Abstract
Nutrition plays a critical role in maternal and fetal health; however, research on error in the measurement of energy intake during pregnancy is limited. The authors analyzed data on 998 women living in central North Carolina with singleton pregnancies during 2001–2005. Second-trimester diet was assessed by food frequency questionnaire. Estimated energy requirements were calculated using Institute of Medicine prediction equations, with adjustment for energy costs during the second trimester. Implausible values for daily energy intake were determined using confidence limits of agreement for energy intake/estimated energy requirements. Prevalences of low energy reporting (LER) and high energy reporting (HER) were 32.8% and 12.9%, respectively. In a multivariable analysis, pregravid body mass index was related to both LER and HER; LER was higher in both overweight (odds ratio = 1.96, 95% confidence interval: 1.26, 3.02; P = 0.031) and obese (odds ratio = 3.29, 95% confidence interval: 2.33, 4.65; P < 0.001) women than in normal-weight counterparts. Other predictors of LER included marriage and higher levels of physical activity. HER was higher among subjects who were underweight, African-American, and less educated and subjects who had higher depressive symptom scores. LER and HER are prevalent during pregnancy. Identifying their predictors may improve data collection and analytic methods for reducing systematic bias in the study of diet and reproductive outcomes.
Keywords: bias (epidemiology), diet, energy intake, nutrition assessment, obesity, pregnancy
Accurately measuring dietary intake in human populations is challenging. Population-based studies typically rely on self-reported dietary assessment, which is subject to considerable measurement error. A growing body of literature has demonstrated that subjects tend to underreport energy and nutrient intakes, and this underreporting occurs more frequently in certain subgroups, such as women and overweight subjects (1). Further, there is accumulating evidence that nutrient risk estimates incorporate this systematic error, which can bias the measure of association (2–4).
Pregnancy is a complex period of human growth for both the mother and the fetus. The course of pregnancy creates some unique physiologic, medical, and psychosocial demands, and these demands affect maternal energy and nutrient needs, appetite, and meal patterns (5, 6). Maternal nutrition plays an important role during this time; however, reported associations between dietary exposures and pregnancy outcomes have been modest or nonexistent (7). One reason for this may be systematic reporting bias in nutritional data, but very little is known about this error in pregnant populations. Therefore, it is possible that the frequency and patterns of measurement error in energy intake may differ between pregnant and nonpregnant populations.
Only a few studies have investigated the misreporting of maternal dietary energy intake (8–10), and to our knowledge, the only population-based study of misreporting was conducted in 490 pregnant Indonesian women. Winkvist et al. (10) found a prevalence of energy underreporting of 16.2% during the second trimester and noted that underreporting was more common among women with a body mass index (BMI; weight (kg)/height (m)2) greater than 25.0. However, this cohort may not be representative of women in developed countries; furthermore, several important predictors were not examined, including certain pregnancy characteristics such as gestational weight gain. Two smaller studies conducted in the United Kingdom also found evidence of energy underreporting among pregnant women but were limited because of small samples (8, 9). Therefore, the prevalence, magnitude, and predictors of measurement error in energy intake among pregnant women remain unclear. These components may be used to improve data collection and analytic methods in order to reduce systematic bias in reproductive studies. For example, dietary assessment tools may be tailored to reduce this type of error in subgroups that are particularly susceptible. Further, stratifying results by low energy reporting and adequate energy reporting can help in interpreting the results of studies of relations between diet and disease outcomes.
To identify measurement error in energy intake, it is necessary to have an objective estimate of energy requirements, which are usually based on total energy expenditure (TEE). Doubly labeled water is generally considered the gold standard for assessment of TEE (1); however, this technique is very costly and is not practical for large population studies. In 2002, prediction equations for calculating estimated energy requirements (EER) were published as part of the Institute of Medicine Dietary Reference Intakes (11). EER equations were developed from an extensive normative doubly labeled water database, which included TEE measurements on adults, children, and pregnant women with a variety of physical activity levels. Dietary Reference Intake equations for EER have been utilized in recent studies on identifying energy underreporting (12, 13), and their accuracy compared with doubly labeled water has been independently corroborated (14). Measurement error in energy intake is typically classified as low energy reporting and high energy reporting. These categories represent implausible energy intakes and are determined using confidence limits of agreement, which account for the within-subject variation expected from the methods used to estimate energy intake and TEE.
The food frequency questionnaire (FFQ) has been shown to be an appropriate method for assessing habitual dietary intake in a wide variety of epidemiologic settings, including studies among pregnant women (15–20). We examined measurement error in daily energy intake during the second trimester from an FFQ among subjects who participated in the third phase of the Pregnancy, Infection, and Nutrition Study (PIN3).
MATERIALS AND METHODS
Study population
PIN3 was a prospective study designed to examine whether certain maternal characteristics, such as maternal physical activity or stress, are associated with preterm birth. Women enrolled in PIN3 were recruited from prenatal clinics included in the University of North Carolina hospital system (UNC Hospitals) at <20 weeks’ gestation from January 2001 through June 2005. Potential subjects were identified by study staff through a review of all medical charts of new prenatal patients. Informed consent was obtained at the time of recruitment. Women were excluded if they were under age 16 years, did not speak English, were not planning to continue care or deliver at the study site, had a multiple pregnancy, or did not have a telephone from which they could complete telephone interviews.
A total of 2,006 women were recruited. Of the 1,446 subjects who completed the FFQ, 319 were missing the survey on restrained eating behaviors, which was added to the study protocol after enrollment began; 8 were missing data on pregravid height and weight; and an additional 119 subjects were missing data for 1 or more other variables of interest. Some women were recruited into the cohort more than once because of additional pregnancies within the recruitment period. In these instances (n = 35), the pregnancy with the most complete information or the first pregnancy (when information was complete for both pregnancies) was included in the analysis. Data from the remaining 988 pregnancies were used in this analysis.
Data collection
The PIN study protocols were reviewed and approved by the institutional review board of the School of Medicine at the University of North Carolina at Chapel Hill. Enrolled women were asked to complete 2 research clinic visits (at <20 and 24–29 weeks’ gestation) and 2 telephone interviews (at 17–22 and 27–30 weeks’ gestation). At each clinic visit, psychosocial domains were assessed via a self-administered questionnaire. Additionally, an FFQ was distributed at the second clinic visit to ascertain dietary intake. Telephone interviews were conducted to collect a variety of data, including demographic characteristics, pregnancy history, and physical activity. Following delivery, data from medical charts were abstracted. Pregnancies were dated using an algorithm based on the first ultrasonogram performed prior to 22 weeks’ gestation (up to 21 weeks, 6 days). If no ultrasonogram was performed or none was performed prior to the start of week 22, then the last menstrual period was used to date the pregnancy.
