Abstract
Social– cognitive and behavioral theories of change disagree on what the relevant controlling variables for initiating behavior change are. Correlations between baseline smoking cessation self-efficacy and the changes in breath carbon monoxide (CO) and the reduction in breath CO and increases in smoking cessation self-efficacy from baseline were obtained from a contingency management smoking cessation procedure. A test of the difference between the cross-lag correlations suggested a nonspurious causal relationship between smoking cessation self-efficacy and changes in breath CO. Path analyses showed that decreases in breath CO (reductions in smoking) predicted later increases in smoking cessation self-efficacy. Baseline self-reports of smoking cessation self-efficacy were not significantly correlated with subsequent changes in breath CO. Rather, significant correlations were found between reductions in breath CO and later increases in smoking cessation self-efficacy. These results suggest that self-efficacy may be a cognitive response to one’s own behavior, and are inconsistent with a social– cognitive view of self-efficacy’s role in behavior change. Implications for the development of smoking cessation programs and health-promoting behavior changes in general are discussed.
Keywords: behavior modification, Bandura, Skinner, correlation, health psychology
Cigarette smoking causes approximately 440,000 deaths per year and is the leading cause of preventable death in the United States (Centers for Disease Control, 2002). Thus, smoking is a socially relevant behavior in need of an effective treatment. The U.S. Department of Health and Human Services (2000) has endorsed therapies for smoking cessation that use behavioral, cognitive, pharmacological, or multifaceted approaches to treatment.
The development and maintenance of healthy behaviors is central to reducing morbidity and mortality and to decreasing health care costs. However, there has not always been general agreement as to the most effective way to initiate behavior change in an individual. Behaviorally based approaches specify environmental contingencies as the main determinant of behavior (Skinner, 1974). They posit that behavior change can be achieved simply by altering the environmental contingencies acting on the behavior without regard for cognitive factors.
Bandura (1977, 1982, 1997) offered an alternative explanation concerning the necessary basis of behavior change. In his social– cognitive theory, self-efficacy, defined as an individual’s perception of their personal capabilities in a given situation, is an important component of later behavior change. Individuals who believe that they can initiate behavior change in a certain situation (i.e., high self-efficacy) will be more likely to change their behavior than those who have little belief in their ability to effect change in their own behavior (i.e., low self-efficacy). There is considerable evidence that contemporaneous measures of abstinence self-efficacy are positively associated with greater posttreatment smoking abstinence (Condiotte & Lichtenstein, 1981; DiClemente, 1981; DiClemente, Prochaska, & Gilbertini, 1985; Gulliver, Hughes, Solomon, & Dey, 1995; McIntyre, Lichtenstein, & Mermelstein, 1983; Stuart, Borland, & McMurray, 1994) and lower rates of subsequent relapse (Gwaltney et al., 2001).
However, the relationship between pretreatment abstinence self-efficacy and treatment success is less clear (Baer, Holt, & Lichtenstein, 1986). Experiments conducted to test the causal link between baseline levels of self-efficacy and achievement of smoking abstinence have generally failed (Nicki, Remington, & McDonald, 1984; Sperry & Nicki, 1991). Other studies have only managed to report an indirect link between self-efficacy and smoking cessation (Manfredi, Cho, Crittenden, & Dolecek, 2007). Thus, the importance of increasing an individual’s self-efficacy for smoking abstinence to increase the initiation of smoking abstinence is uncertain.
Recently, contingency management (Higgins & Silverman, 1999) has shown a great deal of effectiveness for treating substance abuse within the domain of behaviorally based treatments (Higgins, Silverman, & Heil, 2008). In a contingency management procedure, reinforcement, often in the form of either vouchers or cash, is contingent on reducing or abstaining from a specified substance of abuse. More specifically, in contingency management smoking cessation programs, reinforcement is contingent on the reduction of markers of smoking, such as breath carbon monoxide (CO) or cotinine (Sigmon, Lamb, & Dallery, 2008). Early behaviorally based studies showed that smoking could be reduced by providing money dependent on lower breath CO levels (Stitzer & Bigelow, 1982). Participants have also been shown to be sensitive to the incentive magnitude provided for lower CO levels (Stitzer & Bigelow, 1983). To date, contingency management has increased smoking abstinence in pregnant women, individuals diagnosed with a serious mental illness, chronic drug abusers, adolescents, and smokers without definite plans to quit (see Sigmon et al., 2008, for review).
