Abstract
Objective: The purpose of this study was to Investigate the effect of 810-nm low level laser therapy (LLLT) on dendritic cells (DC) in vitro. Background data: LLLT can enhance wound healing and increase cell proliferation and survival, and is used to treat inflammatory conditions. However there are reports that LLLT can stimulate leukocytes and could therefore be pro-inflammatory. Recently, DC have been found to play an important role in inflammation and immune response. Methods: Murine bone-marrow-derived DC were isolated, stimulated with lipopolysaccharide (LPS) or CpG oligodeoxynucleotide and treated with 810-nm laser, using fluences of 0.3, 3, and 30 J/cm2 delivered at irradiances of 1, 10, and 100 mW/cm2 respectively. Confocal microscopy, flow cytometry for DC markers, viability using propidium iodide, enzyme-linked immunosorbent assays (ELISA) for secreted interleukin-12 (IL-12), and bioluminescence measurements in cells transduced with a reporter for toll-like receptor (TLR)-9/nuclear factor kappa B (NF-κB) activation, were performed. Results: LLLT changed the morphology of LPS-stimulated DC, increased their viability, and altered the balance of DC activation markers (major histocompatibility complex [MHC] class 2 up and CD86 down). LLLT reduced IL-12 secretion from DC stimulated by either LPS or CpG. LLLT reduced NF-κB activation in reporter cells stimulated with CpG. There was no obvious light dose response observed. Conclusions: Taken together, these data suggest that 810-nm LLLT has an anti-inflammatory effect on activated DC, possibly mediated by cyclic adenosine monophosphate (cAMP) and reduced NF-κB signaling.
Introduction
Dendritic cells (DC) are known to be efficient stimulators of T and B-lymphocytes, and they play a crucial role as professional antigen-presenting cells (APC) in initiating and modulating the immune response. DC are sometimes described as the orchestrators of the immune response1. Langerhans cells were the first type of DC discovered in the skin, in 18682, but modern understanding of DC only started approximately 25 years ago.
A human has about 109 Langerhans cells located above the proliferating keratinocytes in the skin, and most of the DC remain in an immature state, characterized by a lack of migration mobility and their inability to stimulate T cells.3 Although they lack co-stimulatory molecules for T or B lymphocyte activation, including CD40, CD54, CD80, and CD86, immature DC are capable of capturing antigens and expressing them in the context of major histocompatibility complex (MHC) class 2. Only a few DC are necessary to provoke strong T-cell response. Accumulating evidence suggests that DC play an increasingly complex role in both the beneficial and the detrimental effects of inflammation and immunity. Many diseases caused by either overactive immune responses or sub-optimal immune responses are manifested by DC dysfunction. Among the many signals that DC respond to, toll-like receptor (TLR) ligands are perhaps the best known and most powerful.4 TLR ligands are members of a broad class of pattern-recognition molecules that warn the immune system of the presence of potentially dangerous microbial pathogens, and rapidly mobilize a cellular defense system.5 Nine principal TLR members6 are now known, each of which recognizes a different microbial molecular motif.6 Although TLR are expressed on many different cell types, DC are one of the most effective responders. In the present study, we used two TLR ligands: lipopolysaccharide (LPS) from the outer cell wall of Gram-negative bacteria recognized by TLR4,7 and CpG oligodeoxynucleotide containing a non-methylated CpG motif typical of bacterial DNA that is recognized by TLR9.8
Photobiomodulation (PBM) or low-level light therapy (LLLT) has been proven to have many significant effects in enhancing healing and preventing tissue death.9,10 Karu's laboratory, among others, has reported that mitochondria are a principal intracellular target of red and near-infrared light,11 and cytochrome c oxidase is proposed to be a photoreceptor that absorbs light as far into the infrared as 1000 nm.12 There have been reports of increased cytochrome c oxidase activity after low level light,13 and by stimulating the mitochondrial electron transport train, increased intracellular adenosine triphosphate (ATP) after light delivery to isolated mitochondria14 has been observed.
