Abstract
Mental health is increasingly defined not only by the absence of illness but by the presence of subjective well-being (SWB). Previous cohort studies have consistently shown that indicators of SWB predict favorable life outcomes including better mental and somatic health, including longevity. The favorable effects associated with SWB have prompted new research aimed at raising happiness and well-being through individual interventions and public health initiatives. Standard observational studies of individual-level associations, however, are subject to potential confounding of exposure and outcome by shared genes and environment. The present study explored the association between SWB and increased longevity, using twin pair analyses to determine whether the association is consistent with causality or is due to genetic or environmental confounding. The study sample of 3,966 twins aged 70 or older followed for a median time period of 9 years was drawn from the population-based Longitudinal Study of Aging Danish Twins (LSADT). The association between the exposure of subjective wellbeing, operationalized as affect and life satisfaction, and the outcome of all-cause mortality risk, was examined using between-individual and within-pair survival analyses. As expected, at the individual level (without regard to twin pair membership), SWB predicted increased longevity. Exposure effects were also present in unadjusted and adjusted within-pair analyses of 400 dizygotic (DZ) pairs and 274 monozygotic (MZ) pairs, indicating that SWB is associated with increased longevity independent of familial factors of genes and shared environment.
Keywords: well-being, life satisfaction, aging, longevity, mortality, cotwin control
Subjective well-being (SWB) is a broad construct generally considered to include positive and negative affect and a cognitive evaluation of one’s life (Lucas et al., 1996). Measures of the emotional and cognitive components of SWB show discriminant validity (Lucas et al., 1996). Yet distinct measures of SWB, such as quality of life, life satisfaction, happiness, and optimism are moderately to highly correlated at the phenotypic level, with much of the overlap among similar constructs accounted for by genetic effects (Bartels & Boomsma, 2009; Mosing et al., 2009). Distinct measures of phenotypic SWB used in different studies reflect the same latent genetic factors and yield results that can be meaningfully compared (Bartels & Boomsma, 2009; Mosing et al., 2010).
The association between SWB and reduced morbidity and mortality is well established although the underlying explanatory mechanisms are not understood (Collins et al., 2009; Lyubomirsky et al., 2005; Pitkala, et al., 2004; Pressman & Cohen, 2005). Prospective cohort studies have consistently shown that SWB predicts favorable life outcomes including better mental and somatic health and longevity in samples with identified disease and in generally healthy samples (Chida & Steptoe, 2008). The effect of SWB on mortality risk is similar for measures of positive affect (Koopmans et al., 2010; Pitkala et al., 2004), life satisfaction (Chida & Steptoe, 2008; Collins et al., 2009), negative affect, overall affect, and quality of life (Steel et al., 2008). Variously operationalized, SWB has been consistently related to better somatic and psychological health, and a range of positive life outcomes (Diener, 2000; Lyubomirsky et al., 2005). Conversely, it has been shown that low well-being, dissatisfaction with life (Koivumaa, et al., 2000), negative affect, and depression are associated with morbidity (Schoevers et al., 2000; Schulz et al, 2000), functional disability (Oldehinkel et al., 2001), and mortality (Schoevers et al., 2000; Schulz et al., 2000).
Given the favorable outcomes associated with SWB, there have been calls for interventions to raise happiness at the individual level (Diener, 2000; Lyubomirsky, 2007; Wood & Tarrier, 2010) and at the population level through manipulation of social and environmental conditions (Diener & Seligman, 2004). Successful interventions could be particularly important for the elderly. The oldest-old is the fastest growing segment of the population (Christensen et al., 2009; Vaupel et al., 1998), the most susceptible to disease and disability, and may be increasingly vulnerable to depressive symptoms with age (McGue & Christensen, 2003; Takkinen et al., 2004). Efforts directed at promoting and even raising psychological well-being imply that associations with positive outcomes are causal. Yet without an understanding of the causal mechanisms underlying the association between SWB and longevity interventions may be largely ineffective. Determining whether SWB is a cause of longevity is therefore an important practical and theoretical research question.
Prospective cohort studies provide some evidence of causality through the sequencing of exposure and outcome. However twin survival models are unique in that they provide powerful control for genetic and shared environmental confounding (McGue et al., 2010). The LSADT, one of the largest genetically informative investigations of longitudinal aging, provided a reasonably large and unselected sample with sufficient mortality data for the present investigation.
