Abstract
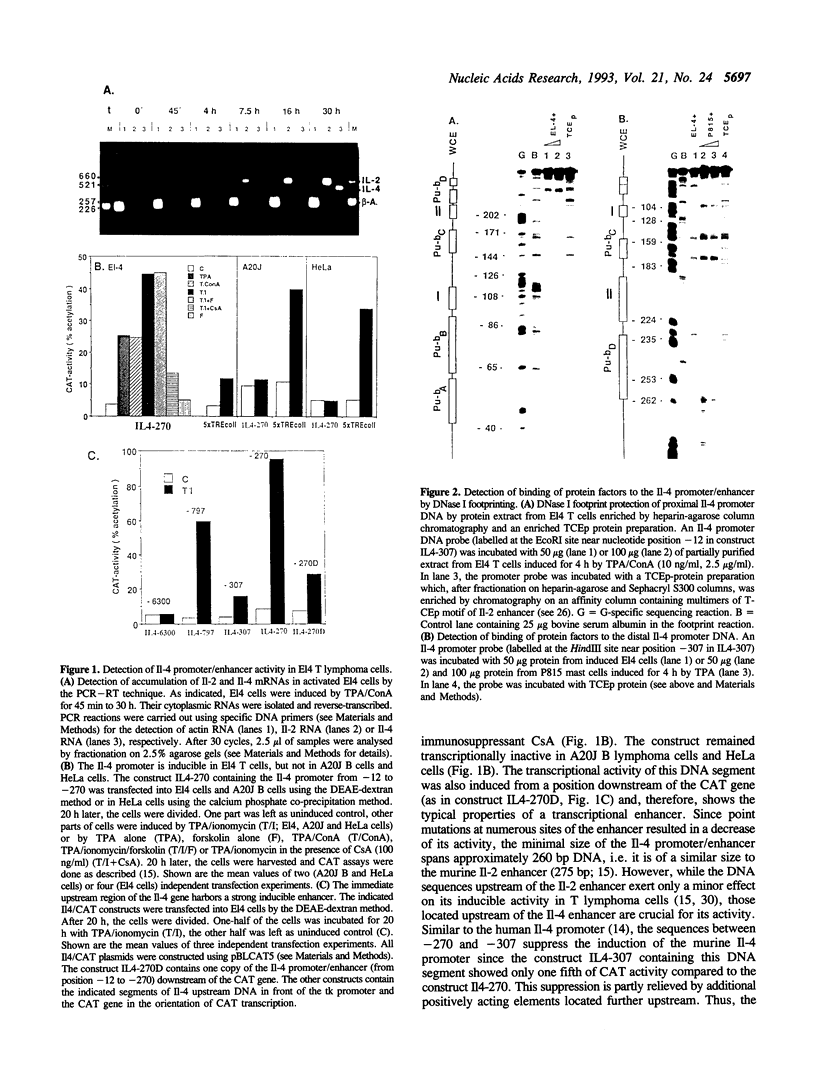
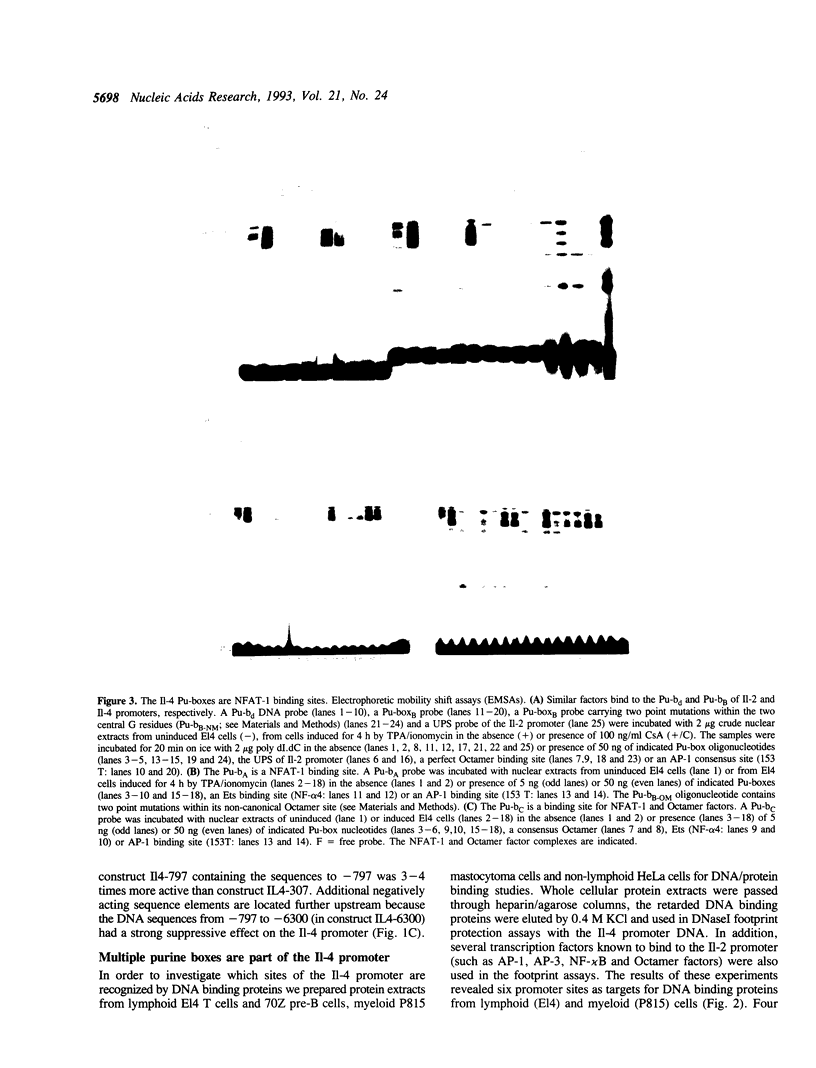
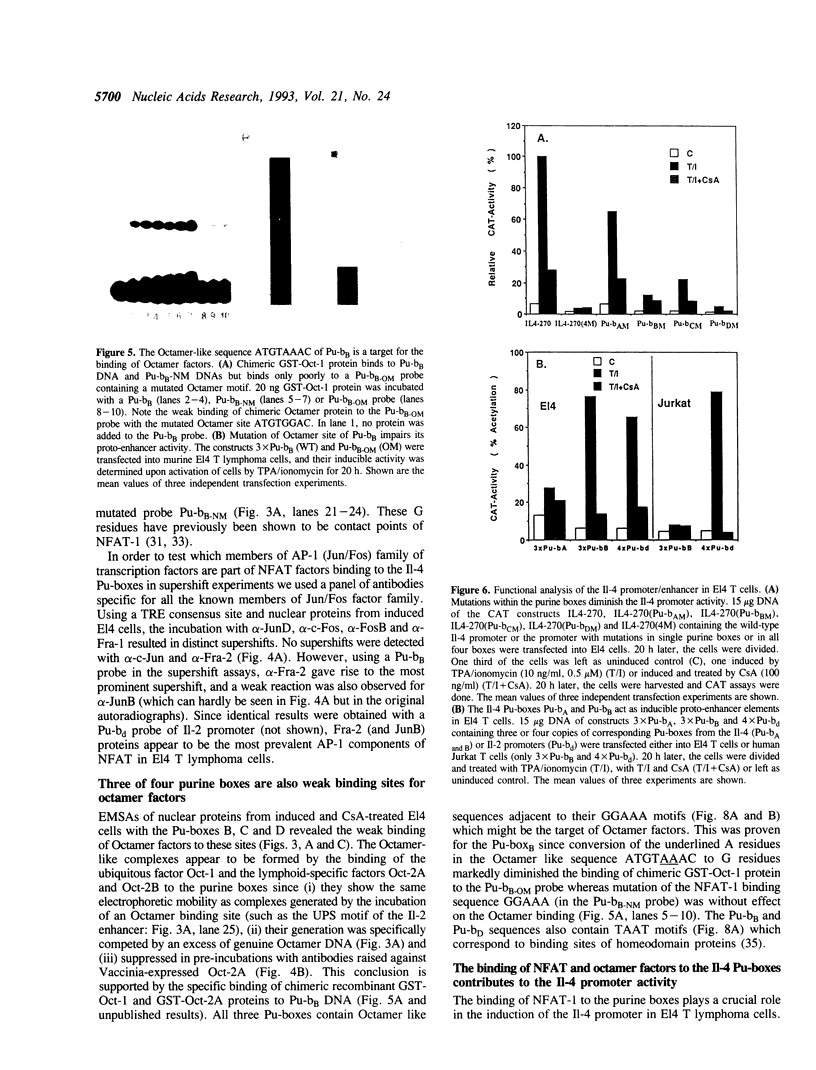
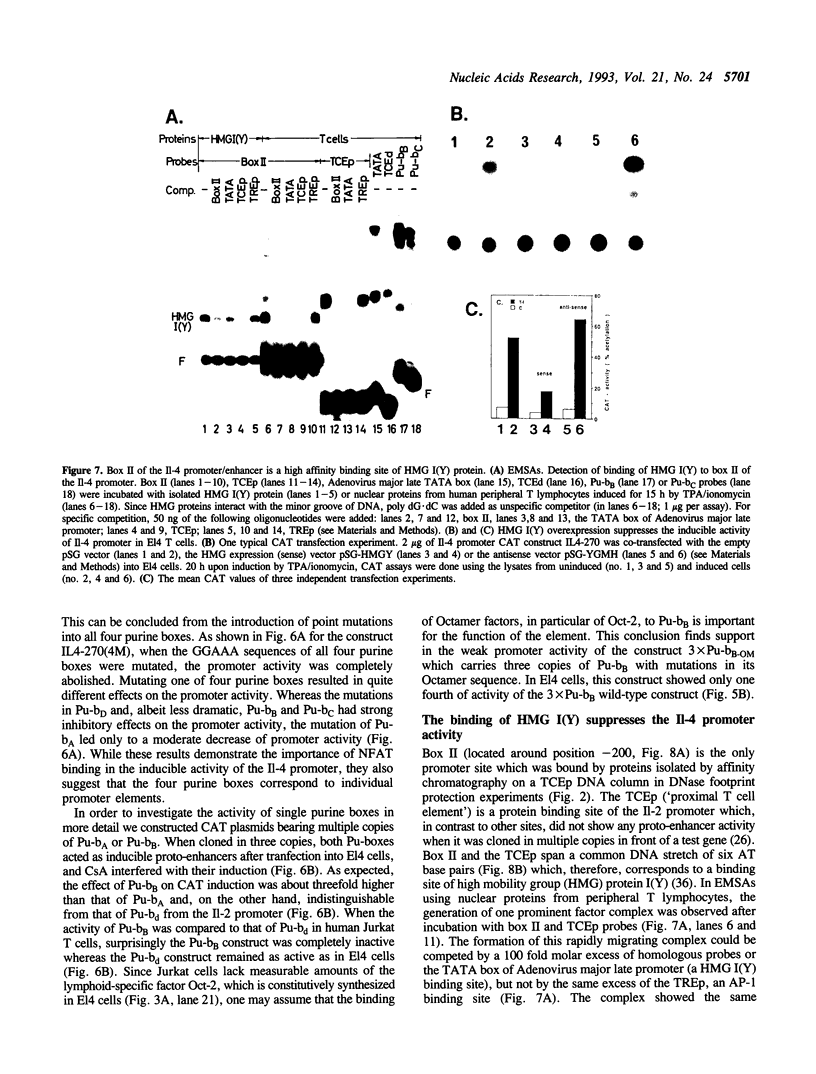
We show here that the immediate upstream region (from position -12 to -270) of the murine interleukin 4 (Il-4) gene harbors a strong cell-type specific transcriptional enhancer. In T lymphoma cells, the activity of the Il-4 promoter/enhancer is stimulated by phorbol esters, Ca++ ionophores and agonists of protein kinase A and inhibited by low doses of the immunosuppressant cyclosporin A. The Il-4 promoter/enhancer is transcriptionally inactive in B lymphoma cells and HeLa cells. DNase I footprint protection experiments revealed six sites of the Il-4 promoter/enhancer to be bound by nuclear proteins from lymphoid and myeloid cells. Among them are four purine boxes which have been described to be important sequence motifs of the Il-2 promoter. They contain the motif GGAAA and are recognized by the inducible and cyclosporin A-sensitive transcription factor NFAT-1. Three of the Il-4 NFAT-1 sites are closely linked to weak binding sites of Octamer factors. Several purine boxes and an AT-rich protein-binding site of the Il-4 promoter are also recognized by the high mobility group protein HMG I(Y). Whereas the binding of NFAT-1 and Octamer factors enhance the activity of the Il-4 promoter, the binding of HMG I(Y) suppresses its activity and, therefore, appears to be involved in the suppression of Il-4 transcription in resting T lymphocytes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., De Waal Malefyt R., Matsuda I., Arai K., Arai N. An 11-base-pair DNA sequence motif apparently unique to the human interleukin 4 gene confers responsiveness to T-cell activation signals. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2864–2868. doi: 10.1073/pnas.89.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N., Naito Y., Watanabe M., Masuda E. S., Yamaguchi-Iwai Y., Tsuboi A., Heike T., Matsuda I., Yokota K., Koyano-Nakagawa N. Activation of lymphokine genes in T cells: role of cis-acting DNA elements that respond to T cell activation signals. Pharmacol Ther. 1992;55(3):303–318. doi: 10.1016/0163-7258(92)90054-4. [DOI] [PubMed] [Google Scholar]