Many genes have been shown to have their transcription upregulated (or downregulated) after illumination of cells with various wavelengths and fluences of light. For example, illumination of human fibroblasts with a 628-nm light-emitting diode led to altered expression of 111 genes of 10 functional groups.15 Studies have shown that one mechanism of light-mediated gene regulation is related to the activation of the pleiotropic transcription factor nuclear factor kappa B (NF-κB),16 probably via generation of mitochondrial reactive oxygen species.17
NF-κB is a transcription factor regulating multiple gene expression, and has been shown to govern various cellular functions, including inflammatory and stress-induced responses and survival.18 NF-κB activation is governed by negative feedback by inhibitor of NF-κB (IκB), an inhibitor protein that binds to NF-κB, but can undergo ubiquitination and proteasomal degradation,19 thus freeing NF-κB to translocate to the nucleus and initiate transcription.20
In addition, NF-κB also plays an important role in activation of inflammation. Many studies have shown that red or near-infrared light can reduce inflammatory conditions such as arthritis21,22 or gingival inflammation.23 The goal of this study was to investigate the effect of LLLT mediated by an 810-nm laser on murine bone- marrow-derived DC.
Materials and Methods
Bone-marrow-derived DC culture
All animal procedures were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital and met the guidelines of the National Institutes of Health. Bone-marrow-derived DC were prepared from 5- to 7-week-old male C57BL/6 mice purchased from Jackson Laboratories (Bar Harbor, ME).
Femurs from mice were dissected, and muscle and tissue were removed. Cleaned bones were washed twice with Hanks Buffered Salt Solution, and placed into culture (murine DC) media composed of RPMI-1640 (Gibco-Invitrogen, Carlsbad, CA) with 1% penicillin–streptomycin (Cellgro-Mediatech, Manassas, VA), and 0.1% 2-mercaptoethanol (Invitrogen), with 10% heat inactivated fetal bovine serum (Invitrogen). Bones were cut and bone marrow was flushed with at least 5 mL of media. The bone marrow suspension was strained with a 70-mm cell strainer (Becton Dickinson, Franklin Lakes, NJ), cells were collected by centrifugation at 1500 rpm for 5 min, and erythrocytes were lysed with ammonium chloride buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). Bone marrow cell suspension was re-suspended at 1.5 x 105 /mL in murine DC media with 20 ng/ mL GM-CSF (Sigma, St Louis, MO). Cells were plated at 3 mL/well in six well plates (Corning, Corning, NY), and incubated at 37°C with 95% relative humidity and 5% CO2. On day 3, cells were fed by exchanging half of the media with fresh murine DC media. Purity and yield of CD3- and CD4-positive cells were measured by flow cytometry. In addition, cell death was quantified by propidium iodide staining and by flow cytometry. To assure the consistency of cell population, plates with >85% purity and <10% cell death were selected for experiments. On day 8, loosely adherent and nonadherent cells were collected and washed twice in phosphate-buffered saline (PBS), centrifuged and re-suspended at 2 ×105/mL in 60-mm cell-culture-treated dishes (Corning), and incubated at 37°C with 95% relative humidity and 5% CO2 overnight.
HEK 293 TLR9 NF-κB reporter cells
TLR9/NF-κB/SEAPorter™ cell line was obtained from Imgenex (San Diego, CA). HEK 293 cells were stably co-transduced with DNA coding for human toll-like receptor 9 (TLR9) and a NF-κB luciferase reporter gene to produce a cell line originally designed to perform high throughput screening for TLR agonists. The cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (Hyclone, Waltham, MA) and 1% penicillin–streptomycin in 37°C incubator. The luciferase assay system (Promega, Madison, WI) was used to measure bioluminescence according to manufacturers' instructions. Part of the cell lysate was measured in a plate luminometer (Wallac Tri-Lux beta, PerkinElmer Life and Analytical Sciences, Waltham, MA). Reading time was set to be 10 sec and each sample was read twice. The remainder of the cell lysate was used to measure cell protein (BCA assay, Pierce Biotechnology Inc., Rockford. IL), in order to normalize the relative light units to mg of protein.
LPS and CpG treatment
Lipopolysaccharide from Escherichia coli purified by gel chromatography (Sigma-Aldrich) was added to DC at a concentration of 1 or 10 μg/mL. Light was delivered at two time points, either immediately after LPS or 12 h after LPS addition. Two different types of CpG oligodeoxynucleotide were employed. CpG 1826 has the sequence (5′-TCCATGACGTTCCTGACGTT-3′) and is recognized by the murine TLR9.24 Because the TLR9 transduced in the HEK293 cells was of human origin, we used CpG ODN2006 (5′- TCGTCGTTTTGTCGTTTTGTCGTT-3′).25 Both the CpG ODN 2006 and 1826 were purchased from Invivogen (San Diego, CA) and were used at concentrations of 10 μg/mL. Incubation times for both LPS and CpG were 24 h except for the HEK 293 TLR9 NF-κB reporter cells, where four time points were used: 0, 8, 16, and 24 h.
