Abstract
Objectives
Although low birthweight is associated with elevated blood pressure (BP) levels, whether the strength of this relationship is amplified with age is still debated. This study tested the hypothesis that the magnitude of the birthweight–BP association increases with age from childhood to adulthood.
Methods
The study cohort included 6251 individuals (64.5% whites and 35.6% blacks, 50.0% males) enrolled in the Bogalusa Heart Study. Individuals were examined 1–15 times for BP from childhood to adulthood, with 24 363 observations. Information on birthweight and gestational age was obtained from Louisiana State birth certificates.
Results
After adjusting for race, sex, age and gestational age, low birthweight (kg) was associated with higher SBP levels (mmHg) in adolescence (aged 12–17 years, regression coefficient β=−0.80, P=0.004) and adulthood (aged 18–50 years, β=−1.34, P=0.010). Adjustment for current BMI yielded considerably stronger association. Importantly, the magnitude of the birthweight–SBP relationship, measured as standardized β(unit=SD), was significantly amplified with increasing age, regardless of adjustment for current BMI and race. Further, the strengthened association (the increase in standardized β ranging 0.02–0.12) by adjustment for current BMI was closely related to the BMI–SBP and birthweight–BMI correlations, especially noted in childhood.
Conclusion
These findings on the potentiating effect of increasing age on the birthweight–BP relationship suggest that the fetal programming and the increasing cumulative burden with age of unhealthy lifestyle behaviors affect the development of adult hypertension in a synergistic manner.
Keywords: age-related trend, birthweight, black–white, blood pressure, BMI
Introduction
The relationship between low birthweight, an indicator of intrauterine growth retardation, and elevated blood pressure (BP) levels at various stages in postnatal life has been well established and extensively replicated in epidemiologic studies [1-12]. Further, in a pooled analysis of four cohorts, the magnitude of the birthweight–BP association was found to increase with increasing age from childhood to adulthood [10]. Although a meta-regression analysis [11] and a large cross-sectional study [12] have recently provided more evidence for the age-amplification hypothesis among adults, this hypothesis was not strongly supported by results from other studies [9,13-15]. One of the major concerns in this regard is that the age-related trend of the association may be largely resulted from the adjustment for current body size [9,14,15].
Researchers have recently outlined substantive challenges to the birthweight–BP association [16-20]. One of the criticisms is the inappropriate use of statistical methodology and improper interpretation of epidemiologic analyses in support of the fetal origins hypothesis [15-17]. It is suggested that many of the inverse associations between birthweight and adult BP might be the consequence of inappropriate adjustment for measures of current body size [14,15-20] by generating a statistical artifact known as the ‘reversal paradox’ [16,17]. To examine the timing of the effect of prenatal growth and the impact of the adjustment for current body size, longitudinal studies spanning the whole growth period, with serial measurements of growth parameters and BP, are appropriate to test the age-amplification hypothesis.
As part of the Bogalusa Heart Study, a long-term biracial (black–white) community-based epidemiologic study of the early natural history of cardiovascular disease beginning in childhood since 1973 [21], the current study examines the birthweight–BP relationship in preadolescence, adolescence and adulthood and the age-related trend of the magnitude of the association from childhood to adulthood in black and white individuals.
Methods
Study cohort
In the biracial (65% whites, 35% blacks) community of Bogalusa, Louisiana, nine cross-sectional surveys of children aged 4–17 years between 1973 and 1994 and 10 cross-sectional surveys of adults aged 18–50 years between 1977 and 2009 were conducted for cardiovascular risk factors. This panel design of repeated cross-sectional examinations conducted approximately every 2–3 years has resulted in serial observations (1–15 times) from childhood to adulthood. Children surveys had short intervals and larger sample size. Birthweight and gestational age of 6251 individuals (64.5% whites, 50.0% males) were obtained from birth certificates in the State Office of Health Statistics in New Orleans, Louisiana, USA. These 6251 individuals who had 24 363 BP observations formed the cohort of the current study. A majority (84.0%) of the study cohort had more than one [2-15] repeated BP measurements.
All individuals in this study had informed consent at each examination. For those under 18 years of age, consent of a parent/guardian was obtained. Study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center.
