 |
| 5 (97 cases) | 10.50 | 5.61, 19.64 | 3.16 | 1.49, 6.73 | 3.57 | 1.73, 7.37 |
| P for trend | <0.0001 | 0.0004 | <0.0001 | |||
Abbreviations: CI, confidence interval; RR, relative risk.
Model 1: included smoking status with nicotine quintile and physical activity.
Model 2: included pack-years with nicotine quintile and physical activity.
Cases matched on age and on date of return of toenail samples.
In the multivariate analyses, which included the biomarker with one of the reported tobacco exposure variables, all relative risks were decreased, but the tests for trend were still highly significant for both the toenail nicotine levels and smoking variables. The highest relative risk in either of the models was for current users who smoked 25 cigarettes or more per day (RR = 20.59, 95% CI: 8.52, 49.75; refer to Table 3, model 1). Men in the highest quintile of nicotine had a relative risk of 3.57 (95% CI: 1.73, 7.37) compared with men in the lowest quintile when pack-years was included in the model and a relative risk of 3.16 (95% CI: 1.49, 6.73) when smoking status and intensity were included in the model.
The log-transformed nicotine levels were able to significantly predict lung cancer risk (RR = 2.10, 95% CI: 1.80, 2.45) in the univariate analyses. In the multivariate analyses, the relative risks for an increase in log-transformed nicotine levels were 1.60 (95% CI: 1.34, 1.91) with the inclusion of pack-years and 1.53 (95% CI: 1.26, 1.87) with the inclusion of smoking status and intensity in the model.
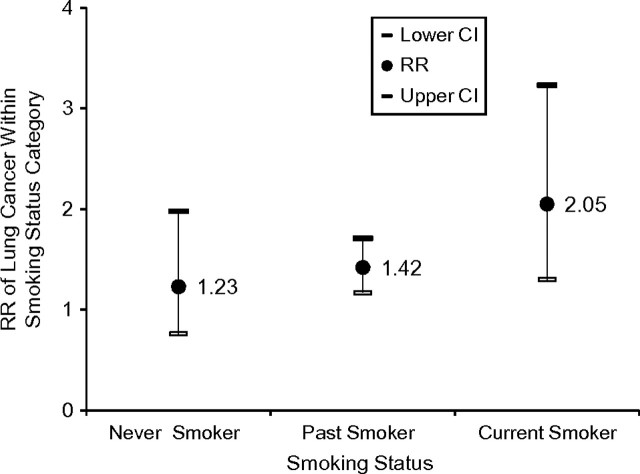
We also assessed the association between log-transformed toenail nicotine levels and lung cancer risk as an interaction term for each smoking status with toenail nicotine level in the same model while adjusting for physical activity. The toenail nicotine levels had a similar influence in predicting cancer regardless of smoking status, and we found no interaction between toenail nicotine levels and smoking status (P = 0.90). In the stratified multivariate analyses according to smoking status (and adjusting for age and for date of nail return in the model as the matching variables), the association between log-transformed nicotine levels and cancer risk was consistently positive, although it did not reach statistical significance for never smokers (Figure 1). When we adjusted for pack-years in the model for current smokers, the cancer risk from log-transformed nicotine levels was still significant (RR = 1.72, 95% CI: 1.06, 2.80).
Figure 1.
Relative risk (RR) and 95% confidence interval (CI) from multivariate analyses (adjusted for age and date of nail return) of lung cancer for log-transformed toenail nicotine levels (ng/mg) according to smoking status stratum of US men participating in the Health Professionals Follow-up Study between 1986 and 2000.
DISCUSSION
We found that levels of nicotine in toenail samples from our study population independently predicted lung cancer risk, with a clear dose-response relation for men with higher toenail nicotine levels having a higher risk of lung cancer. Our data indicate that toenail nicotine levels reflect exposure burden of tobacco not captured entirely through reliance on reported smoking history. Established smokers tend to maintain their level of tobacco use that is driven by their addiction to nicotine. As demonstrated in controlled laboratory experiments, smokers can reduce their number of cigarettes smoked but still maintain their level of nicotine through topographic behavior of smoke-puff ratio and depth of inhalation (4, 26). Such higher intake of nicotine and tobacco carcinogens cannot be estimated when relying on reported number of cigarettes smoked. Similarly, for past smokers, our study demonstrates that they are still being exposed to substantial nicotine levels after they have quit, although fewer of them were exposed to the highest level of nicotine. This finding suggests that some are being exposed to low secondhand smoke levels while others are being exposed to higher levels, presumably from their fellow smokers who have not quit. It could also indicate that some of them are still occasionally smoking or that the measure of toenail nicotine reflects residual nicotine for recent quitters.