Low level light irradiation
An 810-nm laser (HOYA Conbio, Fremont, CA) was used as the light source, and the illumination time was set at 5 min. The laser had an adjustable total power output with a maximum of 5W. The spot size was adjusted via a Fresnel lens to cover a 6-cm dish (28 cm2). The power output was measured using a Lasermate power meter (Coherent, Inc., Santa Clara, CA) and the homogeneity of the spot was checked by moving the power meter detector over the area of the spot. Fluences of 30, 3, and 0.3 J/cm2 were delivered at irradiances of 100, 10, and 1 mW/cm2, respectively, to individual 6-cm dishes. A wide range of fluences was chosen because previous studies have suggested that many features of LLLT effects on cells exhibit a biphasic dose response, in other words, fluences that are too low or too high may both have a reduced effect.26 The irradiance was varied in order to keep the illumination time constant. Light- treated dishes were incubated in the incubator for 24 h before analysis.
IL12 measurement
Quantitative enzyme-linked immunosorbent assays (ELISA) was performed with a kit (Cat No. 88-7120, eBioscience Inc., San Diego, CA) that measures mouse interleukin-12 (IL-12) (total p40) in cell culture supernatants according to the manufacturers' instructions.
Cell morphology and quantification of protein expressions
Twenty-four hours' incubation after light treatment, the cell morphology was monitored by using confocal microscopy. Cells were stained with anti-murine fluorescent antibodies (Invitrogen) against MHC class 2 (IA-b, FITC-labeled, MM3401) CD80 (B7-1, R-PE labeled, MR6504) and CD11c (APC-labeled, MCD11c05) 15 min before microscopy. In the confocal fluorescence micrographs, the fluorescence from these antibodies was false-colored red, green, and blue, respectively, to make superimposition more visible. The same antibodies were used to quantify cell-membrane protein expression levels in live cells by flow cytometry. Propidium iodide was added to distinguish live cells from dead cells.
Statistical analysis
All assays were performed in duplicate, and each sample was read twice and averaged. Excel software was used to perform Single-Factor ANOVA to evaluate the statistical significance of experimental results (p < 0.05).
Results
LLLT affects DC morphology and marker expression by confocal microscopy
Lipopolysaccharide is a potent stimulator for DC activation and maturation. No other cells exhibit the shape and motility typical of mature DC. With proper stimulation, DC display fine long dendrites (>10 μm) and the size of the cell body also enlarges from the immature stage. A fully mature DC also displays MHCII, CD86 and CD11c on its membrane. This mature state allows it to capture antigens and stimulate antigen-specific T cells. The level of CD11c does not change with levels of maturation and/or activation of DC, therefore allowing this marker to be used as an invariant fluorescent marker of DC purity.
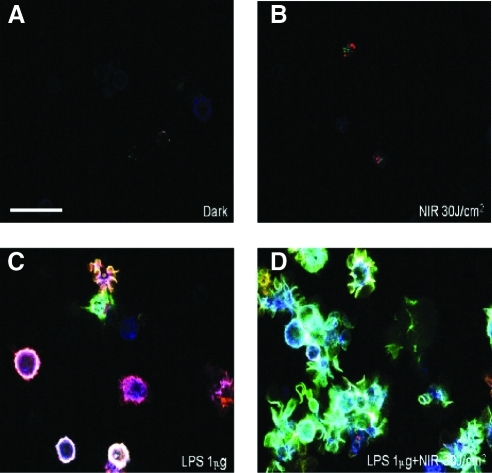
Many studies have shown that low-level light has diminishing positive or even negative effects at higher fluence, such as 30 J/cm2, because of its biphasic dose response.26 This high fluence of “low-level” light together with more usual fluences such as 3 and 0.3 J/cm2 were selected to test if bone-marrow-derived DC responded to the 810-nm continuous wave laser. In the present study, we did not observe any significant dose response effects. After 24 h incubation after light irradiation, which allows DC to process activation and maturation, confocal microscopy imaging showed that the laser alone did not change either of the membrane marker's expression compared with the untreated DC (Fig. 1A and B). However, if laser was delivered to the DC continuously incubated with lipopolysaccharide, the surface MHCII was downregulated, whereas CD86 was upregulated (Fig. 1C and D). Furthermore, the dendrites of DC with LPS plus light were more developed than those in the LPS-only group.
