Abstract
The aim of this feasibility study was to evaluate the in vitro remineralization effects of four dentifrice systems using microhardness and fluoride uptake analyses. In vitro testing for the potential remineralization of the white-spot lesions in bovine and human enamel was performed using a 10-day pH cycling model. The study involved the following NaF silica-based dentifrices: 1) placebo (0 ppm F), 2) 500 ppm F, 3) 1150 ppm F, and 4) 500 ppm F plus functionalized tricalcium phosphate (fTCP). Each day consisted of four two-minute treatments, one four-hour acid challenge (pH = 5.0), and immersion in artificial saliva (pH = 7.0) between these events. After cycling, specimens were analyzed for surface microhardness (SMH), enamel fluoride uptake (EFU), and cross-sectional microhardness (CSM). Statistical analyses revealed significant differences (ANOVA, LSD, p<0.05) among the four groups, with the placebo and 500 ppm F dentifrices providing significantly less remineralization relative to the 1150 ppm F and 500 ppm F plus fTCP dentifrices. Notably, while CSM measurements for both enamel types generated similar profiles for the four groups, SMH and EFU revealed human enamel was more sensitive to the 500 ppm F dentifrice groups compared to bovine enamel. This apparent sensitivity may be due to the inherent structural differences between the two substrates.
Keywords: Fluoride, fTCP, caries, microhardness, remineralization
INTRODUCTION
The development of a low-fluoride dentifrice (e.g. 500 ppm F) that delivers comparable anti-caries efficacy relative to over-the-counter (OTC) fluoride dentifrices (e.g. 1100 ppm F) may have significant potential in reducing the risk of dental fluorosis in children. But due to concerns over adequate caries protection, for instance, the US Food and Drug Administration (FDA) anticaries monograph specifies that fluoride levels in OTC toothpaste shall not lie below 850 ppm (FDA, 1995). Further to this point, clinical data have shown the risk of caries progression increases over time when low-fluoride toothpastes are used relative to typical OTC levels of fluoride toothpaste (Stookey et al., 2004; Lima et al.,2008). Most recently, the Cochrane Oral Health Group reviewed clinical trials and opined that fluoride dentifrices with less than 1000 ppm F do not provide sufficient protection against the caries process (Walsh et al., 2010).
Attempts to improve efficacy from dentifrices containing less than 1000 ppm F have included combinations of fluoride and other mineralizing agents, with the hypothesis that non-fluoride mineralizing agents, such as calcium, phosphate, strontium, etc, might work synergistically with fluoride (Feagin et al., 1971; Dedhiya et al., 1974; LeGeros, 1999; Pfarrer and Karlinsey, 2009) to sufficiently elevate efficacy to a clinically-acceptable level. A dentifrice comprising several mineralizing agents, however, should not compromise fluoride bioavailability and should provide similar protection against dental caries relative to clinically-proven safe and effective standard OTC fluoride dentifrices (e.g. 1100 or 1450 ppm F). Recently we introduced functionalized tricalcium phosphate (fTCP) as an agent that can enhance fluoride’s efficacy without compromising fluoride bioavailability (Karlinsey and Mackey, 2009; Karlinsey et al., 2009a; 2009b; 2010a; 2010b; 2010d). Commercially, this combination is found in the 3M ESPE Clinpro® 5000 dentifrice available from the dentist, and contains 5000 ppm F plus 800 ppm fTCP. Our previous experiments, including infrared spectroscopy investiga-tions, suggest the calcium oxide polyhedra manifested in the tricalcium phosphate lattice, which are protected with specific organic molecules such as fumaric acid or sodium lauryl sulfate (SLS) (Karlinsey, 2010c; 2010d), cooperate with fluoride to bond with loosely-bound or broken enamel lattice constituents, including orthophosphate (Karlinsey et al., 2009c; 2010c; 2010d). Microhardness measurements demonstrate the ensuing mineralization produces stronger and greater acid-resistant tooth enamel relative to fluoride-free and fluoride-only controls. Separately, studies are underway to further understand the nature of the fluoride plus fTCP mechanism.
In this work, the principle purpose was to evaluate the in vitro remineralization potential of a 500 ppm F plus fTCP dentifrice compared to a similar 500 ppm F dentifrice lacking fTCP, as well as to a placebo and an 1150 ppm F dentifrice. As pH cycling performance models are helpful in screening for initial efficacy (ten Cate et al., 1988; Featherstone et al., 1990; White, 1992; ten Cate et al., 2006), we utilized a model with sensitivity to fluoride to evaluate the silica-based NaF dentifrices (Schemehorn et al., 1990; Karlinsey et al., 2009b). This model serves as a critical feasibility step in our pursuit of developing a promising dentifrice formulation, which is supported by the National Institutes of Health’s Small Business Innovation Research program. In addition, since bovine and human enamel are not structurally identical (Spitzer and ten Bosh, 1975; Arends et al., 1978; Edmunds et al., 1988), the second aim of this research was to learn whether sensitivities exist in how these two enamel types respond to the NaF dentifrices.