- Arai N., Nomura D., Villaret D., DeWaal Malefijt R., Seiki M., Yoshida M., Minoshima S., Fukuyama R., Maekawa M., Kudoh J. Complete nucleotide sequence of the chromosomal gene for human IL-4 and its expression. J Immunol. 1989 Jan 1;142(1):274–282. [PubMed] [Google Scholar]
- Benoist C., Mathis D. Regulation of major histocompatibility complex class-II genes: X, Y and other letters of the alphabet. Annu Rev Immunol. 1990;8:681–715. doi: 10.1146/annurev.iy.08.040190.003341. [DOI] [PubMed] [Google Scholar]
- Betz M., Fox B. S. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991 Jan 1;146(1):108–113. [PubMed] [Google Scholar]
- Boise L. H., Petryniak B., Mao X., June C. H., Wang C. Y., Lindsten T., Bravo R., Kovary K., Leiden J. M., Thompson C. B. The NFAT-1 DNA binding complex in activated T cells contains Fra-1 and JunB. Mol Cell Biol. 1993 Mar;13(3):1911–1919. doi: 10.1128/mcb.13.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T., Pfeuffer I., Schorr E., Siebelt F., Wirth T., Serfling E. Transforming growth factor beta and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol Cell Biol. 1993 Feb;13(2):1155–1162. doi: 10.1128/mcb.13.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T., Pietrowski I., Serfling E. The immunosuppressives FK 506 and cyclosporin A inhibit the generation of protein factors binding to the two purine boxes of the interleukin 2 enhancer. Nucleic Acids Res. 1991 Jan 11;19(1):61–67. doi: 10.1093/nar/19.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel K., Hentsch B., Pfeuffer I., Serfling E. One base pair change abolishes the T cell-restricted activity of a kB-like proto-enhancer element from the interleukin 2 promoter. Nucleic Acids Res. 1991 Nov 11;19(21):5929–5936. doi: 10.1093/nar/19.21.