FIG. 1.
Confocal microscopy. Twenty-four hours after specified treatments (the combination light immediately followed addition of LPS) DC membrane protein expression was analyzed by antibody staining. Blue, CD11c; red, MHCII; and green, CD86. (A) control; (B) 30 J/cm2 810-nm laser; (C) LPS (1μg/mL); (D) combination of LPS (1μg/mL) plus 30 J/cm2 810-nm laser. Laser treatment of LPS treated DC downregulated surface MHCII whereas CD86 was upregulated, and the dendrites were more developed than those in the LPS- only group. LPS, lipopolysaccharide; DC, dendritic cells.
Laser affects DC membrane marker expression and viability by FACS analysis
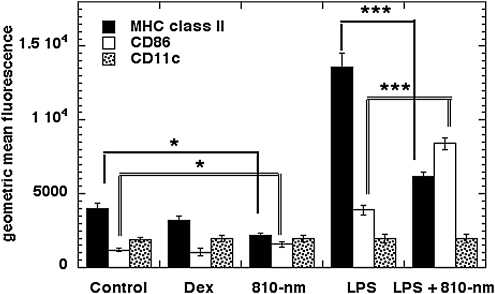
Confocal microscopy imaging indicated that 24 h after irradiation was a suitable time point at which to distinguish changes of membrane marker expression. At the same time point, fluorescence-activated cell sorting (FACS) analysis on the same three markers was quantified as shown in Fig. 2 (BD FACSAria Cell-Sorting System, BD Biosciences, San Jose, CA). Dexamethasone is a potent synthetic compound of the glucocorticoid class of steroid hormones, and acts as an anti-inflammatory and immunosuppressive agent. FACS showed that dexamethasone downregulated MHCII without significantly affecting expression levels of CD81 and CD11c. On the other hand, LPS upregulated both MHCII and CD86 after 24 h. Results with 810-nm laser confirmed the confocal microscopy imaging data that laser alone did not significantly change MHCII or CD86, but in the presence of LPS, 810-nm laser reduced levels of expression of membrane MHCII but increased expression of CD86.
FIG. 2.
FACS analysis of DC membrane markers. Twenty-four hours after specified treatments (the combination light immediately followed addition of LPS), 10,000 DC were stained with antibodies against CD11c, MHCII, and CD86. Laser treatment of LPS-treated DC significantly downregulated surface MHCII whereas CD86 was upregulated. There was a similar significant change but at much smaller levels in non-LPS-treated DC. LPS, lipopolysaccharide; DC, dendritic cells; MHC, major histocompatibility complex; dex, dexamethasone.
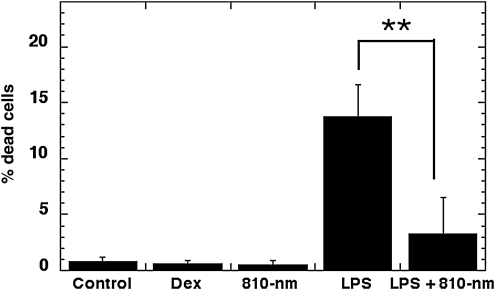
LPS treatment of DC caused a small but significant amount of cell death. Figure 3 shows that incubation with 0.1 ug/mL LPS led to 13 + /- 1.2% cell death after 24 h as judged by propidium iodide staining. Delivery of 3 J/cm2 of 810-nm laser immediately after LPS addition significantly reduced this cell death to 3 + /- 1.3% (p < 0.01). The reduction of LPS-induced cell death was less when the laser was delivered 12 h after addition of LPS (data not shown).
FIG. 3.
FACS determination of cell death. Twenty-four hours after specified treatments (the combination light immediately followed addition of LPS), 10,000 DC were stained with propidium iodide. Laser treatment significantly reduced the number of dead DC in the LPS-treated group. LPS, lipopolysaccharide; DC, dendritic cells; dex, dexamethasone.
