Abstract
Heat shock proteins (HSP) are a family of highly conserved proteins, whose expression increases in response to stresses that may threaten cell survival. Over the past decade, heat shock protein 90 (Hsp90) has emerged as a potential therapeutic target for cancer as it plays a vital role in normal cell maturation and acts as a molecular chaperone for proper folding, assembly, and stabilization of many oncogenic proteins. To date, a majority of Hsp90 inhibitors that have been discovered are macrocycles. The relatively rigid conformation provided by the macrocyclic scaffold allows for a selective interaction with a biological target such as Hsp90. This review highlights the discovery and development of nine macro-cycles that inhibit the function of Hsp90, detailing their potency and the client proteins affected by Hsp90 inhibition.
Keywords: Hsp90, Hsp90 inhibitors, macrocycles, client protein, geldanamycin, 17AAG, 17-DMAG, IPI504, clinical trials, cancer, herbimycin radicicol, pochonin, radanamycin, SanA, di-SanA
1. INTRODUCTION to Hsp90
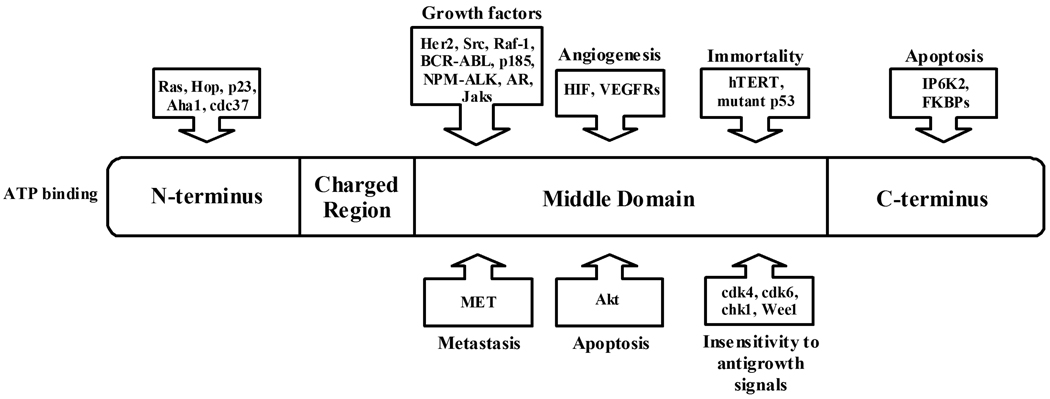
Heat Shock Proteins (HSP) are a set of highly conserved proteins that are activated in response to heat, nutrient deprivation, oxidative conditions, and other stresses that may threaten a cell’s survival [1, 2]. These proteins are identified by their molecular weight. Accordingly, there are five mammalian Hsps - Hsp100, Hsp90, Hsp70, Hsp60, and small Hsp families [3]. The small Hsp family includes heat shock proteins of small molecular weight (14kD - 40kD). Small Hsps are activated in response to the same stresses that threaten the cell’s survival as the other four mammalian Hsps [3]. For the past decade heat shock protein 90 (Hsp90) has been an attractive target for anticancer therapy because it plays an important role in facilitating cell growth. It functions as a molecular chaperone for folding, assembling, and stabilizing many oncogenic proteins. Hsp90 accounts for 1–2% of protein in a normal, unstressed cell [4]. However, as with any heat shock protein, Hsp90 levels change with the stress level of the cell. When cells become stressed, the level of Hsp90 increases. Not surprisingly, cancer cells typically have elevated levels of Hsp90, accounting for 3–5% of all protein in these cells [5]. There are usually 6 characteristics displayed by a cancerous cell: (1) growth factor independence, (2) resistance to antigrowth signals, (3) unlimited replicative potential, (4) tissue invasion and metastasis, (5) avoidance of apoptosis and (6) sustained angiogenesis [6]. Cells displaying these traits have increased levels of Hsp90, which helps sustain their growth via its stabilization and interaction with client proteins. Hsp90’s client proteins that are currently thought to be involved in the development of these six characteristics include HIF-1α, Her2, Raf-1, hTERT, VEGFR, MET, Akt, BRAF, and RAF-1 (Fig. 1). However, this list is frequently updated as new proteins and pathways are discovered and their connection to Hsp90 is revealed [7]. Hsp90 facilitates cell growth by protecting these client proteins from a degradation pathway, allowing their continued function, and maintaining the cell rather than directing it to the appropriate apoptotic pathway [8]. Hsp90 requires a variety of co-chaperones to function properly, including p23, Aha1, cdc37, Hip, HOP, and Hsp70. These co-chaperones assist in Hsp90’s protein folding cycle facilitating Hsp90’s maintenance of its client proteins (Figs. 1 and 2).
Fig. 1.
Hsp90 and its associated oncogenic client proteins.
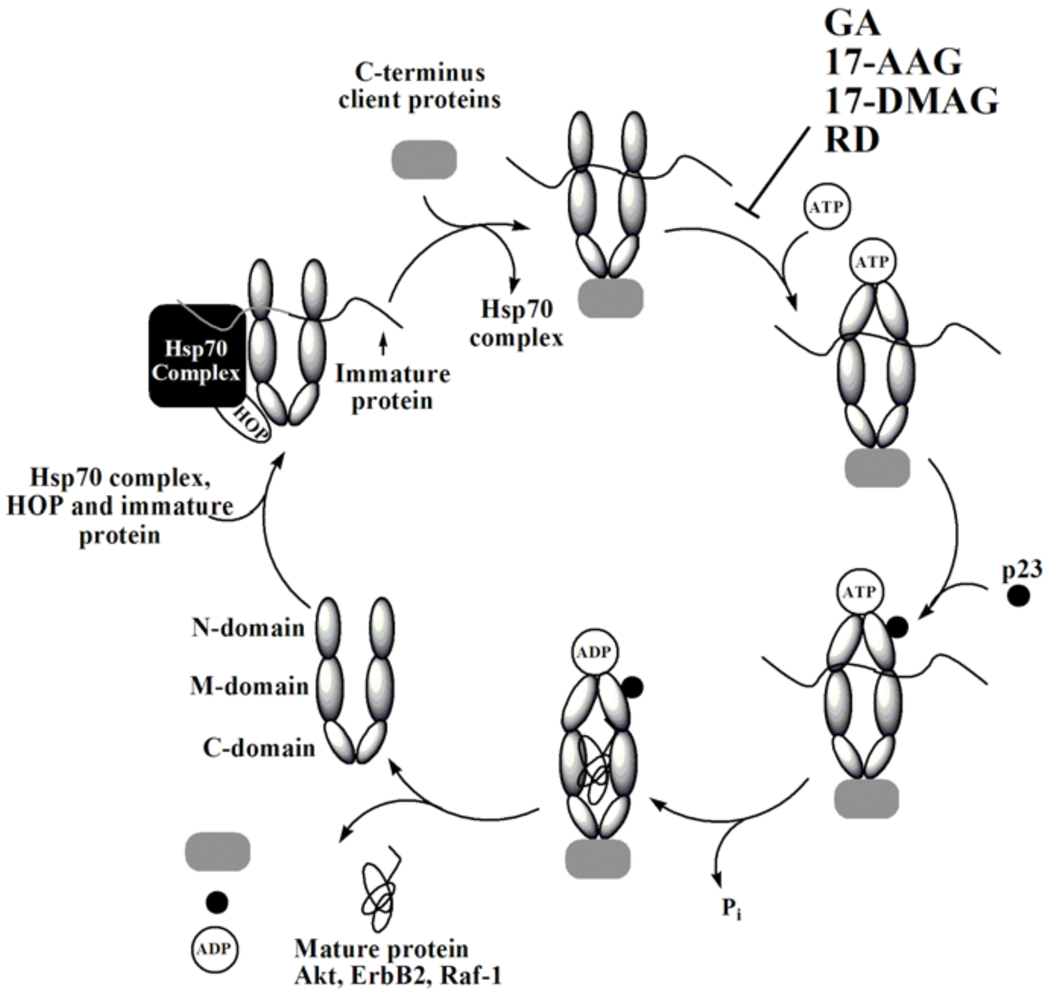
Fig. 2.
Hsp90 cycle.
There are five known isoforms of Hsp90 in humans: the cytoplasmic isoforms Hsp90α, Hsp90β, and Hsp90N, the endoplasmic reticulum isoform Grp94, and the mitochondrial isoform Trap-1 [9–12]. Hsp90α and Hsp90β are the primary focus of cancer therapeutics and in cancer research, both are referred to as Hsp90, and as such these two Hsp90 isoforms are the focus of this review. These two cytoplasmic proteins operate as homodimers; either α/α or β/β and have 85% structural homology. Their identical N-terminal structures make them difficult to separate, and therefore anticancer therapeutics are typically tested against both of these Hsp90 isoforms. Grp94 is the most abundant endoplasmic reticulum protein, but does not play a major role in oncogenic pathways as it has few client proteins with whom it is associated (immunoglobulins, several integrins and Toll-like receptors, plant CLAVATA proteins, and insulin-like growth factor II) and its role in regulating them is unknown [11]. Further, Grp94 does not associate with any of the co-chaperones that are associated with Hsp90. Trap-1 exists in the mitochondria [13], and does not appear to be associated with any cancer-related client proteins or co-chaperones [12]. With the exception of Hsp90N, the four isoforms of Hsp90 have similar structures and contain three domains, the N-terminal, middle and C-terminal domain (Fig. 1) [10, 14]. The N-terminal domain (24–28 kDa), is known to bind ATP, and upon hydrolysis to ADP the Hsp90 dimer switches from the open to closed conformation (Fig. 2). This hydrolysis and subsequent structural change plays a role in Hsp90’s ability to regulate the function of several oncogenic client proteins [15] (Fig. 2). Hsp90N exists in the cytoplasm with Hsp90α and Hsp90β. Although it was first reported in 1988, little has been investigated on its role in cell signaling pathways or in cell growth [16]. However it is known that it lacks the N-terminal domain, and therefore molecules that bind and inhibit ATPase activity via this domain, which are most Hsp90 inhibitors, do not bind to Hsp90N [16]. In contrast, Hsp90N contains a hydrophobic 30 amino acid sequence unique to this isoform. Hsp90N has shown to interact and activate Raf, an oncogenic protein, via this 30 amino acid sequence [10]. However, no other oncogenic client proteins appear to interact with Hsp90N. The middle domain (38–44 kDa) is where most client proteins bind, and this domain plays a key role in stabilizing numerous cell-signaling proteins. By stabilizing and/or refolding these proteins, Hsp90 protects these clients from being degraded, and thus promotes cell growth via these protected pathways. Finally, the C-terminal domain (11–15 kDa) is where the two monomers of Hsp90 dimerize and it is this domain where several apoptotic-inducing proteins, including IP6K2 and FKBP38, bind [9, 14].
Molecules that block either the ATPase activity of the N-terminal domain or interfere with the binding between Hsp90 to its co-chaperones are of interest as potential anticancer therapeutics. Indeed, Hsp90’s role in the maturation and activation of such a large number of proteins involved in oncogenic pathways highlights its outstanding potential as a target for anticancer agents. That is, given that the efficacy of target-specific anti-cancer drugs may decrease or even be lost over time due to the high epigenetic variation within cancer cells, blocking a protein that affects numerous cancer-related pathways, such as Hsp90, can be an effective and efficient means of treating drug-resistant cancers [17–19].
A majority of Hsp90 inhibitors discovered to date are macrocycles and there are a vast number of successful macrocyclic drugs currently in the market, including the immunosuppressant Cyclosporin A, antifungal Casopfungin, antibiotic Vancomycin, and anticancer agent Aplidine to name a few [20, 21]. Macrocyclic molecules exhibit many advantages over their acyclic counterparts [22]. Compared to acyclic molecules, macrocycles generally have more constrained conformations. This controlled conformational flexibility allows macrocycles to be more selective when interacting with a biological target such as Hsp90 [23]. In addition, macrocycles are also less susceptible to proteolytic degradation, which increases their lifetime in the body [23]. This review will examine a number of macrocycles that interact with Hsp90 and their action as anticancer therapeutics.
2. NATURAL PRODUCT MACROCYCLE HSP INHIBITORS
Geldanamycin (GA) and Radicicol (RD) are two natural product inhibitors of Hsp90, both of which bind to the N-terminal ATP binding pocket. Although these natural products are potent cell growth inhibitors, GA suffers from severe hepatotoxicity and insolubility in aqueous media, while RD is inactive in the body because it is metabolically unstable. Therefore, considerable efforts have gone into the alteration of the structures of these two macrocycles in order to improve hepatotoxicity, solubility, and stability. Some derivatives of GA are in clinical trials, while potent RD derivatives are still being explored. Meanwhile, GA, RD, and their analogs have been excellent tools for exploring the function of Hsp90 and its role in stabilizing oncogenic client proteins.
2.1. Geldanamycin (GA)
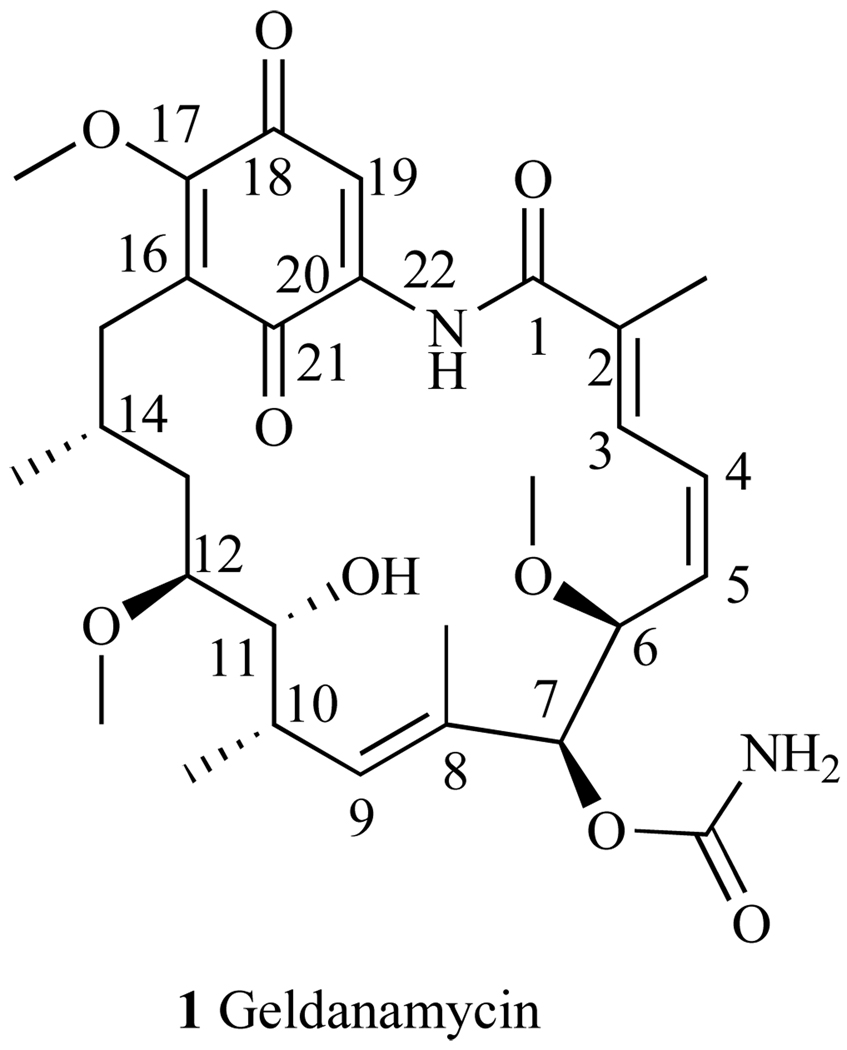
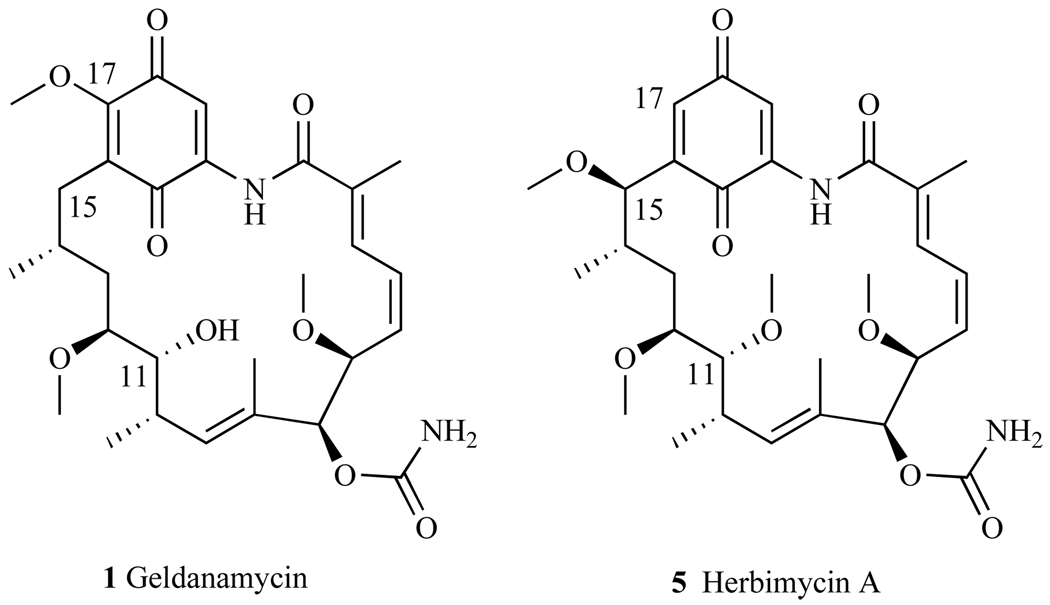
Geldanamycin was the first macrocycle found to inhibit Hsp90 at the N-terminal ATP binding pocket [24] (1, Fig. 3). Discovered in 1970 in the culture filtrates of Streptomyces hygroscopicus var. geldanus, GA exhibits antibiotic activity against protozoa [25]. It is a benzoquinone ansamycin composed of a quinone moiety linked to a macrocycle by an ansa bridge between C-16 and C-20 (1, Fig. 3) To assess this natural product’s capacity as an anticancer agent, GA was tested against the National Cancer Institute (NCI) 60 tumor cell lines and it demonstrated a mean GI50 of 180nM across the panel; notably, GI50 = 0.1nM for prostate cancer cell lines PC3 and DU-145 [26].
Fig. 3.
Natural trans amide structure of Geldanamycin.
GA demonstrates activity against several kinases, (fyn, lck, bcr-abl, and erbB2 [27]), and it was initially hypothesized to be a src-family tyrosine kinase inhibitor [28]. However, Whitesell and coworkers later immobilized a GA derivative on solid support, and identified the major cellular proteins with which GA interacts [29]. By immunoblot analysis it was determined that GA does not bind to v-src proteins directly, but rather it binds to Hsp90 and modulates the src kinase activity via GA’s interaction with Hsp90 [29]. That is, GA binds to Hsp90, blocks the binding of src kinases, leading to degradation and subsequent decrease in src kinase activity, thus it was actually the disruption of the Hsp90-v-src heteroprotein complex by GA that lead to the change in src kinase activity [30].