The method used in this study requires that twins differ on a heritable exposure. Twin studies have shown that SWB is a stable and moderately heritable trait, with about 50% of the variance attributable to stable genetic influences (Lykken & Tellegen, 1996; McGue et al., 1993; Nes et al., 2006; Røysamb et al., 2002; Stubbe et al., 2005; Tellegen et al., 1988). Human lifespan is modestly heritable, with approximately 20–30% of the variance in lifespan attributable to genetic factors (Christensen & Vaupel, 1996; Herskind et al., 1996; Hjelmborg et al., 2006; Martin et al., 2007). The heritability of both SWB and longevity raises the possibility that the association is confounded by shared genes and therefore not causal.
The two members of a twin pair share aspects their environments, especially experiences shared due to their common rearing. In addition, dizygotic (DZ) twins inherit 50% of their segregating genes whereas monozygotic (MZ) twins inherit all of their segregating genes. Therefore within-DZ pair analyses control for common environmental influences and partially for genetic influences, and within-MZ pair analyses control for both shared genes and environment; the within-pair effect of SWB on mortality is independent of the familial factors shared by twins. Thus, if SWB is causal of increased longevity, we expect to find an exposure effect within both DZ and MZ twin pairs. We expected to confirm an association at the individual level, and we explored potential confounding of this association by familial factors, using within-pair analyses in a sample of elderly twins.
Materials and Methods
Sample
The Longitudinal Study of Aging Danish Twins (LSADT) based on the Danish Twin Registry (Kyvik et al., 1996) is a cohort-sequential study begun in 1995 with twins aged 75 or older, followed at 2-year intervals. The LSADT assessment is conducted in participants’ homes by trained interviewers. The survey encompasses demographic background and measures of medical health, physical and cognitive functioning and depression; cotwins are assessed separately by different interviewers. Zygosity in same sex pairs is determined by a self-report questionnaire concerning the similarity between cotwins, a procedure validated by genetic testing, with error rates less than 5% (Hauge, 1981). Information on survival status through January 1, 2010 was obtained from the Danish Central Population Register, which is continuously updated (Pedersen et al., 2006). The ascertainment and sampling of these cohorts has been described in detail by Christensen et al. (1999).
The sample for the present study was based on the 4,731 twins who entered the LSADT between 1995 and 2001. Twins were included if they participated in an intake assessment, had complete data on the depression section of the interview, and survived at least 18 months post-intake. An 18 month lag was introduced to exclude participants who died shortly after intake and whose self-report of well-being at that time may have been influenced by ill health or terminal decline. This left 3,966 twins of whom 2,054 were singletons (whose cotwin did not participate), and 1,912 were members of complete twin pairs (800 DZ, 548 MZ). This sample of 3,966 participants followed for 35,270 person-years (median = 9 years) was used for individual-level analyses.
Informative within-pair analyses could include only those pairs in which one or both twins died before the last follow-up in January, 2010. Excluding doubly-censored twin pairs left 1,348 twins, 548 MZ (274 pairs) and 800 DZ (400 pairs) followed for 11,725 person-years (median = 9 years). Figure 1 illustrates the selection process.
Figure 1.
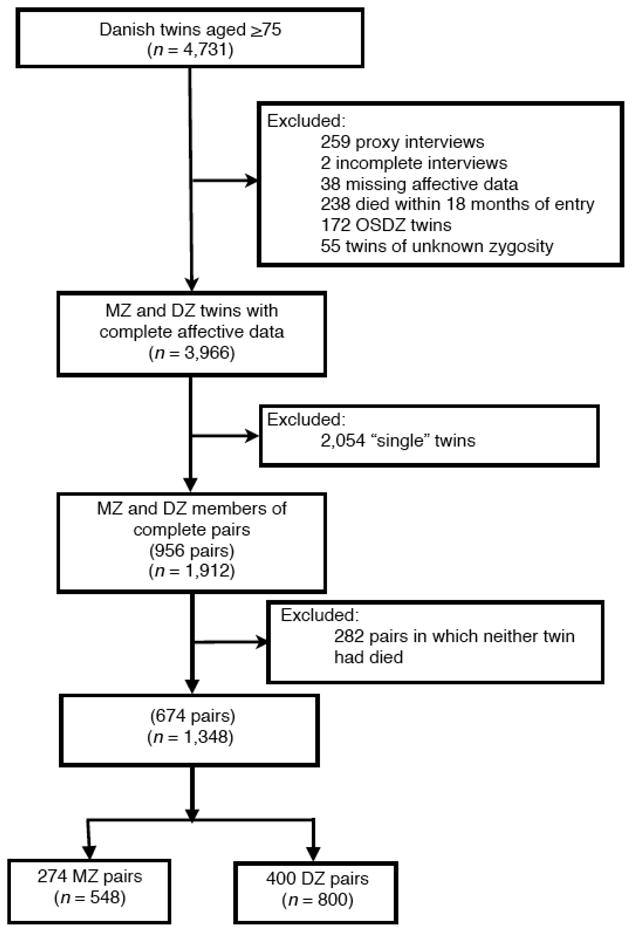
Study population
Measures
We measured SWB using a modified version of the depression scale from the Cambridge Mental Disorders in the Elderly (CAMDEX; Roth et al., 1986). McGue and Christensen (1997) factor analyzed the 21-item CAMDEX depression section to construct affective and somatic subscales. The somatic scale indexes primarily psychomotor slowing and loss of energy, while the affective scale reflects negative affect and lack of well-being. Items on the affective scale are rated on a mostly 3-point scale with responses options 1 = no, 2 = sometimes, and 3 = most of the time. Two items have a dichotomous response format, 1= no, 2 = yes (items 8 and 6, Table 1), and item 3 has a 5-option response format as described below for the life satisfaction item (see Table 1 for scale items). Internal consistency for the affective scale was reported to be 0.78 and two-year stability 0.64 (McGue & Christensen, 1997). We used the affective scale to operationalize psychological well-being on two continuous scales: the absence of depressive affect (the affective scale reversed) and the presence of life satisfaction. Life satisfaction was indexed by a single item from the affective scale, “Are you happy and satisfied with your life at present?” rated from 1, “yes, always” to 5, “no, never.” Both measures were scaled so that higher scores reflected greater SWB. The two measures were correlated at 0.72.