Self-reported pregravid weight and measured height were recorded at the first prenatal visit. Weight measurements taken at the first prenatal clinic visit were compared with the self-reported pregravid weights to identify biologically implausible weight gains. In such cases, an imputed weight was calculated using the measured weight at the first prenatal visit (if taken prior to 16 weeks) minus the recommended amount of weight to be gained in the first and second trimesters as defined by the 1990 Institute of Medicine recommendations (21). Pregravid BMI was then calculated by using either reported or imputed pregravid weight and measured height. The rate of gestational weight gain during the second trimester was calculated as the difference between the first clinically measured weight following 12 weeks’ gestation and the last clinically measured weight recorded prior to week 27, divided by the number of weeks between measurements. Cutpoints to determine inadequate and excessive weight gains were based on the 1990 Institute of Medicine BMI-specific recommendations previously used in the literature (21).
Dietary information was collected at 26–29 weeks of gestation using a self-administered 110-item Block-98 FFQ. This FFQ was modified to assess intake over the previous 3 months. Daily energy and nutrient intakes were estimated from all foods and beverages. The Block FFQ has been validated in several populations (22–24), including participants in the first 2 phases of the PIN Study (PIN1 and PIN2). Deattenuated Pearson correlation coefficients for the correlation of total energy intakes between the FFQ and multiple 24-hour dietary recalls was 0.32 for PIN1 and 0.33 for PIN2. A more detailed description of the PIN FFQ has been published elsewhere (25).
Physical activity data were captured using a 1-week recall questionnaire specifically designed for PIN3, which was administered by telephone at 17–22 weeks’ gestation. This instrument assessed the frequency, duration, and intensity of a variety of reported physical activities over the past 7 days at either a moderate or a vigorous intensity level. Domains incorporated the following settings and/or roles: activity at work, for recreation, for transportation, during care-giving, and as a part of indoor and outdoor household tasks. The criterion validity of this questionnaire was examined in 177 pregnant women who wore an accelerometer for 1 week, kept a daily structured diary, and, following these 2 measures, completed a 1-week recall of the PIN3 physical activity questionnaire. The Spearman correlation coefficient was 0.67 (95% confidence interval (CI): 0.55, 0.78) for total activity in metabolic equivalent-hours per week.
The Revised Restraint Scale (26) was administered to assess preconception dieting and restrained eating behaviors. It consists of 10 questions in a multiple-choice format: 5 that pertain to diet and weight history and 5 that pertain to concern with food and eating. Responses to questions regarding dieting behaviors were based on the Likert scale. The wording of the Revised Restraint Scale was changed so that it was clear that the questions focused on the period prior to pregnancy and not on weight changes associated with pregnancy. An overall score for restrained eating was calculated by summing the scores for all of the questions. Comparisons were made between subjects above and below the median score (26).
The Center for Epidemiologic Studies Depression Scale (27) was administered to assess psychological disposition or generalized distress. The 20-item scale has Likert-type response categories assessing feelings and activities the respondent has experienced during the past week. The scale ranges from 0 points to 60 points. A score of 25 or higher on the scale was considered to indicate a significant number of depressive symptoms.
The EER for each subject was calculated using the 2002 Dietary Reference Intake equations, which are sex- and age-specific and are based on age, weight, and height (11). For pregnant women, the Dietary Reference Intake equations include an additional 340 kcal/day, which was found to be the average energy cost of pregnancy during the second trimester. However, recent evidence has shown that TEE during pregnancy is dependent on pregravid weight status. Using doubly labeled water, Butte et al. (28) estimated the energy requirements of a group of healthy underweight, normal-weight, and overweight pregnant women. Values for the energy costs of pregnancy for the second trimester were 163 kcal/day for subjects with a low BMI (defined as ≤19.8), 356 kcal/day for subjects with a normal BMI (defined as >19.8–<26.0), and 441 kcal/day for subjects with a high BMI (defined as ≥26) (28). We applied these values to our calculation of TEE, which justified our use of the 1990 Institute of Medicine cutpoints for pregravid BMI.
EER equations also allow for 4 levels of physical activity: sedentary, low activity, active, and very active, with a corresponding physical activity coefficient. Each subject was assigned an activity level based on her average daily minutes of moderate-to-vigorous physical activity from the PIN 7-day physical activity recall questionnaire.
Statistical analysis
To identify physiologically implausible self-reported energy intakes, 95% confidence limits of agreement were calculated for the ratio of reported energy intake (EI) to EER (EI:EER) using the Goldberg method described by Black and Cole (29) and further adapted by Huang et al. (30). The combined within-subject coefficient of variation (CVw) was calculated as CVw = √(CV2wEI/d + CV2mTEE + CV2pER). Because the FFQ measures habitual intake, the number of days (d) is not applicable; thus, the combined CVw is equal to the variation in measured TEE (CVmTEE) and predicted energy requirements (CVpER). Using a compilation of data from doubly labeled water studies, Black et al. (29) estimated the within-subject error in TEE measured by doubly labeled water (CVmTEE) to be 9.6% over a period of 13 weeks, which approximates 1 trimester of pregnancy. The error in predicted energy requirements (CVpER) was estimated from the published Dietary Reference Intakes database using the available data on females aged 18–40 years (27). We conducted least-squares regression of measured TEE on age, height, weight, and physical activity level. CVpER was then calculated by dividing the standard deviation of the residuals by the mean TEE. This was performed separately for 3 strata of BMI, which resulted in CVpER values of 10.9% for women with a low BMI (<19.8), 9.9% for women with a normal BMI (19.8–26.0), and 8.1% for women with a high BMI (>26). Therefore, the lower confidence limits for EI:EER were 0.76, 0.73, and 0.72 and the upper confidence limits were 1.24, 1.27, and 1.28 for low-, normal-, and high-BMI women, respectively. Low energy reporting was defined as EI:EER < lower confidence limit; adequate energy reporting was defined as lower confidence limit ≤ EI:EER ≥ upper confidence limit; and high energy reporting was defined as EI:EER > upper confidence limit.
