Abstract
This paper summarizes the evidence that the contribution of backyard poultry flocks to the on-going transmission dynamics of an avian influenza epidemic in commercial flocks is modest at best. Nevertheless, while disease control strategies need not involve the backyard flocks, an analysis of the contribution of each element of the next generation matrix to the basic reproduction number indicates that models which ignores the contribution of backyard flocks in estimating the effort required of strategies focused one host type (eg commercial flocks only) necessarily underestimate the level of effort to an extent that may matter to policy makers.
Keywords: Avian influenza, model, backyard flocks
Introduction
The 2003 avian influenza (H7N7) outbreak in the Netherlands involved large commercial flocks and some backyard flocks (Stegeman et a., 2004; Thomas et al., 2004; Le Menach et al., 2006; Boender et al., 2007; Bavinck et al., 2009). However, Thomas et al. (2004) argued that the contact structure and the small size of the backyard flocks meant that their role in this epidemic was “probably negligible”. This conclusion was buttressed by a later analysis of the next generation matrix for a two-type SEI model of a portion of the outbreak, which led Bavinck et al. (2009) to conclude that “from an epidemiological perspective” backyard flocks played only a marginal role. On face value, this has obvious implications for control of avian influenza in Europe and North America and, indeed, for the architecture and data requirements of future models of this disease. For example, Bavinck et al. (2009) suggest that “if, in a future epidemic, backyard flocks appear to be less susceptible than commercial flocks, as shown in our study, preemptive culling might not be necessarily applied to backyard poultry flocks, as the probability of becoming infected appears to be much lower.” While not necessarily disagreeing with Bavinck et al. (2009), it will be argued here that it would be unwise to further conclude that we can ignore the contribution of backyard flocks to future epidemics even if they can be shown to be less susceptible than commercial flocks. This is especially true when we are attempting to estimate the effort required to curtail an epidemic using strategies directed at only one type (sensu Roberts and Heesterbeek, 2003; Diekmann et al., 2010) of host.
This paper will be set out as follows. First, we shall consider the rather difficult problem of defining what we mean by “backyard flocks” in the context of commercial poultry operations in Western Europe and North America. Next we shall summarize the evidence for and against the notion that backyard flocks contribute rather little to the overall transmission of avian influenza virus in commercial operations. Finally, we shall compare the analysis of Bavinck et al. (2009) with our own analysis of the 2004 highly pathogenic avian influenza (H7N3) outbreak that occurred in the Fraser Valley of British Columbia, Canada (Anon., 2004), and, specifically, address the question of how we can use such analyses to estimate the effort required for targeted interventions (that is, strategies directed against commercial flocks only).
Backyard flock – definitions
One of the difficulties we have in dealing with backyard flocks is the difficulty of defining what we mean by a “backyard flock”. The phrase is in common use but as the OIE (World Health Organization for Animals) points outs there is no accepted definition (Anon., 2009). Common criteria include the number of birds in the flock frequently conflated with whether or not the flock is included in some register of commercial flocks: for example, the Dutch Ministry of Agriculture, Nature and Food Quality defines a backyard flock as consisting of fewer than 500 birds or as not having a unique farm number (Bavinck et al., 2009); the National Animal Health Monitoring System “Poultry ’04” study in the USA defined backyard flocks as residences with fewer than 1000 birds other than pet birds (Garber et al., 2007); the Canadian Food Inspection Agency defines backyard flocks as flocks that are smaller than 1000 birds that are not registered as commercial poultry operations (Anon., 2004). Sometimes, by default, backyard flocks are simply those flocks which by reason of their small size are not obligated to be recorded in national databases: models of avian influenza in Britain do not included flocks less than 50 birds because these flocks are nor reported in The Great Britain Poultry Register (Truscott et al., 2007; Sharkey et al., 2008; Dent et al., 2008). Capua et al., (2002) suggest that backyard flocks should be defined as those having no functional connections with industrial establishments. If we understand the word functional to indicate any contact or process that could plausibly lead to transmission between commercial and backyard flocks (as Capua et al. clearly intend), then obviously, by definition, flocks with no functional connection to industrial premises have no role in the epidemiology of transmission. But setting aside the tautology implicit in the definition how would we know that there was no functional connection? A recent study in the USA, found that only 3.5% of all backyard flocks (range 0.9 – 8.5%) had someone in the household who worked for a commercial poultry operation, only 2.5% of backyard flocks received veterinary care and only 2.8% of backyard flocks were vaccinated (Anon. 2005). But even for those flocks in which there were no obvious commercial or social contacts (e.g. shared personnel, equipment or breeding birds) we could never be sure that wind-blown virus (for example) did not constitute a functional connection between industrial and backyard premises. In the 2004 highly pathogenic avian influenza (H7N3) outbreak that occurred in the Fraser Valley of British Columbia, Canada, not only was there epidemiological evidence of wind borne spread of the virus, but air sampling techniques detected small quantities of wind borne virus up to 800 metres from infected premises (Power 2005; Schofield et al., 2005). Maps of the affected area show that commercial and backyard flocks were intimately intermingled and although the average distance between commercial flocks was about 2–3 km, the distance between commercial flocks and backyard flocks was frequently 1km or less (Hudson and Elwell, 2004). The air sampling studies did not demonstrate whether the viruses found some distance from the infected premises were infectious (Power, 2004) and so our argument is circumstantial - but most of the arguments about backyard flocks are circumstantial.
Risk factors for infection
Several case-control and other studies in Western Europe and North America have identified risk factors for poultry flocks being infected with avian influenza virus. In the 2004 (H7N3) outbreak in British Columbia the odds of commercial flocks (>1000 birds) being infected were 5.6 times greater that the odds of backyard flocks being infected, and infected backyard flocks were always discovered after nearby commercial flocks had been infected (Lees, 2004). In the large, low pathogenicity (H7N2) outbreak in Virginia, USA in 2002 not a single backyard flock was reported to be infected (Akey, 2003) despite the usual intermingling of commercial and backyard flocks. With respect to the risk that commercial flocks will be infected, at least two studies found that proximity to backyard flocks was not a risk factor (McQuiston et al., 2005; Thomas et al., 2005). Flock size (number of houses or number of birds) was identified by Thomas et al. (2005) as a risk factor in the 2003 (H7N7) outbreak in the Netherlands. Flock type is often cited as an additional risk factor and, although the specific type referred to in each study is different, it seems that the birds in the longer production cycles are at more risk (McQuiston et al. 2005; Thomas et al., 2005; Mannelli et al., 2006). Proximity to another infected commercial farm was shown to be important in the 1999 (H7N1) outbreak in northern Italy (Mannelli et al., 2006) and in the 2003 (H7N7) outbreak in the Netherlands (Boender et al., 2007). McQuiston et al. (2005) did not include proximity to other infected commercial premises in their case-control study, perhaps because Akey (2005) had already stated that the spread of the “H7N2 LPAI outbreak in [Virginia] was most consistent with transmission primarily by [the movement of] fomites, people, and equipment contaminated with virus rather than by airborne transmission.” This kind of movement between farms is by far the most entrenched explanation for the between-farm transmission of avian influenza virus despite the fact that rapid imposition of movement bans means that the opportunity for the formal evaluation of the hypothesis is very limited indeed. McQuiston et al. (2005) found that the use of rendering to dispose of euthanized birds was a risk factor for infection and suggested that the use of a common vehicle to transport the dead birds to the rendering site was an important early means of potentiating transmission, but most other arguments are based upon, first, the observation that long distance transmission occurs and, second, the conviction that long distance transmission is more easily explained by the movement of people and vehicles than by the mechanisms usually invoked to explain local or contiguous spread (Akey, 2003; McQuiston et al. 2005; Mannelli et al., 2006; Boender, 2007). The several models of avian influenza that examine the impact of various kinds of control strategies on transmission attributable to the movement of people, vehicles and other fomites between farms are based on the supposition that this kind of transmission is important and depend upon expert opinion or analogy with other animal diseases transmitted by the fecal oral route (but not subject to automatic movement bans) for their estimates of the probability of transmission given contact (Truscott et al., 2007; Dent et al., 2008; Sharkey et al; 2008, Dorea et al. 2010). The important point though, is that none of these studies included backyard flocks in their analyses of the network of premises, feed mills, rendering plants and markets. Like Capua et al. (2002), implicitly or explicitly, they assume that backyard flocks have no functional connection with the industrial network and are therefore irrelevant. Nevertheless, some backyard flocks do become infected during some avian influenza outbreaks (Anon, 2005; Bavinck et al; 2009) and policy makers continue to insist that pre-emptive culling of backyard flocks is an appropriate component of an infectious avian disease control strategy despite recommendations to the contrary (Capua et al., 2002; Bavinck et al., 2009). If we were to shift to control strategies for avian influenza in which infected backyard flocks and their dangerous contacts were depopulated but all the rest of the control effort (depopulation or vaccination) were focused on the commercial flocks would models that omitted backyard flocks altogether fairly represent the control effort required to curtail the outbreak? How big must be the contribution of backyard flocks to transmission before we feel obligated to include them in our models? This question is addressed in the next section.