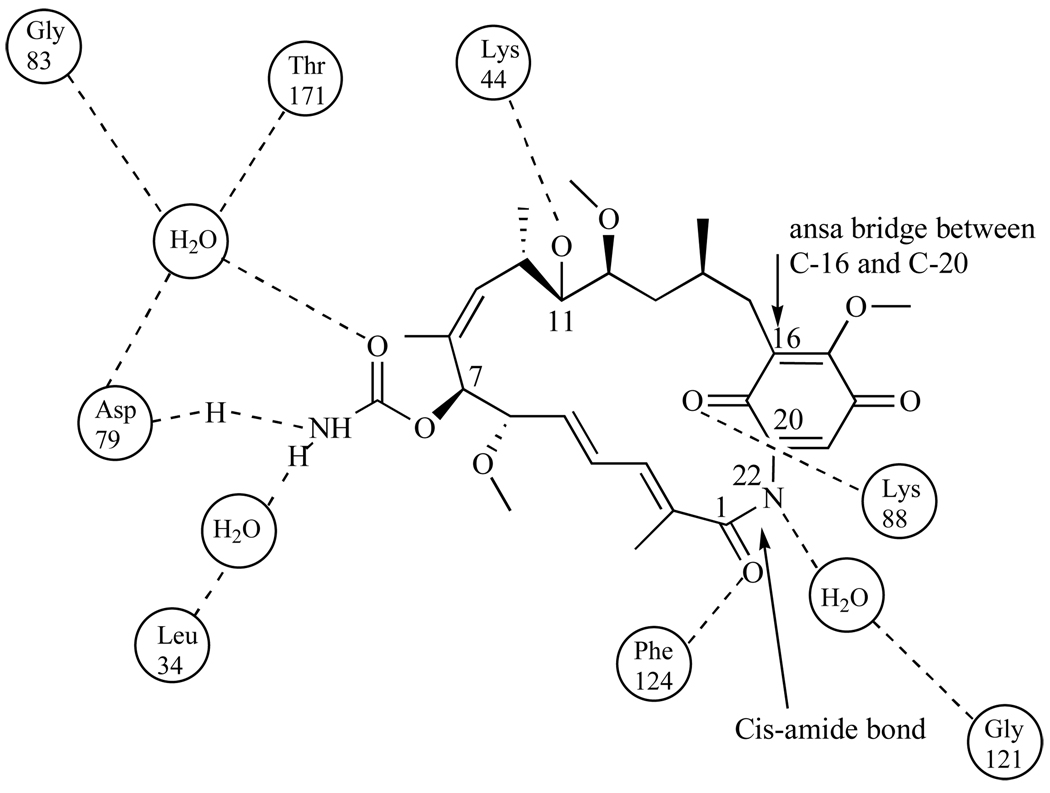
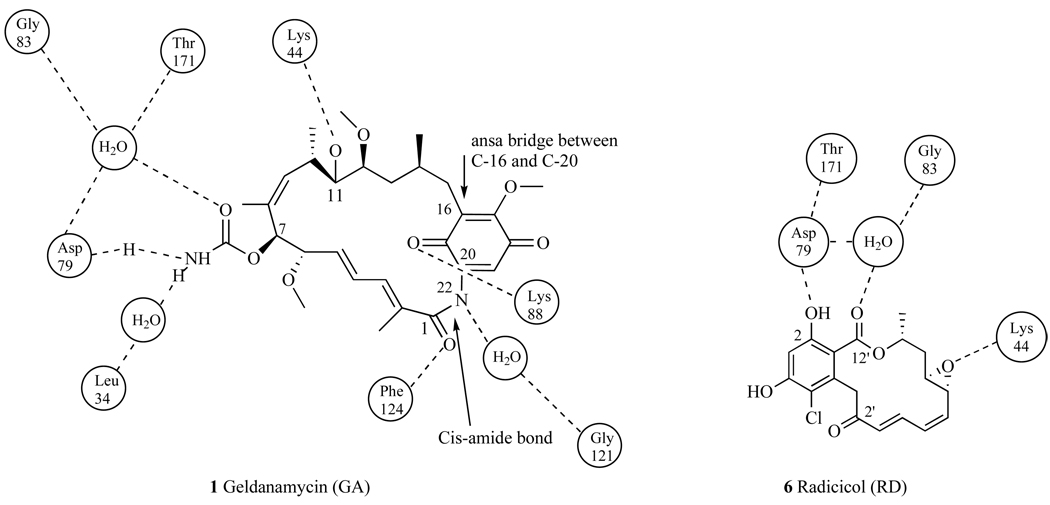
Pearl and coworkers then showed via crystal structure that GA bound to Hsp90 at the N-terminal domain and in the ATP binding site (Kd=1.2µM) [31, 32]. By blocking ATP binding, (Fig. 2), GA disrupts the conversion between the open and closed conformations of the Hsp90 dimer. When bound GA converts from its natural trans-amide shape (Fig. 3) to a cis-amide (Fig. 4) where the benzoquinone ring is directed toward the entrance of the N-terminal ATP binding pocket and the ansa ring is directed towards the bottom of this binding pocket (Fig 4). When bound to Hsp90, the C-7 carbamate of GA is stabilized in the pocket by hydrogen bonding directly to amino acid residue Asp79, and indirectly to Leu 34, Gly83, and Thr171 via water molecules [31]. Hsp90’s resulting conformation is then unable to bind to a number of crucial client proteins, which leads to the degradation of these proteins via the ubiquitin-proteasome pathway [33].
Fig. 4.
Cis-amide conformation of Geldanamycin and the major interactions occurring in the N-terminal ATP-binding pocket of Hsp90.
Although this data indicates that GA is an excellent candidate for advancement into clinical studies, it has many pharmacological drawbacks, the most severe of which are poor solubility and metabolic instability [26]. In addition, therapeutic doses to mice and dogs in pre-clinical studies showed severe hepatotoxicity, which was thought to be associated with the benzoquinone ring [26]. When the quninone moiety is metabolized by liver microsomes it generates free radicals, which induces hepatotoxicity [34]. GA’s poor preclinical data has resulted in many efforts to improve its pharmacological properties by modifying its structure and studying its structure-activity relationship (SAR) with Hsp90’s ATP pocket.
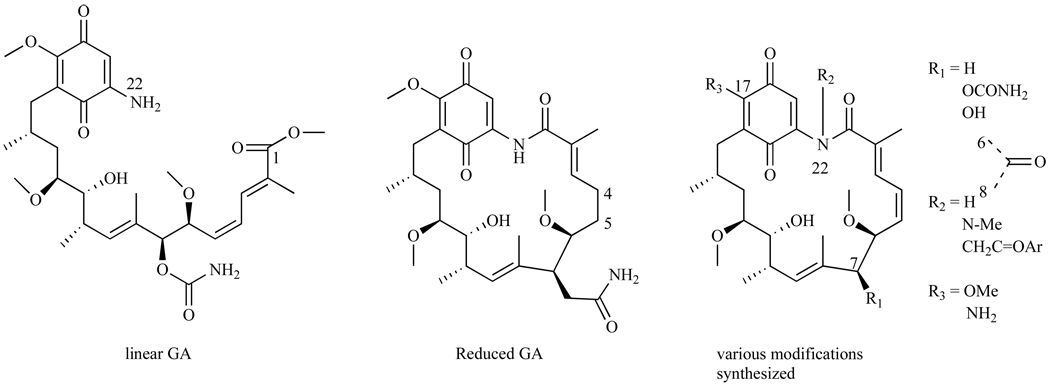
Schnur, et al. examined the SAR of GA, they modified various positions on GA and assessed in the depletion of p185, an Hsp90 client protein, in breast cancer cell line SKBr3 [35]. Cleavage of the GA amide bond between N-22 and C-1 (Fig. 5) generates linear GA that has significantly decreased in vitro activity compared to the macrocyclic GA structure (IC50 > 3200nM and IC50=70nM for linear and cyclic respectively) [35]. These data indicate that the rigid cyclic structure is critical for binding to Hsp90. Reducing the double bond between C-4 and C-5 (Fig. 5) in the backbone of the macrocycle resulted in about a 3-fold decrease in activity (IC50 = 230nM) compared to its parent GA, again suggesting that a rigid macrocycle is important for tight binding to Hsp90. Equally important is the carbamate moiety at position 7, where alterations at this position resulted in a 1000-fold decrease in the compound’s activity and deletion of this group generated a compound that had no activity (IC50 > 3900nM). Schnur et al. also found that small alkyl moieties at N-22, such as an N-methyl, led to compounds with over a 100 fold less activity (IC50 > 3500nM). However, when phenacyl moieties were substituted at the N-22 position, the IC50s were comparable to that of GA (IC50s=70–80nM). This phenomenon was explained by examining the structure of the compound that is active in cell culture, and it was determined that the acyl group is readily cleaved under these conditions, leaving the parent structure before acylation. Thus, this type of modification is not an improvement. In summary, some of these derivatives showed depletion of p185 to the same level as GA, however, these derivatives were not nearly as active as GA in in vivo studies, which Schnur et al. monitored using FRE/erbB-2 tumors in nude nu/nu mice and found them all showing limited potency [35]. The in vivo activity of GA was not determined, as it was inactive in the assay and lethal at doses above 200mg/kg. However, the analogues that were active in vitro, and had improved IC50s as compared to GA, were also inactive in vivo [35].
Fig. 5.
Derivatives synthesized by Schnur and co-workers.
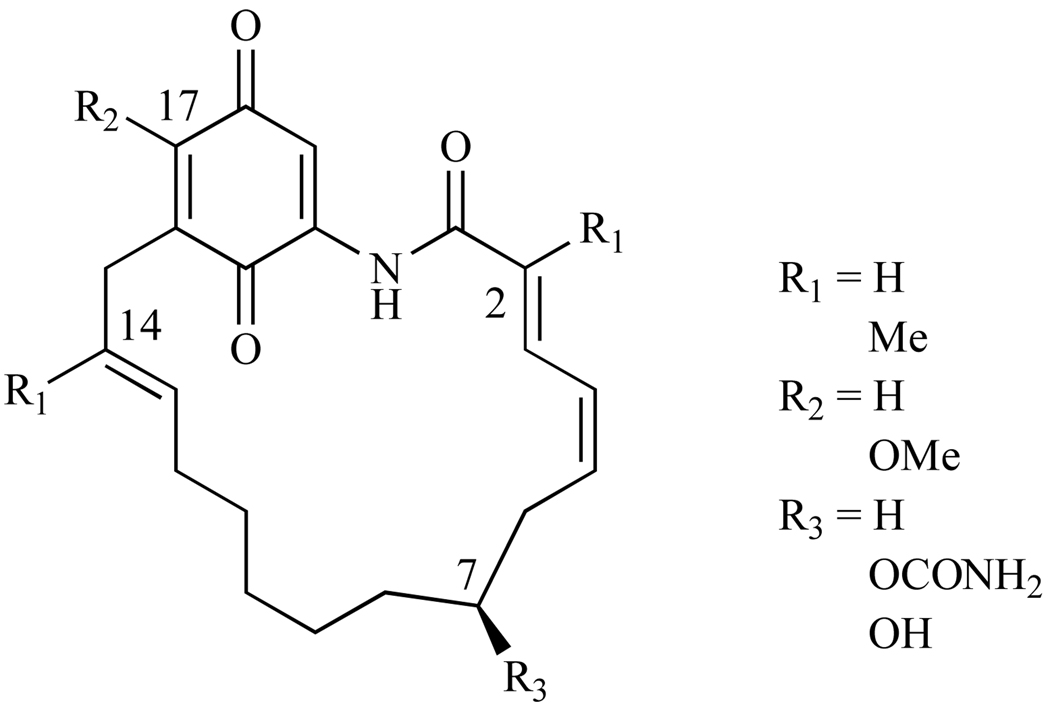
In a different study on the SAR of GA, McErlean et al. synthesized derivatives where only a few substituents were present on GA’s backbone [36]. Thus, derivatives containing only the C-2, C-14 methyl, C-17 methoxy, or C-17 carbamate were made (Fig. 6). For all of these simplified derivatives of GA, the binding affinities to Hsp90 were severely decreased. This can be attributed to the lack of hydrogen bonding networks between the amino acids within the N-terminal ATP binding pocket and the substituents on GA’s macrocycle. It is interesting to note that these basic stripped down derivatives exhibited micromolar potency in the drug-resistant HCT-116 colon cancer cell line, however this is attributed to the compounds acting via a different mechanism other than through modulating Hsp90’s activity [36].
Fig. 6.
Simplified versions of GA.
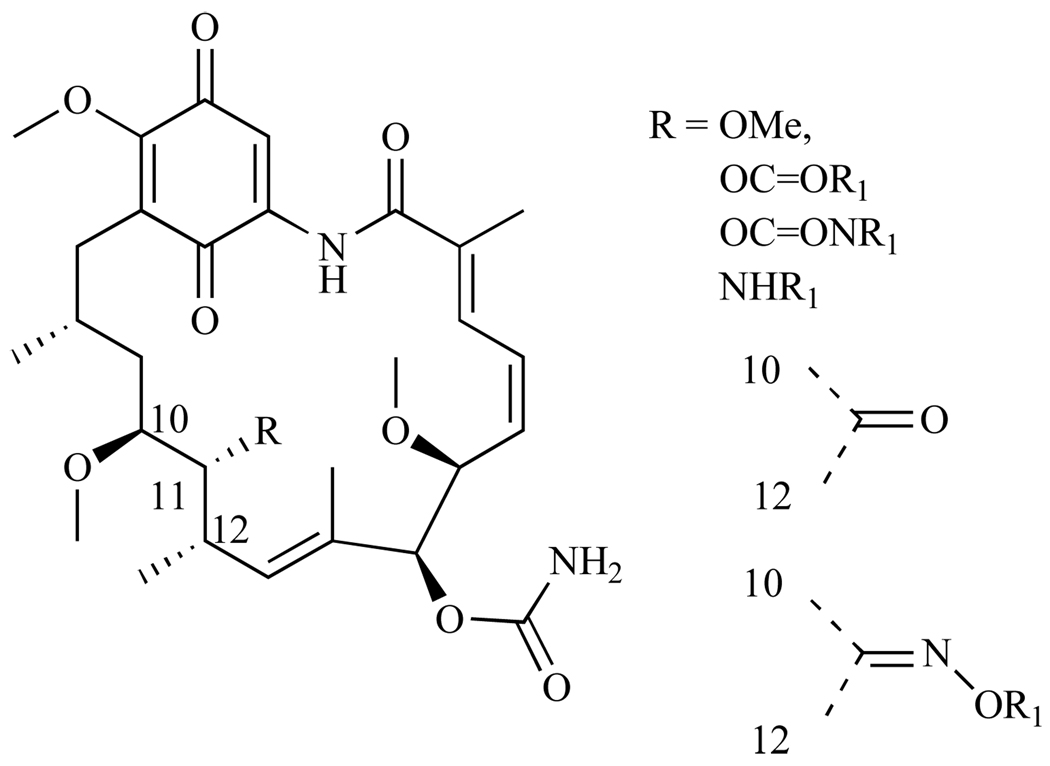
Tian and coworkers, to examine its overall purpose in the macrocycle biological activity, studied position C-11 of GA (Fig. 7) extensively. C-11 was modified with ethers, esters, carbamates, ketones, and oximes; and activity was assessed by measuring their binding affinity for Hsp90 as well as their cytotoxicity in the human breast cancer cell line SKBr3 [37]. All ether group substitutions at C-11, with the exception of O-methyl, gave compounds that had a 2–3 fold decrease in binding affinity for Hsp90. O-methyl had comparable Kd values to GA. All esters at the C-11 position had weak activity in all the cell lines tested (IC50 = 350–1000µM), which can be attributed to hydrolysis of the 11-ester regenerating the parent compound GA. However, they showed zero to weak binding affinity for Hsp90. Conversion of the hydroxyl moiety at C-11 to a ketone or oxime gave a compound that also had no binding affinity for Hsp90, while derivatives with amino groups substituted at C-11 lacked biological activity possibly because of steric interactions with the Hsp90 ATP binding pocket. Since bulky groups attached to C-11 significantly decreased cytotoxicity and binding affinity for Hsp90, and smaller groups did not, this study concluded that in order for a molecule to maintain modulation of Hsp90, it is crucial to have small functional groups at this position [37].
Fig. 7.
GA derivatives with modifications at C-11.
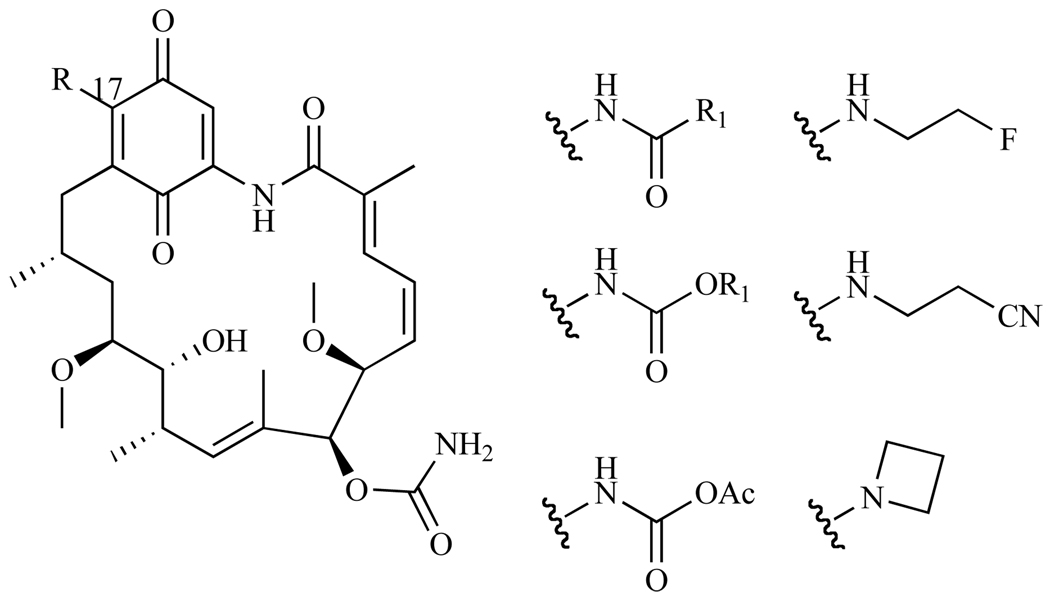
Based on crystallization studies, position C-17 of GA appears to be ideal for modification (Fig. 8). Since groups at this position do not appear to be associated with GA’s binding to Hsp90, unlike other substitutions, functional groups replacing the methoxy moiety should not interfere with the hydrogen bonding network, and should thus show high binding affinity and cytotoxicity via the Hsp90 pathway. It was also anticipated that conversion of the C-17 methoxy group to amino groups, would increase the molecule’s solubility in aqueous media, improving pharmacological properties of GA, while not compromising its potency [38–40]. Numerous derivatives of GA have been synthesized in order to determine which moieties at C-17 would be the most ideal for increasing solubility while maintaining cytotoxicity. Derivatives that incorporated amides, carbamates, ureas, and aryl moieties were synthesized and activities were determined by measuring the depletion of Her-2 client protein in the breast cancer cell line MCF7. It is expected that, if any of the derivatives are actively binding to Hsp90 and inhibiting the interaction between Her-2 and Hsp90, degradation of Her-2 will occur via the ubiquitin-proteasome pathway. Within the amide derivatives, aromatic functional groups had better potencies than their aliphatic counterparts (IC50 = 200–1000nM and 1700µM for aromatic versus aliphatic respectively). Compounds that contained benzylalkylamino groups were three times more active than dialkylamino groups. Interestingly, alkyl carbamate derivatives had similar activity to the amides, while aryl carbamates were too chemically unstable to isolate [38]. Derivatives that incorporated a small, sterically unconstrained, and non-polar alkyl amino group at C-17 exhibited the best activity; these included (fluoroethyl) amino groups (IC50= 12nM), (cyanoethyl) amino (IC50 = 17 nM), and azetidinyl (IC50 = 23 nM) groups. [40].
Fig. 8.