More than 10% of men with the highest levels of toenail nicotine in our study were never smokers. In a previous study (27), never smokers exposed to heavy secondhand smoke had levels of nicotine equivalent to those of active smokers, although misclassification was much higher than in this study. Thus, previous risks of lung cancer due to smoking have likely been underestimated. The same conclusion applies to all other tobacco-related diseases for which assessment of risk has relied on questionnaire measures only.
Two recent studies determined 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in urine and serum of heavy smokers and found it predictive of lung cancer risk (13, 14). To our knowledge, the first study to assess biomarkers of tobacco and lung cancer risk was a case-control study from Norway by Boffetta et al. (28), in which frozen serum samples from lung cancer cases and their matched controls were analyzed for cotinine levels. They found a significant association with cotinine but did not adjust for reported smoking history in any of their analyses; thus, it is not clear whether serum cotinine was able to predict lung cancer risk independent of reported smoking.
Another recent nested case-control study of lung cancer from the Shanghai Cohort Study assessed the ability of cotinine in urine to predict lung cancer risk and found a significant association in the unadjusted model (29). However, when the authors adjusted for reported smoking history, the highest tertile of the urine cotinine biomarker failed to significantly predict lung cancer risk (odds ratio = 2.1, 95% CI: 0.9, 5.0; P for trend = 0.08). Cotinine is a metabolite of nicotine and is highly variable because of interindividual metabolic and excretion differences and a short half-life of 17 hours (9, 30). A greater stability over time could explain the ability of the toenail nicotine biomarker to predict lung cancer independent of reported smoking history in our study.
A previous study reported on the ability of toenail nicotine levels to predict coronary heart disease among women independent of reported smoking history (31). There was a dose-response association, and the women in the highest quintile of toenail nicotine level had a 42% increase in the risk of coronary heart disease compared with women in the lowest quintile (RR = 1.42, 95% CI: 1.33, 1.52).
Although toenail nicotine levels can add to the prediction of lung cancer risk independent of smoking history, as shown in our study, reported number of cigarettes smoked and pack-years of smoking were strongly associated with risk independent of toenail nicotine. Cumulative exposure, which is most relevant to lung cancer, may suffer less misclassification than current or period-specific average exposure. However, in our results, high intensity of smoking of 25 cigarettes or more per day predicted a higher risk of lung cancer than the highest pack-years of cumulative exposure. Pack-years does not differentiate between high-intensity shorter duration of smoking and low-intensity longer duration and may explain the lower risk.
The toenail nicotine biomarker should reflect factors such as passive smoking and depth of inhalation not captured by reported smoking habits. However, nicotine is not carcinogenic; therefore, using it as a proxy for the carcinogenic constituents of tobacco is based on the assumption that the internal dose of these constituents delivered to the lung is proportional to nicotine levels measured in nails. This assumption has been demonstrated by comparing toenail nicotine levels with toenail levels of the tobacco-specific-nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; they were well correlated (r = 0.68; P < 0.0001) (32).
Nevertheless, toenail nicotine levels represent exposure during the past year, which does not reflect the total cumulative exposure assessed by pack-years of smoking. Notably, however, the relative risk of toenail nicotine in relation to lung cancer was not substantially influenced when replacing pack-years with current smoking level in the model. In both models, there was an additional 50%–60% risk of lung cancer with each natural log unit of toenail nicotine, reflecting additional unmeasured risk that is independent of reported smoking. This finding was consistent even when limiting the analyses to smokers in multivariate analyses adjusting for pack-years.
By using toenail nicotine levels, we are assuming that they reflect usual exposure over time, with less error than existing biomarkers that reflect only a few hours or days of exposure. We have shown that toenail nicotine levels are closely correlated with smoking status 6 years prior to collection of samples (22), despite the expected variability in smoking and tobacco exposure over this period. This finding indicates that toenails are a stable biomarker of average exposure over time. Toenail nicotine levels could also reflect the interindividual variability in nicotine metabolism in relation to lung cancer risk.