Epidemic model of the 2004 Abbotsford (H7N3) avian influenza outbreak
The first recorded outbreak of highly pathogenic avian influenza in Canada occurred near Abbotsford in the Fraser Valley, British Columbia. During the course of 91 days, 42 out of 410 commercial flocks and 11 out of 553 backyard flocks were infected (Pasik et al., 2009). Control measures included movement bans, active surveillance, and an increasingly draconian program of depopulation beginning with the depopulation of known infected farms progressing to the pre-emptive culling of all farms within 3, 5 or 10 km of the infected farms (depending on the date) and culminating in a strategy (beginning about 20–22 days into the outbreak) intended to depopulate all the flocks (whether infected or not) within the affected area (Anon., 2004).
Most infected flocks were detected as the result of active surveillance; some were detected as the result of responses to reports of sick birds or increased rates of mortality in flocks. The epidemic data comprise the date that the samples were taken (for PCR), the date of positive diagnosis and the date of depopulation (Anon., 2004). In formulating our model, we focused on the transmission of avian influenza virus between the distinct sets of premises housing the birds. Each set of premises often contains several flocks but, for ease of expression, we shall use the word “flock” to represent the premises as a whole and, in doing so, we shall follow the usual convention of referring to the flocks as being in “susceptible”, “latent” or “infectious” states (although, of course, it is the condition of the birds that confer these properties on the flocks). We assumed that an infected flock passed through a latent (infected but not yet infectious) period that lasted 2 days and was thereafter infectious until depopulation (Boender et al., 2007). We followed the conventional assumption that takes no account of temporal changes in the threat presented by any given infectious flock that might plausibly be attributed to the changing number of infected birds in the flock or the imposition of a quarantine following the confirmation of infection (Stegeman et al., 2004; Le Menach et al., 2006; Bavinck et al., 2009). We divided the host population into two host types (sensu Roberts and Heesterbeek, 2003; Diekman et al., 2010). The two host types are commercial and backyard flocks denoted by the subscripts 1 and 2 respectively. We further subdivided each type into susceptible (Si) flocks, latently infected flocks (Ei) and infectious flocks (Ii).