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catron K. M., Iler N., Abate C. Nucleotides flanking a conserved TAAT core dictate the DNA binding specificity of three murine homeodomain proteins. Mol Cell Biol. 1993 Apr;13(4):2354–2365. doi: 10.1128/mcb.13.4.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Shannon M. F., Bert A. G., Ryan G. R., Vadas M. A. The granulocyte-macrophage colony-stimulating factor/interleukin 3 locus is regulated by an inducible cyclosporin A-sensitive enhancer. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2466–2470. doi: 10.1073/pnas.90.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman M. J., Montgomery R. A., Larsen C. P., Wanders A., Wells A. F. Cytokine gene expression: analysis using northern blotting, polymerase chain reaction and in situ hybridization. Immunol Rev. 1991 Feb;119:163–179. doi: 10.1111/j.1600-065x.1991.tb00583.x. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989 Dec;19(12):2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Fashena S. J., Reeves R., Ruddle N. H. A poly(dA-dT) upstream activating sequence binds high-mobility group I protein and contributes to lymphotoxin (tumor necrosis factor-beta) gene regulation. Mol Cell Biol. 1992 Feb;12(2):894–903. doi: 10.1128/mcb.12.2.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel G., Weiss D. L., McCoy R., Deloughery T., Tara D., Brown M. A. A DNase I-hypersensitive site in the second intron of the murine IL-4 gene defines a mast cell-specific enhancer. J Immunol. 1992 Nov 15;149(10):3239–3246. [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Valge-Archer V. E., Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992 Apr 30;356(6372):801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- Jain J., Valge-Archer V. E., Sinskey A. J., Rao A. The AP-1 site at -150 bp, but not the NF-kappa B site, is likely to represent the major target of protein kinase C in the interleukin 2 promoter. J Exp Med. 1992 Mar 1;175(3):853–862. doi: 10.1084/jem.175.