General examinations
All surveys since 1973 followed the same protocols, and procedures for the general examination were described elsewhere [22]. Height and weight were measured twice to ±0.1 cm and to ±0.1 kg, respectively. BMI (weight in kilograms divided by the square of the height in meters) was used as a measure of overall adiposity. In all surveys, BP levels were measured at 0800–1000 h in the morning on the right arm of individuals in a relaxed, sitting position by two trained observers (three replicates each). SBP and DBP were recorded at the first and fourth Korotkoff phases, respectively, using a mercury sphygmomanometer. The fourth Korotkoff phase was used for DBP for children and adults because our earlier study [23] showed that the fourth phase is more reliably measured in children and more predictive of adult hypertension. Further, the fifth phase is often recorded as zero at young ages. For individuals who were on medications for hypertension at the time of examination, the recorded BP values (n=405) were adjusted by adding 10 mmHg to SBP and 5 mmHg to DBP, based on average treatment effects [24,25]. We tried to include these individuals because they represent a subgroup with the highest BP.
Statistical methods
Differences in mean values of study variables between race and sex groups were tested by analysis of covariance models in preadolescence (aged 4–11 years), adolescence (aged 12–17 years) and adulthood (aged 18–50 years). In order to remove the influence of correlation between repeated measurements, for individuals who had multiple measurements of BP, only the first measurement in preadolescence and the last in adolescence and adulthood were used within growth period groups and each of the 18 age groups (see below).
Only full-term (gestational age ≥37 weeks) births were included in this analysis. The rate of fetal growth was calculated as birthweight (kg)/gestational age (week) for each individual, and then multiplied by the mean gestational age of the total sample. Thus, the adjusted birthweight solely depends on the rate of growth instead of the length of gestation period. The gestational age-adjusted birthweight was used in all subsequent analyses. The effect of birthweight on BP was examined by multiple linear regression models, adjusting for race, sex and age in preadolescence, adolescence and adulthood. Two types of regression analyses were performed, with and without adjustment for BMI, to examine the impact of BMI adjustment on the relation between birthweight and BP. The race–birthweight and sex–birthweight interaction effects on BP were tested using an interaction regression model by including respective interaction terms.
For analyses of birthweight effect on BP by age groups, all 24 363 observations were stratified into 18 groups with an equal number of individuals in each age group by race and sex. The age stratum-specific regression coefficients of BP on birthweight were first derived in separate regression models in 18 age groups with and without adjustment for current BMI. Then the two sets of the effects, measured as regression coefficients, were regressed on the midpoints of the 18 age groups to examine the age-related trend in the effect size. A significant, negative slope of the effect size with increasing age suggests an amplification of the birthweight–BP association with age. We noted that an unstandardized regression coefficient (β) solely depends on variation of the dependent variable (Y=BP) when variation of the independent variable (X=birthweight) is fixed, that is, β=Σ(X − mean)(Y − mean)/Σ(X − mean)2. BP levels and variations are well known to increase with age. The unstandardized β is expected to increase with age. For this reason, standardized βs were estimated in each of the 18 age groups for the age-amplification analyses of the birthweight effect.
Results
Characteristics of the study cohort
Table 1 shows the mean levels of study variables by race, sex and age groups. Preadolescence SBP showed a significant sex difference (males > females) in whites and a significant race difference (whites > blacks) in males. SBP in adolescence and adulthood showed significant race (blacks > whites) and sex (males > females) differences; DBP showed significant sex difference. Significant race (whites > blacks) and sex (males > females) differences were noted for birthweight for both before and after gestational age adjustment.
Table 1.
