Abstract
The field of biomedical optics has matured rapidly over the last decade and is poised to make a significant impact on patient care. In particular, wide-field (typically > 5 cm), planar, near-infrared (NIR) fluorescence imaging has the potential to revolutionize human surgery by providing real-time image guidance to surgeons for tissue that needs to be resected, such as tumors, and tissue that needs to be avoided, such as blood vessels and nerves. However, to become a clinical reality, optimized imaging systems and NIR fluorescent contrast agents will be needed. In this review, we introduce the principles of NIR fluorescence imaging, analyze existing NIR fluorescence imaging systems, and discuss the key parameters that guide contrast agent development. We also introduce the complexities surrounding clinical translation using our experience with the Fluorescence-Assisted Resection and Exploration (FLARE™) imaging system as an example. Finally, we introduce state-of-the-art optical imaging techniques that might someday improve image-guided surgery even further.
Keywords: Near-Infrared Fluorescence, Image-Guided Surgery, Clinical Translation, Optical Imaging Systems, Near-Infrared Fluorescent Contrast Agents
INTRODUCTION
There is often a disconnect between new technology and the clinicians who will ultimately use it. Even the most exciting and elegant technology will fail to find clinical use unless it meets three critical criteria: 1) a true clinical need exists, 2) the new technology solves the problem, and 3) the new technology does not impede normal clinical workflow. This helps explain why, after 100 years, there are only five imaging modalities used routinely in patient care: x-rays (plain film, fluoroscopy, and computed tomography [CT]), magnetic resonance imaging (MRI), ultrasound (US), single-photon emission computed tomography (SPECT) and positron emission tomography (PET) [reviewed in 1]. It also helps explain why only two of these (x-ray fluoroscopy and ultrasound) are used even occasionally for image-guided surgery. The clinical need is there (criterion 1) and workflow is relatively unimpeded because imaging is in real-time (criterion 3), but existing imaging modalities do not solve most surgical problems (criterion 2).
The unmet clinical need in surgery stems from the fact that visible light cannot penetrate into blood and tissue more than a few hundred microns, due to high photon attenuation from absorbance and scatter. Thus, when a surgeon looks at the surgical field, he/she only sees surface features. The implications of this are significant since there are over 40 million surgical procedures performed in the United States (U.S.) each year, and even small improvements in outcome, or reductions in morbidity, will affect large patient populations. For example, with modern surgical techniques, 20 to 25% of breast cancers are still resected incompletely2,3 and local recurrence remains unacceptably high at 12-28%.4 Moreover, nerve damage during many types of surgery, resulting in post-surgical neuralgia and loss of function, occurs in 20,000 to 600,000 patients per year in the U.S. alone.5 Being able to see structures that need to be resected, such as malignant cells, and structures that need to be avoided, such as blood vessels and nerves, is a profound unmet clinical need.
One relatively new technology that has the potential to guide surgeons in real-time is the use of invisible near-infrared (NIR) fluorescent light within the “NIR window” of 700 to 900 nm.6 Within this NIR window, the absorption of most biomolecules (i.e., deoxyhemoglobin, oxyhemoglobin, water, and lipid) reaches local minima, scattering is relatively low, and tissue autofluorescence is relatively low. Thus, photon penetration into, and out of, tissue is relatively high (on the order of several millimeters), and the addition of exogenous NIR fluorophores produces “bright stars on a black background,” or more formally, a high signal-to-background ratio (SBR). Since NIR fluorescent light is essentially invisible to the human eye, special imaging systems are required to excite the NIR fluorophore(s) within the surgical field and to collect emitted photons. Well-designed NIR fluorophores are needed to highlight the specific structures desired by the surgeon. And, finally, training tools are needed to train surgeons how to use the technology. This latter issue has been addressed elsewhere and will not be discussed further.7
Recognizing the potential of optical imaging in general, and NIR fluorescent light in particular, to improve human surgery, the Cancer Imaging Program (CIP) of the National Cancer Institute (NCI), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and the National Science Foundation (NSF) have invested heavily in these technologies over the last decade. As described in this review, the return on this investment has been rather rapid pre-clinical validation, first-in-human clinical trials, and now commercial availability of NIR fluorescence imaging systems for image-guided surgery. NIR fluorophore development is proceeding at a slower pace, but is also on-target for clinical translation in the near future. This review will detail the key issues surrounding the development and clinical translation of continuous wave (CW), reflectance NIR fluorescence image-guided surgery systems and their associated NIR fluorophores in the hopes of establishing a strong foundation upon which the field can build the next-generation of advances.
NEAR-INFRARED FLUORESCENCE IMAGING SYSTEMS
Principles of Operation
NIR diffuse optical imaging takes advantage of the deep photon penetration of NIR light into living tissue to provide fast, quantitative, relatively inexpensive imaging of endogenous and/or exogenous contrast at depths < 1 cm.8,9 Within this field, NIR fluorescence imaging focuses on the detection of exogenous contrast agents that emit fluorescence between 700 to 900 nm. The typical fluorescence imaging system has been described in detail in various references.10-15 Briefly, it consists of a spectrally-resolved light source (filtered broadband source, light-emitting diode, or laser diode) exciting a fluorophore within a turbid medium. The light emitted from this fluorophore is then imaged onto a charge-coupled device (CCD) camera, with special care taken to filter out the powerful excitation light.
Key Design Parameters
Although conceptually simple, virtually every design parameter will impact system performance and thus SBR.
Field-of-View (FOV)
An adjustable FOV is mandatory for surgical imaging and can be accomplished using either zoom or fixed magnification lensing (see below). In general, maximum FOV should be at least 10 cm (≈ 4”) in diameter, and preferably 20 cm (≈ 8”) in diameter to accommodate various surgical applications. For resection of small tumors, or avoidance of thin nerves, a minimum FOV should also be defined, preferably in the range of 1 to 2 cm. The major tradeoffs with FOV are excitation fluence rate, collection optics, and final resolution. The larger the maximum FOV, the more total power will be required by the light source, the higher the magnification needed to achieve the desired minimum FOV, and the lower the final resolution since the CCD camera(s) will have a finite number of pixels.
Fluorescence Excitation Light Source
There are three major technologies capable of producing light for excitation of NIR fluorophores: filtered broadband sources, light emitting diodes (LEDs), and laser diodes. The choice is not straightforward because each has advantages and disadvantages as follows:
Filtered-Broadband Sources
The overriding problem is efficiency. Most of the photons produced by the source are discarded in order to filter excitation light to a narrow enough band, and in most cases excessive heat is produced that must be dissipated. These sources also have a relatively large solid angle, meaning that only a small fraction of the optical power propagates towards the surgical field.10 Concentrating optical power inside light guides using mirrors and lenses is also difficult. Because of these issues, there are few applications in image-guided surgery where broadband sources would be optimal.
LEDs
LEDs provide a good compromise between power (tens of mW each), spectral confinement (typical full-width at half maximum, FWHM, < 50 nm), efficiency, and cost. Moreover, their use in consumer markets has resulted in steady improvements in performance and reductions in package size. Working with LEDs for surgical imaging, however, can be challenging, and an in-depth discussion has recently been published elsewhere.16 The key points are that heat dissipation is a major challenge for dense arrays, an excitation filter must be used to reduce the spectral FWHM, collimators must be used on each LED to restrict solid angle to the acceptance angle of the excitation filter (if needed), and lensing (either inherent to epoxy LEDs or external) must be used to concentrate optical power within the FOV.
Laser Diodes
Laser diodes are the most confined, spatially and spectrally, but can be difficult to integrate, are expensive at high power, and can trigger safety concerns related to maximal permitted exposure (MPE) and the need for personal protective equipment such as laser goggles. Laser diodes also require precise control in current and in temperature (using a proportional-integral-derivative [PID] feedback control loop) as temperature variations induce changes in wavelength and high temperatures reduce the lifetime of the diode. Recently, NIR diodes in the 1 to 2 W range have become available, although not every wavelength is available in high power packages. In some situations, a major problem with coherent sources like laser diodes is speckle, which may have to be removed prior to illuminating the surgical field by either rotating a diffuser or vibrating a fiber at frequencies much higher (typically 10-times) the camera frame rate.