Laser reduces secretion of IL12 in DC stimulated by TLR ligands
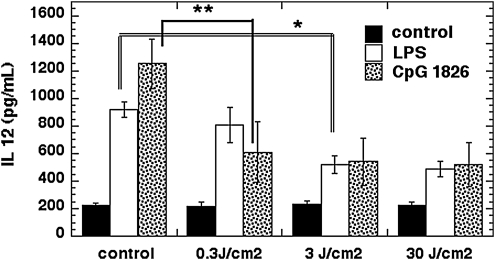
IL-12 is a cytokine secreted primarily by DC in response to pro-inflammatory stimuli such as TLR ligation.27 Its role is to increase inflammation and to stimulate T-cell responses to particular antigenic threats.28 As can be seen in Fig. 4, the baseline IL-12 secretion level from DC was increased fivefold by CpG and increased fourfold by LPS. The CpG-induced IL-12 level was reduced by >50% after 0.3 J/cm2 810-nm laser, and only a little bit more by fluences of 3 and 30 J/cm2. The light response of the IL-12 induced by LPS was broadly similar to that of the IL-12 induced by CpG, but required a higher light dose (3 J/cm2) to achieve a major reduction. Laser had no effect on the background unstimulated level of IL-12 at any fluence.
FIG. 4.
ELISA measurement of IL-12 secretion. Twenty-four hours after specified treatments (the combination light immediately followed addition of LPS), IL-12 in the DC medium was measured by ELISA kit. Laser treatment significantly reduced IL-12 secretion in DC simulated with either LPS or CpG. ELISA, enzyme-linked immunosorbent assays LPS, lipopolysaccharide; IL-12, interleukin-12 ;DC, dendritic cells.
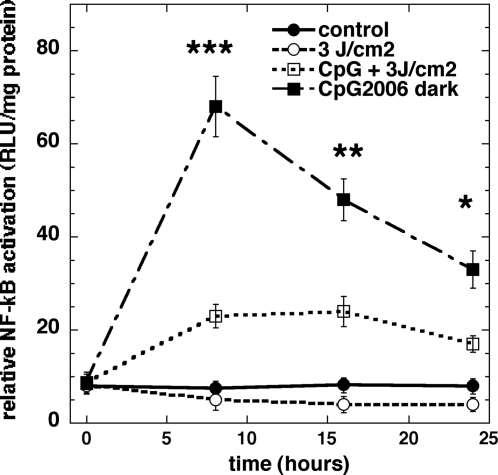
Laser reduces NF-kB activation stimulated by CpG in TLR9/NF-κB/SEAPorter™ cells
The TLR9/NF-kB/SEAPorter cell line was created to allow high throughput screening of TLR9 agonists and antagonists, but it also allowed us to examine the effect of 810-nm laser on TLR9 mediated signaling. Figure 5 shows that there was no significant reduction in NF-κB activation in the unstimulated HEK293 cells at all three time points after 3 J/cm2 of 810-nm laser. In the cells stimulated with CpG, there was a dramatic sixfold increase in NF-κB activation at 8 h that reduced to fourfold at the 16 h time point, and reduced even more to threefold at 24 h. When these cells were treated with 3 J/cm2 810-nm laser, the NF-κB activation caused by CpG was reduced by two thirds at 8 h (p < 0.01) and by half at later time points (p < 0.05).
FIG. 5.
NF-κB activation in TLR9/NF-κB/SEAPorter™ cells. At specified time points after addition of CpG and/or illumination, cells were lysed and NF-κB activity was measured by bioluminescence assay. Laser treatment significantly reduced NF-κB activation in cells stimulated with CpG at all time points. NF-κB, nuclear factor kappa B.
Discussion
This study has demonstrated for the first time that DC respond to 810-nm NIR laser with significant morphology changes and modulation of membrane marker expression levels. LLLT prevented DC cell death caused by LPS stimulation as in other reports of its anti-apoptotic and pro-survival effects in various cell types. LLLT significantly reduced secretion of the pro-inflammatory cytokine, IL-2 in TLR stimulated DC. Furthermore, our data taken together confirm previous reports and clinical trials from other laboratories that NIR laser indeed has effects on reducing inflammation.29–32
There is evidence that light acts on cells via the NF-κB pathway among others,16,33 and many reports have demonstrated that light could have different impacts on different cells and tissues, specifically as regards inflammation. Various studies have reported that the immediate effects of light can be regarded in some cases as pro-inflammatory34 and immunostimulatory,35 and in other cases as anti-inflammatory36 and immunosuppressive. The fact that NIR laser alone in our study did not significantly change immature DC but showed significant changes after the addition of LPS or CpG had induced DC maturation, might imply that light acting on DC requires TLR signaling.