A univariate analysis was conducted to compare values of energy intake, EI:EER, low energy reporting, and high energy reporting across maternal characteristics. Continuous covariates, which included age, education, pregravid BMI, and gestational weight gain, were additionally coded into discrete ordinal categories. Differences in EI:EER were tested using an independent-samples t test or analysis-of-variance F test. Independence between proportions of low energy reporting and high energy reporting was tested using a chi-square test. Multiple logistic regression models were developed separately for low energy reporting and high energy reporting. First, all maternal baseline characteristics, including gestational weight gain, were considered one at a time in each model. Any variable with a P value less than 0.25 was considered for inclusion. Each multivariable model was fitted using backward elimination, with inclusion of all of the potential predictor variables and evaluation of variables one at a time in order of the smallest Wald χ2 test. A variable was removed if the change in deviance via likelihood ratio test was not statistically significant (P < 0.05). Interactions between predictor variables were also considered; however, none were identified. Smooth scatterplots were used to determine linearity on the logit scale for continuous variables.
To examine whether nutrient intakes varied by energy reporting status, mean nutrient densities (intake expressed as a percentage of total energy) for macronutrients and micronutrients were compared between low energy reporting, adequate energy reporting, and high energy reporting using analysis of variance with Bonferroni adjustment for multiple comparisons. Values for each nutrient were log-transformed beforehand to improve normality. The threshold for statistical significance was a P value less than 0.05. All analyses were performed using SAS software (version 9.1.3; SAS Institute Inc., Cary, North Carolina).
RESULTS
This pregnancy cohort consisted of mostly white women who were married and had completed at least some college education (Table 1). Average maternal age was 29 years (standard deviation, 5.5). According to the 1990 Institute of Medicine cutpoints, 11.2% of the participants were overweight prior to pregnancy and 22.0% were obese. Median energy intakes were 1,483 kcal/day (interquartile range, ±451), 2,182 kcal/day (interquartile range, ±583), and 3,801 kcal/day (interquartile range, ±1,213) for low, adequate, and high energy reporting, respectively. The median EI:EER ratio was 0.85, indicating that most subjects underreported their energy intake. The prevalences of implausible intakes—low energy reporting and high energy reporting—were 32.8% and 12.9%, respectively. Univariate analysis also demonstrated that EI:EER, low energy reporting, and high energy reporting varied according to several maternal characteristics (Table 1). The prevalence of low energy reporting differed by education, pregravid BMI, gestational weight gain, physical activity, and restrained eating behavior. Like low energy reporting, the prevalence of high energy reporting differed by pregravid BMI, education, and restrained eating behavior but also varied by race/ethnicity, marriage, adequacy of gestational weight gain, and depressive symptoms.
Table 1.
Energy Intake, Ratio of Energy Intake to Estimated Energy Requirement, and Prevalences of Low and High Energy Reporting According to Maternal Characteristics in Phase 3 of the Pregnancy, Infection, and Nutrition Study, North Carolina, 2001–2005
| Characteristic | No. of Subjects | % | Energy Intake |
EI:EER Ratioa |
Low Energy Reporting |
High Energy Reporting |
|||||||
| Median | IQR | Median | IQR | P Valueb | No. | % | P Valuec | No. | % | P Valuec | |||
| Total | 988 | 100.0 | 2,008 | 878 | 0.85 | 0.87 | 324 | 32.8 | 127 | 12.9 | |||
| Age, years | |||||||||||||
| <25 | 188 | 19.0 | 2,164 | 1,195 | 0.87 | 0.48 | 0.0508 | 55 | 29.3 | 0.1673 | 42 | 22.3 | 0.0001 |
| 25−<30 | 288 | 29.1 | 1,985 | 874 | 0.84 | 0.37 | 107 | 37.2 | 25 | 8.7 | |||
| 30−<35 | 349 | 35.3 | 1,995 | 868 | 0.86 | 0.33 | 105 | 30.1 | 39 | 11.2 | |||
| ≥35 | 163 | 16.5 | 1,929 | 800 | 0.84 | 0.40 | 57 | 35.0 | 21 | 12.9 | |||
| Race/ethnicity | |||||||||||||
| White | 750 | 75.9 | 1,971 | 832 | 0.85 | 0.36 | 0.0076 | 246 | 32.8 | 0.7199 | 81 | 10.8 | <0.0001 |
| Black | 155 | 15.7 | 2,344 | 1,431 | 0.89 | 0.55 | 48 | 31.0 | 37 | 23.9 | |||
| Other | 83 | 8.4 | 1,915 | 785 | 0.80 | 0.34 | 30 | 36.1 | 9 | 10.8 | |||
| Married | |||||||||||||
| No | 205 | 20.7 | 2,258 | 1,212 | 0.91 | 0.49 | 0.0013 | 58 | 28.3 | 0.1231 | 45 | 22.0 | <0.0001 |
| Yes | 783 | 79.3 | 1,925 | 844 | 0.84 | 0.35 | 266 | 34.0 | 82 | 10.5 | |||
| Highest level of education | |||||||||||||
| High school | 171 | 17.3 | 2,249 | 1,422 | 0.88 | 0.70 | 0.0157 | 57 | 33.3 | 0.0278 | 49 | 28.7 | <0.0001 |
| College graduation | 469 | 47.5 | 1,948 | 969 | 0.83 | 0.39 | 171 | 36.5 | 46 | 9.8 | |||
| Graduate school | 348 | 35.2 | 1,976 | 716 | 0.86 | 0.29 | 96 | 27.6 | 32 | 9.2 | |||
| Smoked during pregnancy | |||||||||||||
| No | 890 | 90.1 | 1,976 | 859 | 0.85 | 0.37 | 0.8252 | 293 | 32.9 | 0.7965 | 111 | 12.5 | 0.2792 |
| Yes | 98 | 9.9 | 2,145 | 971 | 0.88 | 0.44 | 31 | 31.6 | 16 | 16.3 | |||
| Nulliparous | |||||||||||||