The two strategies for illuminating a surgical field with a light source are local and remote. Local lighting is placed immediately above the patient, and can be achieved with all three sources. However, this requires high power consumption in the imaging head, power loss in transmission lines, and introduction of potential radiofrequency (RF) noise from light source controllers. LEDs can be an excellent choice for local lighting.16 Remote lighting employs a light guide to illuminate the field using a remote source. At the present time, laser diodes are the only reasonable solution for remote lighting because of the large solid angle of broadband and LED sources, resulting in poor light guide coupling efficiency and difficulty in achieving high fluence rates over large FOVs. With respect to light guides, fiber-based guides can offer high transmissivity over longer distances and small bending radii compared to liquid light guides, and the latter also have relatively high numerical aperture (NA; typically > 0.5) and are sensitive to heat and mechanical manipulation.
NIR Excitation Fluence Rate (i.e., Irradiance)
As discussed below, small molecule organic fluorophores typically undergo irreversible photobleaching when excitation fluence rate is > 50 mW/cm2.17,18 Higher fluence rates also run the risk of heating tissue and causing thermal damage. An “adequate” fluence rate for a particular application depends on whether the fluorophore is embedded deep in tissue (highest fluence rate possible required), the f-number (f/#) of the collection optics, and the sensitivity of the CCD camera. The lowest possible fluence rate necessary to achieve NIR fluorescence emission in the middle range of the CCD camera, at its maximal frame rate, should be employed. Although this value will also depend on the concentration of the NIR fluorophore, in general, the fluence rate should be adjustable to accommodate all possible clinical scenarios. In all cases, care must be taken to limit MPE to the skin and the eyes to values defined in the International Electrotechnical Commission (IEC) standard #60825. For NIR wavelengths closer to the visible range, e.g., 670 nm excitation, maximally permitted fluence rate will also be limited by the changes induced in color rendering index of the white light.
White Light Source
For simultaneous imaging of color video and NIR fluorescence emission using the FLARE™ imaging system,15,16 it is important to filter “white” light to 400 to 650 nm. This produces effective isolation of the color video channel from the two NIR fluorescence channels. This is an important point since most surgical luminaries emit significant amounts of light above 650 nm. Two regulatory guidelines from the IEC standard #60601 then come into play. The first is that the minimum central luminance of the surgical field must be at least 40,000 lux at the typical working distance (WD). The second is that that color temperature must be between 3000 and 6700 K with a color rendering index (CRI) ≥ 85. The latter ensures that the surgical field is not preferentially “tinged” with one or more visible colors. Most filtered broadband and LED white light sources can be used for providing white light to the surgical field using local approaches. Supercontinuum laser sources, which are currently cost prohibitive and relatively low power, may eventually be the most robust solution for remote illumination.
Collection Optics
One of the most important components of the optical system is the lensing that relays white and NIR fluorescent emission light to the camera(s). There are multiple, complex tradeoffs among f/# (i.e., amount of light transmitted by the system), depth of field, WD, and FOV that are beyond the scope of this review. In general, one can choose to employ macro zoom optics, optimized for a fixed WD, or fixed magnification optics, where the FOV decreases, but excitation fluence rate increases, as the imaging system is moved closer to the patient. Each choice has advantages and disadvantages, but both require special attention to chromatic aberration correction from 400 to 900 nm, minimization of distortion and vignetting, and parfocality is preferred to ensure that color video and NIR fluorescence images are in focus at all magnifications. It should also be noted that final system resolution may be diffraction-limited or camera-limited depending on f/# and lensing choices. For NIR fluorescent objects embedded below living tissue, the blur caused by scatter will far outweigh small deficiencies in system optics, however, resolution should always be as high as the data storage system can accommodate.
Cameras
Silicon-based CCD cameras are most commonly used for image-guided surgery, but are not ideal. They suffer from low quantum efficiency (QE; typically ≤ 25%) at 800 nm and relatively slow readout times (typically ≈ 15 to 30 fps), but do have low readout noise (≤ 10 e− rms) when cooled and high resolution (512 to 1324 pixels per dimension). An alternative is complementary metal oxide semiconductor (CMOS) sensors, which reduce size and power consumption, and increase frame rate (≥ 25 fps), but also have low QE and high readout noise. The newly introduced scientific CMOS (sCMOS) sensors appear to have all desired features, with readout noise < 3 e− and QE ≈ 33% at 800 nm, but remain expensive. Although not needed for qualitative imaging, pixel bit depths should be 8 to 12 bits for quantitative NIR fluorescence imaging. In general, non-cooled silicon CCDs can achieve 10 bits and Peltier-cooled CCDs can achieve 12 bits. Finally, form factor is a concern in multi-camera imaging systems, with cooled CCDs adding considerable size and weight.
Light Intensification
In some clinical scenarios, where NIR fluorescence emission collection is photon-limited, light amplification using an image intensifier tube (IIT) might be needed. The most commonly used IIT is also used in military night vision systems and consists of a photocathode (PC), which converts photons into electrons, a single- or multi-stage microchannel plate (MCP), which accelerates electrons, and a phosphor screen (PS). Although currents are low (in the nA range), voltages are on the order of ≈ −900 V for the PC to MCP input (ground), ≈ 1000 V from MCP input to MCP output, and ≈ 4000 V from MCP output to PS. IITs add considerable cost to a system, tend to lower resolution, if not cooled will add significant dark noise. If cooled, they are subject to condensation unless hermetically sealed.
Dichroic Mirrors and Filters
High-quality filters are essential for both excitation and emission light paths. Although absorption-type filters are inexpensive to make, they have poor out-of-band rejection and low transition slopes. The alternative is interference filters, with the state-of-the-art being sputtered manufacturing techniques. A modern sputtered filter can achieve up to 98% transmission, extremely high blocking power (OD ≥ 6), and high transition slopes. The main problems with sputtered interference filters are cost and some limitations with respect to extended spectral blocking ranges. Additionally, spectral characteristics vary greatly with incidence angle, and light should always hit the filter at normal incidence or be constrained to within a specified acceptance angle.
Data Display
A ≥ 20” digital display monitor is typically used to provide the surgeon with real-time images, and screen resolution should be chosen to accommodate native camera(s) resolution. The FLARE™ imaging system actually has three monitors, two facing the technologist (one control, one image display) and one (image display) facing the surgeon.15 Leakage current is the main concern with any monitor in the operating room (OR), but using an isolation transformer can mitigate risk.
Software
Imaging system software should be written in a compiled language (e.g., C++ or C#) for robustness. The data archiving structure should provide lossless compression. In most circumstances, metadata, e.g., excitation fluence rates, camera exposure time, etc., should be saved along with the image. In multi-camera imaging systems, seamless camera streaming is a significant challenge, and often requires the use of multi-core computers and/or a graphical processing unit (GPU). Calibration sequences should be employed prior to the start of each surgery, and can help correct for illumination inhomogeneities and lens distortion, if present. Recent work by Themelis et al. also provides real-time light absorption correction.14
Electrical Constraints
The entire imaging system must be compliant with relevant electronics standards, which in the U.S. is defined in IEC #60601, as well as institutional standards. The main considerations with respect to safety are redundant grounding, maintenance of total leakage current < 300 μA, and a maximum power consumption compatible with a standard 15A, 120 V plug.
Physical and Mechanical
Ergonomics is a key to clinical acceptance. In general, OR staffs prefer small devices with great flexibility in positioning and multiple degrees of freedom of the imaging head. To ensure safety, cart-based imaging systems must also comply with minimum tilt standards.
Sterility
Because of the delicate nature of CCD cameras and electronics, it is typically not possible to sterilize the entire imaging head. As an alternative, a sterilized shield/drape combination15,16 can be attached to the imaging head under sterile conditions, and used to cover the entire apparatus to within 18” of the floor.
Existing Intraoperative NIR Fluorescence Imaging Systems:
Several complete intraoperative NIR fluorescence imaging systems are now available for pre-clinical and clinical studies (Table 1). The Novadaq SPY™ system (www.novadaq.com) is already approved by the U.S. Food and Drug Administration (FDA) for use during a variety of surgical procedures including coronary artery bypass grafting, plastic and reconstructive surgeries, and organ transplant, and is available in narrow [n] or wide [w] FOV models. Fluoptics’ (www.fluoptics.com) Fluobeam®, Hamamatsu’s (www.hamamatsu.com) Photodynamic Eye (PDE™) and the Frangioni Laboratory’s (www.frangionilab.org) FLARE™ imaging systems are presently available for experimental use, and are also eligible for 510(k) approval based on Novadaq’s predicate device.