MATERIALS AND METHODS
Specimen preparation and white-spot lesion formation
Keeping the type of enamel substrates completely separate from one another, 3 mm enamel cores were drilled from the lingual and labial surfaces of bovine and human molars using a bench top drill press (Power Glide, China) affixed with a hollow core drill bit. The cores were embedded into hollowed out acrylic rods using DuraBase resin (Reliance Dental Mfg. Co., Worth, IL, USA). Each specimen was ground by hand with 600 grit SiC sandpaper under water cooling for 30 s using a Leco Spectrum System 1000 Grinder/Polisher (St. Joseph, MI, USA) set to 300 rpm. Then, with the unit set to 200 rpm, each specimen was polished by hand for one minute using 3µ m diamond compound in conjunction with microid extender solution (Leco). After rinsing with distilled (DI) water, Vickers surface microhardness was performed with a 200 gf load and 15 s dwell time (Leco LM247AT microhardness tester) to confirm the presence of sound enamel in the polished specimen as indicated by a Vickers hardness number (VHN) of 300 VHN or greater for bovine and 320 VHN or greater for human enamel. Acceptable sound specimens were then immersed in vials containing a 0.2 wt. % 450 kDa MW polyacrylic acid (Sigma-Aldrich, St. Louis, MO, USA) and 0.1 M lactic acid (Sigma-Aldrich) solution partially saturated with hydroxyapatite (BioRad, Hercules, CA, USA) and adjusted to a pH of 4.9 (White, 1987). These vials were subsequently loaded into an incubator at 37°C to establish ‘white-spot’ (non-cavitated) lesions, the characteristics and histology of which have been discussed in detail previously by White (White, 1987). Briefly, the caries-like lesions produced by this solution have a dense surface mineral zone approximately 15 microns thick (via polarized light microscopy) (White, 1987), while lesion depth extends to about 70 µm (via microradiographic analysis) (White, 1987) and reflective microscopy). Upon white-spot formation, acceptable baseline surface microhardness ranged from 25 to 50 VHN (200 gf for 15 s made using a Leco 247AT indenter).
Treatment groups and study protocol
Human (N=24) and bovine (N=24) enamel specimens were then grouped into four silica-containing dentifrices (GlaxoSmithKline, Weybridge, England) listed below:
Dentifrice A: fluoride-free toothpaste (placebo);
Dentifrice B: 500 ppm fluoride (NaF) toothpaste;
Dentifrice C: 1150 ppm fluoride (NaF) toothpaste;
Dentifrice D: 500 ppm fluoride (NaF) toothpaste plus 50 ppm fTCP;
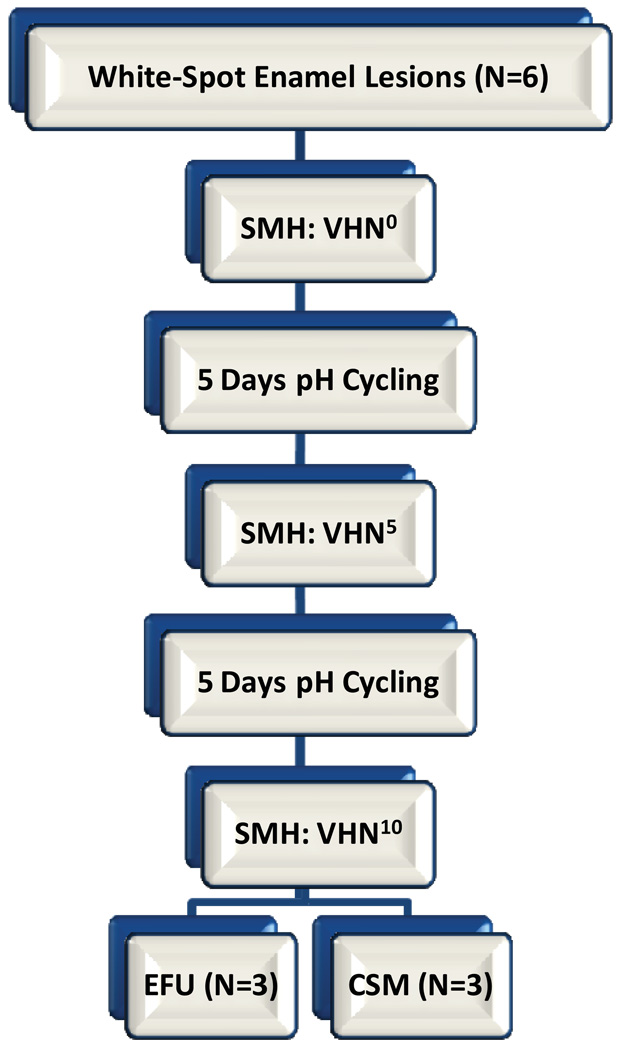
As outlined in Figure 1, the four groups of bovine and human enamel specimens were then cycled through a remin/demin pH cycling model and analyzed using Vickers surface microhardness (SMH) after 5 and 10 days of cycling. For each dentifrice group, the 12 specimens (six human, six bovine) were organized into three subgroups of four specimens each (two human, two bovine). Because of the relatively small number of specimens used in this study, the subgrouping increased the overall exposure to treatments and acid challenges in order to help reduce potential random and systematic errors over the course of the pH cycling study. The daily cycling model includes two two-minute treatment events (15 mL) performed an hour apart in the morning, followed by one four-hour carbopol-lactic acid challenge (15 mL, pH=5.0), and finally two more two-minute treatment events (15 mL) in the afternoon. In between the daily treatments and acid challenge, specimens were immersed in artificial saliva (15 mL) (ten Cate et al., 1988). The treatments were diluted three-fold with DI water (5 g dentifrice:10 mL DI water). The treatments and saliva events were magnetically agitated at 300 rpm, while the acid challenge was static. After each treatment and acid challenge, the specimens were rinsed with DI water prior to placement into artificial saliva. Fresh treatment slurries and acid solution were used daily, with the artificial saliva solution changed once daily during the acid challenge.
Figure 1.
Outline of protocol used for both human (N=6) and bovine (N=6) enamel substrates treated with each one of the four Dentifrice groups.
After 10 days of cycling half of the enamel specimens from each substrate (that is, bovine and human) were further analyzed for enamel fluoride uptake (EFU, N=3 per substrate) and other half for cross-sectional microhardness (CSM, N=3 per substrate). For the cycling regimen and the post-cycle analysis, the analysts were blinded to the identity of the treatments and enamel substrates.