We have previously shown that in murine embryonic fibroblasts (MEF), NIR laser activates NF-κB via generation of mitochondrial reactive oxygen species (ROS).16 This effect occurred in the absence of any TLR stimulation. One difference between our previous study and the present report that could explain the apparently conflicting results, is ROS generation. We showed in the MEF study that addition of antioxidants such as ascorbic acid and N-acetylcysteine abrogated both the ROS and NF-κB activation caused by NIR laser. DC are primary cells and the DC medium contains 0.1% β-mercaptoethanol (BME) as recommended for primary cell isolates (see Materials and Methods). This BME may have been sufficient to quench any ROS produced in the mitochondria of DC. In fact we did not see ROS production after 810-nm laser of DC using dichlorodihydrofluorescein diacetate as a fluorescent ROS indicator as previously described (data not shown). Therefore it may be the case that LLLT has the potential to be pro-inflammatory in the absence of antioxidants, but acts as an anti-inflammatory stimulus in the presence of sufficient antioxidants.
The reduction in levels of IL-12 by 810-nm laser in DC that have been stimulated with either LPS (TLR4 agonist) or by CpG (TLR9 agonist) is consistent with the anti-inflammatory effect of LLLT. A clue to the possible mechanism of this effect is provided by a report37 that compounds that elevate intracellular levels of cyclic adenosine monophosphate (cAMP) can also reduce secretion of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6 and, especially, IL-12. This phenomenon was thought to be because of blockage of phosphorylation of p38 mitogen-activated protein kinase (MAPK) by increased cAMP levels, but the NF-κB binding to the IL-12 promoter was not affected. It should be noted that there are several papers38–40 from multiple different laboratories that show that cellular levels of cAMP are increased by in vitro LLLT.
It has been recently discovered that there are (at least) two NF-κB activation pathways.41 The canonical NF-κB-signaling pathway activated in response to infections (TLR signaling) and cytokines is based on degradation of IkB inhibitors. This pathway depends upon the IkB kinase (IKK), which contains two catalytic subunits, IKKα and IKKβ. IKKβ is essential for inducible IkB phosphorylation and degradation, whereas IKKα is not. IKKα is involved in processing of the NF-kB2 (p100) precursor. IKKα preferentially phosphorylates NF-κB2, and this activity requires its phosphorylation by upstream kinases, one of which may be NF-κB-inducing kinase (NIK). IKKα is therefore a pivotal component of a second NF-κB activation pathway based on regulated NF-κB2 processing, rather than IkB degradation.
Whereas light irradiation took place immediately after addition of LPS, this was an early time point in the process of maturation of DC. Photo-induced signaling could be interfering with signaling from the TLR, and this interference could determine the relative gene expression levels of different DC markers such as MHCII and CD86. It is at present unclear why the levels of MHCII and CD86 moved in different directions after laser treatment. Both these markers are considered to be upregulated during the process of DC maturation and activation. However, it is likely that the overall result of unbalancing the coordinated expression levels of MHCII and CD86 would lead to less effective DC function, in other words to lower immune response levels to various stimuli. This may in fact be a partial explanation of the anti-inflammatory effect that is known to be one of the many benefits of LLLT. Reduced DC activation would tend to lessen the degree of inflammation produced by both the innate and adaptive arms of the immune system in response to diverse insults and injuries.
Conclusion
Taken together, the present data suggest that 810-nm LLLT has an anti-inflammatory effect on activated DC, possibly mediated by cAMP and reduced NF-κB signaling. Further work is needed to fully explore the determinants and mechanistic pathways of LLLT on DC in various states of maturation and activation.
Acknowledgments
This work was supported by NIH grant R01AI050875, Center for Integration of Medicine and Innovative Technology (DAMD17-02-2-0006), CDMRP Program in TBI (W81XWH-09-1-0514), and Air Force Office of Scientific Research (FA9950-04-1-0079).