GA derivatives with modifications at C-17
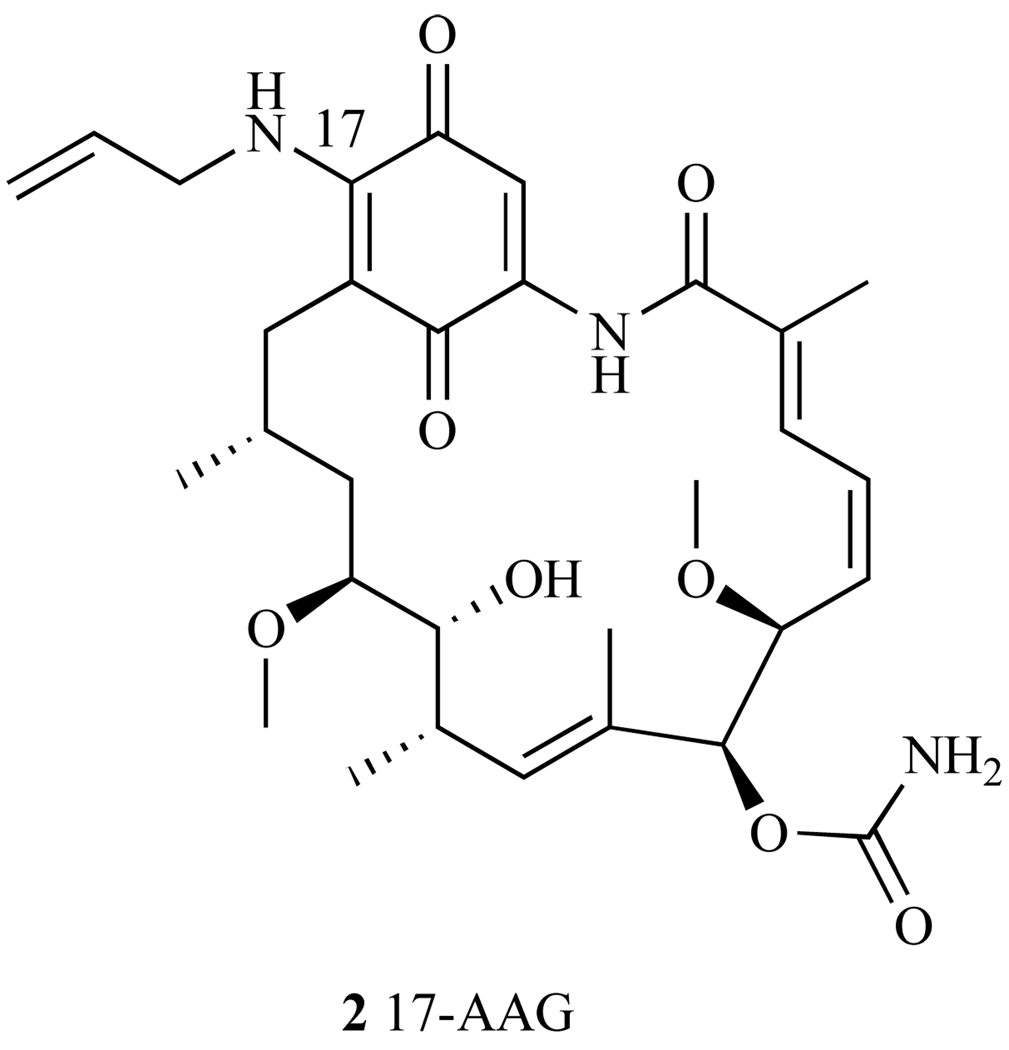
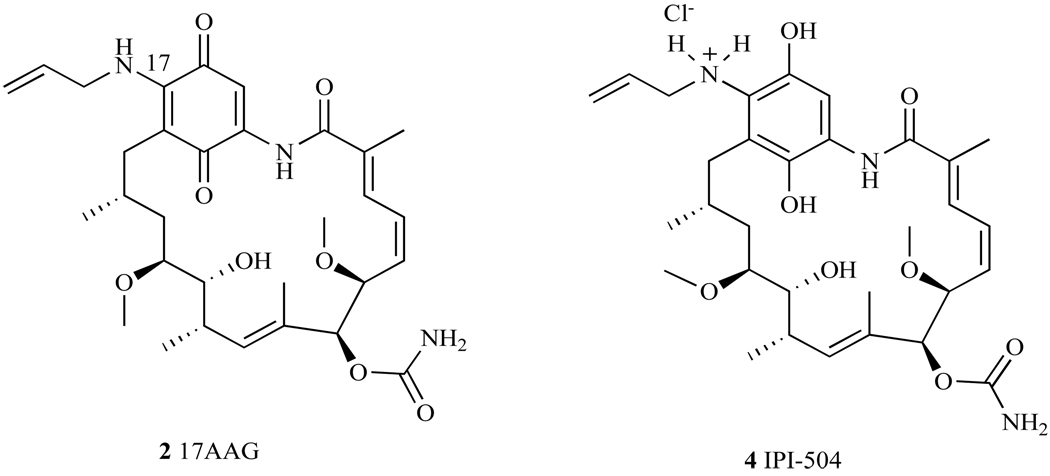
Overall, the SAR studies resulted in the follow up of two GA derivatives. Both have single modifications at the C-17 position and both demonstrated increased cytotoxicity over GA in the NCI 60-cell line panel. These two derivatives are 17-Allylamino-17-demethoxygeldanamycin (17-AAG) (Fig. 9), with an average GI50 = 123 nM in the 60 cell line panel and 17-(Dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG, discussed later), GI50 = 53nM [41]. 17-AAG is currently the most studied derivative of GA (discussed below), and is now in Phase I and Phase II clinical trials for treatment of several different types of cancer.
Fig. 9.
Structure of 17-AAG.
2.2. 17-Allylamino-17-demethoxygeldanamycin (17-AAG)
17-AAG is an allyl amino derivative of GA (Fig. 9), and it was hoped that this C-17 modification would show decreased liver toxicity and improved aqueous solubility and metabolic stability over its parent compound, GA. Like GA, 17-AAG binds to the N-terminal domain of Hsp90, blocking the binding of numerous client proteins, which results in the degradation of these proteins [29], thereby impairing their ability to induce cell growth. In a binding assay using lysed v-src transformed NIH/3T3 fibroblasts cells and GA-affinity beads, 17-AAG competed effectively with GA for Hsp90, inhibiting src from binding to Hsp90 in this assay. However, higher concentrations of 17-AAG were needed to effectively block src from binding to Hsp90 than were needed for the parent compound, GA (EC50 = 7.2 µM and 0.17 µM, respectively, ~50 fold difference). Although these data indicate that 17-AAG has a lower affinity for Hsp90 than GA, 17-AAG has demonstrated increased cytotoxicity compared to GA in several cancer cell lines and has been used as a valuable tool to establish which client proteins were affected by 17-AAG’s binding to Hsp90 [29].
Preclinical Data-GA and 17-AAG Macrocycles
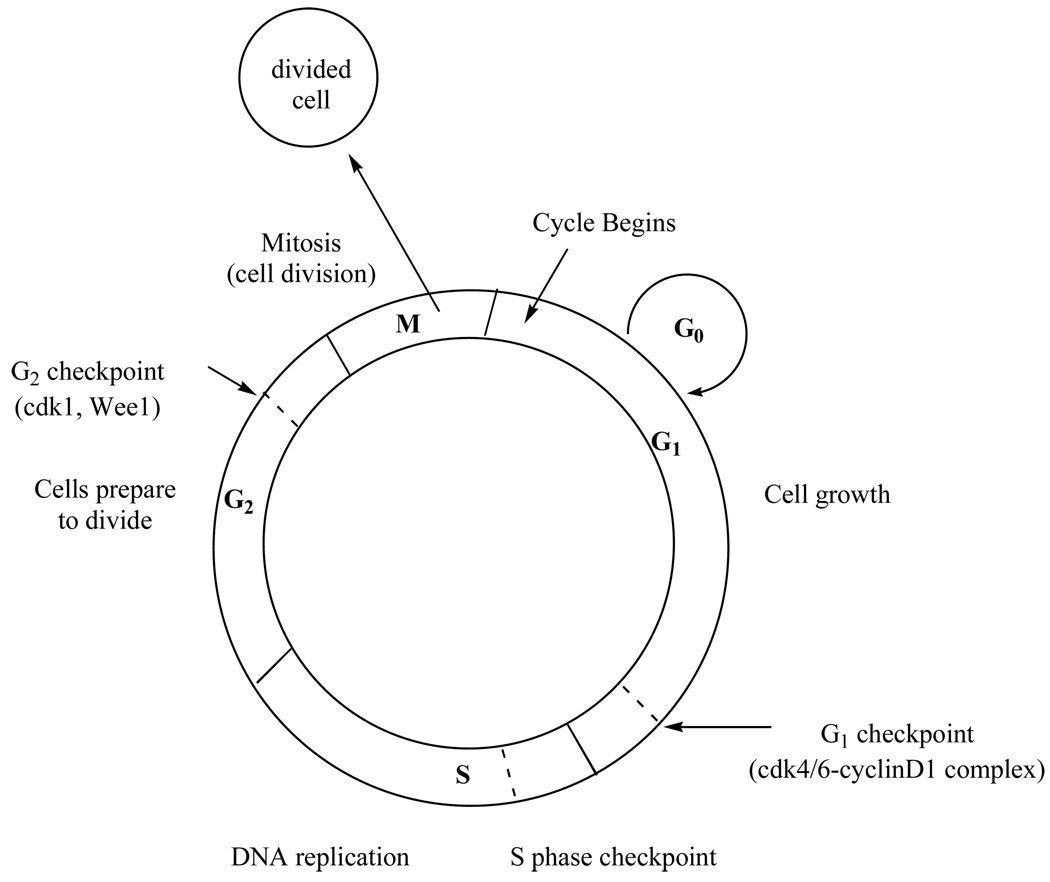
Passage through the cell cycle is regulated by specific proteins that must be expressed at various checkpoints within each phase (Fig. 10). Proteins required at the G1 or G2 checkpoint rely on Hsp90 to function. Therefore, inhibition of Hsp90 leads to a decrease in the amount of checkpoint proteins produced, causing potential problems for the cell during its growth and division phases. By halting cell division at these checkpoints due to a lack of checkpoint proteins that facilitate this process, the cell is unable to complete its replication cycle, which leads to apoptosis. Described below are studies showing how 17-AAG halts the cell cycle at either the G1/S (involving checkpoint proteins cdk4/6 and cyclin-D) or the G2/M (involving cdk1 and Wee1) phase in multiple cell lines, presumably by inhibiting the function of Hsp90, causing a depletion in their checkpoint proteins.
Fig. 10.
The cell cycle: Hsp90 client proteins required at G1 and G2 checkpoint are shown.
Melanoma
Grbovic and coworkers [42] determined that the mutated form of B-Raf, a protein kinase that is a member of the Raf gene family, relies on Hsp90 (Fig. 1). B-Raf is involved in cell signaling and promoting cell growth. Elevated activity stimulates constitutive signaling, proliferation, and survival, thus B-Raf has been established as a human oncogene [43]. Mutated forms of B-Raf activate the Ras/Raf /MAKT signaling pathways, which are typically activated in most melanomas. The most common mutated form of B-Raf is named V600EBraf for the glutamic acid replacement of valine at amino acid 600. Over 90% of all B-Raf mutants found in melanoma cancers have this glutamic acid substitution [44]. In nearly 70% of melanomas, V600EBraf is up-regulated. It was found that when 17-AAG was used to treat melanoma cell line SK-Mel-31, 17-AAG did not affect the level of wild type B-Raf protein, indicating that wild-type B-Raf does not need Hsp90 to function. However, in the V600EBraf mutated cell line SK-Mel-28, treatment of 17-AAG caused depletion of V600EBraf in as little as 12 hours [42]. In addition, treatment of SK-Mel-28 tumor xenographs in mice with a non-toxic dose of 17-AAG (100 mg/kg) resulted in over 80% V600EBraf depletion compared to control mice, who received a vehicle treatment with no drug [42]. These data establish that B-Raf plays an important role in melanoma, and that once mutated to V600EBraf, it relies heavily on Hsp90 for stabilization [45].
Lymphoma
17-AAG appears to affect certain pathways associated with Hsp90 in lymphoma. The P13K/Akt pathway plays a critical role in cell survival by preventing apoptosis and inducing cell proliferation and growth [46]. Akt is a client protein of Hsp90, and its function is to maintain the P13K pathway, thus facilitating the cell’s ability to survive. Disrupting the Hsp90-Akt association leads to the dephosphorylation of Akt and induces apoptosis. The dephosphorylation event occurs because Akt no longer protects the cells from apoptotic stimuli, thus, making the disruption of the Hsp90-Akt interaction an appropriate target in cancer therapy [47]. The inhibition of the P13K/Akt pathway using 17-AAG was observed in the NK/T lymphoma cell line (NKLT), where the PI3K/Akt pathway is constantly activated [48]. Specifically, NKLT cell lines HANK-1 and NK-YS were significantly more vulnerable to 17-AAG relative to the control cell line NK-L, indicating that NKLT was more dependent on Hsp90 via Akt than the control cell line.
In classical Hodgkin’s lymphoma (cHL), the Jak-STAT pathway relies on Hsp90. Janus kinases (Jak) are activators of Signal Transducer and Activator Transcription (STAT) proteins, where permanent activation of STAT is one indicator that a cell has become cancerous [49] (Fig. 1). Specifically, STAT3 and STAT6 are associated with cell proliferation in cHL. In cHL cell lines L428, L1236, and HDLM2, 17-AAG successfully deactivated the Jak-STAT pathway, linking this deactivation to the inhibition of binding between Hsp90 and Jak proteins. This pathway deactivation was indicated by the loss of STAT3 and STAT6 tyrosine phosphorylation, and the inability to detect Jak1 and Jak3 proteins [50]. Further, it was also observed that Akt is necessary for the survival of cHL cells, and 17-AAG rapidly depleted Akt from the HD-LM2 and L-428 cell lines [51, 52].
Mantle Cell Lymphoma (MCL) is characterized by an over expression of cyclin-D1, which is regulated by Hsp90’s client proteins cdk4 and cdk6. Cyclin D1 forms a complex with cdk4/6, which drives the cell from G1 to S phase (Fig. 10) [53]. In the G1 phase of the cell cycle, the cell does the majority of its growth in preparation for DNA synthesis, which occurs in the next phase of the cell cycle, the S phase. Before entering the S phase, the cell must go though a G1 checkpoint, where the cdk4/6-cyclin D1 complex must be expressed to prepare the cell for the S phase. Therefore, inhibition of Hsp90 leads to decreased activity of cdk4/6 and decreased levels of cyclin D1, causing cell cycle arrest at this G1/S transition. Since decreased levels of cyclin D1 can be associated with depletion of Hsp90’s client proteins cdk4/6, MCL cell lines Jek1, Mino, and SP53 were treated with 17-AAG and the level of cyclin D1 was monitored. Decreased levels of cyclin D1 occurred as the cells entered apoptosis via a G1 cell cycle arrest, which led to cell death. It was also observed that client protein Akt was down regulated, suggesting that 17-AAG was directly involved in inhibiting Hsp90 from binding and/or stabilizing Akt, thus perhaps providing an additional apoptotic pathway [54].
Hsp90 also chaperones a number of chimeric proteins that are essential for tumor survival [55]. Chimeric proteins occur when two or more genes are fused together because of an error in chromosomal translocation and can act as oncogenic proteins in cancer. Anaplastic large cell lymphoma (ALCL) arises from the chimeric oncogenic protein NPM-ALK, an Hsp90 client protein [56]. NPM-ALK originates from the fusion of nucleophosmin (NPM) and the membrane receptor anaplastic lymphoma kinase (ALK) genes. When this chimeric kinase is active, it is responsible for the malignancy of lymphomic tumors. Studies show that 17-AAG increases apoptosis, down-regulates NPM-ALK [57], and causes G0/G1 cell cycle arrest in ALCL cells [55]. Thus, by regulating the protein responsible for the cancerous phenotype, 17-AAG may be a potential therapeutic to treat ALCL.
Breast Cancers
In client protein degradation assays, 17-AAG and GA had similar activities in breast cancer cell lines SKBr8 and MCF7. In SKBr3, Hsp90 client proteins include p185, Raf-1 and mutant p53. It was observed that 17-AAG had lower EC50 values for blocking these three client proteins from binding than GA: the EC50 values for 17-AAG were 45nM, 80nM, and 62nM and for GA were 90nM, 170nM, and 210nM for p185, Raf-1, and mutant p53, respectively. In addition, the IC50 values for SKBr8 cells were both 4.1nM for GA and 17-AAG, while in MCF7 cells, 17-AAG had an IC50 of 5.2nM, while GA had an IC50 of 10.7nM, suggesting that GA and 17-AAG act via blocking the binding of these 3 client proteins in breast cancer [58].
Prostate
Multiple Hsp90 client proteins are up-regulated in prostate cancer, with the primary one being the androgen receptor (AR) [59]. ARs are responsible for tumor growth and survival, and Hsp90 seems to be essential for their function and stability in prostate cancer. Studies show that 17-AAG decreased the levels of the AR receptor and additional client proteins such as Akt and Her-2 in the prostate cancer cell line LNCaP [60]. In xenograph animal models using the prostate cancer cell line DU-145, 17-AAG demonstrated reduced tumor growth in 86% of the mice [61].
Leukemia
High expression of Hsp90 is observed in numerous leukemia cell lines [62]. In chronic myeloid leukemia (CML), the chimeric Hsp90 client protein BCR-ABL is depleted after administration of either GA or 17-AAG [63]. As observed in other cell lines, 17-AAG increased apoptosis in the chronic lymphatic leukemia (CLL) cell line, by inhibiting the Akt pathway [64]. Further, when 17-AAG and kinase C inhibitors, such as UCN-01, were used together, they exhibited a synergistic relationship by increasing apoptosis in several leukemia cell lines [65]. Thus, dramatic increase in apoptosis was observed when leukemia cell lines were exposed to combined treatment of 1.5 µM of 17-AAG and 75nM of UCN-01, while no significant apoptotic activity was observed when these cell lines were treated with either UCN01 (75nM) or 17AAG (400nM) alone. Interestingly, Hsp90 client proteins Raf-1 and Akt are the primary client proteins that undergo degradation in these cell lines [65, 66].
Lung Cancer
As is observed in most cancer cells, Hsp90 expression is up-regulated in non-small cell lung cancer (NSCLC) cells, specifically: A549, H226B and ChaGo-K1. With the high levels of Hsp90, several Hsp90 client proteins have been identified as important proteins for promoting growth in NSCLC cells. These up-regulated and activated client proteins include a mutated EGF receptor, a receptor kinase that activates and promotes cell proliferation and survival, Akt, and a mutated p53, whose normal function is to regulate the cell cycle and suppress tumor growth. These three client proteins are found to play a key role in the NSCLS cell survival. The EGF receptor is a tyrosine kinase receptor which is frequently mutated in non-small cell lung cancer (NSCLC) and it is over expressed in 40–80% of NSCLC tissues [67]. The activation of Akt is seen in 51% of NSCLC cell lines [68], and p53 is mutated in 50% of NSCLC tissues [69]. 17-AAG inhibited cell growth with IC50 values 124nM, 61nM, and 110nM for A549, H226B, and ChaGo-K1 cell lines, respectively [70]. As with other cell lines, it was observed that addition of 17-AAG led to degradation and decrease in Akt and p53, thus explaining the potent IC50s observed against this cell line. These data suggest that 17-AAG is a possible therapeutic option for treating NSCLC.
Gastrointestinal Cancers
Colon Cancer
17-AAG depletes Hsp90 client protein Raf-1 in four human colon adenocarcinoma cell lines - HT29, HCT116, KM12 and HCT15. Of the four cell lines, HT29, a cell line that responds well to the drug treatment of choice (5-FU) was the most sensitive to 17-AAG (IC50=0.5µM). HCT116 and KM12, which are established as drug-resistant cancer cell lines [71–73], were moderately sensitive to 17-AAG (IC50=0.9µM and 0.8µM for HCT116 and KM12 respectively). HCT15 cells, also drug resistant, were the least sensitive to 17-AAG treatment (IC50=46µM). In order to determine if the cytotoxic effects of 17-AAG were related to a mechanism involving Hsp90, Raf-1 levels were monitored in these four cell lines. In HT29, Raf-1 levels were not restored, even after 48 hours of 17-AAG treatment. However, a moderate recovery of Raf-1 was observed in HCT116 and KM12 cells [74]. Finally, HCT15 cells showed full restoration to control levels of Raf-1. These data link the potency of 17-AAG in colon cancer cell lines to the recovery of the Hsp90 client protein, Raf-1. More recently it was observed that treatment of HCT116 cells with 17-AAG induced a G2 checkpoint arrest. In the G2 phase of the cell cycle, the cell continues to grow in preparation for mitosis, which occurs in the final step of the cell cycle, the M (mitotic) phase. Before entering the M phase, the cell must go though a G2 checkpoint to ensure the cell is prepared to divide. Wee1 and cdk1 are two proteins that are required to drive the cell from G2 to M phase. Wee1 is responsible for maintaining the cell in G2 phase, and cdk1 triggers mitosis [53]. It has been suggested that both of these two proteins are dependent on Hsp90. Addition of 17AAG to the cell decreases the accumulation of these proteins, ultimately leading to the arrest of the cell in the G2/S phase and subsequently apoptosis [75]. This suggests that both of these are Hsp90 client proteins, and implies that their binding is inhibited by 17-AAG [75]. This discovery has now promoted the investigation of these proteins as potential new therapeutic targets and inhibition of their pathways is being explored as a plausible approach for treating drug resistant cancers.
Esophageal
Esophageal cancer is currently only treatable by radiation and chemotherapeutics [76]. Normal esophageal tissue shows little to no expression of Hsp90, while esophageal cancer tissue from patients shows high levels of Hsp90 expression [76]. In cytotoxicity and protein degradation studies, Wu and coworkers treated esophageal cancer cell lines, Kyse 70 and Kyse 450, with 500nM 17-AAG for 48 hours [76]. They reported 80% and 84% growth inhibition in Kyse70 and Kyse450 cell lines respectively. Further, as observed in other cancer cell lines, there was a down-regulation of Hsp90 client proteins Akt and Erk [76]. These data suggest that 17-AAG is a potential chemotherapeutic for treating esophageal cancer.
Liver Cancer
Watanabe et al. [77] observed a decrease in the G2/M phase when hepatocellular carcinoma (HCC) cell lines, Hep3B and Huh7, were treated with 17-AAG. The cells were arrested in G2/M phase, resulting in an increase in apoptosis over that observed in normal cells. When comparing these two cell lines as xenographs in mice, Hep3 xenographs showed no notable tumor decrease, while Huh7 xenographs demonstrated a marked improvement in tumor growth upon 17-AAG exposure [77]. These data suggest that Huh7 tumors rely heavily on Hsp90 modulation of growth factors, while Hep3 tumors do not.