Surface microhardness (SMH)
Each 3 mm enamel core manifested sufficient space to allow for multiple Vickers diamond-shaped indents onto the flat surface. After 5 and 10 days of cycling, the enamel specimens were examined for Vickers surface hardness (200 gf, 15 s dwell time). The change in Vickers hardness number (ΔVHN) was determined as the difference between the 5 or 10 day and baseline values. Compared to the penetration depth of of a Knoop indenter (~ 3 µm) the Vickers indenter penetrates into the white-spot lesion about twice as much; thus, surface microhardness was assessed using the Vickers indenter in order to help bridge the subsequent cross-sectional microhardness analysis, which could not be effectively measured at depths below 10 µm without extensive cracking.
Enamel fluoride uptake (EFU)
Inspired by Stearns (1970), EFU was determined in units of micrograms of fluoride per unit enamel area (µg F/cm2) (Faller and Best, 1995). Each of the six (three human, three bovine) enamel specimens was immersed and continuously agitated in 0.5 ml of 1 N HClO4 (Fisher Scientific, Pittsburgh, PA, USA) for 15 s. To determine fluoride concentration, 0.25 ml of this etchant was combined with 0.25 ml of 1 N NaOH (MP Biomedicals, Solon, OH, USA) and 0.5 ml of TISAB II (Red Bird Service, Batesville, IN, USA). This solution was magnetically stirred, and a fluoride ion specific electrode (Thermo Fisher, Pittsburgh, PA, USA) was used to measure the free fluoride ion potential. These measurements were converted to fluoride concentrations by using a fluoride electrode calibration curve constructed from known standards prepared with NaF (Thermo Fisher).
Cross-sectional microhardness (CSM)
CSM was performed on the remaining six (three human, three bovine) specimens as follows. A Lapcraft Lil’ Trimmer circular saw (Powell, OH, USA) was used to section the enamel specimens. The sections were then mounted with ClaroCit methylmethacrylate-based cold mounting resin (Struers, Cleveland, OH, USA) with the freshly cut surfaces exposed. The mounted specimens are serially ground with 180, 1000, and 1500 grit sandpaper (3M, St. Paul, MN, USA), and then serially polished with 9, 3, and 1 µm polycrystalline diamond suspension (Buehler, Lake Bluff, IL, USA). This procedure is consistent with that reported previously (Meredith et al., 1996). Longitudinal microhardness was then performed with the Knoop indenter fitted on the microhardness tester. Due to the delicacy of the enamel in the white-spot lesion zone, which can lead to undesirable cracking upon indentation, along with the spatial limitations of multiple indents, we selected the Knoop indenter instead of the Vickers indenter. The Knoop indenter does not penetrate as deeply into the enamel relative to the Vickers indenter, and therefore helps to reduce the risk of enamel cracking. A series of five indentation lanes per specimen are made under a load of 10 gf at 12.5 µm, 25 gf at 25, 37.5, and 50 µm, and 50 gf at 75, 100, 125 and 150 µm below the specimen surface. Measurements closer to the enamel were not feasible at the given load limits due to the delicacy of the specimens. This resulted in a total of 40 indents per specimen. The Knoop indentation lengths were then converted to Knoop Hardness Numbers (KHN).
Sample size and statistics
Our selection of six specimens per enamel type was based on our extensive experience in using this in vitro pH cycling model as a tool to discriminate among treatments having a range in fluoride from 0 to 5,000 ppm F. Previously, we have used up to 18 specimens per group in this in vitro cycling study (Karlinsey and Mackey, 2009; Karlinsey et al., 2009a; 2009b; 2010a; 2010b; 2010d). By virtue of our screening efforts in optimizing concentrations of fTCP with various levels of fluoride, we have found that using fewer specimens per group (e.g. three or six) can still provide statistically important data. Specifically, our criteria justifying the use of fewer specimens lie in the ability to achieve statistically significant differences between the placebo and fluoride-containing treatments as well as a fluoride dose response.
All statistics were performed using SPSS PASW Statistics 17.0 software. The data were analyzed for normality using the Kolmogorov-Smirnov test with p=0.05. One-way ANOVA indicated statistical differences existed among the group means with 95% confidence and least significant difference (LSD) testing was then used to compare group means (p<0.05).
RESULTS
SMH results for the two enamel substrates (N=6 persubstrate) when treated with the dentifrice group after 5 and 10 days of pH cycling are shown in Table 1. For bovine enamel white-spots, the most effective treatment was dentifrice C, which was statistically significant relative to the other three groups (p<0.05). The least effective was dentifrice A, the placebo. No significant differences were found between the two 500 ppm F dentifrices, groups B and D; but between these two, only dentifrice D statistically split from the placebo group. On the other hand, human enamel white-spots were very sensitive to treatment: all groups were significantly different from one another at the end of 10 days of cycling, with the placebo group (A) the least effective, while the 500 ppm F group containing fTCP was the most effective (D).
Table 1.
Summary of surface microhardness results from bovine and human enamel with artificial white-spot lesions after cycling through a remin/demin model lasting 10 days.