Alternatively, social– cognitive theory would suggest that an effective way to reduce CO levels in a smoker would be to increase that person’s self-efficacy to cease smoking (Collins & Lapp, 1991; Sitharthan & Kavanagh, 1991; Solomon & Annis, 1990). That is, for two individuals with identical motivation to quit smoking, social– cognitive theory would predict that the individual who had a greater belief in his or her ability to quit smoking (higher cessation self-efficacy) would be more likely to actually quit smoking.
One contingency management study recently reported subjects’ smoking cessation self-efficacy along with markers of smoking (Lamb, Morral, Galbicka, Kirby, & Iguchi, 2005). Subjects involved in this study were considered “hard to treat” by virtue of their lack of plans to quit smoking in the next 6 months. Reinforcement for breath CO levels was based on a percentile schedule. In a percentile schedule, reinforcement is contingent on behavior that is at or above a certain performance criterion based on past behavior. By making the percentile schedule more responsive to recent behavior, Lamb et al. (2005) showed that the amount of smoking could be substantially reduced even in these hard-to-treat individuals. As a result, subjects’ self-reported smoking cessation self-efficacy also increased as smoking decreased. However, like the earlier studies showing associations between behavior change and smoking cessation self-efficacy, no causal attribution could be inferred because the relationship between smoking cessation self-efficacy and changes in smoking was not explicitly examined.
The present study further examined the association between smoking cessation self-efficacy and smoking cessation in smokers without explicit plans to quit from the Lamb et al. (2005) data set. Because of the longitudinal nature of the study, data were amenable for looking at the correlations between the following variables: baseline breath CO, baseline smoking cessation self-efficacy, changes in breath CO after the contingency management procedure was implemented, and changes in self-efficacy after the contingency management procedure was implemented. If a spurious relationship between changes in breath CO and changes in smoking cessation self-efficacy could be successfully ruled out, then additional analyses could be used to test the directionality of a relationship between changes in breath CO and changes in smoking cessation self-efficacy. Thus, we sought to determine which, if any, measure of smoking cessation self-efficacy correlated with breath CO change, and if or how breath CO change influences changes in smoking cessation self-efficacy.
According to behavioral theory, there may be a significant negative correlation between changes in smoking cessation self-efficacy and changes in breath CO if smoking cessation self-efficacy is merely a byproduct of behavior change. That is, once a person’s smoking behavior declines (as measured by breath CO), that person subsequently feels better about his or her chances of quitting. Social– cognitive theory may also predict this correlation. However, in this case the changing smoking cessation self-efficacy would precede changes in smoking behavior. Changes in smoking cessation self-efficacy were not collected on a day-to-day basis. Therefore, path analysis was used to disentangle the predictions of the two theories. The predictions made for the correlation between baseline smoking cessation self-efficacy and the change in breath CO levels are also different for each theory. Social– cognitive theory predicts a significant negative correlation between baseline smoking cessation self-efficacy and breath CO. That is, the higher a person’s smoking cessation self-efficacy before treatment, the better the outcome (lower levels of CO) during treatment. Behavioral theory does not necessarily predict a significant correlation between baseline smoking cessation self-efficacy and breath CO.
Method
Participants
Adult men and women at least 18 years of age who reported smoking at least 15 cigarettes per day, had a breath CO sample of ≥15 ppm at intake, and had no concrete plans to quit smoking in the next 6 months were eligible for participation. Participants were recruited by word-of-mouth, fliers, newspaper advertisements, and a brief TV segment. Monetary incentives contingent on smoking reduction were the primary motivation for participants to participate in the study. Seventy-one participants were enrolled in the study, 35 of whom were women.
Study Timeline and Groups
The total duration of the study was 70 visits, which occurred each weekday, including 10 pretreatment visits. Each visit included delivery of a breath CO sample, reporting the number of cigarettes smoked in the previous 24 hr, and signing a receipt for any money earned. This took approximately 5 min. Absences were generally not excused. Each participant earned $1 per visit during the initial 10 pretreatment visits. Following the 10th visit, participants were randomized into two conditions, which were in effect for the remaining 60 visits.