Here β11, β12, β21 and β22 are the transmission parameters whose values were to be estimated from the epidemic data. We assumed that until day 21, the turnover rates (μ1 and μ2) of the susceptible commercial and backyard flocks were best represented by those rates normally commensurate with the sector to which they belonged. We assumed that the 410 depopulated commercial flocks consisted of 96 that produced commercial table eggs, 61 that produced broiler hatching eggs and 47 that produced turkey meat. The data in Anon. (2004) are consistent and unequivocal with respect to the numbers of flocks in each of these sectors. However, the document contains conflicting reports of how many “chicken meat” flocks there were in the Fraser Valley (Anon., 2004). The quoted figures ranged between 235 to 286. Given that not all flocks in the Valley were depopulated we simply assumed that the 206 flocks of the 410 flocks not yet assigned to a sector were “chicken meat” flocks. Average production cycle time for all of these sectors combined was estimated as (63*96/410) + (43*61/410) + (14*47/410) + (7*206/410) = 26.39 weeks. Converting this average cycle time to days (185 days), and following standard reasoning, we estimated μ1 = 1/185 = 0.0054/flock/day. Backyard flocks do not undergo regular cycles of depopulation and replacement (Anon., 2005) and so we conservatively estimated the turnover rate of backyard flocks as μ2 = 0.001/flock/day. We rather crudely mimicked the increasing pace of pre-emptive culling of susceptible flocks by replacing μ1 and μ2 with a constant value ρ/flock/day from day 21 of the outbreak. Day 21 was chosen to most closely mimic the date on which the increased pre-emptive culling began (Anon., 2004); we estimated the value of ρ from the epidemic data. The rate at which latently infected flocks became infectious was given by δ = 0.5/flock/day. Two connected, problematic issues remained. First, the epidemic data indicate only the day on which the flock was sampled. Sometimes, sampling was carried out because the flock was experiencing a greater than expected mortality. In the face of an epidemic it is likely that producers will be rather sensitive to any increases in mortality and so it seems reasonable to assume that these flocks moved out of the latent phase 6–7 days previously (Bos et al., 2007). However, most infected flocks were detected as the result of active surveillance suggesting that the move from latency to infectiousness had occurred at some unknown time less than 6–7 days before the sampling date. We therefore constructed a new data set from the epidemic data in which the move from latency to infectiousness for all detected flocks was set by subtracting a random number between 1 and 7 from the sampling date. Given our assumptions, the date of infection was 2 days prior to that. The second problematic issue was that the depopulation rate of infected flocks (α) increased during the course of the outbreak. Figure 1a shows the time (y) between sampling and depopulation plotted by the day of sampling (x). A simple least squares fit demonstrated that y = 9.56 – 0.17x. If we assume that an average of 3.5 days had elapsed between the move from latency to infectiousness we estimate that α ≈ 1/(13.00 – 0.17x)/flock/day. The maximum likelihood algorithms available in Berkeley Madonna (version 8.3.9) were used to fit the model to the data. Two models were fitted to the data. In the full model it was assumed initially that transmission was possible within and between host types (ie β11, β12, β21 and β22 were all greater than zero). In the reduced model, it was assumed that infected backyard flocks were an example of “spill over” and that the backyard flocks played no part in transmission (ie β12 = β22 = 0). The best fit as judged by the root mean square deviation was obtained using a model in which β11 = 0.000505, β12 = 0.00238, β21 = 0.000166 and β22 = 0 (Figure 1b).
Figure 1.
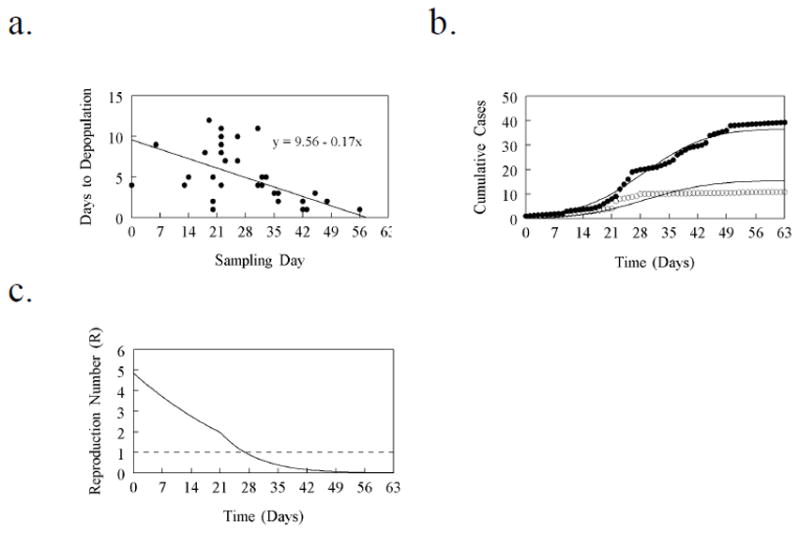
The 2004 highly pathogenic avian influenza (H7N3) outbreak in the Fraser Valley of British Columbia, Canada. a. Number of days (y) between the date of sampling and the date on which the flock was depopulated plotted by sampling day (x); b. Best fit model (solid lines), cumulative number of new cases in commercial flocks (solid circles), cumulative number of new cases in backyard flocks (open circles); c. Changes in the reproduction number during the course of the epidemic.