| Substrate | Dentifrice | VHN0 | VHN5 | ΔVHN5 | VHN10 | ΔVHN10 |
|---|---|---|---|---|---|---|
| Bovine Enamel | A | 35.6 ± 3.0a | 51.1 ± 7.7a | 15.5 ± 5.9a | 53.0 ± 8.3a | 17.4 ± 6.0a |
| B | 35.5 ± 2.7a | 59.5 ± 7.7a | 24.0 ± 5.4a,b | 75.6 ± 13.6a,b | 40.2 ± 11.9a,b | |
| C | 35.5 ± 2.7a | 98.3 ± 7.7b | 62.8 ± 7.6c | 119.8 ± 9.2c | 84.3 ± 10.5c | |
| D | 35.6 ± 2.6a | 69.9 ± 4.6a | 34.3 ± 5.0b | 83.4 ± 8.2b | 47.8 ± 9.0b | |
| Human Enamel | A | 40.6 ± 1.91 | 57.7 ± 4.41 | 17.1 ± 4.31 | 66.2 ± 4.91 | 25.6 ± 5.31 |
| B | 40.6 ± 1.41 | 76.5 ± 4.42 | 35.9 ± 4.32 | 107.4 ± 7.82 | 66.8 ± 7.42 | |
| C | 40.8 ± 1.21 | 102.6 ± 6.53 | 61.8 ± 6.53 | 127.8 ± 4.23 | 87.0 ± 4.73 | |
| D | 40.8 ± 1.11 | 91.6 ± 5.42,3 | 50.9 ± 5.42,3 | 146.9 ± 7.34 | 106.2 ± 7.44 |
In each VHN column, superscripts indicate significant differences (ANOVA, p<0.05, LSD) with ranking listed as a<b<c (for bovine enamel) and 1<2<3<4 (for human enamel).
VHN0 = mean baseline Vickers Hardness Number (VHN) ± SEM (N=6 per enamel type); VHN5 = mean VHN ± SEM (N=6 per enamel type) after 5 days of cycling; ΔVHN5 = difference between mean VHN5 ± SEM (N=6 per enamel type) and VHN0 after 5 days of cycling; VHN10 = mean VHN ± SEM (N=6 per enamel type) after 10 days of cycling; ΔVHN10 = difference between mean VHN10 ± SEM (N=6 per enamel type) and VHN0 after 10 days of cycling.
EFU results for the two substrates (N=3 per substrate) after 10 days of cycling are summarized in Table 2. For bovine enamel, significant differences were only observed between the 500 and 1150 ppm F dentifrice groups (B and C) (p<0.05). For human enamel, no significant differences were found between dentifrices A and B; however, they were inferior to the 1150 ppm F and 500 ppm F plus fTCP dentifrices C and D, respectively.
Table 2.
Summary of mean (± SEM) enamel fluoride uptake (EFU) results from bovine and human enamel with artificial white-spot lesions after cycling through a remin/demin model lasting 10 days.
| Substrate | Dentifrice | EFU (µg F/cm2) |
|---|---|---|
| Bovine enamel | A | 2.0 ± 0.8 |
| B | 1.9 ± 0.5* | |
| C | 4.1 ± 2.1* | |
| D | 3.1 ± 0.3 | |
| Human enamel | A | 1.5 ± 0.1a |
| B | 3.0 ± 0.4a | |
| C | 5.0 ± 0.4b | |
| D | 4.7 ± 1.3b |
The asterisks (for bovine enamel) indicate the only significant difference (ANOVA, p<0.05, LSD) exists between Dentifrices B and C, while significant differences (ANOVA, p<0.05, LSD) are ranked with a<b (for human enamel).
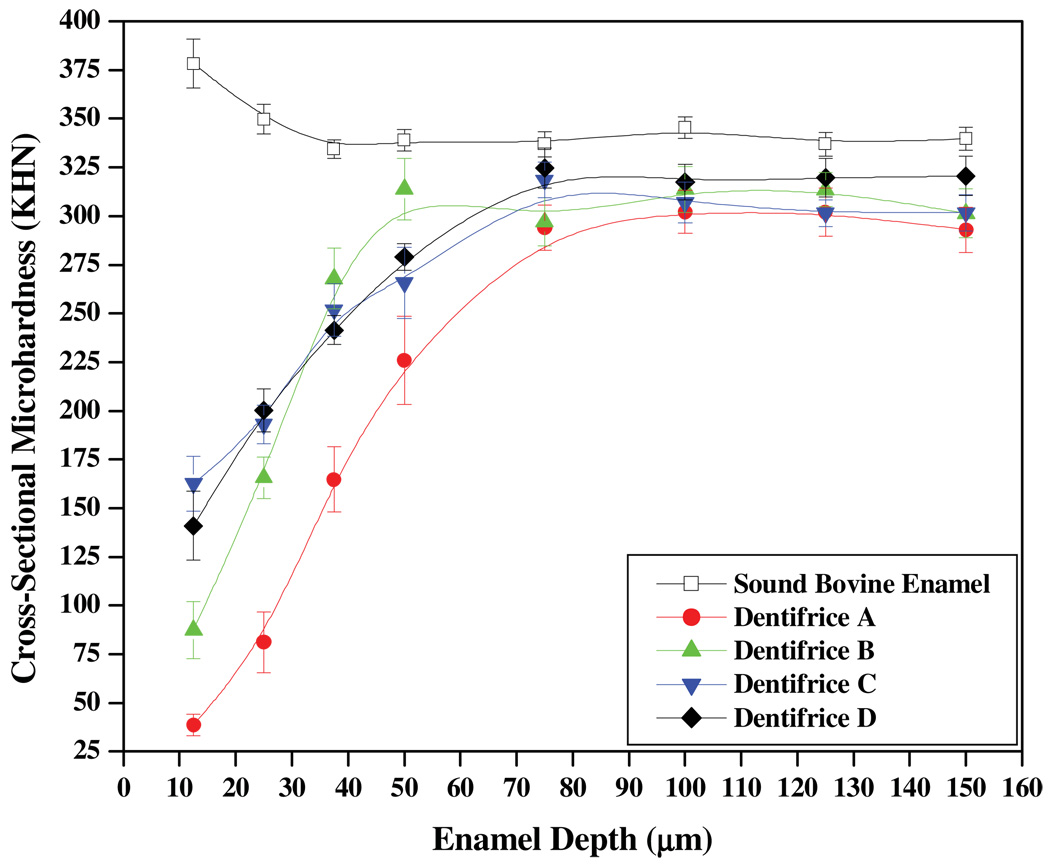
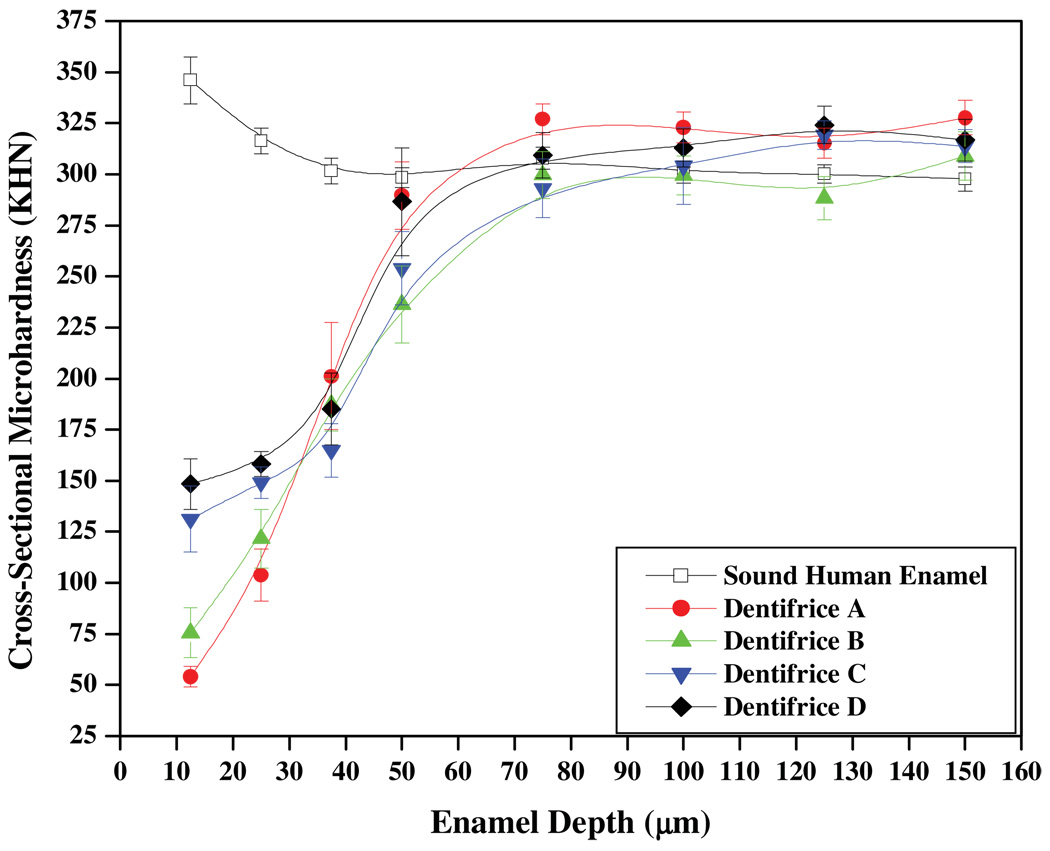
The remaining three specimens for each enamel type were then assessed for CSM. Figures 2 and 3 depict the trends observed in CSM (mean ± SEM KHN) as a function of enamel depth for bovine and human enamel, respectively. These trends are based on the analyses of five measurement lanes extending from 12.5 to 150 µm per specimen (N=3 per substrate), which results in 15 CSM measurements per enamel depth. For bovine enamel, significant differences are observed in the trending, with the dentifrice A imparting the weakest remineralization effects. The greatest remineralization effects are observed for