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. R., Lehn D. A., Elton T. S., Barr P. J., Reeves R. Complete murine cDNA sequence, genomic structure, and tissue expression of the high mobility group protein HMG-I(Y). J Biol Chem. 1988 Dec 5;263(34):18338–18342. [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Kang S. M., Beverly B., Tran A. C., Brorson K., Schwartz R. H., Lenardo M. J. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992 Aug 21;257(5073):1134–1138. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M. Transcriptional regulation during T-cell development: the alpha TCR gene as a molecular model. Immunol Today. 1992 Jan;13(1):22–30. doi: 10.1016/0167-5699(92)90200-q. [DOI] [PubMed] [Google Scholar]
- Li-Weber M., Eder A., Krafft-Czepa H., Krammer P. H. T cell-specific negative regulation of transcription of the human cytokine IL-4. J Immunol. 1992 Mar 15;148(6):1913–1918. [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lohoff M., Schmitt E., Reske-Kunz A. B., Röllinghoff M. Different response of TH1 cells for stimulation with anti-CD3 antibodies. Eur J Immunol. 1990 Mar;20(3):653–658. doi: 10.1002/eji.1830200328. [DOI] [PubMed] [Google Scholar]
- Lohoff M., Sommer F., Solbach W., Röllinghoff M. Coexistence of antigen-specific TH1 and TH2 cells in genetically susceptible BALB/c mice infected with Leishmania major. Immunobiology. 1989 Oct;179(4-5):412–421. doi: 10.1016/S0171-2985(89)80045-2. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Mouzaki A., Weil R., Muster L., Rungger D. Silencing and trans-activation of the mouse IL-2 gene in Xenopus oocytes by proteins from resting and mitogen-induced primary T-lymphocytes. EMBO J. 1991 Jun;10(6):1399–1406. doi: 10.1002/j.1460-2075.1991.tb07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz E., Zubiaga A. M., Merrow M., Sauter N. P., Huber B. T. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990 Jul 1;172(1):95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop J. P., Crabtree G. R., Mattila P. S. Negative regulation of interleukin 2 transcription by the glucocorticoid receptor. J Exp Med. 1992 May 1;175(5):1235–1245. doi: 10.1084/jem.175.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop J. P., Ullman K. S., Crabtree G. R. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem. 1993 Feb 5;268(4):2917–2923. [PubMed] [Google Scholar]