The population we studied was a group of health professionals with lower than average exposure to tobacco smoke, which could explain the inability of lower quintiles of toenail nicotine levels to significantly predict lung cancer risk. We would expect a higher predictive ability of toenail nicotine levels in other more exposed populations. Nevertheless, we were still able to demonstrate a relative risk of 10.5 for the highest quintiles in the univariate analyses and a relative risk of 3.6 in the multivariate analyses. Categories of pack-years of less than 20 were also not significantly related to lung cancer risk. It will be interesting to see the results replicated in studies from other populations.
When we stratified according to smoking status, toenail nicotine levels were still able to predict lung cancer risk. For never smokers, this would reflect passive smoking exposure. A limitation of our study is that no information was collected about passive smoking. Another source of nicotine is from nicotine replacement therapy. However, in 1987, when the toenails were collected, use of nicotine gum was negligible, and such a source is unlikely to have influenced the results of this study (33).
Development of the toenail nicotine biomarker as a clinical tool to assess future risk may provide motivation for smokers to quit and for individuals heavily exposed to secondhand smoke to limit their exposure. Toenails are easily collected by participants and can be stored at room temperature for an extended period of time, making it a feasible tool for large population studies.
In conclusion, our study demonstrated that toenail nicotine levels provide a biomarker that can predict the risk of lung cancer independent of reported smoking history. Similarly, we found that smoking history predicted lung cancer risk independent of toenail nicotine level. The risk according to toenail nicotine level could be predicted for never, past, and current smokers, suggesting that previous studies that have determined risk of lung cancer from tobacco use by using only reported active smoking may have underestimated the true effects of tobacco smoke.
Acknowledgments
Author affiliations: Department of Family and Preventive Medicine, School of Medicine, University of California, San Diego, San Diego, California (Wael K. Al-Delaimy); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Walter C. Willett); Channing Laboratory, Department of Medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, Massachusetts (Walter C. Willett); and Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Walter C. Willett).
This study was funded by Flight Attendants Medical Research Foundation grant 12548. The Health Professionals Follow-up Study is funded by grant CA55075 from the National Cancer Institute.
The authors acknowledge Graeme Mahoney for carrying out the laboratory analyses.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- RR
relative risk
References
- 1.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health. 1978;32(4):303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, US Department of Health and Human Services; 2004. [Google Scholar]
- 3.US Department of Health and Human Services. Rockville, Maryland: Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, US Department of Health and Human Services; 1989. Reducing the Health Consequences of Smoking: 25 Years of Progress: A Report of the Surgeon General. (DHHS publication (CDC) 89-8411) [Google Scholar]
- 4.Patterson F, Benowitz N, Shields P, et al. Individual differences in nicotine intake per cigarette. Cancer Epidemiol Biomarkers Prev. 2003;12(5):468–471. [PubMed] [Google Scholar]
- 5.Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl) 1999;145(1):1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong B, White E, Saracci R. Monographs in Epidemiology and Biostatistics. Oxford: United Kingdom; Principles of Exposure Measurement in Epidemiology. Oxford University Press; 1992. [Google Scholar]
- 7.Dolcini MM, Adler NE, Lee P, et al. An assessment of the validity of adolescent self-reported smoking using three biological indicators. Nicotine Tob Res. 2003;5(4):473–483. [PubMed] [Google Scholar]
- 8.Etzel RA. A review of the use of saliva cotinine as a marker of tobacco smoke exposure. Prev Med. 1990;19(2):190–197. doi: 10.1016/0091-7435(90)90020-k. [DOI] [PubMed] [Google Scholar]
- 9.Wall MA, Johnson J, Jacob P, et al. Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health. 1988;78(6):699–701. doi: 10.2105/ajph.78.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant MS, Skipper PL, Tannenbaum SR, et al. Hemoglobin adducts of 4-aminobiphenyl in smokers and nonsmokers. Cancer Res. 1987;47(2):602–608. [PubMed] [Google Scholar]