Mean levels (±SD) of study variables by race, sex and age groups
| Whites |
Blacks |
Race difference |
||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| Preadolescence (4–11 years) | n=1561 | n=1591 | n=905 | n=919 | ||
| Age (years) | 7.8±1.9 | 7.7±1.9 | 7.9±1.9 | 7.8±1.9 | 0.507 | 0.933 |
| BMI (kg/m2) | 16.9±2.8 | 16.8±3.0 | 16.7±2.8 | 16.7±3.0 | 0.097 | 0.496 |
| SBP (mmHg) | 97.0±8.5 | 96.1±9.0** | 96.1±8.8 | 95.5±9.3 | 0.006 | 0.078 |
| DBP (mmHg) | 57.8±8.2 | 58.1±8.2 | 58.1±8.0 | 57.7±8.6 | 0.496 | 0.182 |
| Adolescence (12–17 years) | n=1450 | n=1376 | n=847 | n=859 | ||
| Age (years) | 15.1±1.7 | 15.2±1.7 | 15.4±1.7 | 15.5±1.6 | <0.001 | <0.001 |
| BMI (kg/m2) | 21.7±4.4 | 21.4±4.5 | 21.6±4.6 | 22.7±4.9** | 0.263 | <0.001 |
| SBP (mmHg) | 109.7±9.8 | 107.3±8.7** | 111.5±10.7 | 109.7±9.2** | 0.005 | <0.001 |
| DBP (mmHg) | 66.4±8.0 | 68.4±7.7** | 66.9±8.6 | 69.3±7.9** | 0.976 | 0.063 |
| Adulthood (18–50 years) | n=833 | n=947 | n=455 | n=536 | ||
| Age (years) | 32.4±9.3 | 32.1±9.2 | 28.5±9.6 | 30.3±9.4** | <0.001 | <0.001 |
| BMI (kg/m2) | 28.0±6.1 | 27.0±7.4** | 26.2±6.3 | 29.0±8.3** | 0.007 | <0.001 |
| SBP (mmHg) | 117.3±12.7 | 110.5±11.6** | 120.6±16.5 | 116.5±16.9** | <0.001 | <0.001 |
| DBP (mmHg) | 78.0±10.0 | 73.9±9.0** | 77.4±13.3 | 75.9±11.8** | 0.001 | <0.001 |
| Birth registry information | n=1991 | n=1978 | n=1134 | n=1148 | ||
| Gestational age (weeks) | 39.9±1.9 | 39.8±1.9 | 39.5±2.0 | 39.4±1.8 | <0.001 | <0.001 |
| Birthweight (kg) | 3.49±0.52 | 3.37±0.50** | 3.23±0.51 | 3.13±0.48** | <0.001 | <0.001 |
| Birthweight (kg)a | 3.46±0.51 | 3.35±0.51** | 3.15±0.50 | 3.08±0.47** | <0.001 | <0.001 |
Sex difference within racial groups:
P<0.05
P<0.01.
Adjusted for gestational age.
Association by age groups
As no significant race–birthweight or sex–birthweight interaction effect was detected, the birthweight–BP association was examined using the combined sample of race and sex groups. As shown in Table 2, in model I without BMI included, race, sex and age were all significant and consistent determinants of SBP in all three age groups. Lower birthweight was significantly associated with higher SBP only in adolescence and adulthood. However, in model II with BMI included, the effect of birthweight on SBP was considerably strengthened and became significant in all three age groups. DBP showed a significant association with birthweight only in adults when BMI was included in the model. In addition, the birthweight–BMI interaction was not significant for both SBP and DBP in all three age groups.
Table 2.
Unstandardized regression coefficients of blood pressure on birth weight, adjusting for covariates, by age groups
| SBP |
DBP |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model I |
Model II |
Model I |
Model II |
|||||
| β | P | β | P | β | P | β | P | |
| Preadolescence (n=4976) | ||||||||
| Black race | −0.78 | 0.003 | −0.98 | <0.001 | 0.06 | 0.800 | −0.06 | 0.798 |
| Female sex | −0.66 | 0.007 | −0.84 | <0.001 | 0.24 | 0.297 | 0.12 | 0.570 |
| Age | 1.24 | <0.001 | 0.59 | <0.001 | 1.15 | <0.001 | 0.74 | <0.001 |
| BMI | – | – | 1.28 | <0.001 | – | – | 0.81 | <0.001 |
| Birthweighta | 0.06 | 0.808 | −1.12 | <0.001 | 0.57 | 0.074 | −0.17 | 0.425 |
| Adolescence (n=4532) | ||||||||
| Black race | 1.37 | <0.001 | 1.05 | <0.001 | 0.37 | 0.130 | 0.26 | 0.288 |
| Female sex | −2.33 | <0.001 | −2.49 | <0.001 | 2.10 | <0.001 | 2.05 | <0.001 |
| Age | 1.65 | <0.001 | 1.51 | <0.001 | 1.32 | <0.001 | 1.27 | <0.001 |
| BMI | – | – | 0.39 | <0.001 | – | – | 0.14 | <0.001 |
| Birthweight* | −0.80 | 0.004 | −1.26 | <0.001 | 0.22 | 0.332 | 0.07 | 0.775 |
| Adulthood (n=2771) | ||||||||
| Black race | 5.71 | <0.001 | 4.95 | <0.001 | 2.27 | <0.001 | 1.70 | <0.001 |
| Female sex | −6.23 | <0.001 | −6.48 | <0.001 | −3.44 | <0.001 | −3.62 | <0.001 |
| Age | 0.50 | <0.001 | 0.36 | <0.001 | 0.55 | <0.001 | 0.45 | <0.001 |
| BMI | – | – | 0.59 | <0.001 | – | – | 0.44 | <0.001 |
| Birthweighta | −1.34 | 0.010 | −2.17 | <0.001 | −0.27 | 0.470 | −0.88 | 0.013 |
Adjusted for gestational age.