Participants received monetary incentives contingent on providing a breath CO sample that was at or better than the 60th percentile of their own most recent breath CO samples. Participants were placed in one of two groups that differed on how many previous samples were used to calculate the 60th percentile: either 4 or 9 samples. Thus, if the breath CO sample was one of the lowest 3 of the last 5 for the 4-sample group (6 of last 10 for 9-sample group), this resulted in an additional incentive payment. In addition, any breath CO sample less than 4 ppm automatically earned the participant the incentive. Incentive payments began at $2.50 and increased by $0.50 for each successive breath CO sample criterion met. Failing to meet the criterion reset the next incentive back to $2.50. Five sequential criterion samples resulted in a $10 bonus incentive and reinstatement of the highest incentive up to that point if the incentive value had been reset.
Measures
Daily breath CO levels were measured with a Vitalograph CO monitor (Vitalograph, Lenexa, KS). At intake and again at Visits 10, 30, 50, and 70, participants completed the Confidence Questionnaire (CQ; Baer & Lichtenstein, 1988). The CQ is a 14-item measure of self-efficacy that asks smokers to rate the probability that they would be able to resist smoking over a range of situations. This was the shortened version of the CQ (Condiotte & Lichtenstein, 1981), which has a high internal reliability (α = .99).
Data Analysis
Data from the CQ completed on Visit 10, the last day of baseline, and Visit 30, the 20th visit postbaseline, were used along with the breath CO sample on Visit 10 and the first 20 visits of breath CO levels postbaseline. Subjects from both experimental conditions showed the same pattern of correlations and were therefore combined for all of the subsequent analysis. Data after Visit 30 are not reported because no further changes in correlation coefficients were observed. Baseline and endpoint data were available on 63 participants. The initial analyses involved testing the cross-lag correlations for spuriousness (Kenny, 1973, 1975; Milburn, 1979). This initial step was necessary to rule out spuriousness (i.e., an unmeasured third variable, such as level of dependence) as an explanation for any correlation between smoking cessation self-efficacy and breath CO before any causal inference could be tested. The subsequent analysis included a path analyses that used structural equation modeling (SEM) to evaluate three alternative causal hypotheses. Also, we created two multiple regressions: one that included breath CO (Visit 10) as a predictor of subsequent smoking cessation self-efficacy (Visit 30), and one that included smoking cessation self-efficacy (Visit 10) as a predictor of subsequent breath CO (Visit 30). All analyses were done using SAS CALIS software (SAS, Version 9.1).
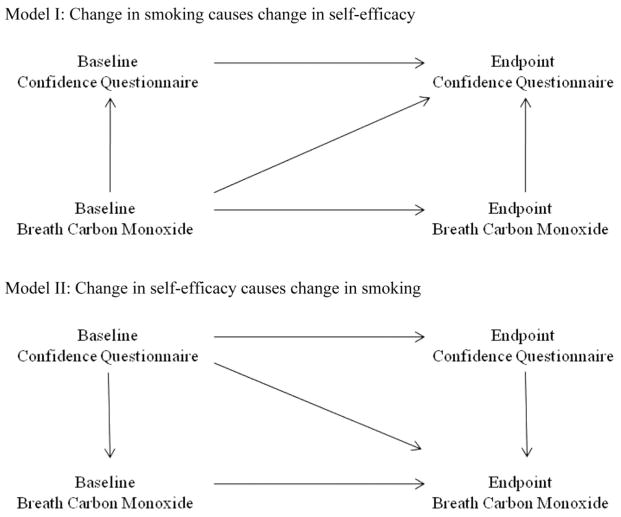
For SEM, two models were evaluated. Autocorrelation paths, that is, the correlation of each measure at baseline with the same measure at endpoint, were included in all models tested. Model I (see Figure 1) posited that changes in smoking cause changes in smoking cessation self-efficacy. The model includes causal paths from breath CO to the CQ measures obtained at the same or subsequent time points. This model has five causal paths and df = 1 to test goodness of fit. Model II (see Figure 1) reverses the directionality of the causal paths between breath CO and the CQ, positing that smoking cessation self-efficacy causes changes in smoking behavior.