Table 1.
Affect scale items (adapted from the CAMDEX depression section)
|
From McGue and Christensen (1997). CAMDEX = Cambridge Mental Disorders in the Elderly Examination.
In multivariable analyses, we controlled for standard measures of health and cognitive functioning used in several previous LSADT studies. Morbidity was assessed with the number of self-reported present illnesses from a list of 31 health conditions, and the number of medications taken regularly at the time of assessment. Cognitive functioning was assessed with a composite of 5 brief cognitive measures designed to tap age-related cognitive changes including tests of verbal fluency, digit span, and immediate and delayed recall (McGue & Christensen, 2001). The cognitive composite has shown an internal consistency of 0.75 and a 2-year stability coefficient of 0.60.
Data Analysis
Cox proportional hazards models were used to analyze our survival data with age as the underlying time scale. To facilitate comparison of estimates across measures, affect, life satisfaction, and cognitive composite scores were standardized with a mean of 0 and standard deviation of 1. Survival time was measured beginning 18 months post-intake with follow-up terminated either at death or censoring in January, 2010. We calculated hazard ratios (HR) and 95% confidence intervals (CI) for mortality risk which may be interpreted as the multiplicative effect on mortality risk associated with a one standard deviation (SD) increase in the exposure. A hazard ratio < 1 indicates a protective effect of exposure on mortality risk. The proportional hazards assumption was tested by examination of scaled Schoenfeld residuals plots and by including an interaction term between SWB covariates and log-transformed follow-up time.
To determine the population (between-individual) effects we used standard proportional hazards regression in the unpaired sample composed of singletons and all like-sex DZ and MZ twins (without regard to twin-pair membership), with age and sex as covariates. Models were estimated using correlated-cluster variance estimates to control for the dependence of observations within twin pairs. Within-pair analyses were conducted using stratified proportional hazards models, which estimate distinct baseline hazards for each twin pair and common values for the hazard ratios across all pairs (Holt & Prentice, 1974). In this way the effect parameter for SWB could be estimated controlling for genetic and environmental factors shared by cotwins. We also modeled the within-pair analyses using shared gamma frailty models, however the results were essentially unchanged and thus are not reported. Statistical tests were made on the two-sided 5% significance level unadjusted for multiple comparisons. All analyses were conducted in Stata 10.1 (StataCorp, College Station, TX).
Results
The individual-level sample had a higher proportion of women than men (p = 0.01) and less mortality over the follow-up period (p < 0.001) than the MZ and DZ twin samples. In the paired samples, mean intrapair death dates were more similar for MZ than DZ twins (p = 0.006). Intrapair affect was also more similar for MZ than DZ twins (intraclass correlation [ICC] = 0.31 vs. 0.12, p < 0.001) however the correlation coefficients for life satisfaction did not differ significantly (ICC = 0.12 vs. 0.08, p = 0.54) (Table 2).
Table 2.