Age-amplification of the association
The trend of the magnitude of the SBP–birthweight association with age derived from two regression models with and without adjustment for BMI is presented in Fig. 1. Because age distributions somewhat varied among the four race–sex groups in the study cohort, the number of individuals of the 18 age groups ranged from 1317 to 1407. The 18 standardized regression coefficients estimated in the model without adjustment for BMI decreased significantly (stronger effects) with increasing age (β=−0.0034, P<0.001). When adjusted for BMI, the 18 age stratum-specific regression coefficients were substantially strengthened, but the trend of the age amplification of the birthweight effect was weakened, although still significant (β=−0.0012, P=0.010). The slopes of the two lines derived from the models=with and without adjustment for BMI differed significantly (P=0.018), suggesting that the adjustment for BMI influenced not only the magnitude of the birthweight–SBP association but also the degree of its age-amplification phenomenon. Regression analyses of SBP by 18 age groups without adjustment for BMI were also performed with BP values (n=405) excluded for individuals who were taking antihypertensive treatment. A substantially similar age-related trend of the standardized regression coefficients (β=−0.0036, P<0.001) to that shown in Fig. 1 was yielded.
Fig. 1.
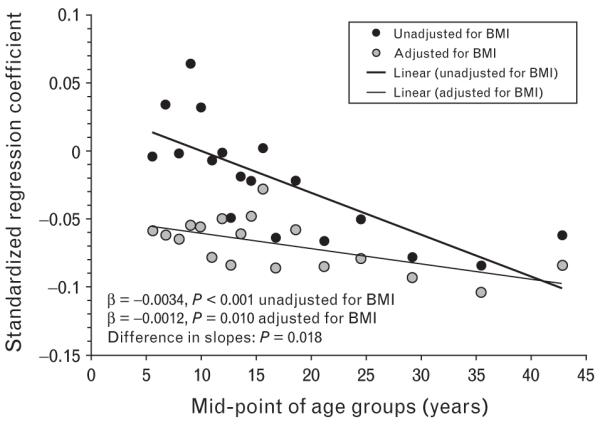
Age-related trend of the relationship between birthweight and SBP with and without adjustment for current BMI by age groups: the Bogalusa Heart Study.
Influence of BMI adjustment
Figure 2 shows the absolute values of the differences in standardized regression coefficients for SBP derived from the two models with and without adjustment for BMI. The values represent the actual distance between the two corresponding data points within each age group shown in Fig. 1. The highest peak of the difference occurred in the age group with a midpoint of 9.1 years, and the differences were greater in preadolescence than in adolescence and adulthood. The increase in the standardized regression coefficients due to adjustment for current BMI ranged from 0.02 to 0.12. Such a pattern indicates that the adjustment for BMI had more impact on the birthweight–SBP relationship in preadolescence. In addition, Pearson correlations among birthweight, current BMI and SBP were calculated in the 18 groups. The 18 BMI–SBP correlation coefficients ranged from 0.167 to 0.503 (P<0.001 for all correlations); the 18 birthweight–BMI correlation coefficients ranged from 0.057 to 0.223 (P=0.036 to P<0.001). Importantly, the two sets of correlations were both higher in preadolescence than in adolescence and adulthood, particularly for the BMI–SBP correlations, which resembled the pattern of the differences shown in Fig. 2. These similar age-related patterns suggest that the strengthened birthweight-SBP association by adjustment for BMI is driven by the strong intercorrelations among birthweight, BMI and SBP, much more in preadolescence.