- Novak T. J., Rothenberg E. V. cAMP inhibits induction of interleukin 2 but not of interleukin 4 in T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9353–9357. doi: 10.1073/pnas.87.23.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak T. J., White P. M., Rothenberg E. V. Regulatory anatomy of the murine interleukin-2 gene. Nucleic Acids Res. 1990 Aug 11;18(15):4523–4533. doi: 10.1093/nar/18.15.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Otsuka T., Villaret D., Yokota T., Takebe Y., Lee F., Arai N., Arai K. Structural analysis of the mouse chromosomal gene encoding interleukin 4 which expresses B cell, T cell and mast cell stimulating activities. Nucleic Acids Res. 1987 Jan 12;15(1):333–344. doi: 10.1093/nar/15.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randak C., Brabletz T., Hergenröther M., Sobotta I., Serfling E. Cyclosporin A suppresses the expression of the interleukin 2 gene by inhibiting the binding of lymphocyte-specific factors to the IL-2 enhancer. EMBO J. 1990 Aug;9(8):2529–2536. doi: 10.1002/j.1460-2075.1990.tb07433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E., Van Brandwijk R., Van Snick J., Siebold B., Rüde E. TCGF III/P40 is produced by naive murine CD4+ T cells but is not a general T cell growth factor. Eur J Immunol. 1989 Nov;19(11):2167–2170. doi: 10.1002/eji.1830191130. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Sher A., Coffman R. L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Strauss F., Varshavsky A. A mammalian high mobility group protein recognizes any stretch of six A.T base pairs in duplex DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1276–1280. doi: 10.1073/pnas.83.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S. J., Gold J. S., Murphy T. L., Murphy K. M. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells: roles for NF-Y and NF-ATc. Mol Cell Biol. 1993 Aug;13(8):4793–4805. doi: 10.1128/mcb.13.