Descriptive statistics for the study variables
| Individual-level sample N = 3,966 | DZ twin sample N = 800 | MZ twin sample N = 548 | ||
|---|---|---|---|---|
| (M, SD) or % | (M, SD) or % | (M, SD) or % | P-value | |
| Positive affect* | 15.7 (2.8) | 15.6 (2.9) | 15.7 (2.8) | 0.67 |
| Life satisfaction* | 4.5 (0.8) | 4.4 (0.9) | 4.5 (0.8) | 0.84 |
| Number of medications | 2.6 (2.4) | 2.6 (2.5) | 2.6 (2.4) | 0.89 |
| Number of illnesses | 2.9 (2.5) | 2.9 (2.4) | 3.0 (2.3) | 0.66 |
| Cognitive composite* | 1.4 (3.5) | 1.2 (3.4) | 1.2 (3.3) | 0.41 |
| Age at entry (years) | 76.6 (4.9) | 76.4 (4.4) | 76.8 (4.6) | 0.19 |
| Female (%) | 59.4 | 64.3 | 64.5 | 0.01 |
| Mortality by January, 2010 (%) | 61.5 | 75.6 | 77.7 | 0.000 |
| Difference in death ages (years) | - | 4.3 (2.9) | 3.6 (3.0) | 0.006 |
| Follow-up time (years) | 8.9 (3.7) | 8.6 (3.9) | 8.8 (3.8) | 0.18 |
Indicates raw values. Significance tests for differences between samples included one-way analysis of variance for normally distributed variables, Kruskal-Wallis rank tests for variables with skewed distributions, and chi-square tests for categorical variables. Difference in death ages are not reported for the individual-level sample because most of the sample was comprised of single twins for whom data on the cotwin was not available. The independence assumption of significance tests was violated because both the MZ and DZ twin samples were included in the individual-level sample.
The complete set of HRs and confidence intervals (CI) are shown in Table 3. In the unpaired sample of 3,966 individuals 2,439 died during follow-up. As expected, at the individual level of analysis, controlling for age and sex, positive affect was associated with a reduction in mortality risk (HR = 0.82, 0.79 – 0.86; adjusted HR [AHR] 0.91, 0.86 – 0.95) as was life satisfaction (HR 0.80, 0.77 – 0.83; AHR 0.87, 0.83 – 0.91). In the unadjusted analyses a one SD increase in positive affect was associated with an 18% reduction in mortality risk and a one-SD increase in life satisfaction was associated with a 20% reduced mortality risk. Adjusting for number of illnesses, number of medications, and cognitive composite score attenuated the effects for positive affect and life satisfaction to 9% and 13% respectively (Table 3).
Table 3.
Hazard ratios for mortality risk associated with affect and life satisfaction
| Individual-level analyses, clustered variance estimators | Within-pair analyses, stratified by twin pair | |||||
|---|---|---|---|---|---|---|
| All twins | DZ twins | MZ twins | ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Affect | N = 3,966 | N = 800 | N = 548 | |||
| Affect | 0.82 (0.79, 0.86) | 0.000 | 0.71 (0.61, 0.83) | 0.000 | 0.76 (0.61, 0.94) | 0.010 |
| Age at intake | 1.12 (1.11, 1.13) | 0.000 | - | - | - | - |
| Sex (Female) | 0.60 (0.55, 0.66) | 0.000 | - | - | - | - |
| Affect adjusted | N = 3,714 | N = 745 | N = 510 | |||
| Affect | 0.91 (0.86, 0.95) | 0.000 | 0.80 (0.67, 0.97) | 0.020 | 0.79 (0.63, 0.99) | 0.040 |
| Age at intake | 1.11 (1.10, 1.12) | 0.000 | - | - | - | - |
| Sex (Female) | 0.57 (0.52, 0.63) | 0.000 | - | - | - | - |
| Number of medications | 1.08 (1.05, 1.10) | 0.000 | 1.02 (0.94, 1.12) | 0.611 | 1.01 (0.91, 1.13) | 0.807 |
| Number of illnesses | 1.04 (1.02, 1.07) | 0.000 | 1.09 (1.00, 1.20) | 0.057 | 1.04 (0.94, 1.15) | 0.478 |
| Cognitive composite | 0.80 (0.76, 0.83) | 0.000 | 0.64 (0.52, 0.79) | 0.000 | 0.78 (0.60, 1.02) | 0.074 |
| Life satisfaction | N = 3,966 | N = 800 | N = 548 | |||
| Life satisfaction | 0.80 (0.77, 0.83) | 0.000 | 0.68 (0.58, 0.80) | 0.000 | 0.78 (0.65, 0.94) | 0.009 |
| Age at intake | 1.12 (1.11, 1.13) | 0.000 | - | - | - | - |
| Sex (Female) | 0.62 (0.57, 0.67) | 0.000 | - | - | - | - |
| Life satisfaction adjusted | N = 3,714 | N = 745 | N = 510 | |||
| Life satisfaction | 0.87 (0.83, 0.91) | 0.000 | 0.77 (0.64, 0.92) | 0.005 | 0.80 (0.65, 0.98) | 0.034 |
| Age at intake | 1.11 (1.10, 1.12) | 0.000 | - | - | - | - |
| Sex (Female) | 0.58 (0.53, 0.63) | 0.000 | - | - | - | - |
| Number of medications | 1.07 (1.05, 1.10) | 0.000 | 1.02 (0.93, 1.11) | 0.638 | 1.01 (0.90, 1.13) | 0.884 |
| Number of illnesses | 1.04 (1.02, 1.07) | 0.000 | 1.09 (1.00, 1.20) | 0.056 | 1.04 (0.93, 1.15) | 0.503 |
| Cognitive composite | 0.80 (0.76, 0.83) | 0.000 | 0.64 (0.51, 0.79) | 0.000 | 0.79 (0.60, 1.04) | 0.093 |
Sample sizes differ for univariate and multivariate models due to missing covariate data. The effects of age and sex are controlled by design in within-pair analyses.