The basic reproduction number and the effort required to render R0 < 1
The next generation matrix (K) for the Abbotsford outbreak model is
Here, N1, N2 and α0 are respectively the number of susceptible commercial and backyard flocks at the start and the initial value of the infectious period. Flocks that were depopulated either because they were infected or as part of the pre-emptive culling processes were not repopulated until the epidemic was over. Furthermore, the rate of preemptive culling increased over time. Thus, as the epidemic progressed, the number of susceptible flocks decreased. In addition, the infectious period (1/α) decreased. As a result, the reproduction number (calculated as the dominant eigenvalue of the matrix, K, Diekmann et al., 2010) also decreased over time (Figure 1c). The basic reproduction number (calculated using the initial values for all parameters) was given by R0 = 4.8, which is higher than a previous estimate (Garske et al., 2006) and generally higher than most estimates for the basic reproduction number of avian influenza (Garske et al., 2006, Bavinck et al., 2009). This arises because infectious period is usually assumed to be about 7 days (thus α = 1/7 = 0.142/flock/day even at the start). However, like Stegeman et al. (2004), we found that, initially, the infectious period was about 13 days and decreased once the outbreak had been recognized and surveillance and detection became more efficient.
We now consider the question of targeted control. Bavinck et al. (2009) suggested that if backyard flocks appear to be less susceptible than commercial flocks it might be sufficient to pre-emptively cull only the commercial flocks. We shall consider this proposition first for the 2004 H7N3 Abbotsford outbreak and then for the 2003 H7N7 outbreak in the Netherlands. Like Bavinck et al. (2009) we acknowledge and then ignore the fact that the infectious period decreased during the course of the outbreak; for the purposes of the arguments that follow, we shall set the value of α to its overall average value (0.143/flock/day). The length of the infectious period reflects the efficiency detection and depopulation. We shall imagine a situation in which all infected flocks (commercial and backyard flocks) can be detected and depopulated (without repopulation) with equal efficiency. This is the default response but it is often not sufficient to curtail the outbreak as rapidly as policy makers would like and pre-emptive depopulation strategies are frequently implemented in addition. In what follows we shall investigate what fraction of the flocks must be depopulated in order to curtail the epidemic. The next generation matrix for the Abbotsford outbreak with the initial flock numbers is thus
The basic reproduction number R0 = 1.70.
In a single host type model, the proportion (p) of susceptible flocks that must be pre-emptively depopulated to ensure that infectious flocks give rise to less than one new infected flock each would be
If pre-emptive culling were applied equally to commercial and backyard flocks alike such that the numbers both host types were reduced to 59 percent of their starting values, the next generation matrix would be
and the reproduction number in the presence of control measures would be R = 1.0, which is the required and expected result. However, the intention is to pre-emptively depopulate only the commercial flocks. If we reduce only the numbers of commercial flocks to 59 percent of their starting values, the next generation matrix becomes
and the reproduction number is R = 1.2, which is not sufficient to eliminate transmission. Even when the backyard flocks do not constitute a reservoir host population (and in this case they clearly do not because k22 = 0), they can contribute to overall virus transmission provided k12 > 0 and K21 > 0. An infectious commercial flock can infect other commercial flocks either directly, or indirectly via spillover to backyard flocks. Roberts and Heesterbeek (2003) and Heesterbeek and Roberts (2007) have pointed out, when there is more than one host type, and targeted control involves only one of them, the overall reproduction number will always lead to underestimates of the effort required to curtail the epidemic. Roberts and Heesterbeek (2003), Hill and Longini, (2003), and Heesterbeek and Roberts (2007) have each described methods for calculating the relevant statistic (Tc, Heesterbeek and Roberts, 2007). For a two host type model,
When k11 = 1.46, k12 = 0.87, k21 = 0.48 and k 22 = 0, then Tc = 1.88. Using this value to estimate the proportion (p) of susceptible commercial flocks that must be depopulated to ensure that infectious flocks give rise to less than one new infected flock gives
Recall that the value of p calculated using R was 0.41. The increase in p that resulted from using Tc rather than R does not seem very big but given the number of susceptible commercial flocks near Abbotsford at the start of the outbreak (410) this represents an additional 25 flocks that must be pre-emptively depopulated in order to curtail the epidemic. This is not trivial. If selective vaccination of commercial flocks were the chosen strategy rather than pre-emptive culling, the same calculations apply. Using the total number of commercial birds killed during the Abbotsford outbreak to estimate the overall average number of birds per flock (Hudson and Elwell, 2004) suggests that a value for p of 0.47 rather than 0.41 represents an additional million doses of vaccine.