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Buckwalter M.R. Albert M.L. Orchestration of the immune response by dendritic cells. Curr. Biol. 2009;19:R355–361. doi: 10.1016/j.cub.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Tamaki K. Stingl G. Katz S.I. The origin of Langerhans cells. J. Invest. Dermatol. 1980;74:309–311. doi: 10.1111/1523-1747.ep12543533. [DOI] [PubMed] [Google Scholar]
- 3.Satthaporn S. Eremin O. Dendritic cells (I): Biological functions. J. R. Coll. Surg. Edinb. 2001;46:9–19. [PubMed] [Google Scholar]
- 4.Pearce E.J. Kane C.M. Sun J. Regulation of dendritic cell function by pathogen-derived molecules plays a key role in dictating the outcome of the adaptive immune response. Chem. Immunol. Allergy. 2006;90:82–90. doi: 10.1159/000088882. [DOI] [PubMed] [Google Scholar]
- 5.Parker L.C. Prince L.R. Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin. Exp. Immunol. 2007;147:199–207. doi: 10.1111/j.1365-2249.2006.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler B. Rehli M. Evolution of the TIR, tolls and TLRs: functional inferences from computational biology. Curr. Top. Microbiol Immunol. 2002;270:1–21. doi: 10.1007/978-3-642-59430-4_1. [DOI] [PubMed] [Google Scholar]
- 7.Divanovic S. Trompette A. Petiniot L.K., et al. Regulation of TLR4 signaling and the host interface with pathogens and danger: the role of RP105. J. Leukoc. Biol. 2007;82:265–271. doi: 10.1189/jlb.0107021. [DOI] [PubMed] [Google Scholar]
- 8.Dalpke A. Zimmermann S. Heeg K. Immunopharmacology of CpG DNA. Biol. Chem. 2002;383:1491–1500. doi: 10.1515/BC.2002.171. [DOI] [PubMed] [Google Scholar]
- 9.Tunér J. Hode L. Laser therapy, clinical practice and scientific background. Grängesberg: Prima Books; 2002. [Google Scholar]
- 10.Karu T.I. The science of low power laser therapy. London: Gordon and Breach Scientific Publications; 1998. [Google Scholar]
- 11.Karu T. Photobiology of low-power laser effects. Health Phys. 1989;56:691–704. doi: 10.1097/00004032-198905000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Szundi I. Liao G.L. Einarsdottir O. Near-infrared time-resolved optical absorption studies of the reaction of fully reduced cytochrome c oxidase with dioxygen. Biochemistry. 2001;40:2332–2339. doi: 10.1021/bi002220v. [DOI] [PubMed] [Google Scholar]
- 13.Pastore D. Greco M. Passarella S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int. J. Radiat. Biol. 2000;76:863–870. doi: 10.1080/09553000050029020. [DOI] [PubMed] [Google Scholar]
- 14.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y. Song S. Fong C.C. Tsang C.H. Yang Z. Yang M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J. Invest. Dermatol. 2003;120:849–857. doi: 10.1046/j.1523-1747.2003.12133.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen A.C.-H. Arany P.R. Huang Y.-Y., et al. Low level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. In: Hamblin M. R., editor; Anders J. J., editor; Waynant R. W., editor. Mechanisms for Low-Light Therapy IV. Bellingham: The International Society for Optical Engineering; 2009. [DOI] [Google Scholar]
- 17.Chen A.C.-H. Huang Y.-Y. Arany P.R. Hamblin M.R. Role of reactive oxygen species in low level light therapy. In: Hamblin M. R., editor; Anders J. J., editor; Waynant R. W., editor. Mechanisms for Low-Light Therapy IV. Bellingham: The International Society for Optical Engineering; 2009. [DOI] [Google Scholar]
- 18.Baichwal V.R. Baeuerle P.A. Activate NF-kappa B or die? Curr. Biol. 1997;7:R94–96. doi: 10.1016/s0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- 19.Henkel T. Machleidt T. Alkalay I. Kronke M. Ben-Neriah Y. Baeuerle P.A. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann A. Levchenko A. Scott M.L. Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 21.Castano A.P. Dai T. Yaroslavsky I., et al. Low-level laser therapy for zymosan-induced arthritis in rats: Importance of illumination time. Lasers Surg. Med. 2007;39:543–550. doi: 10.1002/lsm.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Morais N.C. Barbosa A.M. Vale M.L., et al. Anti-inflammatory effect of low-level laser and light-emitting diode in Zymosan-induced arthritis. Photomed. Laser Surg. 2009;28:227–232. doi: 10.1089/pho.2008.2422. [DOI] [PubMed] [Google Scholar]