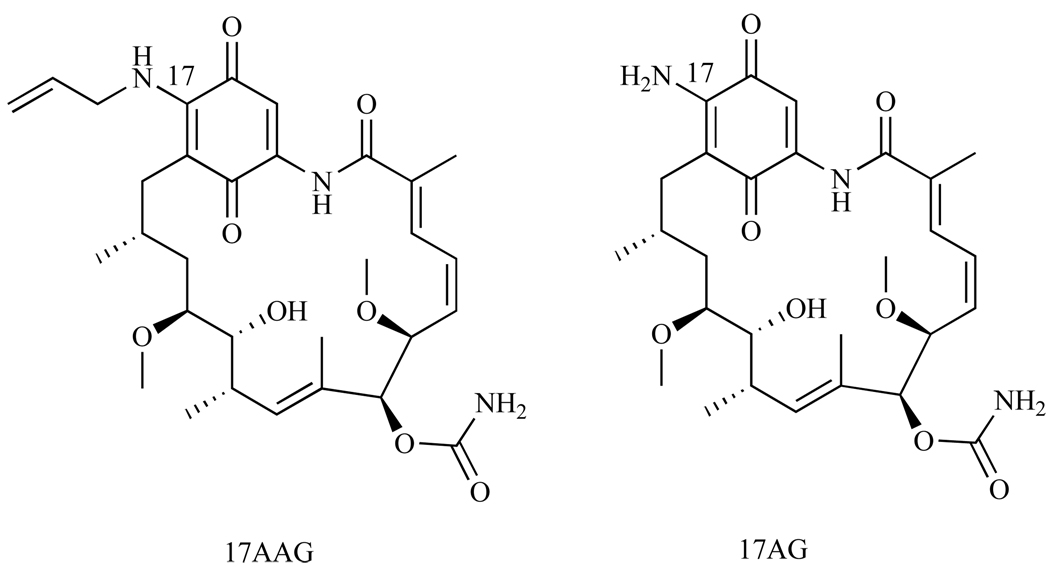
In summary, 17-AAG has proven to be a novel Hsp90 inhibitor in preclinical trials, maintaining potency across numerous cancer cell lines and affecting multiple oncogenic proteins and pathways (Table 1 and Table 2). Since 17-AAG metabolizes to 17-amino,17-demethoxygeldanamycin (17-AG) (Fig. 11), which also inhibits Hsp90, the metabolite may be a contributing factor to 17-AAG’s success [78]. However, 17-AAG is still insoluble in aqueous media, having only 0.10 ± 0.01 mg/mL solubility in a 50mM sodium phosphate buffer at pH 7.0 [79]. Therefore, like GA, its formulation requires dimethyl sulfoxide (DMSO), which can have several adverse side effects [80], including hepatic and cardiac toxicities [81]. In addition, a DMSO-containing vehicle can not be administered orally, which is to most ideal method of administration. Despite these issues, 17-AAG was recommended for clinical trials due to its improved metabolic stability over GA [58]. To date, 17-AAG has passed through Phase I clinical trials and is now in Phase II/III clinical trials and is still being tested against a variety of cancer cell lines including melanoma, breast, prostate and thyroid cancer [82].
Table 1.
Macrocyclic Hsp90 Inhibitors and their Affect on Client Proteins in Cancers
| Hsp90 Inhibitor | Cancer Type | Hsp90 Client Proteins Affected |
|---|---|---|
| Geldanamycin | v-src transformed 3T3 cells | Src kinases, fyn, lck, bcr-abl, and erbB2 |
| 17-AAG | Breast Cancer | erbB-2, Raf-1, mutant p53 |
| Melanoma | V600EBraf | |
| Anaplastic large cell lymphoma | NPM-ALK | |
| NK/T cell lymphoma | Akt | |
| Hodgkins Lymphoma | Jak kinases, Akt | |
| Mantle Cell Lymphona | Cyclin D, Akt | |
| Prostate | Androgen Receptor, Akt, Her-2 | |
| Esophageal Cancer | BCR-ABL, Raf-1, Akt | |
| Non-small cell lung cancer | Akt, mutant p53, EGFR | |
| Colon | c-Raf-1, Chk1, Wee1 | |
| Exophageal | Akt, Erk | |
| 17-DMAG | Melanoma | Akt, cdk4, c-Raf-1 |
| IPI-504 | Non-small cell lung | EGFR |
| Myeloma | ATF6, XBP-1, PERK/eIF-2 | |
| Prostate Cancer | Androgen receptor, Akt, Her-2 | |
| Breast Cancer | Her-2 | |
| Herbimycin | Ehrlich Carcinoma | v-Src and Bcr-abl tyrosine kinases |
| Radicicol | Breast Cancer | Raf-1, Cdk4 |
| Radanamycin | Breast Cancer | Her-2 |
| San A | Colon Cancer | IP6K2, FKBP52, FKBP38 |
| Di-San A | Colon Cancer | IP6K2 |
Table 2.
Clinical Trial Stage and Potencies of Macrocyclic Hsp90 Inhibitors
| Hsp90 Inhibitor | Clinical Trial Stage | Cancer Type | Cell line | IC50 |
|---|---|---|---|---|
| Geldanamycin | Phase I | Prostate Cancer | PC3, DU-145 | 0.1 nM |
| Neuronal | CHP-100 TC-32 SKNMC |
5 nM 5 nM 8 nM |
||
| Breast cancer | SKBR3 | 90 nM | ||
| 17-AAG |
Phase II |
Breast Cancer | SKBR3 | 45 nM |
| Melanoma | MeI270 92.1 TP31 OCM-1 |
97 nM 60 nM 65 nM 77 nM |
||
| Lymphoma | Jeko1 Mino SP53 |
5.02 µM 1.71 µM 3.78 µM |
||
| Prostate Cancer | LNCap LAPC-4 DU-145 PC-3 |
25 nM 40 nM 45 nM 25 nM |
||
| Leukemia | AML | 1.5 µM | ||
| Lung Cancer | A549 H226B ChaGo-K1 |
124 nM 61 nM 110 nM |
||
| Colon Cancer | HT229 HCT116 KM12 HCT15 |
0.5 µM 0.9 µM 0.8 µM 46 µM |
||
| Liver Cancer | Hep3B HuH7 |
2.6 µM 430 nM |
||
| 17-DMAG | Phase I | Lung Cancer | SLR20 | 250nM |
| IPI-504 | Phase III | Myeloma | MM1.s RPMI-8226 |
307 nM 306 nM |
| Herbimycin | Pre-clinical | Leukemia | CLL | 0.31 µM |
| Radicicol | Pre-clinical | Breast Cancer | MCF-7 | 0.03 µM |
| Pochonin D | Pre-clinical | HSP90 | 0.08 µM | |
| Radanamycin | Pre-clinical | Breast Cancer | MCF-7 | 1.2 µM |
Fig. 11.
Structure of 17-AAG compared to 17-AG.
Clinical Trials
Phase I clinical trials for 17-AAG determined that the maximum tolerated dose (MTD) for weekly admission in patients was between 295–450 mg/m2 [83–85]. Side-effects in these studies were primarily related to hepatotoxicity associated with the drug vehicle, DMSO. Although most phase I clinical trials only monitored effectiveness and toxicity, one trial with eleven melanoma patients, specifically monitored Hsp90 client protein degradation using biopsies before and after treatment. At a once-weekly dose of 450mg/m2, two patients with metastatic melanoma were reported to survive in stable condition for 15 and 35 months after treatment [83]. Since the client proteins associated with the Ras/Raf/Mitogen pathway in melanoma are Raf-1 and cdk4, these protein levels were monitored in the patient tissue before and after 17-AAG treatment. Six patients had detectable Raf-1 protein, and depletion of Raf-1 was seen within 24 hours after treatment of 17-AAG. The client protein cdk4 was detectable in nine patients, and depletion of this client protein was seen in 8 out of nine patients. However, at 72 hours, there appeared to be a high level of client protein recovery suggesting that Hsp90 inhibition is short-lived [74, 83].
Phase II clinical trials for 17-AAG have been conducted in patients with melanoma, renal, and prostate cancer. One trial used fifteen metastatic melanoma patients, the majority of whom had the V600EBraf mutation [86]. These patients were monitored for the effects on the Hsp90 client protein Raf-1, however this client protein was not depleted, suggesting that 17-AAG has either a short-lived effect in patients, or its ability to modulate client protein depletion, specifically Raf, in vitro does not translate to in vivo conditions. Given these poor results in Phase II trials, 17-AAG was discontinued as a single treatment [87]. However, there is currently one on-going Phase I clinical trials in which 17-AAG is used in combination with the FDA approved drug Sorafenib to treat solid prostate tumors, in hopes of achieving a synergistic effect (www.clinicaltrials.gov). Given that Hsp90 is up-regulated in these tumors, it is hoped that shutting down pathways linked to this protein, while simultaneously eliminating those associated with Sorafenib, will inhibit Hsp90’s client proteins from recovery. Sorafenib specifically targets the Ras/Raf/Mitogen pathway, inhibiting Raf-1, and EGFRs, which are also Hsp90 client proteins [88]. Thus, unlike the clinical trials where 17-AAG is used alone and the client proteins appear to recover function after a short period of time [83], using 17-AAG in conjunction with drugs that inhibit the same pathways may prevent client protein recovery, leading to an effect that would be similar to that observed in vitro.
Future Directions - GA and 17-AAG
While both GA and its derivative, 17-AAG, effectively alter Hsp90’s function when used alone, using them in conjunction with other treatment therapies can often increase efficacy of this macrocycle. Co-chaperones have recently become of interest as therapeutic targets because they regulate Hsp90’s activity and assist Hsp90 in its protein folding process. It was noted, for example, that when Hsp90 was inhibited from its function of protein folding, Hsp90’s co-chaperone, Hsp70, is up-regulated and has been shown to compensate for Hsp90’s function [89]. This observation may explain why client protein levels in patients are initially low but then recover to normal levels after a short period of time. McDowell et al. [90] have compiled a list of notable co-chaperones that assist in Hsp90’s protein folding cycle. This list was compiled by analyzing the co-chaperones expression in numerous tumors. They reported an increase of at least one Hsp90 co-chaperone protein expression in 10 out of 17 tumors analyzed. Relative to normal cells, all tumors analyzed had increased amounts of co-chaperones Aha1, HSF1, p23, or Tpr2. One study observed that adrenal, liver, and stomach tumors all showed an increased level of HSF1 relative to non-cancerous cells. Lung, ovary, and breast cancer expressed greater than normal levels of Tpr2, and thyroid cancer cells expressed elevated levels of p23 relative to normal cells. Additionally, some cancers had up-regulated levels of more than one co-chaperone; bladder cancer expressed greater than normal levels of Aha1 and Tpr2, while kidney cancer had an increase of Aha1 and HSF1 relative to normal cells [90]. One of the major co-chaperones being studied today is cdc37. A siRNA knockdown of the expression of cdc37 in cells leads to a decrease in client proteins ERK, Akt, and mTOR [91]. Gray and coworkers [92] determined that cdc37 is up-regulated in pancreatic cancer cell lines and they showed that using a knockdown, followed by 17-AAG treatment, resulted in greater tumor growth inhibition than cells that were treated with 17-AAG alone [92, 93]. These data suggest that depletion of the co-chaperone cdc37 in-conjunction with modulation of Hsp90 may limit the cell’s ability to compensate for Hsp90 inhibition alone. Thus, despite the unfavorable pharmacological attributes of GA and 17-AAG, these compounds can still provide beneficial therapeutic effects in patients when used in conjunction with other therapies, potentially exerting a synergistic effect on tumors.
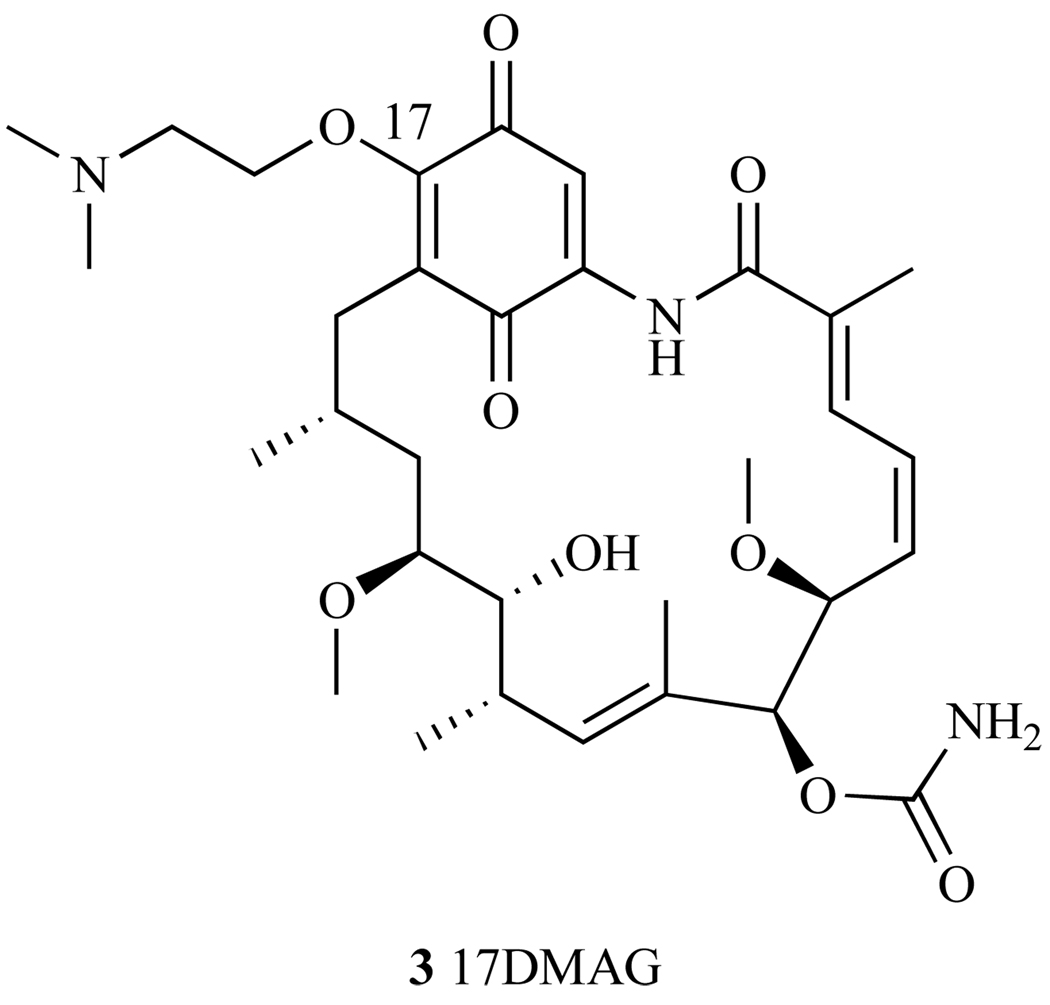
2.3. 17-(Dimethylaminoethylamino)-17-demethoxy-geldanamycin (17-DMAG)
To increase solubility in water, a second generation GA derivative, 17-Dimethyl-aminoethylamino-17- demethoxygeldanamycin (17-DMAG), was developed by Kosan Biosciences. This analog incorporates an ionizable functional group at the C-17 position (3, Fig. 12) and like its predecessors, it binds to the N-terminal ATP pocket of Hsp90 [94]. The NCI 60-cell line panel screening showed an overall GI50 = 51nM, which is over two-fold more potent than 17-AAG (GI50 = 130nM) [95]. In addition, 17-DMAG treated tumors showed reduction in tumor volume in xenographs mouse models of breast, lung, melanoma and leukemia cancer cell lines [96–98]. Further, this hydrophilic analog also showed an increased bioavailability over that of 17-AAG, where in pancreatic carcinoma mouse xenographs, 17-DMAG decreased metastases at doses of 6.7–10mg/kg twice daily for 5 days when administered orally, while 17-AAG had no effect [98]. Thus, the oral activity of 17-DMAG opens up another route of administration that is not possible with 17-AAG. It was observed in mechanistic assays that treatment of several melanoma cell lines with 17-DMAG led to the depletion of Akt, cdk4, and Raf-1 client proteins [41]. However, 17-DMAG has a dose limiting toxicity problem, with high liver and cardiac toxicity. Importantly, 17-DMAG toxicity was significantly higher than that shown by 17-AAG [99]. The recommended MTD to prevent liver damage is 1.3 mg/m2 daily for 5 days, a 30 fold decrease compared to the lowest daily MTD of 17-AAG (40 mg/m2) [99, 100]. In Phase I clinical trials, 3 out of 17 patients with chemotherapy refractory acute myelogenous leukemia had a complete response to therapy, at a twice weekly dose of 8, 16 or 24 mg/m2. However, overall drug related toxicity of this compound was unfavorable, as it caused both liver and cardiac toxicity [101]. Kosan Biosciences ended clinical trials in March 2008 [99].
Fig. 12.
Structure of 17-DMAG.
2.4. IPI-504
17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504, generic name Retaspimycin) was developed as a water-soluble GA derivative by Sydor et al. of Infinity Pharmaceuticals (4, Fig. 13) [102]. It was shown that the hydroquinone was unstable under physiological conditions, and was oxidized to an aniline based aromatic compound [35, 40]. In order to reduce the oxidation potential of the hydroquinone, it was necessary to stabilize this moiety as a hydrochloride salt (Fig. 13) [102, 103]. This salt formation inhibited the oxidation of the hydroquinone under physiologically relevant conditions, while increasing the compound’s aqueous solubility.
Fig. 13.
Structure of 17-AAG and IPI-504.
IPI-504 exhibits five times greater solubility in water than 17AAG (>250mg/ml), allowing other agents besides DMSO to be used for formulation during administration. It was shown in competitive binding assays that IPI-504 had a nearly 2-fold higher binding affinity for Hsp90 than 17-AAG [102]. Thus, the presence of a hydroxyl moiety in IPI-504’s hydroquinone is hypothesized to play an important role in hydrogen-bonding within the binding pocket of Hsp90. IPI-504 also demonstrated comparable IC50 values in cell lines to 17-AAG, and had similar effects on Hsp90 client proteins to those shown by 17-AAG. Given the detail with which the cellular mechanism of 17-AAG was discussed, including the affected client proteins, and the mechanistic similarity of 17-AAG to IPI-504, these details are not replicated for IPI-504, instead they are summarized in Table 1. However, given the improved physiological profile of IPI-504, and its increased stability, IPI-504 has been advanced to Phase I and II clinical trials for numerous cancers, including non-small cell lung cancer (NSCLC), gastrointestinal stromal tumor (GIST), multiple myeloma (MM), castration-resistant prostate cancer (CRPC), and breast cancer [104, 105].
Non-Small Cell Lung Cancer
EGFR is a tyrosine kinase receptor, it is also an Hsp90 client protein, and is frequently mutated in non-small cell lung cancer (NSCLC) [106]. Phase I clinical trials of IPI-504 treatment of NSCLC involved 9 patients with known EGFR mutations [105]. After a four-week, twice-weekly treatment with IPI-504, 7 of the patients had no new tumors appearing and little change in the size of tumors that were already present. IPI-504 was then advanced to Phase II clinical trials for 10 patients with stage IIIB or IV NSCLC and known EGFR mutations [105]. This trial proved successful with 1 out of 10 patients having a complete remission. This positive result led to an expansion of the clinical trials with a patient population of 57 patients with either EGFR mutant, wild-type, or unknown expression [105]. Although the overall response rate to IPI-504 with these 3 different patient populations was 7%, the response rate of those patients with only wild-type EGFR was 14.2%. Further, this 14.2% exhibited a tumor progression-free period of 3.9 months. With this promising data, IPI-504 was advanced to Phase III clinical trials. However, Infinity Pharmaceuticals recently halted the trials when a review of the 46 patients enrolled in the study showed a higher mortality rate among patients treated with IPI-504 than those receiving a placebo [107].
Myeloma
Against multiple myeloma (MM) cells, IPI-504 proved to be an effective Hsp90 inhibitor, disrupting many of the chaperone’s functions [108]. These effects include suppression of cell surface expression and signaling for receptors associated with Hsp90, specifically IGF-1 and IL-6, decreased intracellular levels of several kinases, and finally tumor cell sensitization to other pro-apoptotic drugs. Hsp90 inhibition is unique in MM cells compared to other cancer cells because the client proteins that are inhibited are a part of an unfolded protein response (UPR) pathway [108]. This pathway promotes cell survival by preventing the accumulation of misfolded proteins in the cell [104]. Specific Hsp90 client proteins associated with this pathway include ATF6, XBP-1, and PERK/eIF-2. Blocking these client proteins from binding to Hsp90 allows misfolded proteins to accumulate and triggers apoptosis. Treatment of MM cells with IPI-504 indeed inhibits these client proteins from interacting with Hsp90, thereby inhibiting UPR (allowing misfolded proteins to accumulate), and inducing apoptosis. Thus, it appears that IPI-504 is a promising treatment for myeloma, where the UPR pathway is active.
Prostate
In patients with castration-resistant prostate cancer (CRPC) Hsp90 client proteins AR, Akt, and Her-2 are up-regulated [109]. Phase II clinical trials are currently evaluating treatment of CRPC with IPI-504 [105]. These clinical trials have two groups of male patients: those who have had no prior chemotherapeutic treatment for CRPC (group A: 4 patients) and those who experienced progression of the cancer while being treated with docetaxel (group B: 15 patients). MTD determined was 400mg/m2 on day 1, 4, 8, and 11 for 21-day cycles. Thus far, there have been two deaths for patients in group B, one due to hepatic failure and the other due to hyperglycemic ketoacidosis. This Phase II clinical study is still active and their results appear promising [105, 110].