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992 Nov 27;71(5):777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- Todd M. D., Grusby M. J., Lederer J. A., Lacy E., Lichtman A. H., Glimcher L. H. Transcription of the interleukin 4 gene is regulated by multiple promoter elements. J Exp Med. 1993 Jun 1;177(6):1663–1674. doi: 10.1084/jem.177.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca A., Felli M. P., Farina A. R., Martinotti S., Maroder M., Screpanti I., Meco D., Petrangeli E., Frati L., Gulino A. Glucocorticoid receptor-mediated suppression of the interleukin 2 gene expression through impairment of the cooperativity between nuclear factor of activated T cells and AP-1 enhancer elements. J Exp Med. 1992 Mar 1;175(3):637–646. doi: 10.1084/jem.175.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M., Zastrow G., Klavinius A., Schwender S., Müller F., Luksza H., Hoppe J., Wienberg J., Grummt F. Cis-acting sequences from mouse rDNA promote plasmid DNA amplification and persistence in mouse cells: implication of HMG-I in their function. Nucleic Acids Res. 1989 Dec 11;17(23):9909–9932. doi: 10.1093/nar/17.23.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. M., Moolten D., Burlein J., Romano J., Bhaerman R., Godillot A., Mellon M., Rauscher F. J., 3rd, Kant J. A. Identification of a zinc finger protein that inhibits IL-2 gene expression. Science. 1991 Dec 20;254(5039):1791–1794. doi: 10.1126/science.1840704. [DOI] [PubMed] [Google Scholar]
- Winoto A., Baltimore D. A novel, inducible and T cell-specific enhancer located at the 3' end of the T cell receptor alpha locus. EMBO J. 1989 Mar;8(3):729–733. doi: 10.1002/j.1460-2075.1989.tb03432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen H. F., Rothblum L. I. Purification and characterization of a high-mobility-group-like DNA-binding protein that stimulates rRNA synthesis in vitro. Mol Cell Biol. 1988 Aug;8(8):3406–3414. doi: 10.1128/mcb.8.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]