Figure 1.
Path analysis models showing the predicted relationships between breath carbon monoxide (CO) and smoking cessation self-efficacy if breath CO were the causal factor (top diagram) or smoking cessation self-efficacy were the causal factor (bottom diagram).
The SEM analyses, which provide a comprehensive analysis of the causal models, were supplemented with conventional least-squares multiple regression analyses, regressing the endpoint CQ on the other three measures to assess causation of change in smoking cessation self-efficacy, and then repeating this analysis with breath CO at endpoint as the dependent measure and the other three as independent variables. We also looked at multiple regression analyses based on change scores. As expected, the results of these analyses were very similar to the SEM analyses and they are not reported in detail.
Pearson correlation coefficients between the CQ from Visit 10 and the change in breath CO levels from baseline (Visit 10) for that individual’s mean were analyzed for significance. The change in CQ scores was determined by two points (Visits 10 and 30), and the change in breath CO levels was determined for each visit relative to the baseline measure (Visit 10). Pearson correlation coefficients between the change in CQ scores between Visits 10 and 30 and the change in breath CO levels from baseline were also analyzed for significance.
Results
Table 1 shows the means and standard deviations for each of the four variables used in the analyses. Breath CO is expressed in parts per million, whereas the CQ is measured on a scale from 0% to 100%, where 100% represents that a smoker is sure that he or she can abstain from smoking in a hypothetical situation.
Table 1.
Means and Standard Deviations of Breath Carbon Monoxide and Self-Efficacy During Visits 10 and 30
| Variable | Visit
|
|
|---|---|---|
| 10 | 30 | |
| Mean (SD) breath carbon monoxide (ppm) | 16.94 (9.25) | 5.16 (4.14) |
| Mean (SD) Confidence Questionnaire score | 53.01 (16.29) | 61.33 (18.37) |
The first step in our analyses involved testing the crosslag correlations for spuriousness. The cross-lag correlations are illustrated by the diagonal arrows in each model in Figure 1. The result from the test between the cross-lag correlations was significant (ZPF = 3.26, p < .05),1 suggesting that the relationship between smoking cessation self-efficacy and breath CO was not spurious (Raghunathan, Rosenthal, & Rubin, 1996). Thus, it could be that smoking cessation self-efficacy predicts breath CO or that breath CO predicts smoking cessation self-efficacy. However, based on the significant difference between the cross-lag correlations, there was not a third variable accounting for the relationship.
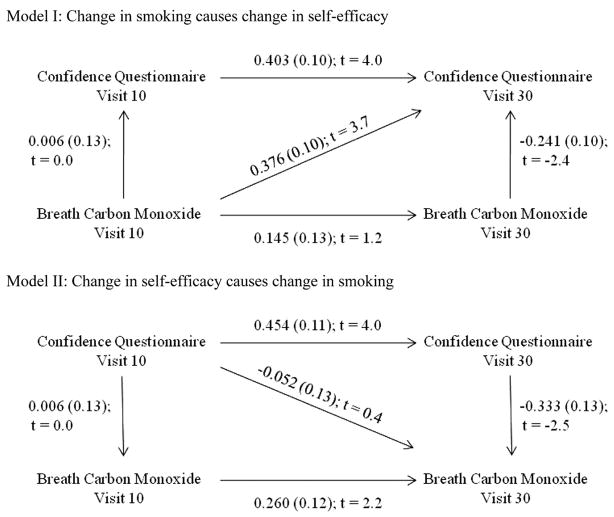
To test for directionality in the relationship between smoking cessation self-efficacy and breath CO, we conducted SEM analyses. Figure 2 shows the path analysis for two models of causality. In the top diagram, changes in smoking were hypothesized to cause changes in smoking cessation self-efficacy. The numbers above each path, or arrow, represent the Pearson correlation coefficient, the standard deviation (in parentheses), and t statistic. This t statistic is like a z statistic and is statistically significant when the value is greater than ~1.95. Model I posited a causal path between Visit 10 breath CO and Visit 30 smoking cessation self-efficacy. In Model I, the goodness-of-fit index (GFI) = 0.98, and a chi-square test showed that this model did an adequate job of fitting the obtained data, χ2(1) = 2.65, p = .103.2 The critical correlation occurred in the path between the mean breath CO level at Visit 10 (baseline) and mean smoking cessation self-efficacy during Visit 30, which was significant (p < .05). This is in contrast to the nonsignificant correlation in the bottom diagram that posited a causal path between Visit 10 smoking cessation self-efficacy and the Visit 30 breath CO level. Model II had a GFI = 0.93, and a chi-square test showed that the model did not do an adequate job of fitting the data, χ2(1) = 9.82, p = .002.