dentifrices C and D, which were not found to be significantly different at the enamel depths considered in this work. In human enamel, the trending was similar to that observed in Figure 2; however, the separation between the four curves is less pronounced. For both substrates, the most striking differences in CSM among groups occurred at enamel depths less than 50 µm. CSM values for sound bovine and human enamel are also provided in each figure for reference. We note that CSM was greater for sound bovine enamel relative to sound human enamel; however, pH cycling significantly weakens sound bovine enamel structure, with CSM diminishing approximately 25 KHN. In contrast, pH cycling appears to at least maintain the same CSM in human enamel.
Figure 2.
CSM analysis of bovine enamel treated with the four Dentifrice groups.
Figure 3.
CSM analysis of human enamel treated with the four Dentifrice groups.
DISCUSSION
Previously, we have used the present pH cycling model to evaluate the potential of fluoride-containing systems (Karlinsey et al., 2009a; 2009b; 2010a) manifesting different NaF concentrations. As evidenced in Tables 1 and 2, a clear dose response among dentifrices A, B, and C is observed; therefore, this outcome helps validate its applicability in assessing the in vitro remineralization potential of dentifrice D. The SMH data in Table 1 reveal that both bovine and human enamel responded to the 500 and 1150 ppm F pastes; however, between the two enamel types, human enamel appears especially sensitive to the extent of surface strengthening achieved by the 500 ppm F dentifrice groups (B and D). In contrast, the surface strengthening of bovine and human enamel appear to be influenced similarly when treated with the placebo or 1150 ppm F paste, respectively. The notable observation that both 500 ppm F dentifrices behaved similarly at the bovine enamel surface yet differed significantly at the human enamel surface may suggest the matrices of the two substrates are innately distinct and may strongly influence the remineralization behavior of fluoride and other mineralizing agents. Although the mineral content and constituent connectivity is quite similar within human and bovine enamel (Bachmann et al., 2003), the mineral densities may contrast depending on the history of the enamel specimens (Fonseca et al., 2008; Tanaka et al., 2008). Structurally, scanning electron microscopy (SEM) has revealed sound bovine enamel manifests crystallites with diameters about 1.5 times larger than that in sound human enamel (Arends and Jongebloed, 1978). Additionally, SEM (Fonseca et al., 2008) and infra-red (Spitzer and ten Bosh, 1975) analyses have revealed significantly more inter-prismatic organic material exists in bovine enamel compared to human enamel. These structural characteristics in conjunction with other factors including mineral distribution and crystallite geometry and shape defects, contribute to the relatively fasterdemineralization rates of bovine enamel in acid solutions compared to human enamel (Featherstone and Mellberg, 1981; Edmunds et al., 1988; Meurman and Frank, 1991).