Fig. 2.
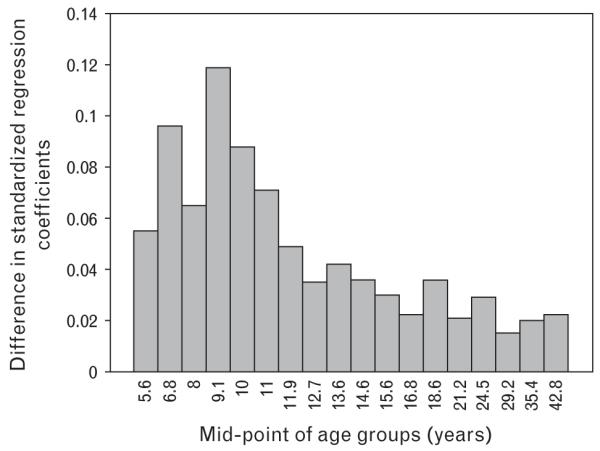
Absolute values of difference in standardized regression coefficients between the two models with and without adjustment for BMI by age groups: the Bogalusa Heart Study.
Age amplification of the association by race groups
Figure 3 shows the age-related trend of the magnitude of the SBP–birthweight association in black and white individuals without adjustment for BMI. The absolute values of standardized regression coefficients were greater in whites than in blacks, consistently for all 18 age groups (P=0.003). However, the two regression lines paralleled (difference in slopes, P=0.960), indicating that the age amplification of the SBP–birthweight association remained almost identical in blacks and whites, although the effect of birthweight on SBP was stronger in whites. Furthermore, a considerably similar pattern of the age-related associations was noted in blacks and whites in terms of the slope of the regression lines (difference in slopes, P=0.963) when BMI was included in the models for 18 age groups, indicating that the current BMI did not account for the observed age amplification of the BP–birthweight association in blacks versus whites.
Fig. 3.
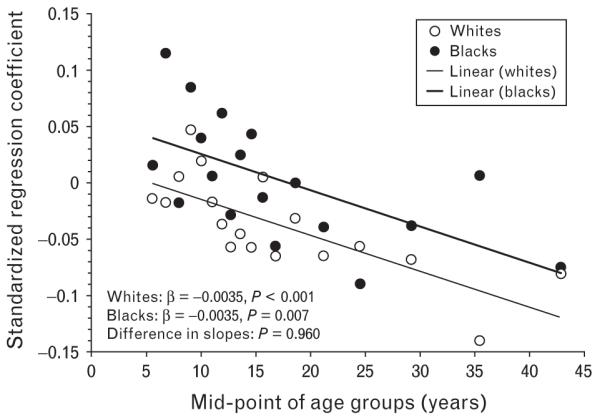
Age-related trend of the relationship between birthweight and SBP without adjustment for current BMI by age and race groups: the Bogalusa Heart Study.
Discussion
There are at least two mechanisms in the cause of essential hypertension: an initiating process in childhood that raises BP and an amplifying process that progressively magnifies the difference throughout life [26,27]. The observation that low birthweight is inversely associated with high BP at various stages in later life could indicate that adult hypertension is initiated in utero. Regarding age amplification of the birthweight effect on BP, the first study was conducted by pooling four studies of children and adults (age range=0–10, 36, 46–54 and 59–71 years) in UK [10]. It is concluded in that study that ‘essential hypertension is initiated in fetal life. A raised BP is then amplified from infancy to old age, perhaps by a positive feedback mechanism’. Recently, evidence for the age-dependent association between birthweight and SBP has been emerging from a meta-regression analysis of 197 954 adults from 20 Nordic cohorts [11]. Further, it is observed in an observational study [12] of 25 874 adults that the inverse association between birthweight and SBP was amplified with age, with a significant interaction of age and birthweight on SBP (P<0.001). On the contrary, a recent meta-analysis [13] concluded that adjusting for potential publication bias weakens the evidence for the age-amplification hypothesis. A study [15] with longitudinal BP measurements during adulthood found weak evidence of the age amplification of birthweight–SBP association, and the association was largely accounted for by current weight. In our study cohort with an age range of 4–50 years, we found that the birthweight–SBP relation was substantially amplified with advancing age from childhood to adulthood, regardless of adjustment for current BMI and race groups. The observation on the age amplification of the effect of birthweight indicates that the fetal programming and the increasing burden of unhealthy lifestyle behaviors affect the development of adult hypertension in a synergistic manner.