Table 1.
Near-Infrared Fluorescence Imaging Systems Available for Pre-Clinical and Clinical Studies.
| Imaging System |
Manufacturer | Excitation Wavelength(s) |
Fluence Rate (mW/cm2) |
FOV (cm × cm) |
Dynamic Range (bits) |
Working Distance (cm) |
Simultaneous Color Video |
Light Source |
Clinical Status |
|---|---|---|---|---|---|---|---|---|---|
| SPY | Novadaq | 806 nm | 31 [n] | 7.6 × 5 | 8 | 30 | No | LASER | FDA-approved |
| 4 [w] | 19 × 12.7 | ||||||||
| PDE | Hamamatsu | 760 nm | 4 | 10 × 6.7 | 8 | 20 | No | LED | Exploratory only |
| Fluobeam® | Fluoptics | 690 nm or 780 nm |
6 (690nm) 6 (780nm) |
12.8 × 9.4 | 12 | 15 | No | LASER | Exploratory only |
| FLARE™ | * | 670 nm and 760 nm |
4 (670nm) 14 (760nm) |
15 × 11.3 | 12 12 |
45 | Yes | LED | Exploratory only |
| Mini- FLARE™ |
* | 670 or 760nm (multiplexed) |
1 (670nm) 7 (760nm) |
12 × 9 | 12 | 32 | Yes | LED | Exploratory only |
[n] = narrow FOV model; [w] = wide FOV model; * = Contact Dr. Frangioni for availability
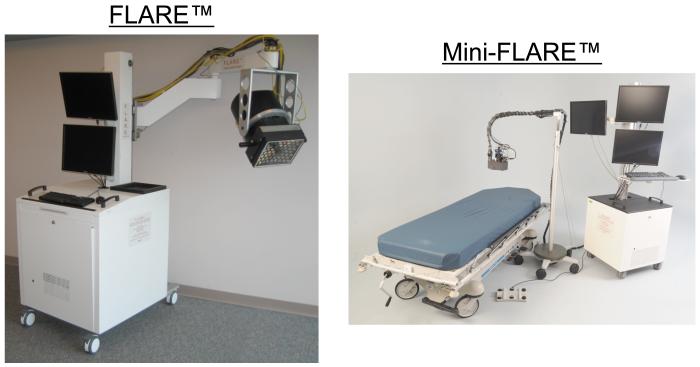
All of these imaging systems utilize either laser diodes or LEDs at fluence rates usually in the range of 1 to 10 mW/cm2. Systems utilize 12-bit dynamic range CCD cameras (FLARE™, Mini-FLARE™, and Fluobeam®) or 8-bit cameras (SPY™ and PDE™). Working distances and FOVs vary. The FLARE™15,16 and Mini-FLARE™ (Troyan et al., manuscript in review) systems (Figure 1) provide simultaneous color video and two independent channels (700 nm and 800 nm) of NIR fluorescence emission.
Figure 1. FLARE™ and Mini-FLARE™ Image-Guided Surgery Systems.
The FLARE™ imaging system (left) is composed of a cart containing control electronics, cooling, and a computer, an articulating providing 6 degrees of freedom, 127 cm lateral reach, and 178 cm vertical reach, and an imaging head containing a custom high power LED light source, 2 NIR and 1 color cameras, and custom optics.15 The Mini-FLARE™ imaging system (right; Troyan et al., manuscript in review) represents a significant reduction in size and improvement in flexibility.
Not listed in Table 1 are surgical microscopes equipped with NIR fluorescence modules, such as the Zeiss INFRARED 800 and Leica FL800. Although widely used in neurosurgical applications, these systems are not optimized for deep tissue imaging due to the use of broadband sources, which result in relatively low excitation fluence rate at large working distances or large fields of view. Nevertheless, the Zeiss Pentero microscope with the INFRARED 800 module is in compliance with the requirements of the European Medical Device directive 93/42/EEC. The Leica M720 microscope with the FL800 module has received FDA 510(k) clearance for heart, plastic, and neurosurgery.
NEAR-INFRARED FLUORESCENT CONTRAST AGENTS
Now that a variety of intraoperative NIR fluorescence imaging systems are available or are about to be available commercially, the development of effective NIR fluorescent contrast agents becomes of paramount importance. A general difficulty in effecting NIR absorption and emission in small molecules is the required number of double bonds. In general, 7 to 10 double bonds are required to reach wavelengths ≈ 700 nm, and 9 to 12 doubles bonds are required to reach ≈ 800 nm, with the range dependent on the influence of nearby chemical substituents. Not only does each double bond increase molecular weight, but it also increases hydrophobicity of the fluorophore. Thus, a core chemical structure that absorbs and emits in the NIR (Figure 2A) is necessarily relatively large and hydrophobic, which presents special problems for in vivo imaging (discussed below), including poor aqueous solubility, non-specific uptake in normal tissues and organs, and, exclusion by the blood-nerve-barrier (BNB) and blood-brain-barrier (BBB).
Figure 2. Contrast Agents for NIR Fluorescence-Guided Surgery.
- General structure of the heptamethine indocyanine class of NIR fluorophores.
- Chemical structures and key optical properties (in serum) of the clinically available NIR fluorophores methylene blue (MB; left) and indocyanine green (ICG; right).
In addition to small molecules, various nanoparticle (NP) systems that generate NIR absorption and emission, including dendrimers, molecular dots, and quantum dots have been described. Because the pre-clinical development and clinical translation of NIR fluorescent NPs are extremely complex, our group has recently reviewed this topic elsewhere.19
In the following section we discuss the physicochemical, optical, targeting, and in vivo properties of NIR fluorescent contrast agents, which are either used clinically now, or have the potential for clinical translation in the near future. The physicochemical and optical properties of commonly used NIR fluorescent small molecules NPs are detailed in Table 2. The reader is also encouraged to read excellent recent reviews on this subject.20-23
Table 2.
Key Physicochemical and Optical Properties in Serum for NIR Fluorescent Contrast Agents Useful for Pre-Clinical and Clinical Imaging.
| Contrast Agent |
MW (Da) |
HD (nm) |
LogD, pH 7.4 |
Net Charge | Peak Abs (nm) |
Peak Em (nm) |
Ext Coeff (M−1cm−1) |
QY* (%) |
Primary Clearance Route | |
|---|---|---|---|---|---|---|---|---|---|---|
| Renal | Hepatic | |||||||||
| MB | 320 | < 1 | −0.2 | +1 | 670 | 690 | 71,200 | 3.8 | + | ++ |
| Cy5.5-CA | 1,069 | < 1 | 1.44 | −4 | 680 | 697 | 166,000 | 21.0 | − | +++ |
| Cy7-CA | 720 | < 1 | 2.05 | −3 | 743 | 767 | 196,000 | 16.8 | − | +++ |
| ZW-1 | 1,149 | < 1 | −3.56 | 0 | 772 | 788 | 249,000 | 15.1 | +++ | − |
| CW800-CA | 1,091 | < 1 | 2.51 | −4 | 786 | 800 | 237,000 | 14.2 | + | ++ |
| ICG | 775 | < 1 | 7.88 | −1 | 807 | 822 | 121,000 | 9.3 | − | +++ |
| Nanoparticles, e.g., Quantum Dots |
N/A | Small** 2 – 6 |
N/A | Variable, depends on final coating (+,−,+/−) |
Variable, depends on nanoparticle size and/or payload of contrast agents (from UV to NIR) |
> 200,000 | 10-30134,135 | +++ | − | |
| (variable, depends on core/shell thickness) |
(depends on surface characteristics) |
|||||||||
| N/A | Large > 6 |
N/A | > 200,000 | 10-40136 | − | +++ | ||||
| (variable, depends on core/shell thickness) |
(RES, depends on coating and size) |
|||||||||
Wavelengths at which absorbance was matched for QY measurements: 655 nm for MB and Cy5.5-CA; 770 nm for Cy7-CA, ZW-1, CW800-CA, and ICG. QY standards included Oxazine 725 in ethylene glycol (QY = 19%30) for 700 nm fluorophores and ICG in DMSO (QY = 13%31) for 800 nm fluorophores.