Figure 2.
Path analysis models with correlation coefficient, standard error, and t value for each correlation. The top diagram hypothesizes breath carbon monoxide (CO) as the causal factor, and the bottom diagram hypothesizes smoking cessation self-efficacy as the causal factor.
In addition, two multiple regression models were created: one with smoking cessation self-efficacy at Visit 30 as the dependent variable, and one with breath CO at Visit 30 as the dependent variable. The regression model with smoking cessation self-efficacy at Visit 30 as the dependent variable was significant, F(2) = 14.28, p < .0001, whereas the model with breath CO at Visit 30 as the dependent variable was not, F(2) = 1.98, p = .147. These results are consistent with the SEM analysis, in which smoking cessation self-efficacy is dependent on changes in breath CO in a contingency management procedure.
Figure 3 shows the correlations between the baseline measure of smoking cessation self-efficacy (Visit 10) and daily change from baseline in subsequent breath CO. It also displays correlations between the overall change in smoking cessation self-efficacy baseline after 20 days (the difference between self-efficacy measured on Visits 10 and 30) and the daily changes from baseline in breath CO for each of the first 20 days of treatment. The upper and lower horizontal dashed lines indicate a 95% confidence interval for statistical significance. Points above the upper dashed line are statistically significant, as are points falling below the lower dashed line. The middle dashed line represents a correlation equal to zero.
Figure 3.
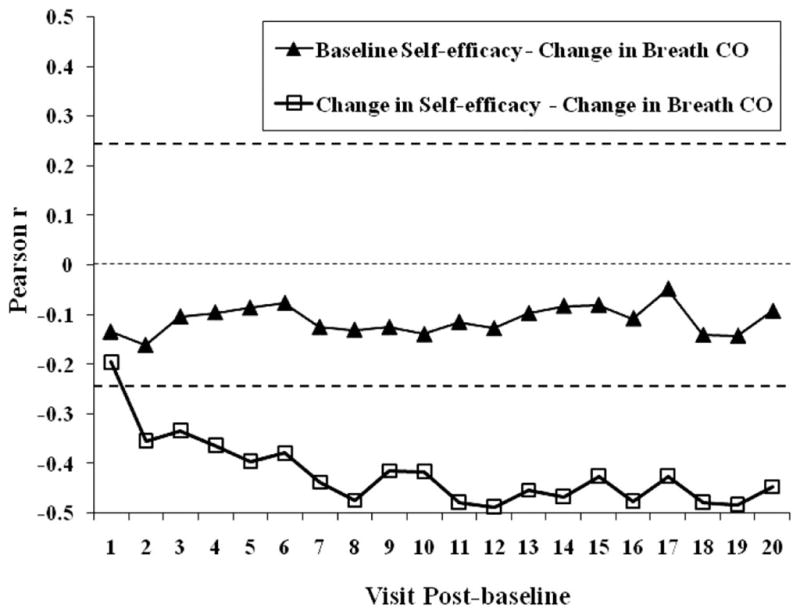
Pearson correlation coefficients as a function of visits (postbaseline) in a contingency management smoking cessation program. Closed triangles represent correlations between the baseline measure of smoking cessation self-efficacy and the change in breath carbon monoxide (CO) levels over 20 consecutive postbaseline visits. Open squares represent correlations between the change in smoking cessation self-efficacy ratings at baseline and postbaseline Visit 20, and the change in breath CO levels over 20 consecutive postbaseline visits. Points above the upper dashed horizontal lines and below the lower dashed horizontal line are statistically significant at p < .05. The middle dashed line represents a correlation coefficient equal to zero.