In previous studies [9-15], the increasing trend of the birthweight–BP association with age was examined by using unstandardized regression coefficients. It is obvious that there is a limitation in using the unstandardized regression coefficients with a unit of mmHg/kg. It is well known that BP levels and variations increase with age. Such an increase was also noted in our study cohort from preadolescence to adulthood as shown in Table 1. In the birthweight–BP association analysis, the mean values and variance of birthweight remained unchanged after birth. Therefore, the effect size of birthweight on BP is expected to increase with age when using unstandardized regression coefficients. In order to eliminate the influence of the age-dependent levels and variations of BP, standardized regression coefficients with a unit of SD for both birthweight and BP levels were applied in the current study. The strengthened association between birthweight and SBP by age observed in this study suggested that the underlying fetal programming mechanisms such as persisting changes in vascular structure, abnormalities of nephrogenesis, effects of glucocorticoid hormones and elevated sympathetic nervous system activity [3,4,28] might be amplified by aging, or individuals born with low birthweights are more susceptible to the exposure of environmental factors.
Although the ‘fetal origins’ hypothesis has been extensively studied, aspects of the methodology used have been questioned. The problem of overestimation of birthweight–BP association by the statistical adjustment for current body size in testing this hypothesis has been specifically addressed [14-20]. A simulation study demonstrated that adjustment for current weight created an inverse association when there was no genuine association between birthweight and BP; the adjustment for current weight exaggerated the magnitude of the association when there was a genuine inverse association. This statistical artifact is known as ‘reversal paradox’. It is concluded that the adjustment for current body mass is inappropriate in the regression analysis model because it is not a true confounder but part of the causal pathways between birthweight and adult BP [16,17]. We examined the birthweight–BP relation in two regression analysis models with and without BMI adjustment in age groups from childhood to adulthood. Although the birthweight–SBP association was substantially attenuated without BMI adjustment, the effect of birthweight on SBP, measured as standardized regression coefficients, was significantly strengthened with age in both models.
Whether current body size adjustment is appropriate and how to interpret the results have been controversial in the analysis of birthweight and BP [14-20]. In spite of the debate, current body size has been adjusted for in most studies. In a meta-analysis of 55 studies, the regression coefficients had been adjusted for current weight in 49 studies, and removal of this adjustment reduced the estimated association to −0.4 mmHg/kg [14]. The impact of the current body size adjustment is considered arising from a close correlation between birthweight and current body size, which are also strongly correlated with BP levels [9,16,17]. The simulation studies have [16,17] shown that the increase in the magnitude of the inverse association was considerably related to the strength of the birthweight–current weight and current weight–BP correlations. Further, adjustment for current BMI and height resulted in a strengthening of the negative association between birthweight and SBP, and the largest impact on the magnitude of the association was seen in the two youngest cohorts of 7 and 16 years; the extent and direction of the change in regression coefficient depended on the size of the correlation between birth-weight and current body size and between current body size and SBP [9]. In this study, the effect size of birth-weight on SBP was strengthened by adjustment for current BMI (Figs. 1 and 2), more in childhood than in adulthood. A substantial resemblance of the two patterns of correlations between current BMI and SBP and between brithweight and current BMI, especially the former, was found in the current study cohort. It provides additional evidence supporting the concept that investigators should consider the potential risk of overestimation of the association due to adjustment for current body weight, particularly in a pediatric population.
This community-based epidemiologic study has certain limitations. First, cross-sectional and longitudinal surveys were combined in this report because large sample size was needed for the analysis by age groups. Second, we tried to include hypertension patients who were taking antihypertensive medications because these patients represent a subgroup with the highest BP. An adjustment of adding 10 and 5 mm Hg to SBP and DBP values, respectively, was used. Furthermore, information on the drugs used for the treatment was not available. Although this approach has been commonly used based on average treatment effects, it is an approximate estimation and may lead to a bias in data analyses to some extent. Third, children and adults are expected to have completely different information on several types of socioeconomic variables such as marital status, household income or education and so on. Such inconsistent information did not allow us to adjust for the socioeconomic status in the age-related trend analysis in the current study.