| No | 481 | 48.7 | 2,018 | 915 | 0.84 | 0.40 | 0.7471 | 164 | 34.1 | 0.3958 | 70 | 14.6 | 0.1202 |
| Yes | 507 | 51.3 | 1,999 | 828 | 0.86 | 0.35 | 160 | 31.6 | 57 | 11.2 | |||
| Pregnancy body mass indexd | |||||||||||||
| <19.8 | 134 | 13.6 | 1,970 | 952 | 0.97 | 0.54 | <0.0001 | 14 | 10.4 | <0.0001 | 37 | 27.6 | <0.0001 |
| 19.8–26.0 | 526 | 53.2 | 1,997 | 779 | 0.86 | 0.35 | 155 | 29.5 | 52 | 9.9 | |||
| >26.0–29.0 | 111 | 11.2 | 2,053 | 837 | 0.82 | 0.37 | 47 | 42.3 | 17 | 15.3 | |||
| >29.0 | 217 | 22.0 | 2,053 | 1,098 | 0.76 | 0.41 | 108 | 49.8 | 21 | 9.7 | |||
| Gestational weight gain, poundse/week | |||||||||||||
| <0.87 | 249 | 25.2 | 1,929 | 930 | 0.80 | 0.38 | 0.0252 | 100 | 40.2 | 0.0091 | 28 | 11.2 | 0.6341 |
| 0.87–1.15 | 241 | 24.4 | 1,940 | 850 | 0.85 | 0.38 | 84 | 34.9 | 36 | 14.9 | |||
| >1.15–1.45 | 234 | 23.7 | 2,018 | 850 | 0.88 | 0.39 | 66 | 28.2 | 28 | 12.0 | |||
| >1.45 | 264 | 26.7 | 2,081 | 858 | 0.87 | 0.36 | 74 | 28.0 | 35 | 13.3 | |||
| Adequacy of gestational weight gain | |||||||||||||
| Inadequate | 207 | 21.0 | 1,885 | 856 | 0.82 | 0.35 | 0.0611 | 75 | 36.2 | 0.1240 | 20 | 9.7 | 0.0093 |
| Adequate | 186 | 18.8 | 1,978 | 895 | 0.88 | 0.46 | 50 | 26.9 | 36 | 19.4 | |||
| Excessive | 595 | 60.2 | 2,034 | 889 | 0.86 | 0.36 | 199 | 33.4 | 71 | 11.9 | |||
| Met physical activity guidelines | |||||||||||||
| No | 810 | 82.0 | 2,042 | 886 | 0.87 | 0.38 | <0.0001 | 245 | 30.2 | 0.0003 | 111 | 13.7 | 0.0888 |
| Yes | 178 | 18.0 | 1,842 | 751 | 0.76 | 0.34 | 79 | 44.4 | 16 | 9.0 | |||
| Restrained eating behavior | |||||||||||||
| No | 430 | 43.5 | 1,976 | 877 | 0.87 | 0.40 | 0.0007 | 120 | 27.9 | 0.0041 | 66 | 15.3 | 0.0397 |
| Yes | 558 | 56.5 | 2,015 | 881 | 0.83 | 0.37 | 204 | 36.6 | 61 | 10.9 | |||
| High depressive symptoms | |||||||||||||
| No | 743 | 75.2 | 1,969 | 841 | 0.85 | 0.36 | 0.0984 | 243 | 32.7 | 0.9180 | 79 | 10.6 | 0.0003 |
| Yes | 245 | 24.8 | 2,125 | 981 | 0.86 | 0.43 | 81 | 33.1 | 48 | 19.6 | |||
Abbreviations: EER, estimated energy requirements; EI, energy intake; IQR, interquartile range.
Statistical testing for the EI:EER ratio was conducted after log transformation.
P value for difference in EI:EER, by maternal characteristic, from analysis-of-variance F test or independent-samples t test.
P value for independence between low energy reporting status (or high energy reporting status) and the maternal characteristic from Pearson's χ2 test.
Weight (kg)/height (m)2.
1 pound = 0.45 kg.
In a multivariable analysis (Table 2), pregravid BMI was related to both low and high energy reporting. Compared with normal-weight women, low energy reporting was higher in overweight (odds ratio (OR) = 1.96, 95% CI: 1.26, 3.02) and obese (OR = 3.29, 95% CI: 2.33, 4.65) women but lower in underweight women (OR = 0.27, 95% CI: 0.15, 0.48; P < 0.01). High energy reporting was higher in underweight women (OR = 4.58, 95% CI: 2.77, 7.60; P < 0.01) and lower in obese women (OR = 0.44, 95% CI: 0.24, 0.82; P < 0.01) than in their normal-weight counterparts.
Table 2.
Predictors of Low and High Energy Reporting in Phase 3 of the Pregnancy, Infection, and Nutrition Study, North Carolina, 2001–2005
| Predictor | Odds Ratio | 95% Confidence Interval |
| Low Energy Reportinga | ||
| Pregravid BMIb | ||
| <19.8 | 0.27 | 0.15, 0.48 |
| 19.8–26.0 | 1.00 | |
| >26.0–29.0 | 1.96 | 1.26, 3.02 |
| >29.0 | 3.29 | 2.33, 4.65 |
| Married | ||
| Yes | 1.86 | 1.29, 2.70 |
| No | 1.00 | |
| Met physical activity guidelines | ||
| Yes | 2.05 | 1.44, 2.91 |
| No | 1.00 | |
| High Energy Reportingc | ||
| Pregravid BMI | ||
| <19.8 | 4.58 | 2.77, 7.60 |
| 19.8–26.0 | 1.00 | |
| >26.0–29.0 | 0.98 | 0.51, 1.88 |
| >29.0 | 0.44 | 0.24, 0.82 |
| Race/ethnicity | ||
| White | 1.00 | |
| Black | 2.77 | 1.62, 4.72 |
| Other | 0.95 | 0.44, 2.03 |
| Highest education | ||
| High school | 3.45 | 2.10, 5.67 |
| College graduation | 1.00 | |
| Graduate school | 0.90 | 0.54, 1.49 |
| High depressive symptoms | ||
| Yes | 1.75 | 1.13, 2.73 |
| No | 1.00 | |
Abbreviations: BMI, body mass index; HER, high energy reporting; LER, low energy reporting.
Odds ratios and 95% confidence intervals were calculated from a logistic regression model of LER vs. non-LER, with adjustment for other significant predictors (pregravid BMI, marital status, and physical activity).
Weight (kg)/height (m)2.
Odds ratios and 95% confidence intervals were calculated from a logistic regression model of HER vs. non-HER, with adjustment for other significant predictors (pregravid BMI, race/ethnicity, education, and depressive symptoms).
Other than pregravid BMI, independent predictors of low and high energy reporting were different. Low energy reporting was more prevalent among married women (P < 0.001) and those who reported higher levels of physical activity (P < 0.001). High energy reporting was more prevalent among African-American subjects (P < 0.001) and those who were less educated (P < 0.001) and had higher depressive symptom scores (P = 0.001). Gestational weight gain in the second trimester and restrained eating behavior were not associated with either low energy reporting or high energy reporting after adjustment for pregravid BMI. Both gestational weight gain and restrained eating scores were moderately correlated with pregravid BMI (−0.31 and 0.47, respectively).