As shown in Figure 3, there was no significant correlation between the baseline measure of smoking cessation self-efficacy and subsequent changes in breath CO (closed triangles). The correlations were never significant over the 20 visits. Thus, participants’ changes in breath CO level over the first 20 visits of treatment were not predictable from baseline smoking cessation self-efficacy. Those participants who started the treatment with high smoking cessation self-efficacy were no better at decreasing their breath CO levels than participants who had moderate or low smoking cessation self-efficacy ratings.
Conversely, Figure 3 shows significant negative correlations between changes in breath CO and ultimate changes in smoking cessation self-efficacy. This negative correlation was statistically significant on postbaseline Visit 2 and maintained significance over the remaining postbaseline visits. Thus, associations between daily decreases in smoking and ultimate increases in self-efficacy began to appear very early and continued throughout the experimental period. The results from the path analysis suggest that the changes in smoking cessation self-efficacy that took place between Visits 1 and 19 postbaseline were caused by changes in breath CO. Between postbaseline Visits 2 and 20 a stable, statistically significant negative correlation was apparent between these two variables.
Discussion
The current study examined the correlations between breath CO and smoking cessation self-efficacy in a contingency management procedure. We were able to rule out a spurious relationship between these two variables, which permitted additional tests to determine the directionality of this relationship. Two models were created, and the model that posited self-efficacy as the dependent variable was consistently superior in relation to the current data through both SEM and multiple regression analyses. In addition, the correlation between baseline smoking cessation self-efficacy and subsequent changes in breath CO was not significant, whereas the correlation between the change in breath CO and the change in smoking cessation self-efficacy was consistently significant after the first contingency management visit. These results strongly support a predictive role for changes in breath CO on changes to smoking cessation self-efficacy, but not vice versa.
Our results are similar to those reported by Wong et al. (2004) in cocaine-dependant outpatients involved in a voucher-based treatment program. Using a combination of bivariate analyses and SEM, Wong et al. showed that increasing rates of cocaine abstinence predicted later changes in coping self-efficacy, as measured by the short form of the Situational Confidence Questionnaire (Annis, 1984). Prior coping self-efficacy made no independent contribution to predicting later changes in abstinence. Thus, along with findings by Baer et al. (1986) and Reilly et al. (1995), there appears to be a growing basis to doubt the predictive validity of self-efficacy in regards to future changes in drug abuse.
One advantage of the current study was the biologically verified levels of smoking in the participants. A portion of the previous studies has based the level of smoking behavior on participants’ self-reports with cross-checks from spouses, relatives, or close friends. This measure of smoking is much less exact. The chances of obtaining a correlation between self-reported measures of smoking and self-efficacy are also much higher, as participants may strive for consistency between their behavior and self-perceptions. Biologically verified smoking levels in the form of breath CO eliminated that risk.
On the other hand, this study was limited by a relatively small sample (n = 63), the lack of daily CQ assessments, and the unique procedure used to initiate smoking abstinence, all of which could limit the generalizability and power of the findings. It could also be argued that because the participants were not seeking treatment for their smoking, the results are inconsequential for the majority of smoking cessation treatments, whose participants are explicitly looking to quit smoking. However, the current study was conducted to test between two competing theories of behavior change, and not smoking cessation treatments. Nonetheless, the fact that robust reductions in smoking can be attained without specifically targeting modifications in smoking cessation self-efficacy argues against self-efficacy’s utility as a key component of initiating behavior change for the current subjects. There has been support for self-efficacy’s role in the maintenance of abstinence (Baer et al., 1986), for which the current results are not applicable.