In summary, the findings of the current study provide strong evidence supporting the hypothesis that the association between birthweight and SBP is amplified with increasing age from childhood to adulthood. The adjustment for current body size considerably strengthens the birthweight–SBP association. These results suggest that the fetal programming related to intrauterine malnutrition and the increasing burden with age of unhealthy lifestyle behaviors affect the development of adult hypertension in a synergistic manner. For studies assessing the birthweight–BP relationship, age has to be taken into account as an indicator of overall cumulative burden of environmental exposures throughout life. On the basis of these observations, further studies on birthweight–lifestyle interaction and the impact of growth patterns during childhood on adulthood BP levels are suggested.
Acknowledgements
This study was supported by grant numbers HD-061437 and HD-062783 from the National Institute of Child Health and Human Development, 0855082E from American Heart Association and AG-16592 from the National Institute on Aging.
Abbreviation
- BP
blood pressure
Footnotes
There are no conflicts of interest.
References
- 1.Barker DJP, Osmond C, Golding J, Kuth D, Wadsworth MEJ. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leon DA, Johansson M, Rasmussen F. Gestational age and growth rate of fetal mass are inversely associated with systolic blood pressure in young adults: an epidemiologic study of 165,136 Swedish men aged 18 years. Am J Epidemiol. 2000;152:597–604. doi: 10.1093/aje/152.7.597. [DOI] [PubMed] [Google Scholar]
- 3.Nuyt AM, Alexander BT. Developmental programming and hypertension. Hypertension. 2009;18:144–152. doi: 10.1097/MNH.0b013e328326092c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner DS, Bell RC, Symonds ME. Fetal mechanisms that lead to later hypertension. Curr Drug Targets. 2007;8:894–905. doi: 10.2174/138945007781386901. [DOI] [PubMed] [Google Scholar]
- 5.Mzayek F, Hassig S, Sherwin R, Hughes J, Chen W, Srinivasan SR, Berenson GS. The relationship of birth weight with the developmental trends of blood pressure from childhood through mid-adulthood. The Bogalusa Heart Study. Am J Epidemiol. 2007;166:413–420. doi: 10.1093/aje/kwm098. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson PM, Östergren P-O, Nyberg P, Söderström M, Allebeck P. Low birth weight is associated with elevated systolic blood pressure in adolescence: a prospective study of a birth cohort of 149 378 Swedish boys. J Hypertens. 1997;15:1627–1631. doi: 10.1097/00004872-199715120-00064. [DOI] [PubMed] [Google Scholar]
- 7.Zhao M, Shu XO, Jin F, Yang G, Li HL, Liu DK, et al. Birthweight, childhood growth and hypertension in adulthood. Int J Epidemiol. 2002;31:1043–1051. doi: 10.1093/ije/31.5.1043. [DOI] [PubMed] [Google Scholar]
- 8.Lurbe E, Carvajal E, Torro I, Aguilar F, Alvarez J, Redon J. Influence of concurrent obesity and low birth weight on blood pressure phenotype in youth. Hypertension. 2009;53:912–917. doi: 10.1161/HYPERTENSIONAHA.109.129155. [DOI] [PubMed] [Google Scholar]
- 9.Hardy R, Sovio U, King VJ, Skidmore PM, Helmsdal G, Olsen SF, et al. EURO–BLCS Study Group Birth weight and blood pressure in five European birth cohort studies: an investigation of confounding factors. Eur J Public Health. 2006;16:21–30. doi: 10.1093/eurpub/cki171. [DOI] [PubMed] [Google Scholar]
- 10.Law CM, de Swiet M, Osmond C, Fayers PM, Barker DJ, Cruddas AM, Fall CH. Initiation of hypertension in utero and its amplification throughout life. BMJ. 1993;306:24–27. doi: 10.1136/bmj.306.6869.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gamborg M, Byberg L, Rasmussen F, Andersen PK, Baker JL, Bengtsson C, et al. NordNet Study Group Birth weight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific results from 20 Nordic studies. Am J Epidemiol. 2007;166:634–645. doi: 10.1093/aje/kwm042. [DOI] [PubMed] [Google Scholar]