- 11.Schäffler G, Betz C, Richter E. Mass spectrometric analysis of tobacco-specific hemoglobin adducts. Environ Health Perspect. 1993;99:187–189. doi: 10.1289/ehp.99-1567014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weston A, Caporaso NE, Taghizadeh K, et al. Measurement of 4-aminobiphenyl-hemoglobin adducts in lung cancer cases and controls. Cancer Res. 1991;51(19):5219–5223. [PubMed] [Google Scholar]
- 13.Church TR, Anderson KE, Caporaso NE, et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18(1):260–266. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan JM, Koh WP, Murphy SE, et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;69(7):2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht SS, Carmella SG, Le KA, et al. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides in the urine of infants exposed to environmental tobacco smoke. Cancer Epidemiol Biomarkers Prev. 2006;15(5):988–992. doi: 10.1158/1055-9965.EPI-05-0596. [DOI] [PubMed] [Google Scholar]
- 16.Lubin JH, Caporaso N, Hatsukami DK, et al. The association of a tobacco-specific biomarker and cigarette consumption and its dependence on host characteristics. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1852–1857. doi: 10.1158/1055-9965.EPI-07-0018. [DOI] [PubMed] [Google Scholar]
- 17.Al-Delaimy WK. Hair as a biomarker for exposure to tobacco smoke. Tob Control. 2002;11(3):176–182. doi: 10.1136/tc.11.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nafstad P, Botten G, Hagen JA, et al. Comparison of three methods for estimating environmental tobacco smoke exposure among children aged between 12 and 36 months. Int J Epidemiol. 1995;24(1):88–94. doi: 10.1093/ije/24.1.88. [DOI] [PubMed] [Google Scholar]
- 19.Wipfli H, Avila-Tang E, Navas-Acien A, et al. Secondhand smoke exposure among women and children: evidence from 31 countries. Am J Public Health. 2008;98(4):672–679. doi: 10.2105/AJPH.2007.126631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pichini S, Garcia-Algar O, Muñoz L, et al. Assessment of chronic exposure to cigarette smoke and its change during pregnancy by segmental analysis of maternal hair nicotine. J Expo Anal Environ Epidemiol. 2003;13(2):144–151. doi: 10.1038/sj.jea.7500264. [DOI] [PubMed] [Google Scholar]
- 21.Al-Delaimy WK, Willett WC. Measurement of tobacco smoke exposure: comparison of toenail nicotine biomarkers and self-reports. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1255–1261. doi: 10.1158/1055-9965.EPI-07-2695. [DOI] [PubMed] [Google Scholar]
- 22.Al-Delaimy WK, Mahoney GN, Speizer FE, et al. Toenail nicotine levels as a biomarker of tobacco smoke exposure. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1400–1404. [PubMed] [Google Scholar]
- 23.Rimm EB, Stampfer MJ, Colditz GA, et al. Effectiveness of various mailing strategies among nonrespondents in a prospective cohort study. Am J Epidemiol. 1990;131(6):1068–1071. doi: 10.1093/oxfordjournals.aje.a115598. [DOI] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 25.Mahoney GN, Al-Delaimy W. Measurement of nicotine in hair by reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl. 2001;753(2):179–187. doi: 10.1016/s0378-4347(00)00540-5. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz NL, Jacob P, III, Kozlowski LT, et al. Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide. N Engl J Med. 1986;315(21):1310–1313. doi: 10.1056/NEJM198611203152102. [DOI] [PubMed] [Google Scholar]
- 27.Al-Delaimy W, Fraser T, Woodward A. Nicotine in hair of bar and restaurant workers. N Z Med J. 2001;114(1127):80–83. [PubMed] [Google Scholar]
- 28.Boffetta P, Clark S, Shen M, et al. Serum cotinine level as predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1184–1188. doi: 10.1158/1055-9965.EPI-06-0032. [DOI] [PubMed] [Google Scholar]
- 29.Stram DO, Yuan JM, Chan KK, et al. Beta-cryptoxanthin and lung cancer in Shanghai, China—an examination of potential confounding with cigarette smoking using urinary cotinine as a biomarker for true tobacco exposure. Nutr Cancer. 2007;57(2):123–129. doi: 10.1080/01635580701273998. [DOI] [PubMed] [Google Scholar]
- 30.Al-Delaimy WK, Crane J, Woodward A. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health. 2002;56(1):66–71. doi: 10.1136/jech.56.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Delaimy WK, Stampfer MJ, Manson JE, et al. Toenail nicotine levels as predictors of coronary heart disease among women. Am J Epidemiol. 2008;167(11):1342–1348. doi: 10.1093/aje/kwn061. [DOI] [PubMed] [Google Scholar]
- 32.Stepanov I, Hecht SS, Lindgren B, et al. Relationship of human toenail nicotine, cotinine, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol to levels of these biomarkers in plasma and urine. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1382–1386. doi: 10.1158/1055-9965.EPI-07-0145. [DOI] [PubMed] [Google Scholar]
- 33.Nicotine gum a boon that the experts say should be used more. New York Times. January 28, 1988. ( http://www.nytimes.com/1988/01/28/us/health-therapy-for-smokers-nicotine-gum-boon-that-experts-say-should-be-used.html) [Google Scholar]