Breast Cancer
The Hsp90 client and oncogenic protein Her2 is up-regulated in breast cancer, and has been shown to be down-regulated when it is inhibited from binding to Hsp90. Thus, IPI-504 has been studied as a possible treatment for breast cancer. Preclinical data shows that IPI-504 degrades Her2 both in vitro and in vivo. In a Phase II clinical trial IPI-504 is now being used in combination with trastuzumab, a current treatment for breast cancer that interferes with the Her3/neu protein receptor [105]. The ongoing trial is examining a three week cycle of IPI-504 at 300mg/m2 for two weeks followed by a single treatment with trastuzumab and one week without treatment [105].
2.5. Herbimycin (HA)
Herbimycin A (HA) was first isolated in 1979 from the fermentation broth of Streptomyces hygroscopicus strain AM-3672 (5, Fig. 14) [22]. The molecule was termed herbimycin A due to its potent herbicidal activity against mono-and di-cotyledonous plants; this molecule also exhibits antifungal, anti-angiogenic and anti-tumor activities [22]. The absolute structure and configuration of HA was confirmed by Omura et al. who reported that HA is a 19-membered macrocyclic lactam with seven stereogenic centers, a carbamate, an isolated tri-substituted (E)-double bond, and (E,Z)-diene and a benzoquinone ring system [111]. Structurally, this molecule resembles GA (Fig. 14), and it was logical to test its ability to modulate Hsp90, perhaps inhibiting its client proteins from binding to Hsp90, as well as its cytotoxicity against cancer cell lines. The first total synthesis of HA was reported in 1991 by Tatsuta et al. [112], with other synthetic routes reported by Panek et al. in 2004 [113] and Cossy et al. in 2007 [111].
Fig. 14.
Structures of Geldanamycin and Herbimycin.
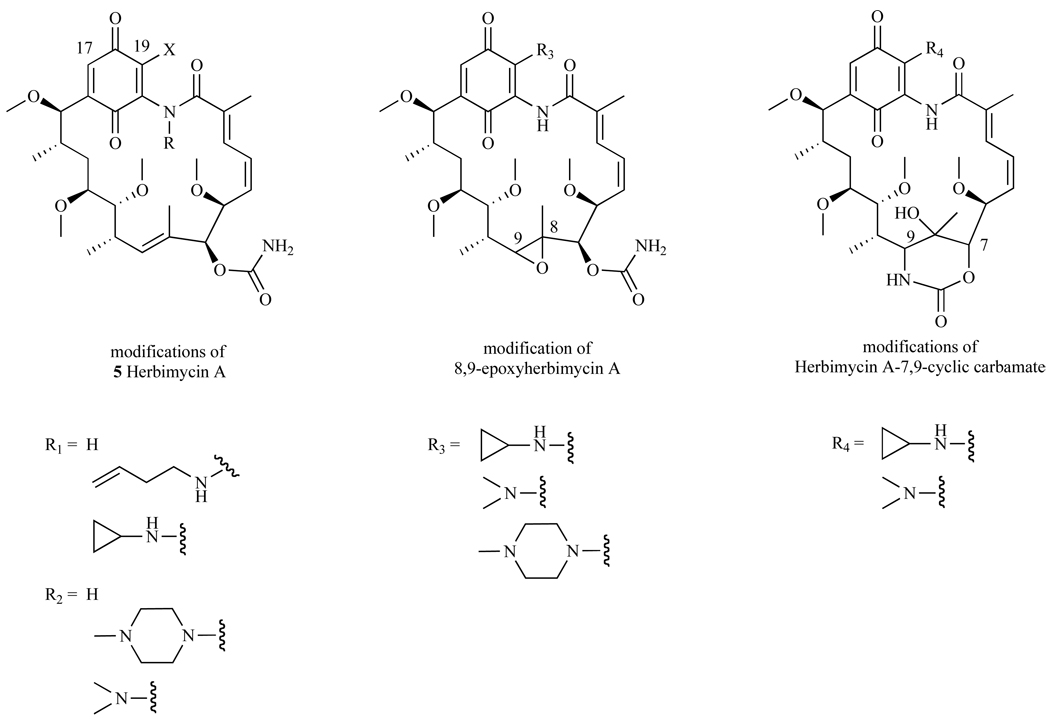
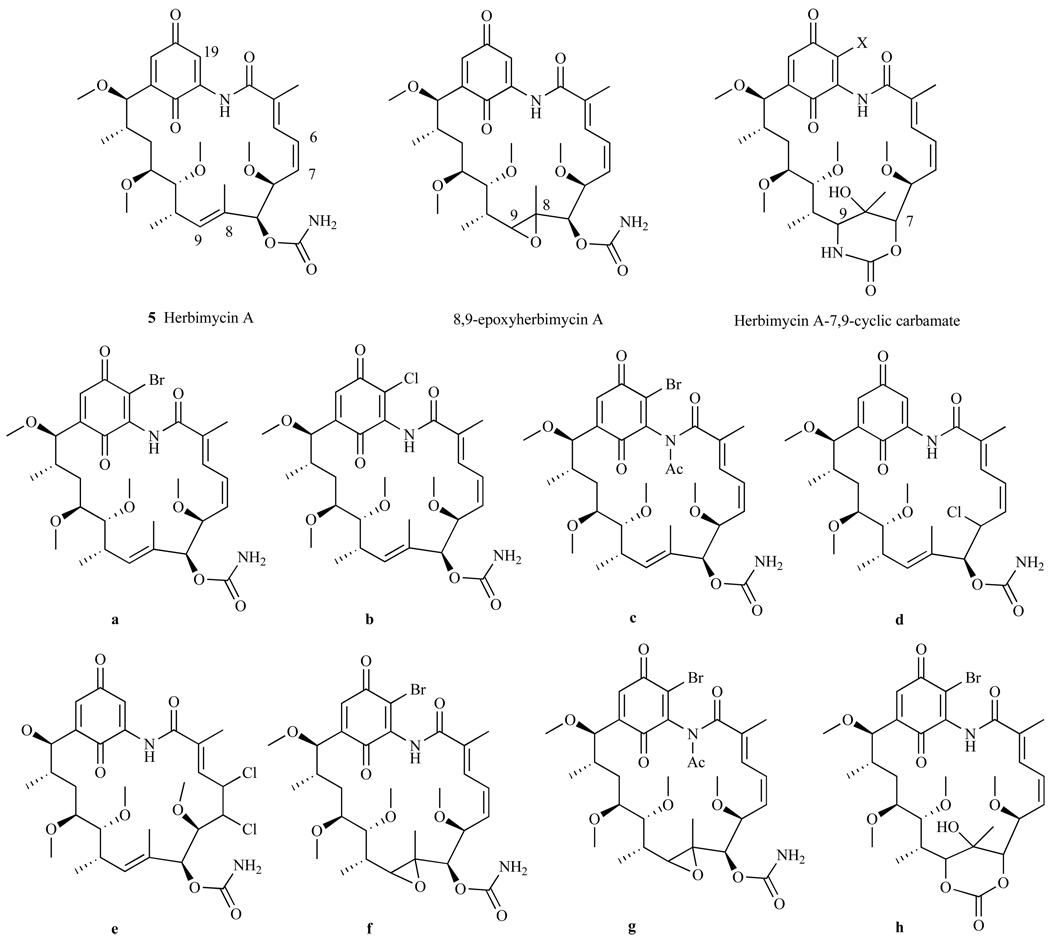
It was noted earlier that modifications to GA at the C-17 position generated potent compounds 17-AAG and 17-DMAG. Thus, Omura et al. synthesized HA derivatives with modifications at the 17 and/or 19-amino position incorporating dimethylamines, allylamines, cyclopropylamines, or methylpiperazines [114]. This effort resulted in three distinct series of derivatives using the HA scaffold: (1) Herbimycin A, (2) 8,9-epoxyherbimycin A, and (3) Herbimycin A-7,9-carbamate (Fig. 15).
Fig. 15.
Synthetic modifications to HA.
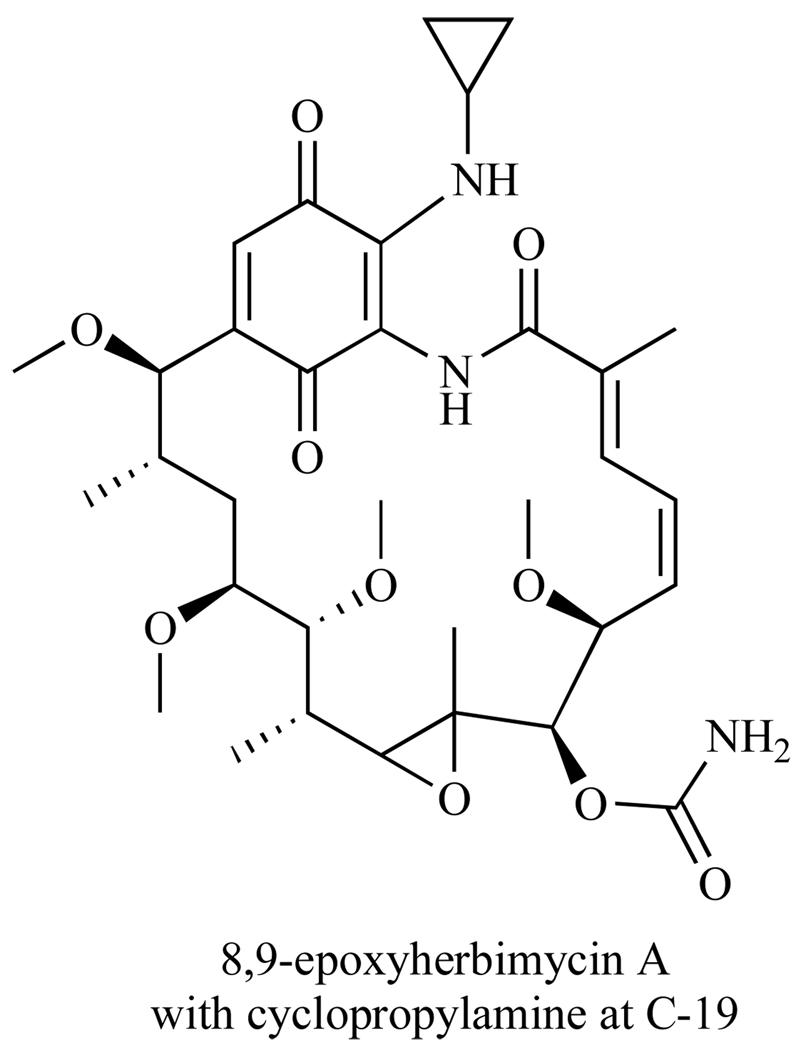
Antitumor activity of these derivatives was measured in Ehrlich ascites carcinoma mice models and expressed in T/C (%), where T is the median survival days of the treated mice and C is the survival days of the control group (receiving no drug treatment). Of the 12 derivatives synthesized, the most promising compound was the 8,9-epoxyherbimycin A with a cyclopropyl amine at C-19 (Fig. 16). This derivative showed significant antitumor activity with 141 T/C and 2/3 mice surviving treatment, compared to HA with 109 T/C and 0/4 mice surviving treatment [114]. There are ongoing investigations of the general anti-tumor activity of this compound.
Fig. 16.
Potent HA derivative.
In another investigation of HA and its derivatives, Omura et al. incorporated Cl or Br moieties into HA, 8,9-epoxyherbimycin A, and Herbimycin A-7,9-carbamate scaffolds (Fig. 17) [115]. In an Ehrlich ascites carcinoma model, these derivatives were tested at doses of 1.3–50 mg/kg for 5 days and derivatives a, d, and e (Fig. 17) were shown to be more effective than HA at treating tumors in mice. These three derivatives a, d, and e had T/C values of 190, 200, 215 respectively, compared to 126 T/C for HA. Further, these derivatives showed less toxicity than HA with 4/4 mice surviving treatment with a, d, and e compared to only 1/4 mice surviving treatment with HA. These HA derivatives are currently being tested in preclinical trials, and are expected to progress into clinical trials if they continue to demonstrate effectiveness with limited toxicity [116].
Fig. 17.
Synthetic modifications to HA incorporating halogens.
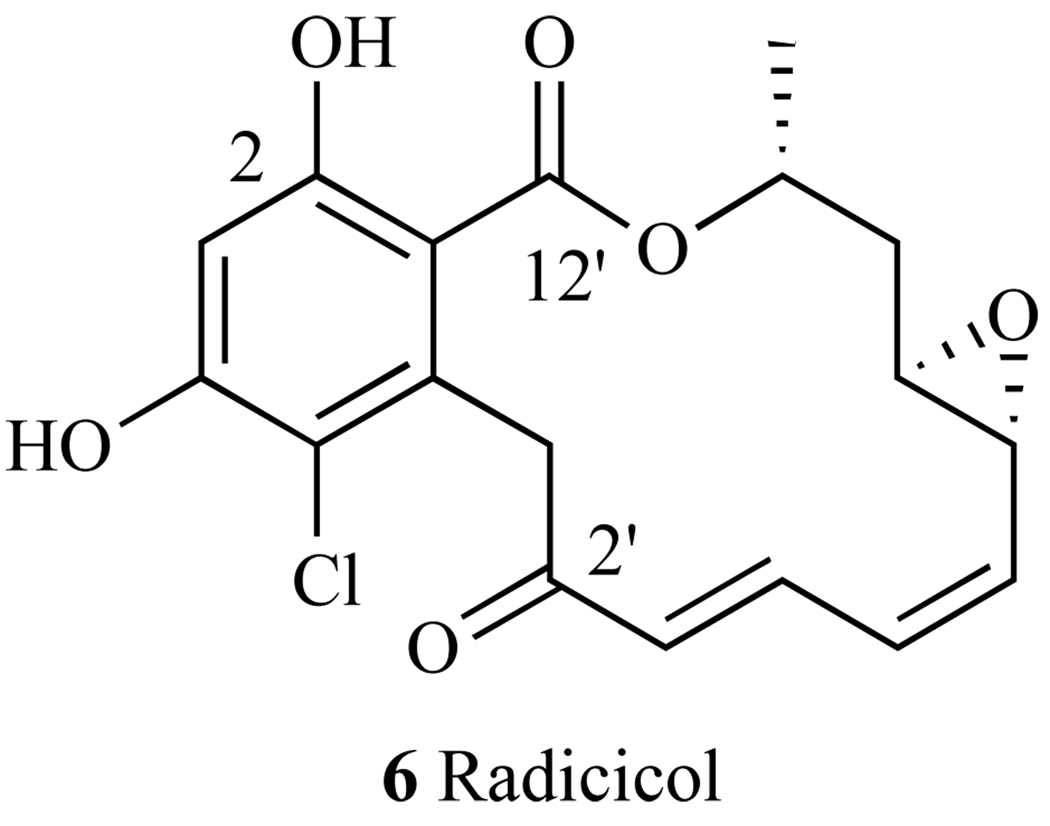
3. RADICICOL
Another macrocyclic Hsp90 inhibitor is Radicicol (RD) (6, Fig. 18), a 14-member macrolide natural product first isolated from M. bonorden [117]. RD is not structurally related to GA and its analogs, but interacts with Hsp90 in a similar manner. Using a biotinylated derivative of the natural product in a series of electrophoresis and immunoblotting experiments [118], RD was shown to bind to the ATP-binding site of the N-terminal domain of Hsp90 [119], and, like GA, adopts a C-shaped conformation that binds tightly with the ATP-binding site of Hsp90 (KD = 19nm) [32]. Similar to the mechanism of GA, it was noted that by preventing ATP from binding, RD destabilizes and inactivates a number of oncogenic client proteins. Specifically, binding of RD to the N-terminal binding site of Hsp90 has led to the decrease of these client proteins: v-src, Raf-1, EGFR, p185, Cdk4, and mutated p53 (Table 1) [120, 121].
Fig. 18.
Structure of Radicicol.
Within the N-terminus of Hsp90, the aromatic ring of RD is directed towards the base of the ATP-binding pocket, while the macrocycle rests on top of the pocket [32]. A co-crystal structure of RD bound to yeast Hsp90 showed that the 2-hydroxy and 12’-carbonyl bind directly to Asp79, and like GA, RD binds to Gly83 via a water molecule. However, it is clear from the crystal structure that the binding mode of RD differs from that of GA because residue Thr171 of the N-terminal ATP-binding pocket interacts with GA via a water molecule, but with RD via Asp79 (Fig. 19). Further, it is noted that epoxide moiety of RD has a unique interaction with Lys44.
Fig. 19.
Geldanamycin and Radicicol interactions with the N-terminal binding site of Hsp90.
RD lacks the toxic hydroquinone moiety of GA and its analogs, and is significantly less hepatotoxic than these analogs. Further, RD possesses nanomolar activity (IC50=20 nM) in cell lysates from ras-transformed mouse fibroblasts, as well as purified human Hsp90 inhibition assays. Despite this success, RD failed to effectively modulate Hsp90 activity during cell-based assays [122, 123]. It was noted that RD was degraded in the presence of DTT. The instability of RD was thought to be due to its conjugated enone moiety which possibly reacts, via Michael addition, with soft nucleophiles such as thiols [124]. Thus, it appeared that RD’s inability to perform in cells is predominantly due to its instability within intracellular environment, where it is degraded in the presence of the reducing environment within the cell. The synthesis of more stable yet active derivatives have become of interest to many organic chemists.
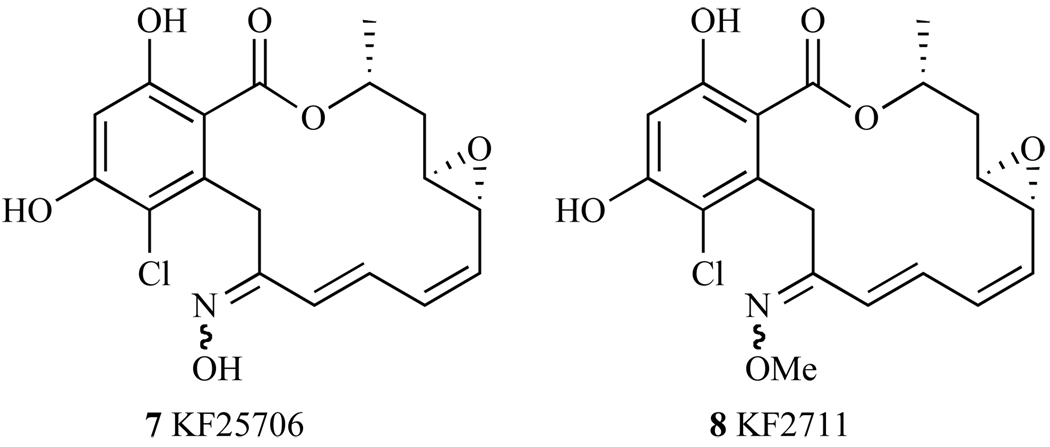
Given that the enone appeared to be responsible for reducing RD’s activity in the cell, the primary modifications to this structure were focused on the carbonyl at the 2 position. The most studied alteration to RD’s original structure is the placement of an oxime group at this 2 position, which reduces the electrophilicity of the Michael acceptor. This oxime group makes derivatives more stable in mouse serum and DTT [125]. The hydroxime derivative KF25706 (7, Fig. 20), inhibits K-ras and v-src signaling through the depletion of Raf-1 and v-src protein expression in v-src-transformed 3Y1 cells (SR-3Y1) [124, 126], a cell line where v-src expression is up-regulated. The cytotoxicity values of KF25706 in K-ras transformed cell line KNRK and v-src-transformed cell line SR-3Y1 were 39 nM and 26 nM, respectively, which is comparable to the activity of RD. Further, KF25706 competes with GA for binding to Hsp90 in vitro, suggesting that it has a similar mode of action to that of GA. For in vivo studies, an effective dose of KF2706 (100mg/kg twice daily for 5 days), has led to decreased levels of Raf-1 and Cdk4 oncogenic client proteins in MX1 human breast cancer cell xenographs in mice [126].
Fig. 20.
Structures of KF25706 and KF2711.
To further increase the potency and water solubility of oxime derivatives, Ikuina and coworkers introduced various carbamoylmethyl groups and studied the SAR of these derivatives in v-src transformed cells (SR-3Y1) and K-ras transformed cells KNRK5.2 [127]. Polar functional groups (hydroxyl, piperidino, and carbamoyl) as well as aromatic moieties, did not significantly influence activity comparing to that of RD. Compound 9 (Fig. 21) was the most potent compound synthesized, as it decreased Raf-1 protein level in the KNRK5.2 cell line and exhibited cytotoxic IC50s of 20–40 nM in SR-3Y1, KNRK5.2, and NRK epithelial cells. The corresponding radicicol activities were found to be 60–110 nM. Thus, it appeared that the oxime derivatives showed tremendous potential for modulating Hsp90 activity in cells.
Fig. 21.