Low energy reporting was most common in pregnant women who were classified as obese prior to pregnancy (49.8%). Figure 1 displays the prevalence of low energy reporting by pregravid BMI status and adequacy of gestational weight gain according to the Institute of Medicine guidelines. Among obese women, we observed a similarly high proportion of low energy reporting in women whose gestational weight gain over the first 2 trimesters was classified as inadequate (45.6%) or excessive (52.6%).
Figure 1.
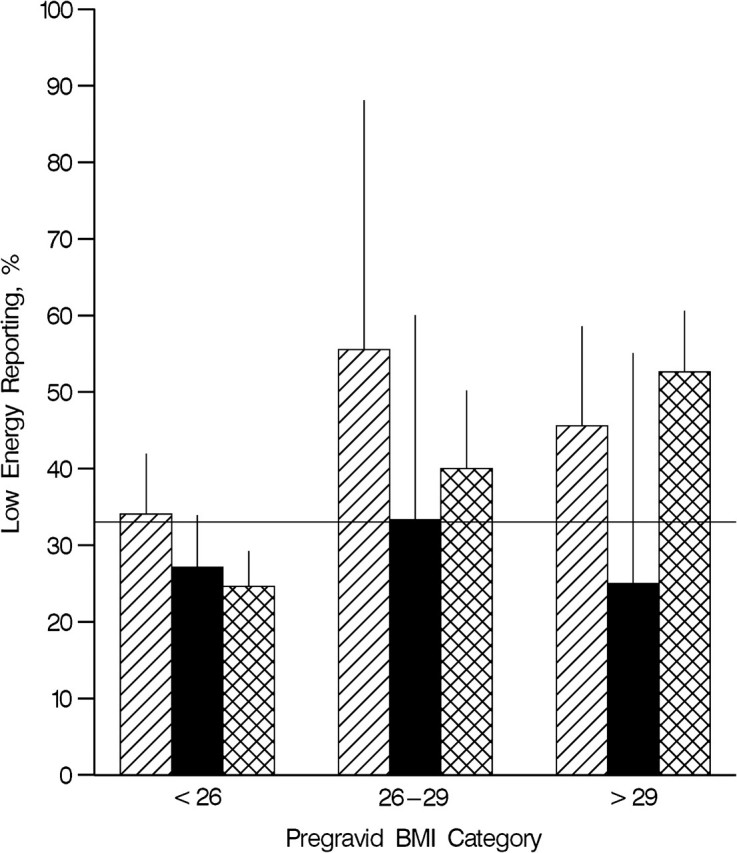
Prevalence of low energy reporting according to pregravid body mass index (BMI) and adequacy of gestational weight gain in phase 3 of the Pregnancy, Infection, and Nutrition Study, North Carolina, 2001–2005. Striped columns, inadequate gestational weight gain; solid columns, adequate gestational weight gain; cross-hatched columns, excessive gestational weight gain. The horizontal line represents the overall prevalence of low energy reporting in the study population (32.8%). Bars, upper confidence limit for binomial proportions using normal approximation.
Table 3 displays median intakes for macronutrients and micronutrients according to category of energy reporting. Nutrient intakes for women with low energy reporting were not significantly different from those for women with adequate energy reporting. However, women with high energy reporting had significantly lower intakes of riboflavin, calcium, and magnesium than women with adequate energy reporting.
Table 3.
Nutrient Densities for Macronutrients and Micronutrients According to Low, Adequate, and High Energy Reporting in Phase 3 of the Pregnancy, Infection, and Nutrition Study, North Carolina, 2001–2005
| Nutrient | Low Energy Reporting |
Adequate Energy Reporting |
High Energy Reporting |
|||
| Median | IQR | Median | IQR | Median | IQR | |
| Protein, % of kcal | 14.5 | 3.70 | 14.2 | 3.37 | 13.7 | 3.32 |
| Carbohydrate, % of kcal | 54.8 | 11.00 | 55.2 | 8.13 | 54.1 | 8.25 |
| Fat, % of kcal | 33.0 | 8.75 | 33.0 | 6.74 | 33.8 | 7.52 |
| Saturated fat, g/1,000 kcal | 11.9 | 3.52 | 12.0 | 3.10 | 12.6 | 2.67 |
| Vitamin A, RE/1,000 kcal | 636.2 | 369.4 | 619.6 | 375.5 | 532.0 | 421.9 |
| Vitamin C, mg/1,000 kcal | 95.8 | 59.23 | 89.0 | 45.49 | 83.7 | 63.16 |
| Vitamin D, μg/1,000 kcal | 96.9 | 103.2 | 89.0 | 80.56 | 82.1 | 68.42 |
| Vitamin E, mg/1,000 kcal | 4.55 | 2.027 | 4.62 | 1.745 | 4.33 | 1.540 |
| Thiamin, mg/1,000 kcal | 0.81 | 0.227 | 0.81 | 0.187 | 0.77 | 0.213 |
| Riboflavin, mg/1,000 kcal | 1.03 | 0.412 | 1.01 | 0.336 | 0.91*a | 0.222 |
| Niacin, mg/1,000 kcal | 9.45 | 2.519 | 9.55 | 2.631 | 9.40 | 3.125 |
| Vitamin B6, mg/1,000 kcal | 0.94 | 0.328 | 0.93 | 0.301 | 0.91 | 0.300 |
| Folate, μg/1,000 kcal | 192.6 | 65.22 | 194.7 | 57.02 | 186.6 | 62.76 |
| Calcium, mg/1,000 kcal | 494.2 | 251.8 | 473.1 | 220.6 | 425.2* | 160.1 |
| Iron, mg/1,000 kcal | 6.95 | 2.553 | 7.16 | 2.153 | 6.90 | 2.587 |
| Zinc, mg/1,000 kcal | 5.13 | 1.794 | 5.08 | 1.677 | 4.86 | 1.755 |
| Magnesium, mg/1,000 kcal | 152.4 | 52.03 | 150.6 | 46.18 | 137.7* | 47.58 |
Abbreviations: IQR, interquartile range; RE, retinol equivalents.
P < 0.05.
Significant difference from adequate energy reporting in analysis of variance with Bonferroni adjustment for multiple comparisons. Values for each nutrient were log-transformed prior to statistical testing.