For small nanoparticles, the thickness of the organic coating and the number of surface-bound targeting ligands significantly effects final HD.
Abs: Absorbance; CA: Carboxylic acid; CW800: IRDye™800-CW; Em: Emission; Ext Coeff: Extinction coefficient; HD: Hydrodynamic diameter; ICG: Indocyanine green; MB: Methylene blue; MW: Molecular weight; N/A: Not applicable; QY: Quantum yield; RES: Reticuloendothelial system; UV: Ultraviolet, ZW-1 (zwitterionic heptamethine indocyanine; Choi et al., manuscript in review).
Physicochemical and Optical Properties
Chemical Structure and Molecular Weight
Even though a relatively large number of double bonds are required to reach the NIR, some compact chemical structures have been described in the literature, including methylene blue, which can reach ≈ 700 nm emission with a molecular weight of only 320 Da (Figure 2B) and styryl pyridinium (FM) fluorophores, which can reach ≈ 800 nm emission with a molecular weight of only 612 Da.24 The compact nature of the NIR fluorophore is important for keeping final molecular weight after targeting ligand conjugation as low as possible. Compactness is also important for nerve targeting, where molecular weight should be ≤ 500 Da for efficient BNB and BBB penetration.25,26
The most widely utilized chemical structure for achieving NIR absorption and emission is the polymethine backbone, comprised of either a pentamethine (≈ 700 nm emission) or heptamethine (≈ 800 nm emission; Figure 2A) core. The heptamethine core, in particular, is remarkably versatile. Simply altering the linkage in R17 position with oxo-, amino-, or sulfur-will shift peak emission to ≈ 800 nm, 760 nm, or 840 nm respectively.27-29 Peak absorption for each of these R17 linkages is not proportional to emission, however, which can be exploited to produce NIR fluorophores with extremely large Stokes shifts.29
Peak Absorbance Wavelength and Extinction Coefficient
In order to preserve “white light” (400 to 650 nm) for color video imaging of the surgical field and efficient separation of fluorescence channels when using the FLARE™ imaging system, peak absorbance wavelength for the ≈ 700 nm NIR fluorophore is typically in the range of 670 to 680 nm, and for the ≈ 800 nm fluorophore is typically in the range of 750 to 770 nm. When exploiting the FLARE™ imaging system’s two independent NIR channels, it is usually preferable to keep the Stokes shift of the ≈ 700 nm fluorophore relatively low (20 to 30 nm) so that peak emission will fall below excitation for the ≈ 800 nm fluorophore (Figure 3).
Figure 3. FLARE™ Imaging System Optical Channels in the Context of Clinically-Available NIR Fluorophores.
The color video, 700 nm NIR fluorescence and 800 nm NIR fluorescence optical channels of the FLARE™ imaging system are shown, along with the absorbance (left axis) and fluorescence (right axis) of 5 μM methylene blue (MB) and 2.5 μM indocyanine green (ICG) in 100% serum, pH 7.4.
In a well-designed NIR fluorophore, the extinction coefficient at peak emission should be ≥ 150,000 M−1cm−1 in serum, pH 7.4. As a frame of reference, the visible fluorophore fluorescein has an extinction coefficient of ≈ 88,000 M−1cm−1 at peak absorption. Since fluorescence photon yield is proportional to the extinction coefficient, it is desirable to achieve the largest possible value in serum. It should be noted, however, that many NIR fluorophores, such as MB and ICG, tend to have a wide absorption curve, which extends towards the blue (Figure 3). This can cause undesired excitation of the ≈ 800 nm NIR fluorophore by the ≈ 700 nm fluorophore’s excitation light if not careful.
Peak Emission Wavelength and Full-Width/Half-Maximum (FWHM)
If only one channel of NIR fluorescence is desired, such as when using the Spy™, Fluobeam®, or PDE™ systems, the emission wavelength simply has to be 20 to 30 nm higher than peak absorption. When using the FLARE™ imaging system, an 80 to 100 nm separation in the peak emission of the two NIR fluorophores is desirable to maximize spectral separation. And, the FWHM of the 700 nm NIR fluorophore emission should be narrow enough to not extend into the emission band of the 800 nm fluorophore. There is less constraint on FWHM emission from the ≈ 800 nm fluorophore, and the wider the emission filter, the better, since the sensitivity of silicon cameras falls off rapidly above 800 nm.
Quantum Yield (QY)
The QY of small molecule NIR fluorophores is highly dependent on chemical environment. In general, a QY of ≈ 10% to 20% in serum, pH 7.4 is typical for heptamethine indocyanines, with higher QYs being rare. The measurement of absolute QY can be difficult, and most reports utilize comparison to published standards under conditions of matched absorbance at single wavelength (i.e., laser) excitation. Our laboratory typically uses Oxazine 725 in ethylene glycol (QY = 19%30) for QY measurements of 700 nm fluorophores and ICG in DMSO (QY = 13%31) for measurements of 800 nm fluorophores.
Fluorescence Lifetime
In general, the fluorescence lifetime of heptamethine indocyanines in serum is in the range of 0.5 to 1.1 ns,32 with lifetime being strongly influenced by chemical environment. A relatively unexplored, but potentially fruitful, area of investigation is how binding of a fluorophore to a target (i.e., cell or protein) changes its lifetime.33 If the change in lifetime upon binding is large enough, it might be possible to discriminate target-bound NIR fluorophore from background NIR fluorophore. Then, again, the use of “modular” ligands, which have an isolating linker between the NIR fluorophore and the targeting ligand,34 may preclude sensing of such binding events. At the very least, measurement of fluorescence lifetime permits separation of NIR fluorophore from tissue autofluorescence.35
Photobleaching Threshold
Fluorescent NPs, such as quantum dots, tend to exhibit extreme photostability at CW excitation fluence rates as high as 600 mW/cm2,17 i.e., high enough to heat water. On the contrary, heptamethine indocyanines undergo irreversible photobleaching at fluence rates > 50 mW/cm2.17,18 This is an important point, because it sets an upper limit to the excitation fluence rate that is needed (or desired) for image-guided surgery. On the FLARE™ imaging system, excitation fluence rate for the 700 nm emission channel is kept to ≤ 5 mW/cm2 so as not move color temperature outside (i.e., too “red”) of IEC #60601 guidelines for a surgical luminary; excitation fluence rate for the 800 nm channel is kept to ≤ 50 mW/cm2 to avoid photobleaching.
Concentration-Dependent Quenching
All fluorophores will exhibit fluorescence quenching if their concentration is too high, and NIR fluorophores are no exception. Previously, we reported that the quenching threshold for heptamethine indocyanines was ≈ 10 μM.36 Recently, we discovered that quenching threshold is a strong function of the geometry of the excitation and emission light paths used for the measurement. As shown in Figure 4, when measured using a traditional 90°, three-sided cuvette geometry, both MB and ICG in serum display quenching thresholds ≈ 10 μM. However, when measured using 0° geometry, as would occur during image-guided surgery, the quenching threshold shifts to ≈ 50 μM, with a much slower rate of decline as concentration is increased further. We believe that this phenomenon is due to differences in the photon path length. Path length in the 90° geometry is longer than in the 0° geometry, and fixed, leading to greater reabsorption of emitted photons. This is a potentially important observation because it suggests that much higher concentrations can be used than previously thought, and much higher signal can be attained, without significant quenching.
Figure 4. Concentration-Dependent Quenching of MB and ICG using Different Optical Geometries.
NIR excitation and emission geometries were either 0° or 90° as indicated (top). Shown at bottom is the concentration-dependent quenching of methylene blue (MB) and indocyanine green (ICG) in 100% fetal bovine serum supplemented with 50 mM HEPES, pH 7.4 under conditions of 0° (open circles) or 90° (closed squares) excitation/emission geometries.