An alternative way to view self-efficacy’s effect on behavior is as a discriminative stimulus for behavior change. A discriminative stimulus is a class of stimuli that changes the likelihood that a class of responses occurs in its presence because of a history (or lack thereof) of reinforcement (Catania, 1997). For example, for those people who feel that they have a greater sense of self-efficacy, attempting to change their own behavior in the past had often been successful. Conversely, for those people who feel that they have low self-efficacy, attempting to change their own behavior had been relatively unsuccessful. The key factor underlying both high and low levels of self-efficacy is the history of consequences for behaving. Hence, altering the probability of success for attempting behavior change will then subsequently affect the state of self-efficacy that has been associated with behavior change in the past. Therefore, contingency management procedures are successful in promoting behavior change because they alter the probability that an attempt to change behavior will contact a reinforcer. On the other hand, changing self-efficacy alters the likelihood that an attempt to change behavior is made. However, the consequences for that behavior may not have changed. If the consequences for behavior change remain poor, that change will not be maintained, no matter the level of self-efficacy. Similar asymmetrical relationships between emitted behavior and subjective effects have been shown in drug discrimination procedures (Lamb & Henningfield, 1994).
In sum, the current results put self-efficacy’s role in initiating behavior for a contingency management procedure in doubt. A more direct and reliable way to promote behavior change may be through altering the probability that a behavior contacts reinforcement.
Acknowledgments
This research was supported by National Institutes of Health Grant DA013304 to R. J. Lamb.
Footnotes
The cross-lag correlations were also tested using the Pearson–Filon method and Olkin z test. Both tests were highly significant (p < .05).
In SEM, the χ2 statistic is described as significant when the p value is greater than .05, not less than .05, as in a traditional nonparametric χ2 test.
References
- Annis HM. Situational Confidence Questionnaire, Short Form. Toronto: Addiction Research Foundation; 1984. [Google Scholar]
- Baer JS, Holt CS, Lichtenstein E. Self-efficacy and smoking reexamined: Construct validity and clinical utility. Journal of Consulting and Clinical Psychology. 1986;54:846–852. doi: 10.1037//0022-006x.54.6.846. [DOI] [PubMed] [Google Scholar]
- Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: An exploration of individual differences. Journal of Consulting and Clinical Psychology. 1988;56:104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy mechanism in human agency. American Psychologist. 1982;37:122–147. [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Catania AC. Learning. Englewood Cliffs, NJ: Prentice Hall; 1997. [Google Scholar]
- Centers for Disease Control and Prevention. Annual smoking- attributable mortality, years of potential life lost, and economic costs—United States 1995–1999. Morbidity and Mortality Weekly Report. 2002;51:300–303. [PubMed] [Google Scholar]
- Collins RL, Lapp WM. Restraint and attributions: Evidence of the abstinence violation effect in alcohol consumption. Cognitive Therapy and Research. 1991;15:69–84. [Google Scholar]
- Condiotte MM, Lichtenstein E. Self-efficacy and relapse in smoking cessation programs. Journal of Consulting and Clinical Psychology. 1981;49:648–658. doi: 10.1037//0022-006x.49.5.648. [DOI] [PubMed] [Google Scholar]
- DiClemente CC. Self-efficacy and smoking cessation maintenance: A preliminary report. Cognitive Therapy and Research. 1981;5:175–187. [Google Scholar]
- DiClemente CC, Prochaska JO, Gibertini M. Self-efficacy and the stages of self-change of smoking. Cognitive Therapy and Research. 1985;9:181–200. [Google Scholar]
- Gulliver SB, Hughes JR, Solomon LJ, Dey AN. Self-efficacy and relapse to smoking in self-quitters. Addiction. 1995;90:767–772. doi: 10.1046/j.1360-0443.1995.9067673.x. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Norman GJ, Paty JA, Kassel JD, Gnys M, et al. Does smoking abstinence self-efficacy vary across situations? Identifying context-specificity within the Relapse Situation Efficacy Questionnaire. Journal of Consulting and Clinical Psychology. 2001;69:516–527. [PubMed] [Google Scholar]