Results of within-pair DZ and MZ analyses were similar to the individual-level unadjusted and adjusted estimates for affect and life satisfaction. In fact, the point estimates were consistently stronger in the within-pair analyses than in the unpaired analyses. In the DZ sample positive affect and life satisfaction were associated with reduced mortality risk (HR 0.71, 0.61 – 0.83) and (HR 0.68, 0.58 – 0.80) respectively. Unadjusted within-pair MZ results were similar for affect (HR 0.76, 0.61 – 0.94) and life satisfaction (HR 0.78, 0.65 – 0.94). The adjusted estimates were attenuated, however the exposure-outcome associations remained significant (Table 3).
The proportional hazards assumption was met for affect and life satisfaction with one exception in the individual-level analyses. A significant interaction with log-follow-up time in the unadjusted model suggested that the effect of positive affect on mortality decreased as a function of time (p = 0.01) although this trend was not present in the adjusted model.
Discussion
The main finding of this study is that the association between SWB and longevity is independent of familial factors such as shared genes and common environment, consistent with a causal link between SWB and longer lifespan. To our knowledge this is the first study to test the association between SWB and longevity for genetic confounding.
We used a multistep method of analysis with a sample of 3,966 elderly Danish twins in order to separate associations among all twins (treated as individuals) from within-pair associations (Dwyer & Blizzard, 2005). An unpaired analysis was conducted to determine if an association between SWB and longevity existed when the twins were treated as individual participants. We found significant associations at the individual-level of analysis, consistent with many previous findings indicating that SWB is associated with reduced risk of all-cause mortality (Chida & Steptoe, 2008; Schoevers et al., 2000; Schulz et al., 2000). Intra-pair differences in exposure and outcome were consistent with the heritability of both factors. MZ twins showed significantly smaller intrapair differences in affect and age at death than DZ pairs. Intrapair life satisfaction did not differ by zygosity, possibly due to the restricted range of possible scores on this single-item measure.
Within-pair analyses in the MZ sample showed that SWB was associated with increased longevity in genetically identical individuals, indicating that the association is not explained by familial factors. The association within DZ pairs was similar, indicating that genetic selection does not even partly explain the association between SWB and longevity. Our findings are consistent with the alternative explanation, that distinct genetic pathways underlie SWB and longevity. While our findings do not establish causality they are consistent with a causal effect of SWB on increased longevity.
Measures of affect and life satisfaction performed similarly in this study, as would be expected because of their strong correlation and previous findings on affect (Koopmans et al., 2010; Pitkala et al., 2004) and life satisfaction (Chida & Steptoe, 2008; Collins et al., 2009). A single-item index of life satisfaction may be a crude index of the experience of SWB. However, despite the simplicity of the measure, life satisfaction was strongly associated with longevity. Single-item measures of quality of life (Zimmerman et al., 2006), life satisfaction (Schimmack & Oishi, 2005), and self-esteem (Robins et al., 2001) have shown acceptable reliability and validity.
Although the association between SWB and longevity is not due to shared genes and environment, the relation between SWB and longevity may be confounded or mediated by other covariates. The relationship between SWB and longevity may be physiological. For example, psychological well-being may directly enhance the functioning of neuroendocrine, immune, and cardiovascular systems (Martin et al., 2007; Ryff et al., 2004; Steptoe et al., 2005) or indirectly by buffering the effects of stress (Pressman & Cohen, 2005).
The association between SWB and longevity may be mediated behaviorally. Individuals higher in well-being may be more socially active and successful at establishing and maintaining social support. Social activity then would confound the association because it may be causal of health and longevity and correlated with SWB (McGue & Christensen, 2007). Individuals reporting more positive affect may be more likely to engage in physical activity and exercise (Carlsson et al., 2007; Stubbe et al., 2007) and avoid smoking and excessive alcohol consumption (Dear et al., 2002; Kujala et al., 2002), which may affect subsequent morbidity and life expectancy. It may be that happier individuals tend to be those with secure access to healthcare and greater education (Rowe & Kahn, 1999; Vaillant & Mukamal, 2001). Both measured and unmeasured (nonshared experience) covariates may underlie the exposure of SWB. Further study should be directed toward determining the “active ingredients” of SWB.