DISCUSSION
Our results indicate that implausible reported energy intakes—both underreporting and overreporting—are prevalent in this cohort of pregnant women. Direct comparison of measurement error between dietary studies is somewhat difficult because of variations in dietary assessment, physical activity assessment, estimation of TEE, and population characteristics. Black (31) conducted a meta-analysis of studies that utilized both doubly labeled water and weighed food records. Among nonpregnant women aged 18–39 years, Black found that 31% underreported their intake and 3% overreported it (31). In larger population studies of nonpregnant females, which utilized prediction equations for energy requirements and a variety of dietary assessment methods, investigators have reported frequencies of low energy reporting ranging from 11% to 52% (32). Although the prevalence of low energy reporting has been shown to vary by dietary assessment method, studies comparing these methods have been inconsistent in their findings. Some have found that the FFQ provided less underreporting than dietary recalls or food records (33–35), while others found the opposite (3, 36–38).
In our study, pregravid BMI was a positive predictor of low energy reporting, which is consistent with most studies in nonpregnant populations (39). It has been proposed that measures of body size and adiposity are likely surrogates for psychosocial characteristics that result in underreporting of energy, such as poor awareness of intake or portion sizes, subconscious biasing toward intake that is perceived to be appropriate, restrained eating behaviors, and fear of weight gain (40–43). However, we found no association between dietary restraint score and underreporting in our cohort after adjusting for pregravid BMI. In addition, there was no independent association between low energy reporting and gestational weight gain. Low energy reporting was similarly prevalent among overweight subjects regardless of whether they were categorized as having excessive or inadequate gestational weight gain.
Low energy reporting was also more common among pregnant women who reported amounts of moderate-to-vigorous physical activity that met the American College of Obstetrics and Gynecology recommendation for exercise during pregnancy (44). However, this finding may have been attributed to measurement error in reported physical activity. One common bias in these data is the overreporting of minutes spent in a given activity (45). Because physical activity data were utilized in the estimation of TEE, an overreporting bias would result in artificially higher EER, thereby reducing the EI:EER ratio and increasing the tendency to be classified as having low energy reporting. In fact, a validation study for PIN3 found that minutes of moderate-to-vigorous physical activity were 85% higher on average when compared with accelerometery, an objective measure of physical activity (46). Further, social desirability bias is a purported reason for both underreporting of energy intake (47) and overreporting of physical activity (45). Therefore, any association between higher physical activity and low energy reporting may also be influenced by correlated error.
To our knowledge, this study was the first to investigate high energy reporting, or overreporting, among pregnant women. We determined that 12.9% of subjects overreported their energy intake, which is higher than is typically seen in nonpregnant populations. High energy reporting was more common among pregnant women who were underweight, were African-American, and had no college education. Additionally, we found that high energy reporting was more prevalent among women with higher depressive symptom scores. Researchers have suggested that depression and anxiety may influence reporting accuracy by impairing cognitive processes such as memory or triggering eating disinhibition (48), and some studies in nonpregnant women have shown a positive association between depression and low energy reporting (43); however, to our knowledge, a relation between depression and high energy reporting has not been previously reported.
An emerging body of literature has demonstrated how both energy-specific and nutrient-specific underreporting can seriously distort measures of association in nutritional epidemiology (2, 49–51). In our cohort, measurement error in energy intake varied significantly according to certain maternal characteristics. However, we did not observe that underreporting bias was associated with variable bias in specific nutrient intakes. This finding suggests that energy underreporting occurred at the whole diet level, which is an important assumption in the analysis of diet and disease, since nutrient intakes are typically adjusted for energy intake to separate the effect of energy intake from the effect of an individual nutrient on a particular health outcome (49). Nevertheless, many researchers agree that energy adjustment alone cannot eliminate the effects of differential reporting bias (2, 49, 50). Additional methods include stratifying results by low energy reporting and adequate energy reporting, as well as a more sophisticated approach of including predictors of low energy reporting (i.e., BMI) in a nutrient measurement-error model (52).
The main limitation of this study was that TEE was estimated rather than directly measured using doubly labeled water. We calculated TEE using Dietary Reference Intake prediction equations for EER, which were derived from doubly labeled water data. These equations have been deemed a valid alternative to doubly labeled water measurements and have also been used in previous studies of low energy reporting. As we noted above, the self-reported physical activity data in our study were biased toward overreporting, which may have resulted in artificially higher EER, thereby reducing the EI:EER ratio and increasing the tendency to be classified as having low energy reporting. Another limitation is that approximately 25% of PIN3 subjects did not complete the FFQ. On average, women that we excluded from the analysis were more likely to be overweight, younger, less educated, African-American, and unmarried. Because some of these characteristics were predictive of low and high energy reporting, it is possible that our results may have differed if complete data had been available for all subjects. Note also that PIN3 subjects were not sampled at random, and all participants received their prenatal care through UNC Hospitals. Therefore, generalizablity of our results to other pregnant populations may be limited.
The primary strength of this project was the prospective design of PIN3. Data were collected from the first trimester of pregnancy through delivery. Moreover, dietary information was ascertained during the second trimester, when intake is less likely to be influenced by nausea.
In conclusion, it appears that levels of low and high energy reporting during pregnancy are not grossly different from those that have been observed in nonpregnant women. Nevertheless, nearly half of all women in our cohort misreported their energy (food) intake. This measurement error was also associated with maternal characteristics, including pregravid BMI, which is a risk factor for several reproductive, perinatal, and pediatric outcomes (53–59). Thus, failure to account for obesity-specific underreporting bias may yield erroneous conclusions in such studies. A few analytical methods of accounting for this error have been proposed; however, more research is needed. Future studies of maternal diet should consider identifying low energy reporting, high energy reporting, and their predictors to assess the level of potential bias and to help adjust for this error in the calculation of nutrient risk estimates.
Acknowledgments
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Eric Nowicki, Anna Maria Siega-Riz, Ka He, Andy Olshan); Department of Nutrition, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Anna Maria Siega-Riz, Ka He); Carolina Population Center, University of North Carolina, Chapel Hill, North Carolina (Anna Maria Siega-Riz); Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Amy Herring); and Department of Obstetrics and Gynecology, School of Medicine, University of North Carolina, Chapel Hill, North Carolina (Alison Stuebe).