- Higgins ST, Silverman K. Motivating behavior change among illicit-drug abusers: Research on contingency management interventions. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- Higgins ST, Silverman K, Heil SH. Contingency management in substance abuse treatment. New York: Guilford Press; 2008. [Google Scholar]
- Kenny DA. Cross-lagged and synchronous common factors in panel data. In: Goldberger AS, Duncan OD, editors. Structural equation models in the social sciences. New York: Seminar Press; 1973. pp. 153–166. [Google Scholar]
- Kenny DA. Cross-lagged panel correlation: A test for spuriousness. Psychological Bulletin. 1975;82:887–903. [Google Scholar]
- Lamb RJ, Henningfield JE. Human d-amphetamine drug discrimination: Methamphetamine and hydromorphone. Journal of the Experimental Analysis of Behavior. 1994;61:169–180. doi: 10.1901/jeab.1994.61-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Galbicka G, Kirby KC, Iguchi MY. Shaping reduced smoking in smokers without cessation plans. Experimental and Clinical Psychopharmacology. 2005;13:83–92. doi: 10.1037/1064-1297.13.2.83. [DOI] [PubMed] [Google Scholar]
- Manfredi C, Cho YI, Crittenden KS, Dolecek TA. A path model of smoking cessation in women smokers of low socio-economic status. Health Education Research. 2007;22:747–756. doi: 10.1093/her/cyl155. [DOI] [PubMed] [Google Scholar]
- McIntyre KO, Lichtenstein E, Mermelstein RJ. Self-efficacy and relapse in smoking cessation: A replication and extension. Journal of Consulting and Clinical Psychology. 1983;51:632–633. doi: 10.1037//0022-006x.51.4.632. [DOI] [PubMed] [Google Scholar]
- Milburn MA. A longitudinal test of the selective exposure hypothesis. Public Opinion Quarterly. 1979;43:507–517. [Google Scholar]
- Nicki RM, Remington RE, MacDonald GA. Self-efficacy, nicotine-fading/self-monitoring, and cigarette-smoking behaviour. Behaviour Research and Therapy. 1984;22:477–485. doi: 10.1016/0005-7967(84)90051-2. [DOI] [PubMed] [Google Scholar]
- Raghunathan TE, Rosenthal R, Rubin DB. Comparing correlated but nonoverlapping correlations. Psychological Methods. 1996;1:18–22. [Google Scholar]
- Reilly PM, Sees KL, Shopshire MS, Hall SM, Delucchi KL, Tusel DJ, et al. Self-efficacy and illicit opioid use in a 180-day methadone detoxification treatment. Journal of Consulting and Clinical Psychology. 1995;63:158–162. doi: 10.1037//0022-006x.63.1.158. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Lamb RJ, Dallery J. Tobacco. In: Higgins ST, Silverman K, Heil SH, editors. Contingency management in substance abuse treatment. New York: Guilford Press; 2008. pp. 99–119. [Google Scholar]
- Sitharthan T, Kavanagh DJ. Role of self-efficacy in predicting outcomes from a programme for controlled drinking. Drug and Alcohol Dependence. 1991;27:87–94. doi: 10.1016/0376-8716(91)90091-c. [DOI] [PubMed] [Google Scholar]
- Skinner BF. About behaviorism. Oxford, England: Knopf; 1974. [Google Scholar]
- Solomon KE, Annis HM. Outcome and efficacy expectancy in the prediction of post-treatment drinking behaviour. British Journal of Addiction. 1990;85:659–665. doi: 10.1111/j.1360-0443.1990.tb03528.x. [DOI] [PubMed] [Google Scholar]
- Sperry JM, Nicki RM. Cognitive appraisal, self-efficacy and cigarette smoking behavior. Addictive Behaviors. 1991;16:381–388. doi: 10.1016/0306-4603(91)90046-k. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for reduced carbon monoxide levels in cigarette smokers. Addictive Behaviors. 1982;7:403–412. doi: 10.1016/0306-4603(82)90010-7. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent payment for carbon monoxide reduction: Effects of pay amount. Behavior Therapy. 1983;14:647–656. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart K, Borland R, McMurray N. Self-efficacy, health locus of control and smoking cessation. Addictive Behaviors. 1994;19:1–12. doi: 10.1016/0306-4603(94)90046-9. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Smoking cessation: Clinical practice guideline. Washington, DC: Public Health Service, Centers for Disease Control and Prevention; 2000. [Google Scholar]
- Wong CJ, Anthony S, Sigmon SC, Mongeon JA, Badger GJ, Higgins ST. Examining interrelationships between abstinence and coping self-efficacy in cocaine-dependent outpatients. Experimental and Clinical Psychopharmacology. 2004;12:190–199. doi: 10.1037/1064-1297.12.3.190. [DOI] [PubMed] [Google Scholar]