With respect to remineralization, while early studies sometimes mixed human and bovine enamel together for investigational purposes (Feagin et al., 1971; Gwinnett et al., 1972), few studies have reported on comparing dedicated human and bovine enamel responses to fluoride (or other mineralizing agents): in one report, human and bovine enamel produced significantly different fluoride uptake from acidulated phosphate-fluoride solutions (Mellberg and Loerstcher, 1974), while no differences in enamel substrate were observed in a pH cycling model assessing remineralization potential of 0, 250 and 1100 ppm F dentifrices (Stookey et al., 2006). In our previous work using the same pH cycling model with bovine enamel white-spot lesions, we did not observe statistical splitting between a 500 ppm F dentifrice and either a 950 ppm F or 1100 ppm F after 10 days of cycling (Karlinsey et al., 2009b). Therefore, coupled with our prior results, we suggest an apparentsurface-sensitivity exists in human enamel at particular fluoride concentrations, especially 500 ppm F (that is, three-fold dilutions of the dentifrice), that may be absent in bovine enamel. The fluoride uptake data reported in Table 2 further demonstrate an overall increase in fluoride sensitivity at the surface of human relative to bovine enamel.
CSM measurements at depths between 12.5 and 150 µm below the enamel surface provide quantitative insight into the extent of remineralization. In Figures 2 and 3, the trends show for all dentifrice treatments a homogeneous mineral matrix is essentially achieved approximately 75 µm from the enamel surface. When compared to human enamel, the remineralization of white-spot bovine enamel lesions does not lead to the recovery of sound enamel strength after 10 days of pH cycling. This result demonstrates the demineralization and remineralization of bovine and human enamel may not be identical and therefore might be influenced by the factors discussed above. In the context of caries formation and repair (Robinson et al., 2000), this is discussed further below.
The placebo group (dentifrice A) in Figures 2 and 3 produced the weakest enamel. Presumably, over the 10-day cycling period, increased porosity and solubility of the enamel would ensue due to repeated exposures to the acid challenge. Additionally, the extent of remineralization from exposure to the fluoride-free artificial saliva would be limited due to the apparently poor mineral nucleation ability of calcium and phosphate of the artificial saliva relative to fluoride. Without effective nucleation and the development of acid-resistant mineral, leaching of apatite constituents (which includes calcium, phosphate, carbonate, etc) from deep within the enamel tissue is likely to occur (Robinson et al., 2000). Based on the trends in Figures 2 and 3, this appears to be the case, with the exception that the mineral leaching in human enamel appears to generate hyper-mineralized regions between 50 and 75 µm. In turn, this may account for the relative difference in remineralization slopes between bovine (5.2 ± 0.4 KHN/µm) and human (6.4 ± 0.6 KHN/ µm) enamel. This might be facilitated in part by the physical packing of the smaller apatite crystals which are bounded by minimal amounts of inter-prismatic organic material. Such reasonsing may help explain why the placebo paste demonstrates higher microhardness relative to the other three dentifrices near the bottom of the lesion and into the sound enamel region in Figure 3. In contrast, the larger porosity and structural features, including more robust inter-prismatic material, may prevent bovine enamel from exhibiting similar behavior.
Remineralization from dentifrice B produces significant strengthening compared to the placebo group, and is especially robust in bovine enamel, where its slope (6.3 ± 0.6 KHN/µm) trends more steeply compared to that for human enamel (4.4 ± 0.2 KHN/µm) between 12.5 and 50 µm. While the surface of human enamel was especially sensitive to 500 ppm F, the bovine enamel subsurface appears to be more sensitive to the same level of fluoride. A possible explanation for this contrast is that the outer layers of human enamel might be stabilized, and therefore condensed, due to incorporation of fluoride; in turn, this may inhibit significant penetration of fluoride, and therefore mineral formation, deeper within the enamel lesion. In contrast and relative to human enamel, the porosity and structure in bovine enamel presumably encourage deeper penetration of fluoride, and therefore robust mineral formation. In turn, this may affect the extent of mineralization near the bovine enamel surface and is similar to the low fluoride uptake observed in bovine enamel relative to human enamel at different fluoride concentrations (Mellberg and Loerstcher, 1974).
Although dentifrices B and D contain 500 ppm F, the remineralization behavior between these two dentifrices contrasts significantly in both enamel types. In Figure 2, although dentifrice D produces twice the CSM at 12.5 µm, the remineralization from dentifrice B spans almost 200 KHN between 12.5 and 50 µm, while dentifrice D spans about 100 KHN within the same range. These trends are similar to that observed in Figure 3, albeit to a much-lesser extent. Despite the same concentration of fluoride, human enamel appears especially sensitive to mineralization behavior by virtue of the inflection near 37.5 µm for dentifrice D in Figure 3, as such an inflection is absent for dentifrice B. Collectively, these differences demonstrate fTCP significantly affects mineralization and improves the manner in which 500 ppm F mineralizes subsurface lesions. But further insight into the mechanism responsible for this mineralization behavior is underway and is reserved for a separate paper.
The remineralization characteristics of dentifrice groups C and D exhibit marked similarities even though dentifrice C contains more than double the fluoride content of dentifrice D. For instance, in Figures 2 and 3 both dentifrices confer statistically greater CSM at depths down to 25 µm compared to dentifrices A and B. Also, in Figure 3 both of these groups yield similar remineralization characteristics throughout the body of the lesion and then deeper within the enamel matrix. In contrast, in human enamel the effects of these two groups are similar down to 37.5 µm, at which point the remineralization trends diverge, with dentifrice D generating sharply more mineralization to about 70 µm. This observation may indicate a shifting in the mechanism of mineralization between 1100 ppm F (dentifrice C) and 500 ppm F plus fTCP (dentifrice D).