This study received support from the National Institute of Child Health and Human Development, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Cancer Institute.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CV
coefficient of variation
- EER
estimated energy requirements
- EI
energy intake
- FFQ
food frequency questionnaire
- OR
odds ratio
- PIN
Pregnancy, Infection, and Nutrition Study
- TEE
total energy expenditure
References
- 1.Hill RJ, Davies PS. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr. 2001;85(4):415–430. doi: 10.1079/bjn2000281. [DOI] [PubMed] [Google Scholar]
- 2.Prentice RL. Measurement error and results from analytic epidemiology: dietary fat and breast cancer. J Natl Cancer Inst. 1996;88(23):1738–1747. doi: 10.1093/jnci/88.23.1738. [DOI] [PubMed] [Google Scholar]
- 3.Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158(1):1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
- 4.Fraser GE. A search for truth in dietary epidemiology. Am J Clin Nutr. 2003;78(suppl 3) doi: 10.1093/ajcn/78.3.521S. 521S–525S. [DOI] [PubMed] [Google Scholar]
- 5.Brown JE. Nutrition Through the Life Cycle. Belmont, CA: Wadsworth/Thomson Learning; 2002. Nutrition during pregnancy. [Google Scholar]
- 6.Picciano MF. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr. 2003;133(6):1997S–2002S. doi: 10.1093/jn/133.6.1997S. [DOI] [PubMed] [Google Scholar]
- 7.Kind KL, Moore VM, Davies MJ. Diet around conception and during pregnancy—effects on fetal and neonatal outcomes. Reprod Biomed Online. 2006;12(5):532–541. doi: 10.1016/s1472-6483(10)61178-9. [DOI] [PubMed] [Google Scholar]
- 8.Derbyshire E, Davies J, Costarelli V, et al. Prepregnancy body mass index and dietary intake in the first trimester of pregnancy. J Hum Nutr Diet. 2006;19(4):267–273. doi: 10.1111/j.1365-277X.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg GR, Prentice AM, Coward WA, et al. Longitudinal assessment of energy expenditure in pregnancy by the doubly labeled water method. Am J Clin Nutr. 1993;57(4):494–505. doi: 10.1093/ajcn/57.4.494. [DOI] [PubMed] [Google Scholar]
- 10.Winkvist A, Persson V, Hartini TN. Underreporting of energy intake is less common among pregnant women in Indonesia. Public Health Nutr. 2002;5(4):523–529. doi: 10.1079/PHN2001317. [DOI] [PubMed] [Google Scholar]
- 11.Food and Nutrition Board . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press; 2005. Institute of Medicine. [Google Scholar]
- 12.Schröder H, Morales-Molina JA, Bermejo S, et al. Relationship of abdominal obesity with alcohol consumption at population scale. Eur J Nutr. 2007;46(7):369–376. doi: 10.1007/s00394-007-0674-7. [DOI] [PubMed] [Google Scholar]
- 13.Johnson L, van Jaarsveld CH, Emmett PM, et al. Dietary energy density affects fat mass in early adolescence and is not modified by FTO variants. PLoS ONE. 2009;4(3):e4594. doi: 10.1371/journal.pone.0004594. (doi: 10.1371/journal.pone.0004594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tooze JA, Schoeller DA, Subar AF, et al. Total daily energy expenditure among middle-aged men and women: the OPEN study. Am J Clin Nutr. 2007;86(2):382–387. doi: 10.1093/ajcn/86.2.382. [DOI] [PubMed] [Google Scholar]
- 15.Baer HJ, Blum RE, Rockett HR, et al. Use of a food frequency questionnaire in American Indian and Caucasian pregnant women: a validation study. BMC Public Health. 2005;5:135. doi: 10.1186/1471-2458-5-135. (doi: 10.1186/1471-2458-5-135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vriese SR, De Henauw S, De Backer G, et al. Estimation of dietary fat intake of Belgian pregnant women. Comparison of two methods. Ann Nutr Metab. 2001;45(6):273–278. doi: 10.1159/000046738. [DOI] [PubMed] [Google Scholar]
- 17.Forsythe HE, Gage B. Use of a multicultural food-frequency questionnaire with pregnant and lactating women. Am J Clin Nutr. 1994;59(suppl 1) doi: 10.1093/ajcn/59.1.203S. 203S–206S. [DOI] [PubMed] [Google Scholar]
- 18.Mouratidou T, Ford F, Fraser RB. Validation of a food-frequency questionnaire for use in pregnancy. Public Health Nutr. 2006;9(4):515–522. doi: 10.1079/phn2005876. [DOI] [PubMed] [Google Scholar]
- 19.Wei EK, Gardner J, Field AE, et al. Validity of a food frequency questionnaire in assessing nutrient intakes of low-income pregnant women. Matern Child Health J. 1999;3(4):241–246. doi: 10.1023/a:1022385607731. [DOI] [PubMed] [Google Scholar]
- 20.Brantsaeter AL, Haugen M, Alexander J, et al. Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa) Matern Child Nutr. 2008;4(1):28–43. doi: 10.1111/j.1740-8709.2007.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institute of Medicine . Nutrition During Pregnancy. Part I: Weight Gain. Washington, DC: National Academies Press; 1990. [Google Scholar]
- 22.Block G, Hartman AM, Dresser CM, et al. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 23.Block G, Thompson FE, Hartman AM, et al. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92(6):686–693. [PubMed] [Google Scholar]
- 24.Boucher B, Cotterchio M, Kreiger N, et al. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9(1):84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- 25.Deierlein AL, Siega-Riz AM, Herring A. Dietary energy density but not glycemic load is associated with gestational weight gain. Am J Clin Nutr. 2008;88(3):693–699. doi: 10.1093/ajcn/88.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polivy J, Herman CP, Warsh S. Internal and external components of emotionality in restrained and unrestrained eaters. J Abnorm Psychol. 1978;87(5):497–504. doi: 10.1037//0021-843x.87.5.497. [DOI] [PubMed] [Google Scholar]
- 27.Roberts RE, Vernon SW. The Center for Epidemiologic Studies Depression Scale: its use in a community sample. Am J Psychiatry. 1983;140(1):41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- 28.Butte NF, Wong WW, Treuth MS, et al. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am J Clin Nutr. 2004;79(6):1078–1087. doi: 10.1093/ajcn/79.6.1078. [DOI] [PubMed] [Google Scholar]
- 29.Black AE, Cole TJ. Within- and between-subject variation in energy expenditure measured by the doubly-labelled water technique: implications for validating reported dietary energy intake. Eur J Clin Nutr. 2000;54(5):386–394. doi: 10.1038/sj.ejcn.1600970. [DOI] [PubMed] [Google Scholar]