Most potent carbamoylmethyl group
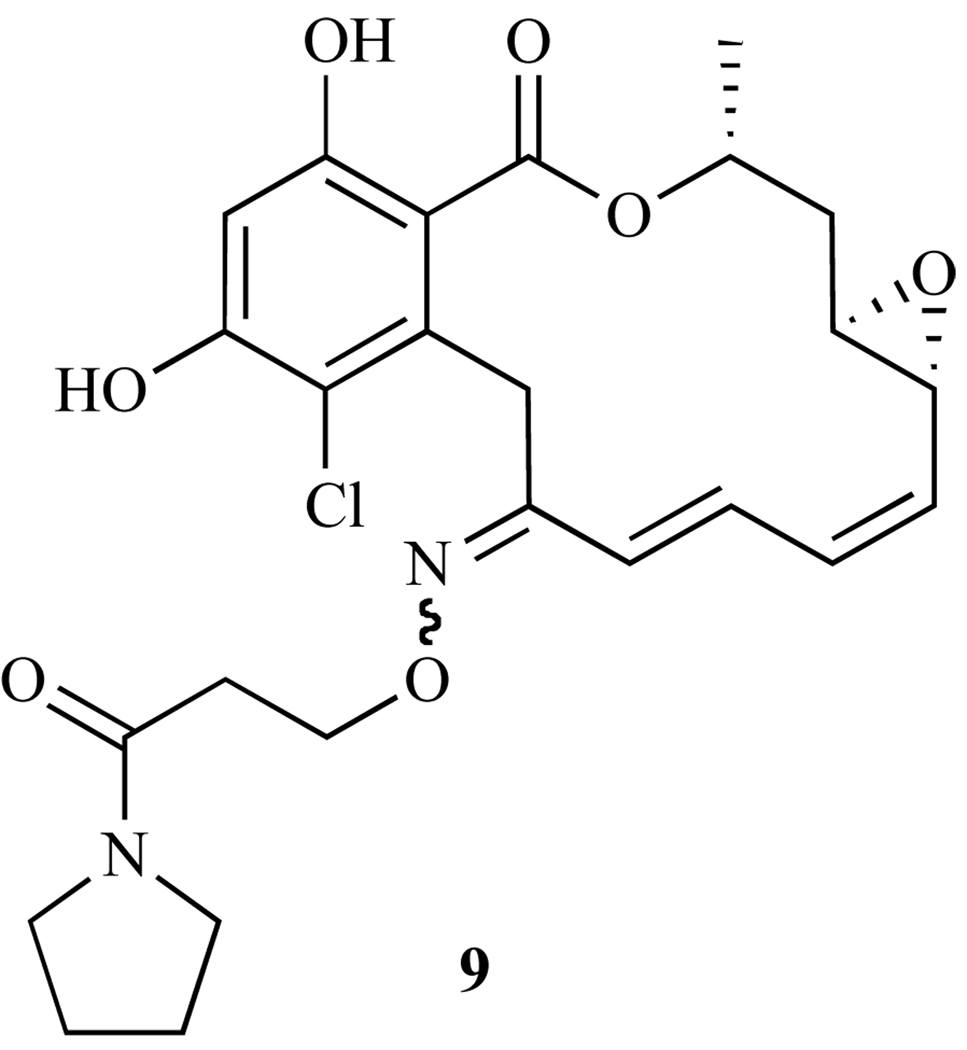
Oxime derivatives 7, 8, and 9, were all synthesized and tested as a mixture of E/Z isomers across the N=C double bond, thus posing the question of whether or not stereochemistry has an effect on potency. Soga and coworkers [128] isolated and tested each isomer separately, and found that the E isomer, KF58333 (10, Fig. 21) was 2–13 times more potent than its Z isomer, KF58332 (11, Fig. 22) in 7 different breast cancer cell lines that express both high and low amounts of Hsp90 client protein ErbB2. In addition, the E isoform showed significant reduction in the tumors of xenografted KPL-4 cells of nude mice (T/C minimum<0.5 at doses of 25 and 50 mg/kg per day), whereas the Z isoform did not [128]. In summary, these oxime derivatives show tremendous potential as Hsp90 inhibitors, and further studies on these molecules are ongoing to investigate their activity in regulating Hsp90 client proteins, as well as to test their activity in xenograph mouse models.
Fig. 22.
Structures of KF58333 and KF58332.
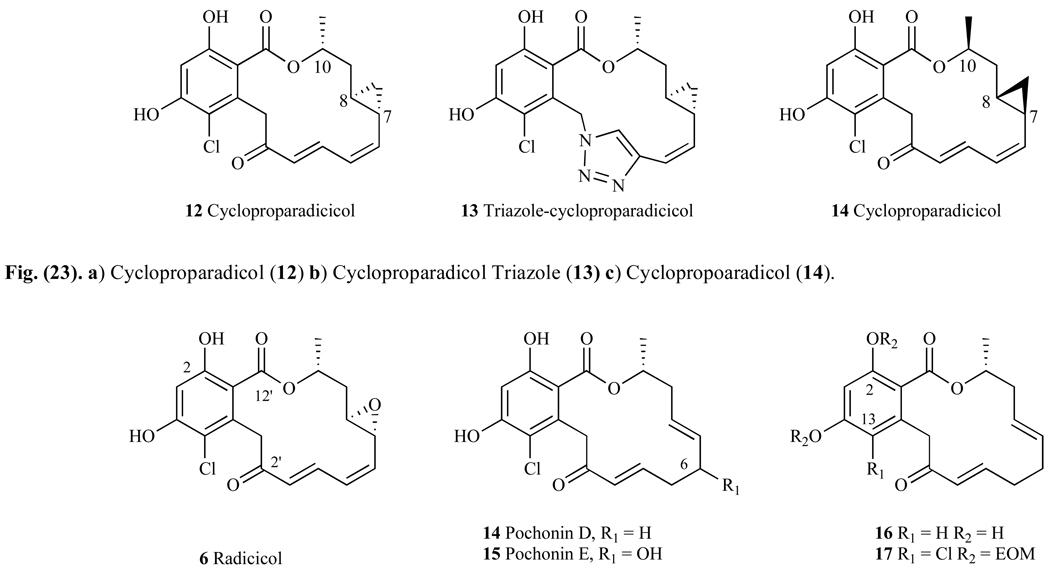
A set of radicicol derivatives has been synthesized by Yamamoto et al. [129] who replaced the labile epoxy group with a cyclopropyl, (12, Fig. 23a). The binding affinity to Hsp90 of this analog was 160nM (ED50), which was about four-fold less than that observed with the natural product RD (ED50= 45nM). Growth inhibition studies using MCF-7 breast-cancer cell line showed RD had a GI50 of 23 nM, whereas cycloproparadicicol (12) had a GI50 = 43 nM. Incorporating a triazole unit in cycloproparadicicol (13, Fig. 23b) gave a compound with significantly weaker binding affinity for Hsp90 than either RD or 12, with an ED50 = 400 nM. Compounds that had alternative stereochemistry of the cyclopropyl moiety at C7 and C8 showed significantly decreased inhibitory effects relative to RD, with ED50 = 2 µM in Hsp90 affinity assay and IC50 = 836 nM in MCF-7 cells (versus 45 nM and 23 nM respectively). Inversion of the stereocenter at C10 gave a compound that also had poor activity, with an ED50 = 5 µM against Hsp90 and IC50 = 2 µM in MCF-7 cells. Inversion of all three stereocenters relative to compound 12 gave compound 14, which not surprisingly had millimolar potency, with an ED50 > 10 mM in an Hsp90 assay, and micromolar potency in a cell-based assay (IC50 = 3.4 µM). Despite these results, the fact that the cyclopropyl analogue 12 still binds in the namomolar range suggests that the interaction between the Lys44 (Fig. 19) of Hsp90’s binding pocket to the epoxy oxygen is not critical. However, the compounds that have altered stereocenters of carbon 7, 8, and 10 (Fig. 23) are significantly less active than those with the natural product stereochemistry, indicating that specific stereochemistry at these positions is critical for binding effectively within the ATP binding pocket of Hsp90 [130].
Fig. 23.
Structures of Radicicol, Pochonin D, E, and additional derivatives.
3.1. Pochonin Derivatives
Pochonin A-F are natural products isolated from Pochonia chlamydosporia and although structurally similar to radicicol (RD), the substitution pattern of the 14-membered macrocyclic lactone ring differs from that of RD. One of the most studied derivatives in this class is Pochonin D (14, Fig. 23), which is similar to RD but only contains one double bond in conjunction with the carbonyl moiety, and a double bond between carbon 7 and 8 rather than the epoxide moiety in RD. When evaluated for Hsp90 affinity in a binding assay, Pochonin D had an IC50 = 80 nM, suggesting that both the epoxide and the conjugated diene moieties are unimportant for binding to Hsp90. Indeed, when Pochonin D was docked into Hsp90, it appeared to have a similar binding mode to that of RD. Pochonin E (15, Fig. 23) is similar to the structure of Pochonin D, but has a secondary alcohol at carbon 6. Very little is known about this molecule, and there are no reported IC50s or EC50s with Hsp90 or its effects on client proteins. Interestingly, the chlorine atom at carbon 13 shows to be crucial for binding to Hsp90 because when it is substituted with a hydrogen atom (16, Fig. 23), this molecule has no affinity for Hsp90 (IC50>50µM). Presumably this is because the chlorine atom has a crucial electronic effect on the aromatic ring, making the hydroxyl at C2 more desirable for hydrogen bonding to Asp 79 (Fig. 19). Not surprisingly, when the phenols are alkylated with ethoxy methyl (EOM) moieties (17, Fig. 23), this derivative shows no affinity for Hsp90 (IC50 > 50µM). Given that the hydroxyl at C2 is critical for hydrogen bonding to the ATP binding site of Hsp90, and the bulky protecting EOM group blocks this event, the lack of potency for compound 17 is hardly surprising [131].
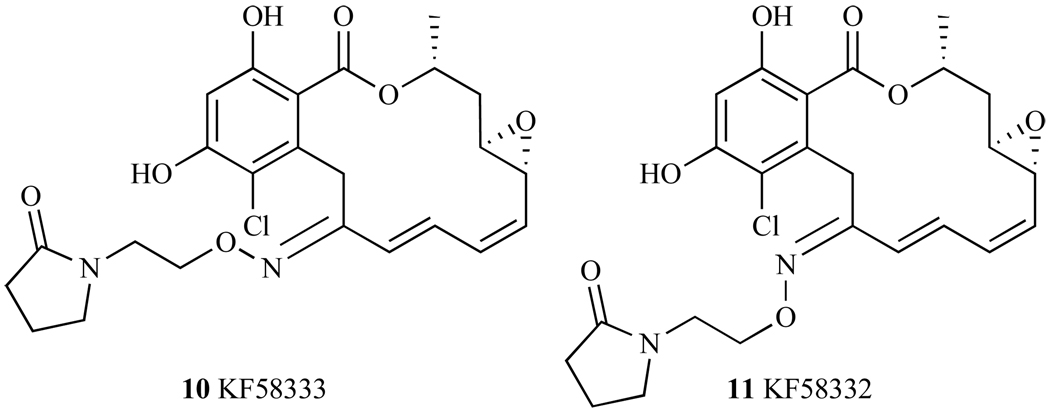
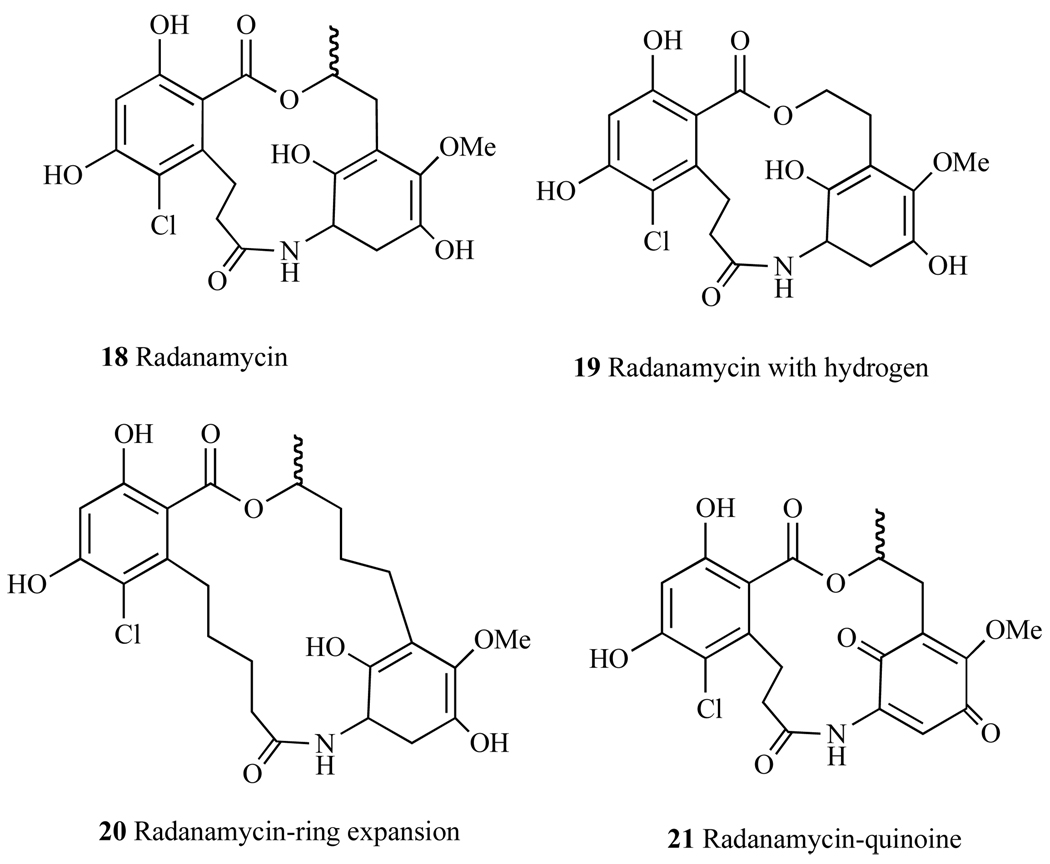
4. CHIMERIC Hsp90 INHBITORS - RADANAMYCIN
Careful evaluation of crystal structures of Hsp90 protein bound to Geldanamycin and Radicicol led Wang et al. [132] to design Radanamycin (RDM) (18, Fig. 24), a macrocyclic-chimera of both natural products. Biological activity studies have revealed that RDM has a significant effect on Hsp90 client protein Her-2 [132], where the addition of RDM to cytosol led to the degradation of Her2. In addition, it was noted that this molecule had an anti-proliferative effect on MCF-7 breast cancer cell line (IC50 = 1.2 ± 0.1 µM) (Table 2).
Fig. 24.
Structure of Radanamycin.
A library of RDM derivatives have been synthesized and tested against breast cancer cell line MCF7 and Hsp90-dependent protein Her2 [133]. Substitution of the phenol with methoxy groups led to a significant decrease in inhibitory activity against Hsp90. Removal of the methyl group on the carbon alpha to the lactone, did not have a significant impact on cytotoxicity of this molecule (19, Fig. 24). Variations of the macrocycle’s size, where the ring was expanded by 4 carbons (20, Fig. 24) also exhibited efficacy comparable to that of RDM. Oxidation of one phenol moiety to the quinone (21, Fig. 24) produced a molecule that had decreased binding to Hsp90 and lower cytotoxicity than RDM. Given that RDM was rationally designed using two Hsp90-inhibiting compounds, additional design methods are being employed to derive a molecule that is favorable in activity as well as in pharmacological aspects. As such, the future of this class of molecules appears to be promising.
5. ALLOSTERIC Hsp90 INHIBITORS- SANSAL-VAMIDE A
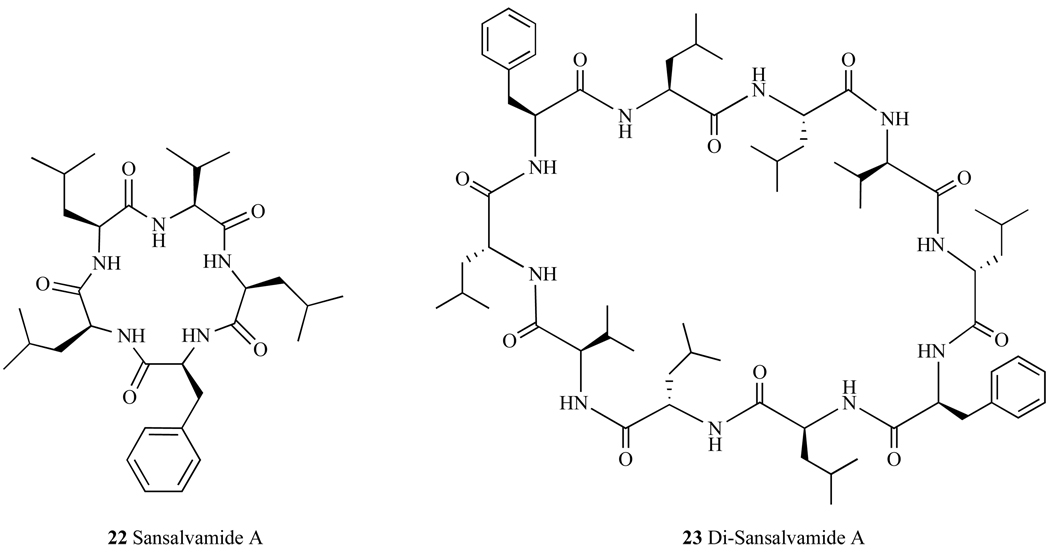
Sansalvamide A (San A) (22, Fig. 25), is a cyclic pentapeptide natural product that was isolated from a marine fungus (Fusarium ssp.)[134] and it exhibits anti-tumor activity at mid micromolar potency. Studies investigating the structure activity of San A derivatives have found a number of compounds that have high nanomolar potency [135]. In addition, the dimerized motif of San A derivatives, Di-Sansalvamide A (Di-San A) (23, Fig. 25) decapeptide also demonstrates cytotoxicity in multiple cancer cells lines with one derivative exhibiting a 1nM IC50 against pancreatic cancer cells [136]. In vitro as well as in vivo mechanistic studies have shown that San A derives its cytotoxic behavior at least in part by binding to Hsp90 and subsequently disrupting protein-protein interactions with specific C-terminal client proteins IP6K2 and FKBP [137], while Di-San A disrupts Hsp90 binding of IP6K2 [138]. Both IP6K2 and FKPB are pro-apoptotic proteins that elicit cell death when they are not bound to Hsp90 [139, 140]. Additional mechanistic studies on these compounds and their effects on Hsp90 client proteins are ongoing.
Fig. 25.
Structures of macrocyclic peptides Sansalvamide A and Di-Sansalvamide A.
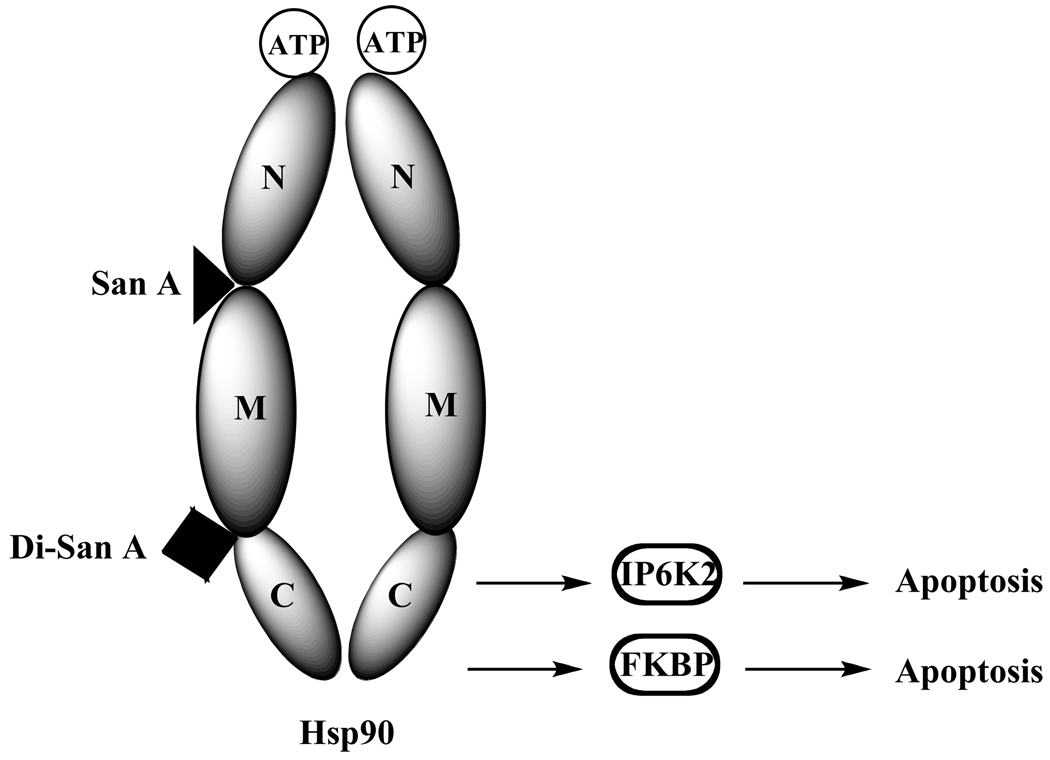
Mechanistic studies on these molecules included in-vitro pull down assays using a biotinylated San A derivative and the N-, middle, C-, middle-C, and N-middle domains of Hsp90. Somewhat surprisingly, they showed that San A binds optimally to the N-Middle domain, indicating that its effects on the apoptotic C-terminal client proteins are via an allosteric effect [138] (Fig. 26). Using the same pull-down techniques, biotinylated Di-San A was found to bind to the Middle-C domain of Hsp90, indicating that its effects on these apoptotic C-terminal client proteins may be due to it physically blocking the binding of these client proteins. In summary, these molecules show potential as therapeutic agents and their impacts on additional client proteins and subsequent oncogenic events are under investigation.