We can apply the same arguments to the H7N7 avian influenza outbreak studied by Bavinck et al. (2009). The next generation matrix for the two host type model in that case was
for which the overall basic reproduction number was given by R0 = 1.33. Were we mistakenly to use this number to calculate p, we would get p = 0.25. The value of Tc for this model is
and
Given the number of commercial farms (984) in the outbreak studied by Bavinck et al. (2009) this represents an additional 10 commercial farms.
Discussion
One of the more important contributions models can make to the decision making process is to provide estimates of the effort required to achieve specific results. One methodology for reducing control effort is to focus on only one host type. In the context of the commercial poultry industry, especially in countries for which there is little information about the location of backyard flocks - and access is difficult, depopulating or vaccinating only the commercial flocks is an appealing prospect. This idea is buttressed by expert opinion (Capua et al., 2002; Akey, 2003) and modeling studies (Bavinck et al., 2009, and the work presented here) both of which suggest that the contribution of backyard flocks to the on-going transmission dynamics of an epidemic is modest at best. However, as we have shown, even this modest contribution may be sufficient to compromise the calculated effort required of targeted control strategies. How much this will matter to decision makers will depend upon how risk averse they are, but they should at least be made aware that effort estimates that do not take account of backyard flocks will probably be underestimates.
Acknowledgments
GS was supported in part by award number 5U01GM-076426 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. SD was supported by a collaborative grant (07-9208-0175-CA) between the University of Pennsylvania and the Center for Epidemiology and Animal Health, USDA, APHIS, VS, Fort Collins, Colorado.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
G. Smith, Email: garys@vet.upenn.edu.
S. Dunipace, Email: dunipace@vet.upenn.edu.
References
- Anonymous. In: Comprehensive Report on the 2004 Outbreak of High Pathogenicity Avian Influenza (H7N3) in the Fraser Valley of British Columbia, Canada. June 30, 2004, minor revisions November 24, 2004. Lees W, Chown L, editors. Animal Disease Surveillance Unit, Canadian Food Inspection Agency; 2004. [Google Scholar]
- Anonymous. USDA, APHIS, CEAH, VS Information Sheet. 2005. Aug, Highlights of NAHMS Poultry ’04 Part I: Reference of Health and Management of Backyard/Small Production Flocks in the United States, 2004. [Google Scholar]
- Anonymous. Report of the meeting of the OIE terrestrial animal health standards commission. Paris: OIE, World Organization for Animal Health; 2009. Sep 7–18, [Google Scholar]
- Akey BL. Low-pathogenicity H7N2 avian influenza outbreak in Virginia during 2002. Avian Dis. 2003;47:1099–1103. doi: 10.1637/0005-2086-47.s3.1099. [DOI] [PubMed] [Google Scholar]
- Bavinck V, Bouma A, van Boven M, Bos ME, Stassen E, Stegeman JA. The role of backyard poultry flocks in the epidemic of highly pathogenic avian influenza virus (H7N7) in the Netherlands in 2003. Prev Vet Med. 2009;88(4):247–254. doi: 10.1016/j.prevetmed.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Boender GJ, Hagenaars TJ, Bouma A, Nodelijk G, Elbers AR, de Jong MC, van Boven M. Risk maps for the spread of highly pathogenic avian influenza in poultry. PLoS Comput Biol. 2007;3(4):704–712. doi: 10.1371/journal.pcbi.0030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busani L, Valsecchic MG, Rossic E, Tosona M, Ferrèa N, Pozzaa MD, Marangona S. Risk factors for highly pathogenic H7N1 avian influenza virus infection in poultry during the 1999–2000 epidemic in Italy. Vet J. 2009;181(2):171–177. doi: 10.1016/j.tvjl.