- 12.Davies AA, Smith GD, May MT, Ben-Shlomo Y. Association between birth weight and blood pressure is robust, amplifies with age, and may be underestimated. Hypertension. 2006;48:431–436. doi: 10.1161/01.HYP.0000236551.00191.61. [DOI] [PubMed] [Google Scholar]
- 13.Schluchter MD. Publication bias and heterogeneity in the relationship between systolic blood pressure, birth weight, and catch-up growth: a meta analysis. J Hypertens. 2003;21:273–279. doi: 10.1097/00004872-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birth weight and subsequent blood pressure? Lancet. 2002;360:659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 15.Hardy R, Kuh D, Langenberg C, Wadsworth ME. Birthweight, childhood social class, and change in adult blood pressure in the 1946 British birth cohort. Lancet. 2003;362:1178–1183. doi: 10.1016/S0140-6736(03)14539-4. [DOI] [PubMed] [Google Scholar]
- 16.Tu YK, West R, Ellison GT, Gilthorpe MS. Why evidence for the fetal origins of adult disease might be a statistical artifact: the ‘reversal paradox’ for the relation between birth weight and blood pressure in later life. Am J Epidemiol. 2005;161:27–32. doi: 10.1093/aje/kwi002. [DOI] [PubMed] [Google Scholar]
- 17.Tu YK, Gilthorpe MS, Ellison GT. What is the effect of adjusting for more than one measure of current body size on the relation between birth weight and blood pressure? J Hum Hypertens. 2006;20:646–657. doi: 10.1038/sj.jhh.1002044. [DOI] [PubMed] [Google Scholar]
- 18.Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease: the hypothesis revisited. BMJ. 1999;319:245–249. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adair L, Dahly D. Developmental determinants of blood pressure in adults. Annu Rev Nutr. 2005;25:407–434. doi: 10.1146/annurev.nutr.25.050304.092538. [DOI] [PubMed] [Google Scholar]
- 20.Paneth N, Ahmed F, Stein AD. Early nutritional origins of hypertension: a hypothesis still lacking support. J Hypertens Suppl. 1996;14:S121–S129. [PubMed] [Google Scholar]
- 21.The Bogalusa Heart Study 20th Anniversary Symposium. Am J Med Sci. 1995;310(Suppl 1):S1–S138. doi: 10.1097/00000441-199512000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Berenson GS, McMahan CA, Voors AW, Webber LS, Srinivasan SR, Frank GC, et al. In: Cardiovascular risk factors in children: the early natural history of atherosclerosis and essential hypertension. Andrews C, Hester HE, editors. Oxford University Press; New York, USA: 1980. pp. 47–123. [Google Scholar]
- 23.Elkasabany AM, Urbina EM, Daniels SR, Srinivasan SR, Berenson GS. Prediction of adult blood pressure by K4 and K5 diastolic blood pressure in children: the Bogalusa Heart Study. J Pediatr. 1998;132:687–692. doi: 10.1016/s0022-3476(98)70361-0. [DOI] [PubMed] [Google Scholar]
- 24.Cui J, Hopper JL, Harrap SB. Genes and family environment explain correlations between blood pressure and body mass index. Hypertension. 2002;40:7–12. doi: 10.1161/01.hyp.0000022693.11752.e9. [DOI] [PubMed] [Google Scholar]
- 25.Neaton JD, Grimm RH, Jr, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, et al. Treatment of Mild Hypertension Study Research Group Treatment of Mild Hypertension Study. Final results. JAMA. 1993;270:713–724. [PubMed] [Google Scholar]
- 26.Voors AW, Berenson GS. Search for the determinants of the early onset of essential hypertension. In: Onesti G, Kim KE, editors. Phasic pressor mechanisms: hypertension in the young and the old. Grune and Stratton; New York, USA: 1981. pp. 43–55. [Google Scholar]
- 27.Lever AF, Harrap SB. Essential hypertension: a disorder of growth with origins on childhood. J Hypertens. 1992;10:101–120. doi: 10.1097/00004872-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Schreuder MF, Nauta J. Prenatal programming of nephron number and blood pressure. Kidney Int. 2007;72:265–268. doi: 10.1038/sj.ki.5002307. [DOI] [PubMed] [Google Scholar]