In conclusion, we conducted a pH cycling study to evaluate the remineralization performance four dentifrices using bovine and human enamel. We observed that significant surface and subsurface strengthening can occur from a 500 ppm F dentifrice containing functionalized tricalcium phosphate, and despite having half the fluoride, this remineralization is comparable to that from an 1150 ppm F dentifrice. Our observations would be strengthened with support from additional studies, including in vitro studies with larger sample sizes as well as an intra-oral study for clinical evaluation. Additionally, separate studies are underway to investigate the mineralization mechanism by the fluoride plus fTCP combination.
ACKNOWLEDGEMENTS
This project was supported by an award (R43DE020998) from the National Institutes of Health’s Office of the Director and National Institute of Dental and Craniofacial Research. We would also like to thank GlaxoSmithKline for providing the dentifrices used in this study.
Footnotes
DISCLOSURE
RL Karlinsey is Nanotech’s CEO and Principle Investigator, AC Mackey is Laboratory and Quality Director, and TJ Walker, KE Frederick, DD Blanken, SM Flaig, and ER Walker are research technicians.
REFERENCES
- Arends J, Jongebloed WL. Crystallite dimensions of enamel. J. Biol. Buccale. 1978;6:161–171. [PubMed] [Google Scholar]
- Bachmann L, Diebolder R, Hibst R, Zezell DM. Infrared absorption bands of enamel and dentin tissues from human and bovine teeth. Appl. Spectrosc. Rev. 2003;38:1–14. [Google Scholar]
- Dedhiya MG, Young F, Higuchi WI. Mechanism of hydroxyapatite dissolution. The synergistic effects of solution fluoride, strontium, and phosphate. J. Phys. Chem. 1974;78:1273–1279. [Google Scholar]
- Edmunds DH, Whittaker DK, Green RM. Suitability of human, bovine, equine, and ovine tooth enamel for studies of artificial bacterial carious lesions. Caries Res. 1988;22:327–336. doi: 10.1159/000261132. [DOI] [PubMed] [Google Scholar]
- Faller RV, Best JM. Anticaries efficacy of an improved stannous fluoride toothpaste. J. Clin. Dent. 1995;6(Spec Issue):89–96. [PubMed] [Google Scholar]
- FDA. Final monograph on anticaries drug products for over-the-counter human use. Federal Register. 1995;60:52474–52510. [Google Scholar]
- Feagin F, Patel PR, Koulourides T, Pigman W. Study of the effect of calcium, phosphate, fluoride and hydrogen ion concentrations on the remineralization of partially demineralized human and bovine enamel sufaces. Archs. Oral Biol. 1971;16:535–548. doi: 10.1016/0003-9969(71)90199-3. [DOI] [PubMed] [Google Scholar]
- Featherstone JDB, Glena R, Shariati M, Shields CP. Dependence of in vitro demineralization of apatite and remineralization of dental enamel on fluoride concentration. J. Dent. Res. 1990;69(Spec Iss):620–625. doi: 10.1177/00220345900690S121. [DOI] [PubMed] [Google Scholar]
- Featherstone JDB, Mellberg JR. Relative rates of progress of artificial carious lesions in bovine, ovine and human enamel. Caries Res. 1981;15:109–114. doi: 10.1159/000260508. [DOI] [PubMed] [Google Scholar]
- Fonseca RB, Haiter-Neto F, Carlo HL, Soares CJ, Sinhoreti MAC, uppin-Rontani RM, Correr-Sobrinho L. Radiodensity and hardness of enamel and dentin of human and bovine teeth, varying bovine teeth age. Archs. Oral Biol. 2008;53:1023–1029. doi: 10.1016/j.archoralbio.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Gwinnett AJ, Buonocore MG, Sheykholeslam Z. Effect of fluoride on etched human and bovine tooth enamel surfaces as demonstrated by scanning electron microscopy. Archs. Oral Biol. 1972;17:271–278. doi: 10.1016/0003-9969(72)90210-5. [DOI] [PubMed] [Google Scholar]
- Karlinsey RL, Mackey AC. Solid-state preparation and dental application of an organically-modified calcium phosphate. J. Mater. Sci. 2009;44:346–349. [Google Scholar]
- Karlinsey RL, Mackey AC, Stookey GK. In vitro remineralization efficacy of NaF systems containing unique forms of calcium. Am. J. Dent. 2009a;22:185–188. [PubMed] [Google Scholar]
- Karlinsey RL, Mackey AC, Stookey GK, Pfarrer AM. In vitro assessments of experimental NaF dentifrices containing a prospective calcium phosphate technology. Am. J. Dent. 2009b;22:180–184. [PubMed] [Google Scholar]
- Karlinsey RL, Mackey AC, Walker ER, Amaechi BT, Karthikeyan R, Najibfard K, Pfarrer AM. Remineralization potential of 5,000 ppm fluoride dentifrices evaluated in a pH cycling model. J. Dent. Oral Hyg. 2010a;2:1–6. [Google Scholar]
- Karlinsey RL, Mackey AC, Walker ER, Frederick KE. Spectroscopic evaluation of native, milled, and functionalized β-TCP seeding into dental enamel lesions. J. Mater. Sci. 2009c;44:5013–5016. [Google Scholar]