Charge and Charge Distribution
Because of the hydrophobicity of polymethine indocyanine cores, solubilizing groups are always required to render the molecules compatible with aqueous solvents and serum. The most commonly used solubilizing group to date is sulfonic acid, imparting a net negative charge to most polymethines. While aqueous solubility is proportional to the number of solubilizing groups, the distribution of charge around the bulky core is equally important. ICG, for example, which has only two sulfonates, has two distinctly different characters. The “top half” of the molecule is extremely hydrophobic, while the “bottom half” is hydrophilic. The former likely contributes to the rapid extraction of ICG by the liver (half-life in blood ≤ 1 min).
Recently, we have discovered that relatively equal spacing of solubilizing groups around a heptamethine indocyanine, and alternation of anion and cation charges to create a zwitterionic (net neutral) molecule, imparts special properties to the NIR fluorophore, especially ultra-low non-specific binding to serum proteins, normal tissues, and organs (Choi et al., manuscript in review).
In Vivo Properties
Injected Dose
It is important to consider the “typical” injected dose of a NIR fluorophore in the context of other imaging modalities (Table 3). In most image-guided surgery applications, NIR fluorophore dose will be ≈ 10 mg. This dose is 60-times lower than the bioavailable dose resulting from ingesting two acetaminophen tablets, 700-times lower than MRI contrast agents, and 5600-times lower than CT contrast agents. The administration of such low doses, in an infrequent fashion, might help to reduce some of the burdens associated with regulatory approval (discussed below).
Table 3.
Typical Doses of Intravenously Administered Diagnostic Agents and Radiotracers from High (CT) to Low (PET).
| Imaging Modality | Human dose (g) |
|---|---|
| CT | 75 cc of 930 mM solution (≈ 800 Da) = 56 g |
| MRI | 15 cc of 500 mM solution (≈ 940 Da) = 7 g |
| 2 regular-strength acetaminophen (bioavailable dose) = 600 mg | |
| Optical | ≈ 10 cc of 1 mM solution (≈1,000 Da) = 10 mg |
| FDA definition of “micro-dosing” = 100 μg | |
| SPECT | 20 mCi (10,000 Ci/mmol; ≈500 Da) = 1 μg |
| PET | 20 mCi (10,000 Ci/mmol; ≈500 Da) = 1 μg |
Biodistribution
The molecular weight, charge, and dispersion of charge of a NIR fluorophore will determine its biodistribution after intravenous injection. In general, NIR fluorophores without any solubilizing groups are lipophilic cations, which readily cross the plasma membrane and are concentrated in mitochondria and/or endoplasmic reticulum.18 When injected into the bloodstream, they act as perfusion tracers, with retention in certain tissues and organs providing “free” targeting without the need for a separate ligand. MB, for example, exhibits preferential retention in cardiomyocytes37 and insulinoma38 after a single intravenous injection. Interestingly, subtle changes to NIR fluorophore chemical structure can result in profound alterations in tissue and organ uptake. Newly synthesized pentamethine indocyanines, for example, provide endocrine gland- and endocrine tumor-specific targeting (Choi et al., manuscript in preparation).
In the presence of two or more solubilizing groups, NIR fluorophores no longer cross the plasma membrane and remain extracellular after intravenous injection. In general, di-sulfonated heptamethine indocyanines exhibit short half-life (as short as 1 min) in blood due to rapid extraction by the liver, and tetra-sulfonated molecules exhibit longer half-lives (on the order of 10 to 30 min). Recent data from our lab suggests that polyanionic NIR fluorophores exhibit high non-specific uptake in normal tissues and organs despite high serum solubility (Choi et al., manuscript in review).
Clearance
The clearance, or elimination, of NIR fluorophores from the body after intravenous injection occurs through the liver (i.e., excretion into bile then feces), the kidney (i.e., excretion into urine), or both. In general, di-sulfonated molecules, which retain both a strongly hydrophobic and strongly hydrophilic character, are cleared into bile. Tetra-sulfonated NIR fluorophores, and molecules such as MB, are excreted roughly equally by the liver and kidney. These natural clearance routes can be exploited to provide high-sensitivity identification of the ureters39,40 and bile ducts.41,42 Zwitterionic NIR fluorophores, on the other hand, are cleared exclusively by the kidney (Choi et al., manuscript in review). The route of clearance and clearance rate are critically important for targeted NIR fluorophores since they control blood half-life, and therefore contact time, with the target of interest, as well as influence background signal. Tetra-sulfonated NIR fluorophores, for example, will always have difficulty in imaging gastrointestinal lesions because excretion into bile increases background in the gut, whereas zwitterionic NIR fluorophores can be used for image-guided surgery of any tissue or organ in the body with the exception of kidneys, ureters, and bladder.
Metabolism
Few data have been published about the metabolic fate of NIR fluorophores after intravenous injection. Some tetra-sulfonated molecules appear to be relatively stable, appearing in bile and urine unchanged after intravenous injection.40,42 However, clinical translation of these molecules will ultimately require a comprehensive understanding of the rate, location, and byproducts of metabolism.43-46
Affinity and Targeting
As mentioned above, some lipophilic NIR fluorophores are capable of being extracted and/or retained by cells in the body, and provide targeting by virtue of their inherent chemical structure (Choi et al., manuscript in preparation and 18). Hydrophilic NIR fluorophores, though, require covalent conjugation of a targeting ligand specific for an extracellular epitope. When using small molecule and peptide ligands, the NIR fluorophore itself can often dominate in vivo behavior. Care must be taken, therefore, to select a NIR fluorophore whose biodistribution, clearance, and non-specific uptake is compatible with the target of interest. The absolute affinity of the targeting ligand necessary for effective image-guided surgery is a key variable that is only now being defined precisely. In general, final NIR fluorescent contrast agent affinity in the picomolar to low nanomolar range is required to generate a SBR adequate for image-guided surgery.
SBR is also a strong function of BMax, i.e., the number of receptors or epitopes per cell. Consider, for example, that most cell surface receptors occur at a frequency of only 103 to 104 per cell, and that an average cell volume is ≈ 1 pL. Thus, the highest achievable concentration of NIR fluorophore bound to a collection of cells would be 2 to 20 nM. Endocytosis can “amplify” this signal two- to five-fold, however, under most circumstances, NIR fluorophore concentration is limiting. This fact serves to highlight why imaging system optimization, and rejection of tissue autofluorescence, are so important for image-guided surgery.
Clinically-Available NIR Fluorophores
With so many groups around the world developing novel NIR fluorophores, and targeted NIR fluorophores, the future of image-guided surgery looks bright. Nevertheless, the reality is that first-in-human testing of a new chemical entity (discussed below) has a minimum lag of ≈ 2 years and commercial availability of a new chemical entity has a minimum lag of 2 to 5 years. For the foreseeable future, there are only two NIR fluorophores approved for other indications by the FDA and thus clinically available for study: methylene blue (MB) and indocyanine green (ICG). Interestingly, both of these molecules have been used for the last 50+ years as visible dyes (blue for MB and green for ICG). Thus, there is an unprecedented body of clinical data regarding their safety when used at millimolar concentrations.
MB (Figure 2B) is a hydrophilic phenothiazine derivative that emits ≈ 700 nm and is compatible with the first NIR channel of the FLARE™ imaging system (Figure 3). Its performance as a NIR fluorophore is only fair, exhibiting an extinction coefficient and QY far below those of the indocyanines (Table 2). Even so, after a single intravenous injection, MB provides contrast for many important surgical procedures, including identification of the ureters,39 identification of extrahepatic bile ducts,41 assessment of cardiac perfusion,37 and importantly, detection of neuroendocrine tumors, such as insulinoma.38
ICG (Figure 2B) is a di-sulfonated heptamethine indocyanine with moderate optical properties (Table 2), which emits ≈ 800 nm. Like MB, there are many clever uses for ICG during image-guided surgery. After intravenous injection, it can be used for NIR angiography of blood vessels,10,37,47 identification of the extrahepatic bile ducts,41 and identification of liver metastases.48 After subcutaneous injection, ICG or ICG adsorbed to human serum albumin (ICG:HSA36) can be used for sentinel lymph node (SLN) mapping15,49-53 and assessing lymphatic function.54
While it is the general consensus that MB and ICG are not ideal NIR fluorophores, they are presently the best the field has to offer. Clearly, the development of new and better-suited fluorophores are needed to provide the best clinical options for both patients and surgeons.