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Bos MEH, Van Boven M, Nielen, Bouma A, Elbers ARW, Nodelijk G, Koch G, Stegeman A, De Jong MCM. Estimating the day of highly pathogenic avian influenza (H7N7) virus introduction into a poultry flock based on mortality data. Vet Res. 2007;38:493–504. doi: 10.1051/vetres:2007008. [DOI] [PubMed] [Google Scholar]
- Capua I, Dalla Pozza M, Mutinelli F, Maragon S, Terrigino C. Newcastle disease outbreaks in Italy during 2000. Vet Rec. 2002;150:565–568. doi: 10.1136/vr.150.18.565. [DOI] [PubMed] [Google Scholar]
- Capua I, Marangon S. The use of vaccination as an option for the control of avian influenza. OIE, World Organization for Animal Health, International Committee Report, 71st General Session; Paris. 18–23 May, 2003; 2003. p. 10. 71 SG/12/CS3 E. [Google Scholar]
- de Jong MCM, Hagenaars TJ. Modelling control of avian influenza in poultry: the link with data. Rev sci tech Off Int Epiz. 2009;28 (1):371–377. doi: 10.20506/rst.28.1.1858. [DOI] [PubMed] [Google Scholar]
- Diekmann O, Heesterbeek JA, Roberts MG. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface. 2010;7:873–885. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent JE, Kao RR, Kiss IZ, Hyder K, Arnold M. Contact structures in the poultry industry in Great Britain: Exploring transmission routes for a potential avian influenza virus epidemic. BMC Vet Res. 2008;4:27. doi: 10.1186/1746-6148-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber L, Hill G, Rodriguez J, Gregory G, Voelker L. Non-commercial poultry industries: surveys of backyard and game fowl breeder flocks in the United States. Prev Vet Med. 2007;80:120–128. doi: 10.1016/j.prevetmed.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Garske T, Clarke P, Ghani A. The transmissibility of highly pathogenic avian influenza in commercial poultry in industrialized countries. PLoS ONE. 2006;2(4):e349. doi: 10.1371/journal.pone.0000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesterbeek JAP, Roberts MG. The type-reproduction number T in models for infectious disease control. Math Biosci. 2007;206:3–10. doi: 10.1016/j.mbs.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Henning J, Pfeiffer DU, Tri Vu L. Risk factors and characteristics of H5N1 Highly Pathogenic Avian Influenza (HPAI) post-vaccination outbreaks. Vet Res. 2009;40:15. doi: 10.1051/vetres:2008053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AN, Longini IM. The critical vaccination fraction for heterogeneous epidemic models. Math Biosci. 2003;181:85–106. doi: 10.1016/s0025-5564(02)00129-3. [DOI] [PubMed] [Google Scholar]
- Hudson R, Elwell L. Report on the Canadian Poultry Industry Forum, Avian Influenza-lessons learned and moving forward. Abbotsford: 2004. Oct 27–28, Report dated December 2004. [Google Scholar]
- Hurd HS, Forsythe K, Trock SC. Foreign Animal Disease Report. USDA, APHIS; 1998. Summer. Risk analysis of potential control options for the 1997 nonpathogenic avian influenza outbreak in Pennsylvania; pp. 32–40. [Google Scholar]
- Lees W. Overview: The Avian Influenza Outbreak in BC (2004). Presentation given to the Canadian Poultry Industry Forum, Avian Influenza-lessons learned and moving forward; Abbotsford. October 27–28, 2004.2004. [Google Scholar]