Several limitations must be noted. Our outcome was all-cause mortality. We expect to see a fairly low rate of accidental death in elderly patients, however exclusion of adventitious deaths would be desirable. To be informative, only twin pairs in which one or both twins died could be included in the twin-pair samples, reducing sample size by 29%. Analysis of a larger sample might yield more precise point estimates of associations between psychological well-being and longevity in within-pair analyses and would permit additional analyses by sex.
Our findings may not extend to other countries and cultures. Twin cohorts are similar to their background population with respect to health, even when considering cause of death (Christensen et al., 1995, 2001). The LSADT sample is relatively homogeneous with respect to ethnicity, socioeconomic status, and access to health care, and levels of life satisfaction in Denmark are the highest in the European Union (Christensen et al., 2006).
The strengths of this study include the use of national register-based data to minimize selection bias. The longitudinal design and use of an 18-month lag between exposure and outcome help to rule out reverse causation.
This study should be replicated in different samples using different measures. However the findings provide encouraging evidence for those in the applied areas of psychology, psychiatry, and public health, working to promote SWB. An important focus for further research concerns the extent to which positive psychological well-being can be changed among those who do not posses the “genetic talent for happiness” (Lykken, 1999, p. 246). The efficacy of such interventions remains to be shown, however the present evidence supports further investigation of exposures, not related to familial factors, that may promote and maintain SWB into old age.
Acknowledgments
This work was supported by a grant from the U.S. National Institute on Aging (PO1-AG08761). The Danish National Aging Research Center is supported by a grant from the VELUX Foundation.
References
- Bartels M, Boomsma DI. Born to be happy? The etiology of SWB. Behavior Genetics. 2009;39:605–615. doi: 10.1007/s10519-009-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S, Andersson T, Lichtenstein P, Michaelsson K, Ahlbom A. Physical activity and mortality: Is the association explained by genetic selection? American Journal of Epidemiology. 2007;166(3):255–259. doi: 10.1093/aje/kwm132. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosomatic medicine. 2008;70:741–756. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- Christensen K, Doblehammer G, Rau R, Vaupel JW. Aging populations: The challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Herskind AM, Vaupel JW. Why Danes are smug: comparative study of life satisfaction in the European Union. British Medical Journal. 2006;333:1289–11291. doi: 10.1136/bmj.39028.665602.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Holm N, McGue M, Corder L, Vaupel JW. A Danish population-based twin study on self-rated health in the elderly. Journal of Aging and Health. 1999;11:49–64. doi: 10.1177/089826439901100103. [DOI] [PubMed] [Google Scholar]
- Christensen K, Vaupel JW, Holm NV, Yashin AI. Mortality among twins after age 6: Fetal origins hypothesis versus twin method. British Medical Journal. 1995;310(6977):432–436. doi: 10.1136/bmj.310.6977.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Vaupel JW. Determinants of longevity: genetic, environmental and medical factors. Journal of Internal Medicine. 1996;240:333–341. doi: 10.1046/j.1365-2796.1996.d01-2853.x. [DOI] [PubMed] [Google Scholar]
- Christensen K, Wienke A, Skytthe A, Holm NV, Vaupel JW, Yashin AI. Cardiovascular mortality in twins and the fetal origins hypothesis. Twin Research. 2001;4(5):344–349. doi: 10.1375/1369052012506. [DOI] [PubMed] [Google Scholar]
- Collins AL, Glei DA, Goldman N. The role of life satisfaction and depressive symptoms in all-cause mortality. Psychology and Aging. 2009;24(3):696–702. doi: 10.1037/a0016777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dear K, Henderson S, Korten A. Wellbeing in Australia–findings from the national survey of mental health and well-being. Social Psychiatry and Psychiatric Epidemiology. 2002;37:503–509. doi: 10.1007/s00127-002-0590-3. [DOI] [PubMed] [Google Scholar]
- Diener E. SWB. The science of happiness and a proposal for a national index. American Psychologist. 2000;55(1):34–43. [PubMed] [Google Scholar]
- Diener E, Seligman M. Beyond money: Toward an economy of well-being. Psychological Science in the Public Interest. 2004;5(1):1–31. doi: 10.1111/j.0963-7214.2004.00501001.x. [DOI] [PubMed] [Google Scholar]