SURGICAL PROCEDURES THAT COULD BENEFIT FROM REAL-TIME NIR FLUORESCENCE-GUIDANCE
Unmet Clinical Needs
NIR fluorescence imaging has the potential to address a variety of unmet clinical needs related to finding structures that need to be resected, such as sentinel lymph nodes, malignant cells, and lumenal calcifications, and avoiding other structures that could cause acute or chronic morbidity, such as nerves, blood vessels, ducts, lymphatics, and normal glands. We now highlight only a few examples from the literature where proof of principle has been established in pre-clinical or clinical studies.
Sentinel-Lymph Node (SLN) Mapping
Visible blue dyes exhibit poor contrast, have a risk of anaphylaxis, and stain the surgical field blue, and radioactive lymphatic tracers expose patient and caregivers to ionizing radiation, increase the cost of the procedure, and complicate logistics. Because of these challenges, many groups around the world have explored the use of NIR fluorescence imaging for SLN mapping.15,55-57 The ideal NIR fluorescent SLN tracer has yet to be identified, but until such an agent receives regulatory approval, our group has suggested that simple adsorption of ICG to human serum albumin (ICG:HSA36), which occurs naturally after injection into the body albeit with slow kinetics, is effective for SLN mapping in breast cancer (Figure 5A).15
Figure 5. Clinical Imaging using the FLARE™ Imaging System.
A. Sentinel lymph node (arrow) mapping in the axilla of a woman with breast cancer after peri-tumoral injection of ICG:HSA as described in 15. NIR fluorescence = 800 nm channel; 67 msec exposure time. The NIR fluorescence image was pseudo-colored in lime green and superimposed on the color video image to produce Color-NIR Merge.
B. Perforator flap NIR fluorescence angiography after intravenous injection of ICG. Dominant perforator artery (dashed circle) identified during dynamic imaging (Lee et al., manuscript in review). NIR fluorescence = 800 nm channel; 67 msec exposure time. The NIR fluorescence image was pseudo-colored in lime green and superimposed on the color video image to produce Color-NIR Merge.
Identification of the Ureters and Urinary Calcifications
The natural clearance of some NIR fluorophores into urine can be exploited to identify the ureters and to assess intralumenal contents (i.e., the presence of stones) and lumenal integrity.39,40 One such agent with immediate clinical relevance is MB.39 Additionally, NIR fluorophores that bind the hydroxyapatite calcium salt, such as Pam800,58 can be used to identify micro-calcifications within the urinary tract.59
Identification of the Extrahepatic Bile Ducts
Natural clearance of some NIR fluorophores into bile can be exploited to identify the extrahepatic bile ducts and to assess lumenal contents and integrity.41,42,60,61 Once again, MB is useful in this regard, but is by no means ideal due to its modest optical properties.41
Identification of Tissue and Breast Cancer Calcifications
The same hydroxyapatite-specific NIR fluorophore that is capable of imaging urinary calcifications can be used to image microcalcifications in breast cancer.62-65 This could be useful in optical tomography to help identify malignant calcifications, or during surgery to help ensure complete resection of breast tumors.
Tumor Margin Detection
Without question, real-time tumor margin detection will someday be the primary application for NIR fluorescence-guided surgery. Even without clinical availability of ideal tumor-specific NIR fluorescent contrast agents, recent reports suggest that the uptake of MB in insulinoma permits highly sensitive, real-time identification and resection of sub-millimeter metastases,38 and uptake of ICG helps identify tumors in the liver.66,67 Newer agents have greatly improved optical and tumor-targeting properties for this application (Choi et al., manuscript in preparation). The literature also suggests that tumor-specific agents will be available for many cancers within the next decade, with prostate cancer agents68,69 and integrin-specific agents70,71 probably being the furthest in their development.
Breast Reconstructive Surgery after Mastectomy
Several pre-clinical47,72-75 and clinical (76,77, Lee et al., manuscript in review; see Figure 5B) studies have demonstrated the utility of NIR fluorescence angiography for perforator artery mapping and assessment of tissue perfusion before, during, and after flap preparation. Ongoing studies are identifying the intraoperative predictive nature of NIR fluorescence imaging towards long-term outcome.
Cardiovascular and Vascular Imaging
NIR fluorescence imaging has several potential uses in cardiovascular surgery including simple angiography,10,11,78 cardioplegia delivery,79,80 and numerous other procedures.81-94 The use of hydroxyapatite-specific NIR fluorophores also permits the non-invasive identification of vascular calcifications.95 Recently, our group has shown that autologous platelets can be made NIR fluorescent without loss of bioactivity, and can be combined with the FLARE™ imaging system to identify intravascular thrombi and vessel patency, non-invasively, and in real-time.37,96 Fluorescence angiography is also widely employed in neurosurgery by using modified surgical microscopes.97-99
Nerves and Intervertebral Discs
Useful image-guidance during spine surgery includes identification of the intervertebral discs and identification of nerves. A recent study demonstrated uptake of NIR-compatible fluorophores by the annulus fibrosus of the intervertebral discs in three species.24 Another described the development of myelinated nerve-specific agents that are approaching NIR emission.100
CLINICAL TRANSLATION
The typical sequence of events for moving a new imaging system or contrast agent from “bench to bedside” in the U.S. includes pre-clinical validation, manufacture under current Good Manufacturing Practices (cGMP), first-in-human clinical trials, clinical trials to support regulatory approval, regulatory approval, marketing, and post-marketing surveillance. In addition, each country has its own set of guidelines that regulate each part of this process. Because most investigators are familiar with regulations surrounding pre-clinical studies, and regulatory approval is a complex subject that is beyond the scope of this review, we will focus on the impact academic investigators can make on the field by performing first-in-human studies with new technology.
Imaging Systems
The details surrounding clinical translation of a new optical imaging system have recently been reviewed by our group.101 Briefly, the key to starting the process is determination of risk status: “non-significant risk” (NSR) or “significant risk” (SR). NSR devices do not require FDA approval of an Investigational Device Exemption (IDE) application, but SR devices do. Preparation of an IDE is a labor-intensive and costly exercise that should only performed when required.
The local Institutional Review Board (IRB) has the authority to make the risk status determination. The problem is that once an IRB has determined the level of risk for an NSR device, the labor-intensive portion of the application has already been completed. The need to backtrack adds delay, cost, and unneeded work to the process. Yet, the IRB is under no obligation to make this binding decision until a full IRB application is submitted to them, i.e., the “risk conundrum.”101 For this reason, we recommend informal (non-binding) discussions with the IRB prior to beginning the translation process. In general, pilot trials of imaging systems for which 1) there is no contact with the patient, 2) an NIR fluorescent contrast agent is being used in a way that is consistent with its indication, and 3) the imaging is not used to diagnose or treat the patient, will be classifiable as NSR.
During clinical translation of the FLARE™ and Mini-FLARE™ image-guided surgery systems, we were careful to design the trials such that all patients received the standard of care, and the NIR fluorescence imaging as performed did not alter or interfere with the standard of care. Moreover, since such optical imaging systems have a long working distance (i.e., no patient contact) and the NIR fluorophores were used at low dose and consistent with previously approved indications, our IRB determined that that these instruments were NSR. This permitted 6-patient pilot trials of each device to be performed in a relatively short period of time, with feedback from surgeons helping our group to develop the next-generation FLARE™ imaging system.
Contrast Agents
Until recently, the model for clinical translation of contrast agents and other drugs was for an academic institution to license the agent to a for-profit company, who would then invest the significant resources necessary for clinical translation. In NIR fluorescence image-guided surgery, however, there is no well-defined market yet, and most companies view clinical translation as both costly and risky. This leaves academic investigators with the burden of introducing new agents into the medical field, thereby minimizing the risks of commercialization by weeding out underperforming agents and identifying potentially successful agents.