- Karlinsey RL, Mackey AC, Walker ER, Frederick KE. Enhancing Remineralization of Subsurface Enamel Lesions with Functionalized β-TCP. In: Bourg H, Lisle A, editors. Biomaterials Developments and Applications. New York: Nova Science Publishers; 2010b. pp. 353–374. [Google Scholar]
- Karlinsey RL, Mackey AC, Walker ER, Frederick KE. Preparation, characterization and in vitro efficacy of an acid-modified β-TCP material for dental hard-tissue remineralization. Acta Biomater. 2010c;6:969–978. doi: 10.1016/j.actbio.2009.08.034. [DOI] [PubMed] [Google Scholar]
- Karlinsey RL, Mackey AC, Walker ER, Frederick KE. Surfactant-modified β-TCP: structure, properties, and in vitro remineralization of subsurface enamel lesions. J. Mater. Sci. Mater. Med. 2010d;21:2009–2020. doi: 10.1007/s10856-010-4064-y. [DOI] [PubMed] [Google Scholar]
- LeGeros RZ. Calcium phosphates in demineralization/remineralization processes. J. Clin. Dent. 1999;10:65–73. [Google Scholar]
- Lima TJ, Ribeiro CCC, Tenuta LMA, Cury JA. Low-fluoride dentifrice and caries lesion control in children with different caries experience: a randomized clinical trial. Caries Res. 2008;42:46–50. doi: 10.1159/000111749. [DOI] [PubMed] [Google Scholar]
- Mellber JR, Loerstcher KL. Comparison of in vitro fluoride uptake by human and bovine enamel from acidulated phosphate-fluoride solutions. J. Dent. Res. 1974;53:64–67. doi: 10.1177/00220345740530013101. [DOI] [PubMed] [Google Scholar]
- Meredith N, Sherriff M, Setchell DJ, Swanson SAV. Measurement of the microhardness and Young's modulous of human enamel and dentine using an indentation technique. Archs. Oral Biol. 1996;41:539–545. doi: 10.1016/0003-9969(96)00020-9. [DOI] [PubMed] [Google Scholar]
- Meurman JH, Frank RM. Progression and surface ultrastructure of in vitro caused erosive lesions in human and bovine enamel. Caries Res. 1991;25:81–87. doi: 10.1159/000261348. [DOI] [PubMed] [Google Scholar]
- Pfarrer AM, Karlinsey RL. Challenges of implementing new remineralization technologies. Adv. Dent. Res. 2009;21:79–82. doi: 10.1177/0895937409335643. [DOI] [PubMed] [Google Scholar]
- Robinson C, Shore RC, Brookes SJ, Strafford S, Wood SR, Kirkham J. The chemistry of enamel caries. Crit. Rev. Oral Biol. Med. 2000;11:481–495. doi: 10.1177/10454411000110040601. [DOI] [PubMed] [Google Scholar]
- Schemehorn BR, Farnham RL, Wood GD, Stookey GK. A Bovine Enamel Model for In vitro Remin/Demin Tests. J. Dent. Res. 1990;69:260. (Abstract #1213) [Google Scholar]
- Spitzer D, Ten Bosh JJ. The absorption and scattering of light in bovine and human dental enamel. Calcif. Tiss. Res. 1975;17:129–137. doi: 10.1007/BF02547285. [DOI] [PubMed] [Google Scholar]
- Stearns RI. Incorporation of fluoride by human enamel: I. Solid-state diffusion process. J. Dent. Res. 1970;49:1444–1451. doi: 10.1177/00220345700490064801. [DOI] [PubMed] [Google Scholar]
- Stookey GK, Izu M, Schemehorn BR. Comparison of bovine and human enamel specimens in a profile hardness pH cycling model. Caries Res. 2006;40:312–313. (Abstr 27) [Google Scholar]
- Stookey GK, Mau MS, Isaacs RL, Gonzalez-Gierbolini C, Bartizek RD, Biesbrock AR. The relative anticaries effectiveness of three fluoride-containing dentifrices in Puerto Rico. Caries Res. 2004;38:542–550. doi: 10.1159/000080584. [DOI] [PubMed] [Google Scholar]
- Tanaka JLO, Medici-Filho E, Salgado JAP, Moraes LC, Moraes MEL, Castilho JCM. Comparative analysis of human and bovine teeth: radiographic density. Braz. Oral Res. 2008;22:346–351. doi: 10.1590/s1806-83242008000400011. [DOI] [PubMed] [Google Scholar]
- ten Cate JM, Exterkate RAM, Buijs MJ. The relative efficacy of fluoride toothpastes assessed with pH cycling. Caries Res. 2006;40:136–141. doi: 10.1159/000091060. [DOI] [PubMed] [Google Scholar]
- ten Cate JM, Timmer K, Shariati M, Featherstone JDB. Effect of timing of fluoride treatment on enamel de- and remineralization in vitro: a pH-cycling study. Caries Res. 1988;22:20–26. doi: 10.1159/000261078. [DOI] [PubMed] [Google Scholar]
- Walsh T, Worthington HV, Glenny AM, Appelbe P, Marinho VCC, Shi X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database of Systemetic Reviews Issue 1 (Art. No. CD0076868) 2010 doi: 10.1002/14651858.CD007868.pub2. [DOI] [PubMed] [Google Scholar]
- White DJ. Reactivity of Fluoride Dentifrices with Artificial Caries I. Effects on Early Lesions: F Uptake, Surface Hardening andRemineralization. Caries Res. 1987;21:126–140. doi: 10.1159/000261013. [DOI] [PubMed] [Google Scholar]
- White DJ. Use of Synthetic Polymer Gels for Artificial Carious Lesion Preparation. Caries Res. 1987;21:228–242. doi: 10.1159/000261026. [DOI] [PubMed] [Google Scholar]
- White DJ. The comparative sensitivity of intra-oral, in vitro, and animal models in the 'profile' evaluation of topical fluorides. J. Dent. Res. 1992;71(Spec Iss):884. doi: 10.1177/002203459207100S19. [DOI] [PubMed] [Google Scholar]