- Dwyer T, Blizzard L. A discussion of some statistical methods for separating within- pair associations from associations among all twins in research on fetal origins of disease. Paediatric and Perinatal Epidemiology. 2005;19(Supp 1):48–53. doi: 10.1111/j.1365-3016.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- Hauge M. The Danish Twin Register. In: Mednich SA, Baert AE, Bachmann BP, editors. Prospective longitudinal research. Oxford, UK: Oxford Medical Publications; 1981. pp. 217–222. [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sorensen TIA, Harvald B, Vaupel JW. The heritability of human longevity: A population-based study of 2872 Danish twin pairs born 1870–1900. Behavior Genetics. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- Hjelmborg JB, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, Kaprio J, Pedersen NL, Christensen K. Genetic influence on human lifespan and longevity. Human Genetics. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- Holt JD, Prentice RL. Survival analyses in twin studies and matched pair experiments. Biometrica. 1974;66:17–30. [Google Scholar]
- Koivumaa H, Honkanen R, Viinamaki K, Kaprio J, Koskenvuo M. Self-reported life satisfaction and 20 year mortality in health Finnish adults. American Journal of Epidemiology. 2000;152:983–991. doi: 10.1093/aje/152.10.983. [DOI] [PubMed] [Google Scholar]
- Koopmans TA, Geleijnse JM, Zitman FG, Giltay EJ. Effects of happiness on all-cause mortality during 15 years of follow-up: The Arnhem Elderly Study. Journal of Happiness Studies. 2010;11:113–124. [Google Scholar]
- Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: The roles of genetics and childhood environment. American Journal of Epidemiology. 2002;156:985–993. doi: 10.1093/aje/kwf151. [DOI] [PubMed] [Google Scholar]
- Kyvik KO, Christensen K, Skytthe A, Harvald B, Holm NV. The Danish twin registry. Danish Medical Bulletin. 1996;43:467–470. [PubMed] [Google Scholar]
- Lucas RE, Diener E, Suh E. Discriminant validity of well-being measures. Journal of Personality and Social Psychology. 1996;17(3):616–628. doi: 10.1037//0022-3514.71.3.616. [DOI] [PubMed] [Google Scholar]
- Lykken D. Happiness. New York: St. Martin’s Griffin; 1999. [Google Scholar]
- Lykken D, Tellegen A. Happiness is a stochastic phenomenon. Psychological Science. 1996;7:186–189. [Google Scholar]
- Lyubomirsky S. The how of happiness: A scientific approach to getting the life you want. London: The Pengu in Press; 2007. [Google Scholar]
- Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: Does happiness lead to success? Psychological Bulletin. 2005;131(6):803–855. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]
- Martin GM, Bergman AV, Barzilai N. Genetic determinants and human health and life-span: Progress and new opportunities. PLoS Genetics. 2007;3(7):1121–1130. doi: 10.1371/journal.pgen.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Bacon S, Lykken DT. Personality stability and change in early adulthood: a behavioral genetic analysis. Developmental Psychology. 1993;29:96–109. [Google Scholar]
- McGue M, Christensen K. Genetic and environmental contributions to depression symptomatology: Evidence from Danish twins 75 years of age and older. Journal of Abnormal Psychology. 1997;106(3):439–448. doi: 10.1037//0021-843x.106.3.439. [DOI] [PubMed] [Google Scholar]
- McGue M, Christensen K. The heritability of cognitive functioning in very old adults: Evidence from Danish twins aged 75 years and older. Psychology and Aging. 2001;16:272–280. doi: 10.1037//0882-7974.16.2.272. [DOI] [PubMed] [Google Scholar]
- McGue M, Christensen K. The heritability of depression symptoms in elderly Danish twins: occasion-specific versus general effects. Behavior Genetics. 2003;33(2):83–93. doi: 10.1023/a:1022545600034. [DOI] [PubMed] [Google Scholar]
- McGue M, Christensen K. Social activity of healthy aging: A study of aging Danish twins. Twin Research and Human Genetics. 2007;10(2):255–265. doi: 10.1375/twin.10.2.255. [DOI] [PubMed] [Google Scholar]
- McGue M, Osler M, Christensen K. Causal inference and observational research: The utility of twins. Perspectives on Psychological Science. 2010;5(5):546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosing MA, Pedersen NL, Martin NG, Wright MJ. Sex differences in the genetic architecture of optimism and health and their interrelation: A study of Australian and Swedish twins. Twin Research and Human Genetics. 2010;13(4):322–329. doi: 10.1375/twin.13.4.322. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Zietsch BP, Shekar SN, Wright MJ, Martin NG. Genetic and environmental influences on optimism and its relationship to mental and self-rated health: A study of aging twins. Behavior Genetics. 2009;39:597–604. doi: 10.1007/s10519-009-9287-7. [DOI] [PubMed] [Google Scholar]