- 30.Huang TT, Roberts SB, Howarth NC, et al. Effect of screening out implausible energy intake reports on relationships between diet and BMI. Obes Res. 2005;13(7):1205–1217. doi: 10.1038/oby.2005.143. [DOI] [PubMed] [Google Scholar]
- 31.Black AE. The sensitivity and specificity of the Goldberg cut-off for EI: BMR for identifying diet reports of poor validity. Eur J Clin Nutr. 2000;54(5):395–404. doi: 10.1038/sj.ejcn.1600971. [DOI] [PubMed] [Google Scholar]
- 32.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133(suppl 3) doi: 10.1093/jn/133.3.895S. 895S–920S. [DOI] [PubMed] [Google Scholar]
- 33.Svendsen M, Tonstad S. Accuracy of food intake reporting in obese subjects with metabolic risk factors. Br J Nutr. 2006;95(3):640–649. doi: 10.1079/bjn20051662. [DOI] [PubMed] [Google Scholar]
- 34.Kroke A, Klipstein-Grobusch K, Voss S, et al. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am J Clin Nutr. 1999;70(4):439–447. doi: 10.1093/ajcn/70.4.439. [DOI] [PubMed] [Google Scholar]
- 35.Sawaya AL, Tucker K, Tsay R, et al. Evaluation of four methods for determining energy intake in young and older women: comparison with doubly labeled water measurements of total energy expenditure. Am J Clin Nutr. 1996;63(4):491–499. doi: 10.1093/ajcn/63.4.491. [DOI] [PubMed] [Google Scholar]
- 36.Scagliusi FB, Ferriolli E, Pfrimer K, et al. Underreporting of energy intake in Brazilian women varies according to dietary assessment: a cross-sectional study using doubly labeled water. J Am Diet Assoc. 2008;108(12):2031–2040. doi: 10.1016/j.jada.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 37.McKeown NM, Day NE, Welch AA, et al. Use of biological markers to validate self-reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort. Am J Clin Nutr. 2001;74(2):188–196. doi: 10.1093/ajcn/74.2.188. [DOI] [PubMed] [Google Scholar]
- 38.Bingham SA, Cassidy A, Cole TJ, et al. Validation of weighed records and other methods of dietary assessment using the 24 h urine nitrogen technique and other biological markers. Br J Nutr. 1995;73(4):531–550. doi: 10.1079/bjn19950057. [DOI] [PubMed] [Google Scholar]
- 39.Rennie KL, Coward A, Jebb SA. Estimating under-reporting of energy intake in dietary surveys using an individualised method. Br J Nutr. 2007;97(6):1169–1176. doi: 10.1017/S0007114507433086. [DOI] [PubMed] [Google Scholar]
- 40.Rennie KL, Siervo M, Jebb SA. Can self-reported dieting and dietary restraint identify underreporters of energy intake in dietary surveys? J Am Diet Assoc. 2006;106(10):1667–1672. doi: 10.1016/j.jada.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Horner NK, Patterson RE, Neuhouser ML, et al. Participant characteristics associated with errors in self-reported energy intake from the Women's Health Initiative food-frequency questionnaire. Am J Clin Nutr. 2002;76(4):766–773. doi: 10.1093/ajcn/76.4.766. [DOI] [PubMed] [Google Scholar]
- 42.Briefel RR, Sempos CT, McDowell MA, et al. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr. 1997;65(suppl 4) doi: 10.1093/ajcn/65.4.1203S. 1203S–1209S. [DOI] [PubMed] [Google Scholar]
- 43.Kretsch MJ, Fong AK, Green MW. Behavioral and body size correlates of energy intake underreporting by obese and normal-weight women. J Am Diet Assoc. 1999;99(3):300–306. doi: 10.1016/S0002-8223(99)00078-4. [DOI] [PubMed] [Google Scholar]
- 44.Committee on Obstetric Practice. American College of Obstetricians and Gynecologists. ACOG committee opinion. Exercise during pregnancy and the postpartum period. Number 267, January. 2002. doi: 10.1016/s0020-7292(02)80004-2. Int J Gynaecol Obstet. 2002;77(1):79–81. [DOI] [PubMed] [Google Scholar]
- 45.Adams SA, Matthews CE, Ebbeling CB, et al. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol. 2005;161(4):389–398. doi: 10.1093/aje/kwi054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evenson KR, Wen F. Measuring physical activity among pregnant women using a structured one-week recall questionnaire: evidence for validity and reliability. Int J Behav Nutr Phys Act. 2010;7:21. doi: 10.1186/1479-5868-7-21. (doi: 10.1186/1479-5868-7-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hebert JR, Clemow L, Pbert L, et al. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol. 1995;24(2):389–398. doi: 10.1093/ije/24.2.389. [DOI] [PubMed] [Google Scholar]
- 48.Maurer J, Taren DL, Teixeira PJ, et al. The psychosocial and behavioral characteristics related to energy misreporting. Nutr Rev. 2006;64(2):53–66. doi: 10.1111/j.1753-4887.2006.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 49.Lissner L. Measuring food intake in studies of obesity. Public Health Nutr. 2002;5(6A):889–892. doi: 10.1079/phn2002388. [DOI] [PubMed] [Google Scholar]
- 50.Freedman LS, Schatzkin A, Thiebaut AC, et al. Abandon neither the food frequency questionnaire nor the dietary fat-breast cancer hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1321–1322. doi: 10.1158/1055-9965.EPI-07-0179. [DOI] [PubMed] [Google Scholar]
- 51.Heitmann BL, Lissner L. Can adverse effects of dietary fat intake be overestimated as a consequence of dietary fat underreporting? Public Health Nutr. 2005;8(8):1322–1327. doi: 10.1079/phn2005750. [DOI] [PubMed] [Google Scholar]
- 52.Prentice RL, Sugar E, Wang CY, et al. Research strategies and the use of nutrient biomarkers in studies of diet and chronic disease. Public Health Nutr. 2002;5(6A):977–984. doi: 10.1079/PHN2002382. [DOI] [PubMed] [Google Scholar]
- 53.Siega-Riz AM, Herring AH, Olshan AF, et al. The joint effects of maternal prepregnancy body mass index and age on the risk of gastroschisis. Paediatr Perinat Epidemiol. 2009;23(1):51–57. doi: 10.1111/j.1365-3016.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 54.Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 55.Stothard KJ, Tennant PW, Bell R, et al. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 56.Chu SY, Kim SY, Lau J, et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007;197(3):223–228. doi: 10.1016/j.ajog.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 57.Rasmussen SA, Chu SY, Kim SY, et al. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198(6):611–619. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 58.Bodnar LM, Siega-Riz AM, Cogswell ME. High prepregnancy BMI increases the risk of postpartum anemia. Obes Res. 2004;12(6):941–948. doi: 10.1038/oby.2004.115. [DOI] [PubMed] [Google Scholar]
- 59.Boney CM, Verma A, Tucker R, et al. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]