- Mannelli A, Ferre N, Marangon S. Analysis of the 1999–2000 highly pathogenic avian influenza (H7N1) epidemic in the main poultry production area in Northern Italy. Prev Vet Med. 2006;73:273–285. doi: 10.1016/j.prevetmed.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mannelli A, Busani L, Toson M, Bertolini S, Marangon S. Transmission parameters of highly pathogenic avian influenza (H7N1) among industrial poultry farms in northern Italy in 1999–2000. Prev Vet Med. 2007;81(4):318–322. doi: 10.1016/j.prevetmed.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Le Menach A, Vergu E, Grais RB, Smith DL, Flahault A. Key strategies for reducing spread of avian influenza among commercial poultry holdings: lessons for transmission to humans. Proc R Soc B. 2006;273:2467–2475. doi: 10.1098/rspb.2006.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston JH, Garber LP, Porter-Spalding BA, Hahn JW, Pierson FW, Wainwright SH, Senne DA, Brignole TJ, Akey BL, Holt TJ. Evaluation of risk factors for the spread of low pathogenicity H7N2 avian influenza virus among commercial poultry farms. JAVMA. 2005;226:767–772. doi: 10.2460/javma.2005.226.767. [DOI] [PubMed] [Google Scholar]
- Mulatti P, Kitron U, Mannelli A, Ferre N, Maragon S. Spatial analysisi of the 1999–2000 highly pathogenic avian influenza (H7N1) epidemi in Northern Italy. Avian Dis. 2007;51:421–424. doi: 10.1637/7549-033106R.1. [DOI] [PubMed] [Google Scholar]
- Power C. An Interim Report February 15, 2005. Animal Disease Surveillance Unit Canadian Food Inspection Agency; 2005. The Source and Means of Spread of the Avian Influenza Virus in the Lower Fraser Valley of British Columbia During an Outbreak in the Winter of 2004. [Google Scholar]
- Roberts MG, Heesterbeek JAP. A new method for estimating the effort required to control an infectious disease. Proc Roy Soc of Lond. 2003;270:1359–1364. doi: 10.1098/rspb.2003.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L, Ho J, Kournikakis B, Booth T. DRDC Suffield TR 2005-032. Defence R&D Canada. Suffield: DRDC; 2005. Avian Influenza Sampling Campaign in the British Columbia Fraser Valley, 9–19 April 2004: Sampling of rare biological events. [Google Scholar]
- Sharkey KJ, Bowers RG, Morgan KL, Robinson SE, Christley RM. Epidemiological consequences of an incursion of highly pathogenic H5N1 avian influenza into the British poultry flock. Proc Biol Sci. 2008;275:19–28. doi: 10.1098/rspb.2007.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegeman A, Bouma A, Elbers AR, de Jong MC, Nodelijk G, de Klerk F, Koch G, van Boven M. Avian influenza A virus (H7N7) epidemic in The Netherlands in 2003: course of the epidemic and effectiveness of control measures. J Infect Dis. 2004;190(12):2088–2095. doi: 10.1086/425583. [DOI] [PubMed] [Google Scholar]
- Thomas ME, Bouma A, Ekker HM, Fonken AJM, Stegeman JA, Nielen M. Risk factors for the introduction of high pathogenicity Avian Influenza virus into poultry farms during the epidemic in the Netherlands in 2003. Prev Vet Med. 2004;69:1–11. doi: 10.1016/j.prevetmed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Truscott J, Garske T, Chis-Ster I, Guitian J, Pfeiffer D, Snow L, Wilesmith J, Ferguson NM, Ghani AC. Control of a highly pathogenic H5N1 avian influenza outbreak in the GB poultry flock. Proc Biol Sci. 2007;274:2287–2295. doi: 10.1098/rspb.2007.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Park C-K, Nam H-M, Wee S-H. Virus spread pattern within infected chicken farms using regression model: the 2003–2004 HPAI epidemic in the Republic of Korea. J Vet Med. 2005;B52:428–431. doi: 10.1111/j.1439-0450.2005.00891.x. [DOI] [PubMed] [Google Scholar]