In January 2006, the FDA issued a very important document titled “Guidance for Industry, Investigators, and Reviewers: Exploratory IND Studies.”102 This guidance document creates the exploratory Investigational New Drug (eIND) application and reduces the cost and complexity of pre-clinical testing by replacing formal lethal-dose studies with no-observed adverse event level (NOAEL) testing. There are two common misconceptions about the eIND mechanism. The first is that it can only be used for micro-dosing (< 100 μg) studies. This is incorrect; Example 2 in the guidance document is consistent with a NIR fluorophore to be used during image-guided surgery. The second is that pre-clinical animal studies must be expensive and complicated. This is exactly the opposite intent of the eIND, and NOAEL testing can be performed cost effectively. Two recent articles help clarify these points.103,104
Nevertheless, the process of clinical translation is still complex and costly, and requires careful forethought. The key steps in the process are: 1) synthesis of the compound under cGMP conditions and preparation of the batch record and quality assurance/quality control (QA/QC) documentation (≈ $100,000 to $200,000, depending on the compound), 2) rodent NOAEL testing and second-species (typically dog) NOAEL confirmatory testing (≈ $20,000 to $75,000 depending on resource availability), 3) additional toxicology tests (if needed, could add additional $10,000 to x$20,000), 4) submission of an eIND application to the FDA, 5) submission of an institutional IRB application after the eIND is approved by the FDA, and 6) conduction of the first-in-human trial. If the pilot clinical trial is designed correctly, it will have enormous value by not only evaluating safety (the primary role of the trial), but also by providing imaging data that will guide future trials.
Prior to embarking on first-in-human testing of a novel diagnostic agent, one must consult with an experienced regulatory specialist. Depending on the agent, the device it is used with, and the indication, the FDA has the right to label the proposed technology a “combination,” which under Title 21 of the Code of Federal Regulations (CFR) Part 3.2, Subpart (e) (i.e., 21 CFR 3.2(e)), will require both an IDE and an eIND.
FUTURE IMAGING SYSTEM IMPROVEMENTS
An important, yet unanswered, question is whether reflectance-based, CW fluorescence imaging is “good enough” for most image-guided surgery. The advantages of CW fluorescence imaging include real-time acquisition and display, no requirement for data post-processing, relatively simple and inexpensive hardware and software, and an ergonomic footprint in the OR. The disadvantages of CW fluorescence imaging include an inability to quantify tissue optical properties or sub-surface depth of NIR fluorophores, or to distinguish between multiple fluorophores of the same peak emission wavelength. There is also a general trend in the field of surgery to replace “open” procedures with minimally-invasive, fiberoptic-based procedures.
Minimally-Invasive Imaging Systems
Endoscopy and laparoscopy are widely used surgical procedures that could benefit from NIR fluorescence imaging. Although all of the principles discussed in this review apply equally to fiberoscopy, certain engineering challenges unique to fiberoscopy are outside the scope of this review. Nevertheless, commercial fiberoscopy systems capable of simultaneous color video and NIR fluorescence are beginning to become available.
Fluorescence Lifetime Imaging (FLIM)
FLIM measures the time-dependent fluorescence intensity decay of a NIR fluorophore and has been widely studied in biomedical optics and microscopy.32,105-108 FLIM consists of modulating, in time, the fluorescence excitation light and extracting the time-dependent decay by means of time-gated fluorescence intensity detection. It can be performed either in the temporal domain or temporal-frequency domain, with the performance of each dependent on the instrumentation used. Since lifetime is dependent on chemical environment (e.g., hydrophobicity, pH, etc.), and even NIR fluorophores with the same peak emission can be discriminated, FLIM has the potential to impact many surgical procedures. Some studies have demonstrated FLIM over large fields of view, under clinically realistic conditions.109-112
Depth-Sensitive Imaging
It has been known for some time that temporal or spatial modulation of a light source could provide depth-sensitive measurement of endogenous and exogenous optical contrast.113-116 However, it is only until recently that developments in spatial frequency-domain imaging have permitted in vivo imaging over large fields of view.117-120 Tomographic absorption measurements have been demonstrated,118,121 and adapted to fluorescence imaging.122 The technique not only provides depth-sensitive fluorescence detection, but also contributes to improving lateral resolution of fluorescent targets within a diffusing medium. Depth-sensitive imaging might help identify critical structures, such as nerves, which are hidden under structures being resected, such as SLNs. Also contributing to the success of these techniques are recent advances in motion gating hardware, which can help isolate optical images from the effects of cardiac, respiratory, and involuntary motions.123
Endogenous Properties and Chromophore Imaging:
As just mentioned, temporal or spatial modulation of light permits extraction of tissue optical properties (i.e., scattering and absorption). By making spectroscopic measurements, i.e., at various wavelengths, a modified Beer Lambert’s law can also be employed to separate out the contributions of oxy-hemoglobin, deoxyhemoglobin, water and lipids.124-126 This technology should someday permit near-real-time assessment of tissue oxygenation during surgery, thus permitting the surgeon to assess tissue ischemia non-invasively, over wide FOVs, without the need for exogenous contrast agents.
FUTURE NIR FLUOROPHORE IMPROVEMENTS
Although few molecules have yet to reach the clinic, the level of innovation in the field of NIR fluorophore chemistry remains high. Particularly exciting are new classes of molecules that could lower fluorescence background by orders of magnitude. The first are “upconverting” fluorophores, which through a two-photon process, absorb light of long wavelength and emit light of a shorter wavelength.127 The second are “smart” NIR fluorophores, which become fluorescent only after encountering a particular external stimulus.128
There is also continual innovation in the structure of NIR fluorophores. A carbon-carbon bond at the R17 position of the polymethine backbone (Figure 2A), introduced using Suzuki-Miyaura coupling,129 improves stability and permits direct conjugation to targeting ligands. Zwitterionic NIR fluorophores (Choi et al., manuscript in review) provide ultra-low non-specific uptake and renal-only clearance, which are ideal for tumor targeting applications. NIR fluorophores with varying fluorescence lifetimes could improve rejection of background caused by tissue autofluorescence.32 Particularly exciting are voltage-sensitive NIR fluorophores, which could be used with the ratiometric capabilities of the FLARE™ imaging system to perform real-time imaging of electrical activity in the heart and brain.130-132 Ultimately, the discovery of disease-specific small molecular targeting ligands will eventually find their way into NIR fluorescent contrast agents for image-guided surgery.
CONCLUSION
Objectively speaking, the impact of NIR fluorescence imaging on patient care is still unknown. Nevertheless, it is easy to forget that just 5 years ago, there wasn’t a single NIR fluorescence-based image-guided surgery system available commercially. Now there are several available, with more options likely in the near future. The availability of imaging systems has finally broken the “chicken and the egg” problem in the field. The challenge, now, is to develop high-performance NIR fluorescence contrast agents that provide surgeons with the guidance they desire, and to translate these agents to the clinic for patient care.
One of the main motivators for this review was to define the state-of-the-art so that academic investigators could build upon it and help develop the next generation of imaging systems and contrast agents for image-guided surgery. The key to future success will be first-in-human trials of new technology, which in turn requires a comprehensive understanding of those design parameters that are important, and those that are not.
If one reviews the history of the other imaging modalities now used in clinical medicine (plain films, CT, MRI, US, SPECT, and PET), one finds that the time between first-in-human studies and routine use of the technology in patient care has a mean and median of 23 and 22 years, respectively. The first description of intravenously injected ICG (aside, as an absorber rather than a fluorophore) for NIR tomographic optical imaging of the breast was in the year 2000.133 If this is chosen as the start of the field, then ten years have passed, and we’re on target for clinical acceptance and widespread use in patient care around the beginning of the next decade. The next ten years will likely see the explosion or the demise of image-guided surgery using invisible NIR light.
ACKNOWLEDGEMENTS
We thank Susan Troyan, M.D., Bernard Lee, M.D., Joshua Winer, M.D., Mireille Rosenberg, Ph.D., Barbara L. Clough, M.A., Summer Gibbs-Strauss, Ph.D., Alan Stockdale, B.S., Rafiou Oketokoun, M.S., Vida Kianzad, Ph.D., Razvan Ciocan, Ph.D., Sunil Gupta, B.S., and Merlijn Hutteman, M.Sc., for their contribution to the FLARE™ and Mini-FLARE™ imaging systems design and clinical trials, Lucjan Strekowski, Ph.D., Gabor Patonay, Ph.D., and Maged Henary, Ph.D. for many helpful discussions on NIR fluorophores, Lorissa A. Moffitt, M.S. for editing, and Eugenia Trabucchi for administrative support. The work presented in this review was funded by NIH Bioengineering Research Partnership (BRP) grant #R01-CA-115296, R01-EB-0005805, and Quick Trials for Imaging grant #R21-CA-130297 to JVF.