Fig. 26.
San A binds to NM domain and Di-San A binds to MC domain, inducing apoptosis.
Overall, there are 9 classes of macrocycles that modulate Hsp90’s activity and affect its client proteins. These compounds and their impact on the specific client proteins are summarized in Table 1 below.
CONCLUSION
In general, natural product macrocycles have proven to be very successful lead structures in the development of chemotherapeutics. Their macrocyclic structure makes them uniquely suited in binding proteins and inhibiting their function. Additionally, they have restricted bond rotations which give them defined 3-D structures that are conformationally constrained [141]. Thus, a relatively small macrocycle will tend to have greater binding affinity for protein targets than their linear counterparts or small molecules, creating a rigid interaction with their protein target, effectively inhibiting other large proteins from binding to this target. In this review, we have outlined the discovery and development of nine classes of Hsp90 inhibitors. Given that Hsp90 is an important target in cancer research because it is over-expressed in almost all forms of cancer and that there are currently no drugs in the market that target this protein, these macrocycles provide exciting new scaffolds worthy of investigation. These macrocycles have all shown that they act by binding directly to Hsp90 and disrupting its function by inhibiting its interaction and/or inducing the degradation of oncogenic client proteins that are associated with Hsp90. These interactions have been shown to lead to upregulation of apoptotic pathways, a favorable event for tumor cell death. A number of these Hsp90-modulating macrocycles are currently in various phases of clinical trials (Table 2), highlighting their successful contribution to the medicinal chemistry community. Finally, a wide range of studies involving these scaffolds have proven that they maintained activity over a variety of cancers and, thus, one or more of these inhibitors may become a universal chemotherapeutic.
ABBREVIATIONS
- Hsp
Heat shock protein
- TRAP1
Tumor necrosis factor receptor-associated protein 1
- Grp94
Glucose-regulated protein 94
- GA
Geldanamycin
- 17-AAG
17allyamino-17-demethylgeldanamycin
- 17-DMAG
17-Dimethylaminoethylamino-17-demethoxygeldanamycin
- SAR
Structure activity relationship
- AR
Androgen receptor
- RD
Radicicol
- HA
Herbimycin A
- MTD
Maximum tolerated dose
REFERENCES
- 1.Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- 2.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol:from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 3.Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 4.Welch WJ, Feramisco JR. Purification of the major mammalian heat shock proteins. J. Biol. Chem. 1982;257(24):14949–14959. [PubMed] [Google Scholar]
- 5.Crevel G, Bates H, Huikeshoven H, Cotterill S. The Drosophila Dpit47 protein is a nuclear Hsp90 co-chaperone that interacts with DNA polymerase alpha. J. Cell Sci. 2001;114(Pt 11):2015–2025. doi: 10.1242/jcs.114.11.2015. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.See www.picard.ch for client protein updates.
- 8.Kim TS, Jang CY, Kim HD, Lee JY, Ahn BY, Kim J. Interaction of Hsp90 with ribosomal proteins protects from ubiquitination and proteasome-dependent degradation. Mol. Biol. Cell. 2006;17(2):824–833. doi: 10.1091/mbc.E05-08-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998;79(2):129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 10.Grammatikakis N, Vultur A, Ramana CV, Siganou A, Schweinfest CW, Watson DK, Raptis L. The role of Hsp90N, a new member of the Hsp90 family, in signal transduction and neoplastic transformation. J. Biol. Chem. 2002;277(10):8312–8320. doi: 10.1074/jbc.M109200200. [DOI] [PubMed] [Google Scholar]
- 11.Argon Y, Simen BB. GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell Dev. Biol. 1999;10(5):495–505. doi: 10.1006/scdb.1999.0320. [DOI] [PubMed] [Google Scholar]
- 12.Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J. Biol. Chem. 2000;275(5):3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- 13.Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol. Ther. 2004;101(3):227–257. doi: 10.1016/j.pharmthera.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr. Cancer Drug Targets. 2003;3(5):301–323. doi: 10.2174/1568009033481877. [DOI] [PubMed] [Google Scholar]
- 15.Wayne N, Bolon DN. Dimerization of Hsp90 is required for in vivo function. Design and analysis of monomers and dimers. J. Biol. Chem. 2007;282(48):35386–35395. doi: 10.1074/jbc.M703844200. [DOI] [PubMed] [Google Scholar]
- 16.Schweinfest CW, Graber MW, Henderson KW, Papas TS, Baron PL, Watson DK. Cloning and sequence analysis of Hsp89alpha DeltaN, a new member of theHsp90 gene family. Bio-chim. Biophys. Acta. 1998;1398(1):18–24. doi: 10.1016/s0167-4781(98)00031-1. [DOI] [PubMed] [Google Scholar]
- 17.Usmani SZ, Bona R, Li ZH. 17 AAG for HSP90 inhibition in cancer - from bench to bedside. Curr. Mol. Med. 2009;9:654–664. doi: 10.2174/156652409788488757. [DOI] [PubMed] [Google Scholar]
- 18.Koga F, Kihara K, Neckers L. Inhibition of cancer invasion and metastasis by targeting the molecular chaperone heat-shock protein 90. Anticancer Res. 2009;29:797–807. [PubMed] [Google Scholar]
- 19.Barginear MF, Van Poznak C, Rosen N, Miodi S, Hudis CA, Budman DR. The heat shock protein 90 chaperone complex: an evolving therapeutic target. Curr. Cancer Drug Targets. 2008;8:522–535. doi: 10.2174/156800908785699379. [DOI] [PubMed] [Google Scholar]
- 20.Le Tourneau C, Raymond E, Faivre S. Aplidine: a paradigm of how to handle the activity and toxicity of a novel marine anticancer poison. Curr. Pharm. Des. 2007;13:3427–3429. [PubMed] [Google Scholar]
- 21.Starzl TE, Klintmalm GB, Porter KA, Iwatsuki S, Schro-ter GP. Liver transplantation with use of cyclosporin a and prednisone. N. Engl. J. Med. 1981;305:266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uehara Y. Natural product origins of hsp90 inhibitors. Curr. Cancer Drug Targets. 2003;3(5):325–330. doi: 10.2174/1568009033481796. [DOI] [PubMed] [Google Scholar]
- 23.Fairlie DP, Abbenante G, March DR. Macrocyclic pepti-domimetics - forcing peptides into bioactive conformations. Curr. Med. Chem. 1995;2(2):654–686. [Google Scholar]
- 24.Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, Krutzsch H, Ochel HJ, Schulte TW, Sausville E, Neckers LM, Toft DO. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J. Biol. Chem. 1997;272(38):23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 25.DeBoer C, Meulman PA, Wnuk RJ, Peterson DH. Gel-danamycin, a new antibiotic. J. Antibiot., (Tokyo) 1970;23(9):442–447. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]
- 26.Supko JG, Hickman RL, Grever MR, Malspeis L. Preclini-cal pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother. Pharmacol. 1995;36(4):305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 27.Uehara Y, Murakami Y, Mizuno S, Kawai S. Inhibition of transforming activity of tyrosine kinase oncogenes by herbimycin A. Virology. 1988;164(1):294–298. doi: 10.1016/0042-6822(88)90649-6. [DOI] [PubMed] [Google Scholar]
- 28.Uehara Y, Murakami Y, Sugimoto Y, Mizuno S. Mechanism of reversion of Rous sarcoma virus transformation by herbimycin A: reduction of total phosphotyrosine levels due to reduced kinase activity and increased turnover of p60v–src1. Cancer Res. 1989;49(4):780–785. [PubMed] [Google Scholar]
- 29.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v–src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA. 1994;91(18):8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchison KA, Brott BK, De Leon JH, Perdew GH, Jove R, Pratt WB. Reconstitution of the multiprotein complex of pp60src, hsp90, and p50 in a cell-free system. J. Biol. Chem. 1992;267(5):2902–2908. [PubMed] [Google Scholar]
- 31.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90(1):65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 32.Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and gel-danamycin. J. Med. Chem. 1999;42(2):260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 33.Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c–erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J. Biol. Chem. 1996;271(37):22796–22801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- 34.Powis G. Free radical formation by antitumor quinones. Free Radic. Biol. Med. 1989;6(1):63–101. doi: 10.1016/0891-5849(89)90162-7. [DOI] [PubMed] [Google Scholar]
- 35.Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, DiOrio CI, Barbacci EG, Miller PE, Pollack DM, Sloan LR, Pustilnik JD, Moyer JD, Moyer MP. erbB-2 oncogene inhibition by gel-danamycin derivatives: synthesis, mechanism of action, and structure-activity relationships. J. Med. Chem. 1995;38(19):3813–3820. doi: 10.1021/jm00019a011. [DOI] [PubMed] [Google Scholar]
- 36.McErlean CS, Proisy N, Davis CJ, Boland NA, Sharp SY, Boxall K, Slawin AM, Workman P, Moody CJ. Synthetic ansamycins prepared by a ring-expanding Claisen rearrangement. Synthesis and biological evaluation of ring and conformational analogues of the Hsp90 molecular chaperone inhibitor geldanamycin. Org. Biomol. Chem. 2007;5(3):531–546. doi: 10.1039/b615378j. [DOI] [PubMed] [Google Scholar]
- 37.Tian ZQ, Wang Z, MacMillan KS, Zhou Y, Carreras CW, Mueller T, Myles DC, Liu Y. Potent cytotoxic C-11 modified geldanamycin analogues. J. Med. Chem. 2009;52(10):3265–3273. doi: 10.1021/jm900098v. [DOI] [PubMed] [Google Scholar]
- 38.Le Brazidec JY, Kamal A, Busch D, Thao L, Zhang L, Timony G, Grecko R, Trent K, Lough R, Salazar T, Khan S, Burrows F, Boehm MF. Synthesis and biological evaluation of a new class of geldanamycin derivatives as potent inhibitors of Hsp90. J. Med. Chem. 2004;47(15):3865–3873. doi: 10.1021/jm0306125. [DOI] [PubMed] [Google Scholar]
- 39.Lee K, Ryu JS, Jin Y, Kim W, Kaur N, Chung SJ, Jeon YJ, Park JT, Bang JS, Lee HS, Kim TY, Lee JJ, Hong YS. Synthesis and anticancer activity of geldanamycin derivatives derived from biosynthetically generated metabolites. Org. Biomol. Chem. 2008;6(2):340–348. doi: 10.1039/b713407j. [DOI] [PubMed] [Google Scholar]
- 40.Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, Moyer JD, DiOrio CI, Barbacci EG, Miller PE, O’Brien AT, Morin MJ, Foster BA, Pollack VA, Savage DM, Sloan DE, Pustilnik LR, Moyer MP. Inhibition of the oncogene product p185erbB-2 in vitro and in vivo by geldanamycin and dihydrogeldanamycin derivatives. J. Med. Chem. 1995;38(19):3806–3812. doi: 10.1021/jm00019a010. [DOI] [PubMed] [Google Scholar]
- 41.Smith V, Sausville EA, Camalier RF, Fiebig HH, Burger AM. Comparison of 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17DMAG) and 17-allylamino-17-demethoxy-geldanamycin (17AAG) in vitro: effects on Hsp90 and client proteins in melanoma models. Cancer Chemother. pharm. 2005;56(2):126–137. doi: 10.1007/s00280-004-0947-2. [DOI] [PubMed] [Google Scholar]
- 42.Grbovic OM, Basso AD, Sawai A, Ye Q, Friedlander P, Solit D, Rosen N. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc. Natl. Acad. Sci. USA. 2006;103(1):57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 2004;5(11):875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 44.Cohen C, Zavala-Pompa A, Sequeira JH, Shoji M, Sexton DG, Cotsonis G, Cerimele F, Govindarajan B, Macaron N, Arbiser JL. Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin. Cancer Res. 2002;8(12):3728–3733. [PubMed] [Google Scholar]
- 45.da Rocha Dias S, Friedlos F, Light Y, Springer C, Workman P, Marais R. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxy-geldanamycin. Cancer Res. 2005;65(23):10686–10691. doi: 10.1158/0008-5472.CAN-05-2632. [DOI] [PubMed] [Google Scholar]
- 46.Neri LM, Borgatti P, Capitani S, Martelli AM. The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim. Biophys. 2002;1584:73–80. doi: 10.1016/s1388-1981(02)00300-1. [DOI] [PubMed] [Google Scholar]
- 47.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Nat. Acad. Sci. 2000;97(20):10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon YK, Park CH, Kim KY, Li YC, Kim J, Kim YA, Paik JH, Park BK, Kim CW, Kim YN. The heat-shock protein 90 inhibitor, geldanamycin, induces apoptotic cell death in Epstein-Barr virus-positive NK/T-cell lymphoma by Akt down-regulation. J. Pathol. 2007;213(2):170–179. doi: 10.1002/path.2219. [DOI] [PubMed] [Google Scholar]
- 49.Shang L, Tomasi TB. The heat shock protein 90-CDC37 chaperone complex is required for signaling by types I and II interferons. J. Biol. Chem. 2006;281(4):1876–1884. doi: 10.1074/jbc.M509901200. [DOI] [PubMed] [Google Scholar]
- 50.Schoof N, von Bonin F, Trumper L, Kube D. HSP90 is essential for Jak-STAT signaling in classical Hodgkin lymphoma cells. Cell Commun. Signal. 2009;7:17. doi: 10.1186/1478-811X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Georgakis GV, Li Y, Rassidakis GZ, Martinez-Valdez H, Medeiros LJ, Younes A. Inhibition of heat shock protein 90 function by 17-allylamino-17-demethoxy-geldanamycin in Hodgkin's lymphoma cells down-regulates Akt kinase, dephosphorylates extracellular signal-regulated kinase, and induces cell cycle arrest and cell death. Clin. Cancer Res. 2006;12(2):584–590. doi: 10.1158/1078-0432.CCR-05-1194. [DOI] [PubMed] [Google Scholar]
- 52.Dutton A, Reynolds GM, Dawson CW, Young LS, Murray PG. Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin's lymphoma cells through a mechanism involving Akt kinase and mTOR. J. Pathol. 2005;205(4):498–506. doi: 10.1002/path.1725. [DOI] [PubMed] [Google Scholar]
- 53.Burrows F, Zhang H, Kamal A. Hsp90 activation and cell cycle regulation. Cell Cycle. 2004;3(12):1530–1536. doi: 10.4161/cc.3.12.1277. [DOI] [PubMed] [Google Scholar]
- 54.Georgakis GV, Li Y, Younes A. The heat shock protein 90 inhibitor 17-AAG induces cell cycle arrest and apoptosis in mantle cell lymphoma cell lines by depleting cyclin D1, Akt, Bid and activating caspase 9. Br. J. Haematol. 2006;135(1):68–71. doi: 10.1111/j.1365-2141.2006.06247.x. [DOI] [PubMed] [Google Scholar]
- 55.Georgakis GV, Li Y, Rassidakis GZ, Medeiros LJ, Younes A. The HSP90 inhibitor 17-AAG synergizes with doxorubicin and U0126 in anaplastic large cell lymphoma irrespective of ALK expression. Exp. Hematol. 2006;34(12):1670–1679. doi: 10.1016/j.exphem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Bonvini P, Gastaldi T, Falini B, Rosolen A. Nucleophosmin-anaplastic lymphoma kinase (NPM-ALK), a novel Hsp90-client tyrosine kinase: down-regulation of NPM-ALK expression and tyrosine phosphorylation in ALK(+) CD30(+) lymphoma cells by the Hsp90 antagonist 17-allylamino,17-demethoxygeldanamycin. Cancer Res. 2002;62(5):1559–1566. [PubMed] [Google Scholar]
- 57.Bonvini P, Dalla Rosa H, Vignes N, Rosolen A. Ubiquitina-tion and proteasomal degradation of nucleophosmin-anaplastic lymphoma kinase induced by 17-allylamino-demethoxygeldana-mycin: role of the co-chaperone carboxyl heat shock protein 70-interacting protein. Cancer Res. 2004;64(9):3256–3264. doi: 10.1158/0008-5472.can-03-3531. [DOI] [PubMed] [Google Scholar]
- 58.Schulte TW, Neckers LM. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998;42(4):273–279. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- 59.Vanaja DK, Mitchell SH, Toft DO, Young CY. Effect of geldanamycin on androgen receptor function and stability. Cell Stress Chaperones. 2002;7(1):55–64. doi: 10.1379/1466-1268(2002)007<0055:eogoar>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, Heller G, Tong W, Cordon-Cardo C, Agus DB, Scher HI, Rosen N. 17-Allylamino-17-demethoxygeldana-mycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8(5):986–993. [PubMed] [Google Scholar]
- 61.Williams CR, Tabios R, Linehan WM, Neckers L. aIntratu-mor injection of the Hsp90 inhibitor 17AAG decreases tumor growth and induces apoptosis in a prostate cancer xenograft model. J. Urol. 2007;178(4 Pt 1):1528–1532. doi: 10.1016/j.juro.2007.05.120. [DOI] [PubMed] [Google Scholar]
- 62.Flandrin P, Guyotat D, Duval A, Cornillon J, Tavernier E, Nadal N, Campos L. Significance of heat-shock protein (HSP) 90 expression in acute myeloid leukemia cells. Cell Stress Chaperones. 2008;13(3):357–364. doi: 10.1007/s12192-008-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shiotsu Y, Soga S, Akinaga S. Heat shock protein 90-antagonist destabilizes Bcr-Abl/HSP90 chaperone complex. Leuk Lymphoma. 2002;43(5):961–968. doi: 10.1080/10428190290021371. [DOI] [PubMed] [Google Scholar]
- 64.Johnson AJ, Wagner AJ, Cheney CM, Smith LL, Lucas DM, Guster SK, Grever MR, Lin TS, Byrd JC. Rituxi-mab and 17-allylamino-17-demethoxygeldanamycin induce synergistic apoptosis in B-cell chronic lymphocytic leukaemia. Br. J. Haematol. 2007;139(5):837–844. doi: 10.1111/j.1365-2141.2007.06878.x. [DOI] [PubMed] [Google Scholar]
- 65.Jia W, Yu C, Rahmani M, Krystal G, Sausville EA, Dent P, Grant S. Synergistic antileukemic interactions between 17-AAG and UCN-01 involve interruption of RAF/MEK- and AKT-related pathways. Blood. 2003;102(5):1824–1832. doi: 10.1182/blood-2002-12-3785. [DOI] [PubMed] [Google Scholar]
- 66.Mesa RA, Loegering D, Powell HL, Flatten K, Arlander SJ, Dai NT, Heldebrant MP, Vroman BT, Smith BD, Karp JE, Eyck CJ, Erlichman C, Kaufmann SH, Karnitz LM. Heat shock protein 90 inhibition sensitizes acute myelogenous leukemia cells to cytarabine. Blood. 2005;106(1):318–327. doi: 10.1182/blood-2004-09-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim DW, Choy H. Potential role for epidermal growth factor receptor inhibitors in combined-modality therapy for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004;59 Suppl 2:11–20. doi: 10.1016/j.ijrobp.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 68.Balsara BR, Pei J, Mitsuuchi Y, Page R, Klein-Szanto A, Wang H, Unger M, Testa JR. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25(11):2053–2059. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 69.Fujita T, Kiyama M, Tomizawa Y, Kohno T, Yokota J. Comprehensive analysis of p53 gene mutation characteristics in lung carcinoma with special reference to histological subtypes. Int. J. Oncol. 1999;15(5):927–934. doi: 10.3892/ijo.15.5.927. [DOI] [PubMed] [Google Scholar]
- 70.Senju M, Sueoka N, Sato A, Iwanaga K, Sakao Y, Tomimi-tsu S, Tominaga M, Irie K, Hayashi S, Sueoka E. Hsp90 inhibitors cause G2/M arrest associated with the reduction of Cdc25C and Cdc2 in lung cancer cell lines. J. Cancer Res. Clin. Oncol. 2006;132(3):150–158. doi: 10.1007/s00432-005-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Otrubova K, Lushington GH, Vander Velde D, McGuire KL, McAlpine SR. A comprehensive study of Sansalvamide A derivatives and their structure-activity relationships against drug-resistant colon cancer cell lines. J. Med. Chem. 2008;51:530–544. doi: 10.1021/jm070731a. [DOI] [PubMed] [Google Scholar]
- 72.Otrubova K, McGuire KL, McAlpine SR. A scaffold targeting drug-resistant colon cancers. J. Med. Chem. 2007;50:1999–2002. doi: 10.1021/jm070088s. [DOI] [PubMed] [Google Scholar]
- 73.Otrubova K, Styers TJ, Pan P-S, Rodriguez R, McGuire KL, McAlpine SR. Synthesis and novel structure-activity relationships of potent Sansalvamide A derivatives. Chem. Commun. 2006:1033–1034. doi: 10.1039/b517434a. [DOI] [PubMed] [Google Scholar]
- 74.Clarke PA, Hostein I, Banerji U, Stefano FD, Maloney A, Walton M, Judson I, Workman P. Gene expression profiling of human colon cancer cells following inhibition of signal transduction by 17-allylamino-17-demethoxygeldanamycin, an inhibitor of the hsp90 molecular chaperone. Oncogene. 2000;19(36):4125–4133. doi: 10.1038/sj.onc.1203753. [DOI] [PubMed] [Google Scholar]
- 75.Tse AN, Sheikh TN, Alan H, Chou TC, Schwartz GK. 90-kDa heat shock protein inhibition abrogates the topoisomerase I poison-induced G2/M checkpoint in p53-null tumor cells by depleting Chk1 and Wee1. Mol. Pharmacol. 2009;75(1):124–133. doi: 10.1124/mol.108.050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu X, Wanders A, Wardega P, Tinge B, Gedda L, Berg-strom S, Sooman L, Gullbo J, Bergqvist M, Hesselius P, Lennartsson J, Ekman S. Hsp90 is expressed and represents a therapeutic target in human oesophageal cancer using the inhibitor 17-allylamino-17-demethoxygeldanamycin. Br. J. Cancer. 2009;100(2):334–343. doi: 10.1038/sj.bjc.6604855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watanabe G, Behrns KE, Kim JS, Kim RD. Heat shock protein 90 inhibition abrogates hepatocellular cancer growth through cdc2-mediated G2/M cell cycle arrest and apoptosis. Cancer Chemother. Pharmacol. 2009;64(3):433–443. doi: 10.1007/s00280-008-0888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelland LR, Sharp SY, Rogers PM, Myers TG, Workman P. DT-Diaphorase expression and tumor cell sensitivity to 17-allylamino, 17-demethoxygeldanamycin, an inhibitor of heat shock protein 90. J. Natl. Cancer Inst. 1999;91(22):1940–1949. doi: 10.1093/jnci/91.22.1940. [DOI] [PubMed] [Google Scholar]
- 79.Tian ZQ, Liu Y, Zhang D, Wang Z, Dong SD, Carreras CW, Zhou Y, Rastelli G, Santi DV, Myles DC. Synthesis and biological activities of novel 17-aminogeldanamycin derivatives. Bioorg. Med. Chem. 2004;12(20):5317–5329. doi: 10.1016/j.bmc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 80.Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem. Pharmacol. 2003;65(7):1035–1041. doi: 10.1016/s0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 81.Davis JM, Rowley SD, Braine HG, Piantadosi S, Santos GW. Clinical toxicity of cryopreserved bone marrow graft infusion. Blood. 1990;75(3):781–786. [PubMed] [Google Scholar]
- 82.See <http://www.clinicaltrials.gov> for current Phase I/II/III trials.