- Ness RB, Roysamb E, Tambs K, Harris JR, Reichborn-Kjennerud T. SWB: genetic and environmental contributions to stability and change. Psychological Medicine. 2006;6(7):1033–1042. doi: 10.1017/S0033291706007409. [DOI] [PubMed] [Google Scholar]
- Oldenhinkel AJ, Boubuys AL, Brilman EI, Ormel J. Functional disability and neuroticism as predictors of late-life depression. American Journal of Geriatric Psychiatry. 2001;9:241–248. [PubMed] [Google Scholar]
- Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Danish Medical Bulletin. 2006;53:441–449. [PubMed] [Google Scholar]
- Pitkala KH, Laakkonen ML, Strandberg TE, Tilvis RS. Positive life orientation as a predictor of 10-year outcome in an aged population. Journal of Clinical Epidemiology. 2004;57:409–414. doi: 10.1016/j.jclinepi.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131(6):925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Robins RW, Hendin HM, Trzesniewski KH. Measuring global self-esteem: Construct validation of a single-item measure and the Rosenberg Self-Esteem Scale. Personality and Social Psychology Bulletin. 2001;27:151–161. [Google Scholar]
- Roth M, Tym E, Mountjoy CQ, Huppert FA, Verma S, Goddard R. CAMDEX: A standardized instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. The British Journal of Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Successful Aging. New York: Dell; 1999. [Google Scholar]
- Røysamb E, Harris JR, Magnus P, Vitterso J, Tambs K. SWB. Sex-specific effects of genetic and environmental factors. Personality and Individual Differences. 2002;32:211–223. [Google Scholar]
- Ryff CD, Singer BH, Dienberg Love G. Positive health: Connecting well-being with biology. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences. 2004;359(1449):1383–1394. doi: 10.1098/rstb.2004.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmack U, Oishi S. The influence of chronically and temporarily accessible information on life satisfaction judgments. Journal of Personality and Social Psychology. 2005;89:395–406. doi: 10.1037/0022-3514.89.3.395. [DOI] [PubMed] [Google Scholar]
- Schoevers RA, Beekman ATF, Deeg DJH, Geerlings MI, Jonker C, Van Tilburg W. Risk factors for depression in later life; results of a prospective community based study (AMSTEL) Journal of Affective Disorders. 2000;59:127–137. doi: 10.1016/s0165-0327(99)00124-x. [DOI] [PubMed] [Google Scholar]
- Schulz R, Martire LM, Beach SR, Scheier MF. Depression and mortality in the elderly. Current Directions in Psychological Science. 2000;9(6):204–208. [Google Scholar]
- Steel P, Schmidt J, Shulz J. Refining the relationship between personality and SWB. Psychological Bulletin. 2008;134(1):138–161. doi: 10.1037/0033-2909.134.1.138. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processed. Proceedings of the National Academy of Sciences. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe JH, de Moor MHM, Boomsma DI, de Geus EJC. The association between exercise participation and well-being: a cotwin study. Preventive Medicine. 2007;44:148–152. doi: 10.1016/j.ypmed.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Stubbe JH, Posthuma D, Boomsma DI, De Geus EJC. Heritability of life satisfaction in adults: a twin-family study. Psychological Medicine. 2005;35:1581–1588. doi: 10.1017/S0033291705005374. [DOI] [PubMed] [Google Scholar]
- Takkinen S, Gold C, Pedersen NL, Malmberg B, Nilsson S, Rovine M. Gender differences in depression: A study of older unlike-sex twins. Aging & Mental Health. 2004;8(3):187–195. doi: 10.1080/13607860410001669714. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Lykken DT, Bouchard TJ, Wilcox KJ, Rich S, Segal NL. Personality similarity in twins reared apart and together. Journal of Personality and Social Psychology. 1988;54:1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- Vaillant GE, Mukamal K. Successful aging. American Journal of Psychiatry. 2001;158:839–847. doi: 10.1176/appi.ajp.158.6.839. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, Iachine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW, et al. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Wood AM, Tarrier N. Positive clinical psychology: A new vision and strategy for integrated research and practice. Clinical Psychology Review. 2010;30:819–829. doi: 10.1016/j.cpr.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Ruggero CJ, Chelminski I, Young D, Posternak MA, Friedman M. Developing brief scales for use in clinical practice: The reliability and validity of single-item self-report measures of depression symptom severity, psychosocial impairment due to depression, and quality of life. Journal of Clinical Psychiatry. 2006;67:1536–1541. doi: 10.4088/jcp.v67n1007. [DOI] [PubMed] [Google Scholar]