- 23.Pejcic A. Kojovic D. Kesic L. Obradovic R. The Effects of Low Level Laser Irradiation on Gingival Inflammation. Photomed. Laser Surg. 2009;28:69–74. doi: 10.1089/pho.2008.2301. [DOI] [PubMed] [Google Scholar]
- 24.Milas L. Mason K.A. Ariga H., et al. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64:5074–5077. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 25.Bauer S. Kirschning C.J. Hacker H., et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. U S A. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y.Y. Chen A.C. Carroll J.D. Hamblin M.R. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bullens D.M. Ceuppens J.L. Influence of Toll-like-receptor ligands on the dendritic cell-T cell interactions: therapeutic options for allergic diseases? Mini-review. Inflamm. Allergy Drug Targets. 2008;7:211–216. doi: 10.2174/187152808786848397. [DOI] [PubMed] [Google Scholar]
- 28.Krumbiegel D. Zepp F. Meyer C.U. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum. Immunol. 2007;68:813–822. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Bjordal J.M. Lopes-Martins R.A. Iversen V.V. A randomised, placebo controlled trial of low level laser therapy for activated Achilles tendinitis with microdialysis measurement of peritendinous prostaglandin E2 concentrations. Br. J. Sports Med. 2006;40:76–80. doi: 10.1136/bjsm.2005.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavish L. Perez L.S. Reissman P. Gertz S.D. Irradiation with 780 nm diode laser attenuates inflammatory cytokines but upregulates nitric oxide in lipopolysaccharide-stimulated macrophages: implications for the prevention of aneurysm progression. Lasers Surg. Med. 2008;40:371–378. doi: 10.1002/lsm.20635. [DOI] [PubMed] [Google Scholar]
- 31.Bjordal J.M. Couppe C. Chow R.T. Tuner J. Ljunggren E.A. A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust. J. Physiother. 2003;49:107–116. doi: 10.1016/s0004-9514(14)60127-6. [DOI] [PubMed] [Google Scholar]
- 32.Yamaura M. Yao M. Yaroslavsky I. Cohen R. Smotrich M. Kochevar I.E. Low level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes. Lasers Surg. Med. 2009;41:282–290. doi: 10.1002/lsm.20766. [DOI] [PubMed] [Google Scholar]
- 33.Haas A.F. Wong J.W. Iwahashi C.K. Halliwell B. Cross C.E. Davis P.A. Redox regulation of wound healing? NF-kappaB activation in cultured human keratinocytes upon wounding and the effect of low energy HeNe irradiation. Free Radic. Biol. Med. 1998;25:998–1005. doi: 10.1016/s0891-5849(98)00135-x. [DOI] [PubMed] [Google Scholar]
- 34.Moriyama Y. Nguyen J. Akens M. Moriyama E.H. Lilge L. In vivo effects of low level laser therapy on inducible nitric oxide synthase. Lasers Surg Med. 2009;41:227–231. doi: 10.1002/lsm.20745. [DOI] [PubMed] [Google Scholar]
- 35.Omi T. Kawana S. Sato S., et al. Cutaneous immunological activation elicited by a low-fluence pulsed dye laser. Br. J. Dermatol. 2005;153(Suppl 2):57–62. doi: 10.1111/j.1365-2133.2005.06971.x. [DOI] [PubMed] [Google Scholar]
- 36.Boschi E.S. Leite C.E. Saciura V.C., et al. Anti-Inflammatory effects of low-level laser therapy (660 nm) in the early phase in carrageenan-induced pleurisy in rat. Lasers Surg. Med. 2008;40:500–508. doi: 10.1002/lsm.20658. [DOI] [PubMed] [Google Scholar]
- 37.Feng W.G. Wang Y.B. Zhang J.S. Wang X.Y. Li C.L. Chang Z.L. cAMP elevators inhibit LPS-induced IL-12 p40 expression by interfering with phosphorylation of p38 MAPK in murine peritoneal macrophages. Cell Res. 2002;12:331–337. doi: 10.1038/sj.cr.7290135. [DOI] [PubMed] [Google Scholar]
- 38.Hu W.P. Wang J.J. Yu C.L. Lan C.C. Chen G.S. Yu H.S. Helium-neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J. Invest. Dermatol. 2007;127:2048–2057. doi: 10.1038/sj.jid.5700826. [DOI] [PubMed] [Google Scholar]
- 39.Karu T.I. Lobko V.V. Lukpanova G.G. Parkhomenko I.M. Chirkov I. Effect of irradiation with monochromatic visible light on the cAMP content in mammalian cells. Dokl. Akad. Nauk SSSR. 1985;281:1242–1244. [PubMed] [Google Scholar]
- 40.Zungu I.L. Hawkins Evans D. Abrahamse H. Mitochondrial responses of normal and injured human skin fibroblasts following low level laser irradiation–an in vitro study. Photochem. Photobiol. 2009;85:987–996. doi: 10.1111/j.1751-1097.2008.00523.x. [DOI] [PubMed] [Google Scholar]
- 41.Senftleben U. Cao Y. Xiao G., et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]