- 83.Goetz MP, Toft D, Reid J, Ames M, Stensgard B, Safgren S, Adjei AA, Sloan J, Atherton P, Vasile V, Salazaar S, Adjei A, Croghan G, Erlichman C. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J. Clin. Oncol. 2005;23(6):1078–1087. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 84.Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M, Walton M, Lakhani S, Kaye S, Workman P, Judson I. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J. Clin. Oncol. 2005;23(18):4152–4161. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 85.Ramanathan RK, Trump DL, Eiseman JL, Belani CP, Agarwala SS, Zuhowski EG, Lan J, Potter DM, Ivy SP, Ramalingam S, Brufsky AM, Wong MK, Tutchko S, Egorin MJ. Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin. Cancer Res. 2005;11(9):3385–3391. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- 86.Solit DB, Osman I, Polsky D, Panageas KS, Daud A, Goydos JS, Teitcher J, Wolchok JD, Germino FJ, Krown SE, Coit D, Rosen N, Chapman PB. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin. Cancer Res. 2008;14(24):8302–8307. doi: 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heath EI, Hillman DW, Vaishampayan U, Sheng S, Sarkar F, Harper F, Gaskins M, Pitot HC, Tan W, Ivy SP, Pili R, Carducci MA, Erlichman C, Liu G. A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin. Cancer Res. 2008;14(23):7940–7946. doi: 10.1158/1078-0432.CCR-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 89.Powers MV, Clarke PA, Workman P. Death by chaperone: HSP90, HSP70 or both? Cell Cycle. 2009;8(4):518–526. doi: 10.4161/cc.8.4.7583. [DOI] [PubMed] [Google Scholar]
- 90.McDowell CL, Bryan Sutton R, Obermann WM. Expression of Hsp90 chaperome proteins in human tumor tissue. Int. J. Biol. Macromol. 2009;45(3):310–314. doi: 10.1016/j.ijbiomac.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 91.Smith JR, Workman P. Targeting CDC37: an alternative, kinase-directed strategy for disruption of oncogenic chaperoning. Cell Cycle. 2009;8(3):362–372. doi: 10.4161/cc.8.3.7531. [DOI] [PubMed] [Google Scholar]
- 92.Gray PJ, Jr, Stevenson MA, Calderwood SK. Targeting Cdc37 inhibits multiple signaling pathways and induces growth arrest in prostate cancer cells. Cancer Res. 2007;67(24):11942–11950. doi: 10.1158/0008-5472.CAN-07-3162. [DOI] [PubMed] [Google Scholar]
- 93.Smith JR, Clarke PA, de Billy E, Workman P. Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene. 2009;28(2):157–169. doi: 10.1038/onc.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jez JM, Chen JC, Rastelli G, Stroud RM, Santi DV. Crystal structure and molecular modeling of 17-DMAG in complex with human Hsp90. Chem. Biol. 2003;10(4):361–368. doi: 10.1016/s1074-5521(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 95.Egorin MJ, Lagattuta TF, Hamburger DR, Covey JM, White KD, Musser SM, Eiseman JL. Pharmacokinetics, tissue distribution, and metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (NSC 707545) in CD2F1 mice and Fischer 344 rats. Cancer Chemother. Pharmacol. 2002;49(1):7–19. doi: 10.1007/s00280-001-0380-8. [DOI] [PubMed] [Google Scholar]
- 96.Smith MA, Morton CL, Phelps DA, Kolb EA, Lock R, Carol H, Reynolds CP, Maris JM, Keir ST, Wu J, Houghton PJ. Stage 1 testing and pharmacodynamic evaluation of the HSP90 inhibitor alvespimycin (17-DMAG, KOS-1022) by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2008;51(1):34–41. doi: 10.1002/pbc.21508. [DOI] [PubMed] [Google Scholar]
- 97.Kaur G, Belotti D, Burger AM, Fisher-Nielson K, Borsotti P, Riccardi E, Thillainathan J, Hollingshead M, Sausville EA, Giavazzi R. Antiangiogenic properties of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin: an orally bioavailable heat shock protein 90 modulator. Clin. Cancer Res. 2004;10(14):4813–4821. doi: 10.1158/1078-0432.CCR-03-0795. [DOI] [PubMed] [Google Scholar]
- 98.Hollingshead M, Alley M, Burger AM, Borgel S, Pacula-Cox C, Fiebig HH, Sausville EA. In vivo antitumor efficacy of 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride), a water-soluble geldanamycin derivative. Cancer Chemother. Pharmacol. 2005;56(2):115–125. doi: 10.1007/s00280-004-0939-2. [DOI] [PubMed] [Google Scholar]
- 99.Glaze ER, Lambert AL, Smith AC, Page JG, Johnson WD, McCormick DL, Brown AP, Levine BS, Covey JM, Egorin MJ, Eiseman JL, Holleran JL, Sausville EA, Tomaszewski JE. Preclinical toxicity of a geldanamycin analog, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG), in rats and dogs: potential clinical relevance. Cancer Chemother. Pharmacol. 2005;56(6):637–647. doi: 10.1007/s00280-005-1000-9. [DOI] [PubMed] [Google Scholar]
- 100.Grem JL, Morrison G, Guo XD, Agnew E, Takimoto CH, Thomas R, Szabo E, Grochow L, Grollman F, Hamilton JM, Neckers L, Wilson RH. Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J. Clin. Oncol. 2005;23(9):1885–1893. doi: 10.1200/JCO.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 101.Lancet J, Baer MR, Gojo I, Burton M, Quinn M, Tighe SM, Bhalla K, Kersey K, Wells S, Zhong Z. In Phase 1, pharmacokinetic (PK) and pharmacodynamic (PD) study of intravenous alvespimycin (KOS-1022) in patients with refractory hematological malignancies; ASH Annual Meeting.2006. [Google Scholar]
- 102.Sydor JR, Normant E, Pien CS, Porter JR, Ge J, Grenier L, Pak RH, Ali JA, Dembski MS, Hudak J, Patterson J, Penders C, Pink M, Read MA, Sang J, Woodward C, Zhang Y, Grayzel DS, Wright J, Barrett JA, Palombella VJ, Adams J, Tong JK. Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc. Natl. Acad. Sci. USA. 2006;103(46):17408–17413. doi: 10.1073/pnas.0608372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo W, Reigan P, Siegel D, Zirrolli J, Gustafson D, Ross D. The bioreduction of a series of benzoquinone ansamycins by NAD(P)H:quinone oxidoreductase 1 to more potent heat shock protein 90 inhibitors, the hydroquinone ansamycins. Mol. Pharmacol. 2006;70(4):1194–1203. doi: 10.1124/mol.106.025643. [DOI] [PubMed] [Google Scholar]
- 104.Sydor JR, Normant E, Pien CS, Porter JR, Ge J, Grenier L, Pak RH, Ali JA, Dembski MS, Hudak J, Patterson J, Penders C, Pink M, Read MA, Sang J, Woodward C, Zhang Y, Grayzel DS, Wright J, Barrett JA, Palombella VJ, Adams J, Tong JK. Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc. Natl. Acad. Sci. USA. 2006;103(46):17408–17413. doi: 10.1073/pnas.0608372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hanson BE, Vesole DH. Retaspimycin hydrochloride (IPI-504): a novel heat shock protein inhibitor as an anticancer agent. Exp. Opin. Investig. Drugs. 2009;18(9):1375–1383. doi: 10.1517/13543780903158934. [DOI] [PubMed] [Google Scholar]
- 106.Shimamura T, Shapiro GI. Heat shock protein 90 inhibition in lung cancer. J. Thoracic Oncol. 2008;3(6):152–159. doi: 10.1097/JTO.0b013e318174ea3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sequist L, Janne P, J S. In Inhibitor IPI-504, in Patients with Relapsed and/or Refractory Stage IIIb or Stage IV Non-Small Cell Lung Cancer (NSCLC) Stratified by EGFR Mutation Status; AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics.2007. [Google Scholar]
- 108.Patterson J, Palombela VJ, Frtiz C, Normant E. IPI-504, a novel hsp90 inhibitor, blocks the unfolded protein response in multiple myeloma cells. Cancer Chemotherapy and Pharmacology. 2008;61(6):923–932. doi: 10.1007/s00280-007-0546-0. [DOI] [PubMed] [Google Scholar]
- 109.Neckers L. Heat shock protein 90 inhibition by 17-allylamino-17-demethoxygeldanamycin: a novel therapeutic approach for treating hormone-refractory prostate cancer. Clin. Cancer Res. 2002;8(5):962–966. [PubMed] [Google Scholar]
- 110.Oh WK, Stadler WM, Srinivas S. In A single arm phase II trial of IPI-504 in patients with castration resistant prostate cancer (CRPC) Genitourinary Cancers Symposium. 2009 [Google Scholar]
- 111.Canova S, Bellosta V, Bigot A, Mailliet P, Mignani S, Cossy J. Total synthesis of herbimycin A. Org. Lett. 2007;9(1):145–148. doi: 10.1021/ol062642i. [DOI] [PubMed] [Google Scholar]
- 112.Nakata M, Osumi T, Ueno A, Kimura T, Tamai T, Tatsuta K. Total synthesis of herbimycin A. Tetrahedron Lett. 1991;32(42):6015–6018. [Google Scholar]
- 113.Carter KD, Panek JS. Total SYnthesis of herbimycin A. Org. Lett. 2004;6(1):55–57. doi: 10.1021/ol036092p. [DOI] [PubMed] [Google Scholar]
- 114.Omura S, Miyano K, Nakagawa A, Sano H, Komiyama K, Umezawa I. Chemical Modification and Antitumor Activity of Herbimycin A. 8,9-epoxide, 7,9-cyclic carbamate, and 17 or 19-amino acid derivatives. J. Antibiot. 1984;37(10):1264–1267. doi: 10.7164/antibiotics.37.1264. [DOI] [PubMed] [Google Scholar]
- 115.Sano H, Komiyama K, Nakagawa A, Omura S. Chemical Modification of Herbimycin A: Synthesis and in vivo antitumor activities of halogenated and other related derivatives of Herbimycin A. J. Antibiot. 1985;39(3):415–423. doi: 10.7164/antibiotics.39.415. [DOI] [PubMed] [Google Scholar]
- 116.Noguchia M, Hirayamab R, Druzhininb S, Okayasu R. Enhanced radiation-induced cell killing by Herbimycin A pre-treatment. Radiat. Phys. Chem. 2009;78(12):1184–1187. [Google Scholar]
- 117.Delmotte P, Delmotte-Plaque J. A new antifungal substance of fungal origin. Nature. 1953;171(4347):344. doi: 10.1038/171344a0. [DOI] [PubMed] [Google Scholar]
- 118.Sharma SV, Agatsuma T, Nakano H. Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene. 1998;16(20):2639–2645. doi: 10.1038/sj.onc.1201790. [DOI] [PubMed] [Google Scholar]
- 119.Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3(2):100–108. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kwon HJ, Yoshida M, Abe K, Horinouchi S, Beppu T. Radicicol, an agent inducing the reversal of transformed phenotypes of src-transformed fibroblasts. Biosci. Biotechnol. Biochem. 1992;56(3):538–539. doi: 10.1271/bbb.56.538. [DOI] [PubMed] [Google Scholar]
- 121.Zhao JF, Nakano H, Sharma S. Suppression of RAS and MOS transformation by radicicol. Oncogene. 1995;11(1):161–173. [PubMed] [Google Scholar]
- 122.Duerfeldt AS, Blagg BS. Hydrating for resistance to radicicol. ACS Chem. Biol. 2009;4(4):245–247. doi: 10.1021/cb9000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prodromou C, Nuttall JM, Millson SH, Roe SM, Sim TS, Tan D, Workman P, Pearl LH, Piper PW. Structural basis of the radicicol resistance displayed by a fungal hsp90. ACS Chem. Biol. 2009;4(4):289–297. doi: 10.1021/cb9000316. [DOI] [PubMed] [Google Scholar]
- 124.Agatsuma T, Ogawa H, Akasaka K, Asai A, Yamashita Y, Mizukami T, Akinaga S, Saitoh Y. Halohydrin and oxime derivatives of radicicol: synthesis and antitumor activities. Bioorg. Med. Chem. 2002;10(11):3445–3454. doi: 10.1016/s0968-0896(02)00260-2. [DOI] [PubMed] [Google Scholar]
- 125.Kwon HJ, Yoshida M, Nagaoka R, Obinata T, Beppu T, Horinouchi S. Suppression of morphological transformation by radicicol is accompanied by enhanced gelsolin expression. Oncogene. 1997;15:2625–2631. doi: 10.1038/sj.onc.1201443. [DOI] [PubMed] [Google Scholar]
- 126.Soga S, Neckers LM, Schulte TW, Shiotsu Y, Akasaka K, Narumi H, Agatsuma T, Ikuina Y, Murakata C, Tamaoki T, Akinaga S. KF25706, a novel oxime derivative of radicicol, exhibits in vivo antitumor activity via selective depletion of Hsp90 binding signaling molecules. Cancer Res. 1999;59(12):2931–2938. [PubMed] [Google Scholar]
- 127.Ikuina Y, Amishiro N, Miyata M, Narumi H, Ogawa H, Akiyama T, Shiotsu Y, Akinaga S, Murakata C. Synthesis and antitumor activity of novel O-carbamoylmethyloxime derivatives of radicicol. J. Med. Chem. 2003;46(12):2534–2541. doi: 10.1021/jm030110r. [DOI] [PubMed] [Google Scholar]
- 128.Soga S, Sharma SV, Shiotsu Y, Shimizu M, Tahara H, Yamaguchi K, Ikuina Y, Murakata C, Tamaoki T, Kurebaya-shi J, Schulte TW, Neckers LM, Akinaga S. Stereospecific antitumor activity of radicicol oxime derivatives. Cancer Chemother. Pharmacol. 2001;48(6):435–445. doi: 10.1007/s002800100373. [DOI] [PubMed] [Google Scholar]
- 129.Yamamoto K, Garbaccio RM, Stachel SJ, Solit DB, Chio-sis G, Rosen N, Danishefsky SJ. Total synthesis as a resource in the discovery of potentially valuable antitumor agents: cycloproparadicicol. Angew. Chem. Int. Ed. Engl. 2003;42(11):1280–1284. doi: 10.1002/anie.200390329. [DOI] [PubMed] [Google Scholar]
- 130.Lei X, Danishefsky SJ. Efficient synthesis of a novel resorcy-clide as anticancer agent based on Hsp90 inhibition. Adv. Synthesis Catalysis. 2008;350:1677–1681. doi: 10.1002/adsc.200800187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moulin E, Zoete V, Barluenga S, Karplus M, Winssinger N. Design, synthesis, and biological evaluation of HSP90 inhibitors based on conformational analysis of radicicol and its analogues. J. Am. Chem. Soc. 2005;127(19):6999–7004. doi: 10.1021/ja043101w. [DOI] [PubMed] [Google Scholar]
- 132.Wang M, Shen G, Blagg BSJ. Radanamycin, a macrocyclic chimera of radicicol and geldanamycin. Bioorg. Med. Chem. Lett. 2006;16:2459–2462. doi: 10.1016/j.bmcl.2006.01.086. [DOI] [PubMed] [Google Scholar]
- 133.Shen G, Wang M, Welch TR, Blagg BSJ. Design, Synthesis, and structuresactivity relationships for chimeric inhibitors of Hsp90. J. Org. Chem. 2006;71(20):7618–7631. doi: 10.1021/jo061054f. [DOI] [PubMed] [Google Scholar]
- 134.Belofsky GN, Jensen PR, Fenical W. Sansalvamide: A new cytotoxic cyclic depsipeptide produced by a marine fungus of the genus Fusarium. Tetrahedron Lett. 1999;40:2913–2916. [Google Scholar]
- 135.Pan PS, Vasko RC, Lapera SA, Johnson VA, Sellers RP, Lin C-C, Pan C-M, Davis MR, Ardi VC, McAlpine SR. A comprehensive study of Sansalvamide A derivatives: their structure-activity relationships and their binding mode to Hsp90. Bio. Org. Med. Chem. 2009;17:5806–5825. doi: 10.1016/j.bmc.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Davis MR, Styers TJ, Rodriguez RA, Pan P-S, Vasko RC, McAlpine SR. Synthesis and cytotoxicity of a new class of potent decapeptide macrocycles. Org. Lett. 2008;10:177–180. doi: 10.1021/ol702403r. [DOI] [PubMed] [Google Scholar]
- 137.Vasko RC, Rodriguez R, Ardi V, Cunningham C, Agard DA, McAlpine SR. Mechanistic studies of Sansalvamide A peptide: an allosteric modulator of Hsp90. 2009 doi: 10.1021/ml900003t. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alexander LD, Sellers RP, Davis ME, Ardi VC, Johnson VA, Vasko RC, McAlpine SR. Evaluation of cyclic de-capeptides: Synthesis, SAR, and mechanism of action. J. Med. Chem. 2009 doi: 10.1021/jm901566c. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chakraborty A, Koldobskiy MA, Sixt KM, Juluri K, Mustafa AK, Snowman AM, van Rossum DB, Patterson RL, Snyder SH. Hsp90 regulates cell survival via inositol hexakisphosphate kinase-2. Proc. Natl. Acad. Sci. USA. 2008;105:1134–1139. doi: 10.1073/pnas.0711168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen SY, Sullivan WP, Toft DO, Smith DF. Differential interactions of p23 and the TPR-containing proteins Hop, Cyp40, FKBP52 and FKBP51 with Hsp90 mutants. Cell, Stress Chaperones. 1998;3:118–129. doi: 10.1379/1466-1268(1998)003<0118:diopat>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wenger RM. Synthesis of cyclosporin and analogues: structural and conformational requirements for immunosuppressive activity. Prog. Allergy. 1986;38:46–64. [PubMed] [Google Scholar]