Footnotes
Financial Disclosure: All intellectual property surrounding FLARE™ imaging technology is owned by the Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. As inventor, Dr. Frangioni may someday receive royalties if products are ever commercialized.
REFERENCES
- 1.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol. 2008;26:4012–21. doi: 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bani MR, Lux MP, Heusinger K, et al. Factors correlating with reexcision after breast-conserving therapy. Eur J Surg Oncol. 2008 doi: 10.1016/j.ejso.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Schiller DE, Le LW, Cho BC, et al. Factors associated with negative margins of lumpectomy specimen: potential use in selecting patients for intraoperative radiotherapy. Ann Surg Oncol. 2008;15:833–42. doi: 10.1245/s10434-007-9711-2. [DOI] [PubMed] [Google Scholar]
- 4.Besana-Ciani I, Greenall MJ. The importance of margins status after breast conservative surgery and radiotherapy in node positive patients: a follow-up of 10-15 years. Int Semin Surg Oncol. 2008;5:13. doi: 10.1186/1477-7800-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke S, Shorten GD. When pain after surgery doesn’t go away. Biochem Soc Trans. 2009;37:318–22. doi: 10.1042/BST0370318. [DOI] [PubMed] [Google Scholar]
- 6.Chance B. Near-infrared images using continuous, phase-modulated, and pulsed light with quantitation of blood and blood oxygenation. Ann. N. Y. Acad. Sci. 1998;838:29–45. doi: 10.1111/j.1749-6632.1998.tb08185.x. [DOI] [PubMed] [Google Scholar]
- 7.De Grand AM, Lomnes SJ, Lee DS, et al. Tissue-like phantoms for near-infrared fluorescence imaging system assessment and the training of surgeons. J Biomed Opt. 2006;11:014007. doi: 10.1117/1.2170579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sevick-Muraca EM, Houston JP, Gurfinkel M. Fluorescence-enhanced, near infrared diagnostic imaging with contrast agents. Curr Opin Chem Biol. 2002;6:642–50. doi: 10.1016/s1367-5931(02)00356-3. [DOI] [PubMed] [Google Scholar]
- 9.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–34. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 10.De Grand AM, Frangioni JV. An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat. 2003;2:553–62. doi: 10.1177/153303460300200607. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama A, del Monte F, Hajjar RJ, Frangioni JV. Functional near-infrared fluorescence imaging for cardiac surgery and targeted gene therapy. Mol Imaging. 2002;1:365–77. doi: 10.1162/15353500200221333. [DOI] [PubMed] [Google Scholar]
- 12.Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13:195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka E, Choi HS, Fujii H, et al. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol. 2006;13:1671–81. doi: 10.1245/s10434-006-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Themelis G, Yoo JS, Soh K, et al. Real-time intraoperative fluorescence imaging system using light-absorption correction. J Biomed Opt. 2009;14:064012-1–9. doi: 10.1117/1.3259362. [DOI] [PubMed] [Google Scholar]
- 15.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE™ intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–52. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gioux S, Kianzad V, Ciocan R, et al. High-power, computer-controlled, light-emitting diode-based light sources for fluorescence imaging and image-guided surgery. Mol Imaging. 2009;8:156–65. [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–7. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama A, Bianco AC, Zhang CY, et al. Quantitation of brown adipose tissue perfusion in transgenic mice using near-infrared fluorescence imaging. Mol Imaging. 2003;2:37–49. doi: 10.1162/15353500200303103. [DOI] [PubMed] [Google Scholar]
- 19.Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: fundamentals of clinical translation. Mol. Imaging. 2010 In Press. [PMC free article] [PubMed] [Google Scholar]
- 20.Kiyose K, Kojima H, Nagano T. Functional near-infrared fluorescent probes. Chem. Asian J. 2008;3:506–515. [Google Scholar]
- 21.Kobayashi H, Ogawa M, Alford R, et al. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem Rev. 2009 doi: 10.1021/cr900263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierce MC, Javier DJ, Richards-Kortum R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int J Cancer. 2008;123:1979–90. doi: 10.1002/ijc.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Te Velde EA, Veerman T, Subramaniam B, Ruers T. The use of fluorescent dyes and probes in surgical oncology. Eur J Surg Oncol. 2009 doi: 10.1016/j.ejso.2009.10.014. In Press. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs-Strauss SL, Vooght C, Fish KM, et al. Molecular imaging agents specific for the annulus fibrosus of the intervertebral disc. Mol Imaging. 2010 In Press. [PubMed] [Google Scholar]
- 25.Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol Imaging Biol. 2003;5:376–89. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Fagerholm U. The highly permeable blood-brain barrier: an evaluation of current opinions about brain uptake capacity. Drug Discov Today. 2007;12:1076–82. doi: 10.1016/j.drudis.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Strekowski L, Lipowska M, Patonay G. Substitution reactions of a nucleofugal group in heptamethine cyanine dyes. Synthesis of an isothiocyanato derivative for labeling of proteins with a near-infrared chromophore. J Org Chem. 1992;57:4578–80. [Google Scholar]
- 28.Narayanan N, Patonay G. A new method for the synthesis of heptamethine cyanine dyes: synthesis of new near-infrared fluorescent labels. J Org Chem. 1995;60:2391–95. [Google Scholar]
- 29.Kiyose K, Aizawa S, Sasaki E, et al. Molecular design strategies for near-infrared ratiometric fluorescent probes based on the unique spectral properties of aminocyanines. Chemistry. 2009;15:9191–200. doi: 10.1002/chem.200900035. [DOI] [PubMed] [Google Scholar]
- 30.Sens R, Drexhage KH. Fluorescence quantum yield of oxazine and carbazine laser dyes. J. Luminesc. 1981;24:709–712. [Google Scholar]
- 31.Benson C, Kues HA. Absorption and fluorescence properties of cyanine dyes. J. Chem. Eng. Data. 1977;22:379–383. [Google Scholar]
- 32.Akers WJ, Berezin MY, Lee H, Achilefu S. Predicting in vivo fluorescence lifetime behavior of near-infrared fluorescent contrast agents using in vitro measurements. J Biomed Opt. 2008;13:054042. doi: 10.1117/1.2982535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakowicz JR, Szmacinski H, Nowaczyk K, Johnson ML. Fluorescence lifetime imaging of free and protein-bound NADH. Proc Natl Acad Sci U S A. 1992;89:1271–5. doi: 10.1073/pnas.89.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humblet V, Misra P, Frangioni JV. An HPLC/mass spectrometry platform for the development of multimodality contrast agents and targeted therapeutics: prostate-specific membrane antigen small molecule derivatives. Contrast Media Mol Imaging. 2006;1:196–211. doi: 10.1002/cmmi.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akers W, Lesage F, Holten D, Achilefu S. In vivo resolution of multiexponential decays of multiple near-infrared molecular probes by fluorescence lifetime-gated whole-body time-resolved diffuse optical imaging. Mol Imaging. 2007;6:237–46. [PubMed] [Google Scholar]
- 36.Ohnishi S, Lomnes SJ, Laurence RG, et al. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging. 2005;4:172–81. doi: 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka E, Chen FY, Flaumenhaft R, et al. Real-time assessment of cardiac perfusion, coronary angiography, and acute intravascular thrombi using dual-channel near-infrared fluorescence imaging. J Thorac Cardiovasc Surg. 2009;138:133–40. doi: 10.1016/j.jtcvs.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winer JH, Choi HS, Gibbs-Strauss SL, et al. Intraoperative localization of insulinoma and normal pancreas using invisible near-infrared fluorescent light. Ann Surg Oncol. 2010 doi: 10.1245/s10434-009-0868-8. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsui A, Tanaka E, Choi HS, et al. Real-time near-infrared fluorescence-guided identification of the ureters using methylene blue. Surgery. 2010 doi: 10.1016/j.surg.2009.12.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka E, Ohnishi S, Laurence RG, et al. Real-time intraoperative ureteral guidance using invisible near-infrared fluorescence. J Urol. 2007;178:2197–202. doi: 10.1016/j.juro.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsui A, Tanaka E, Choi HS, et al. Real-time intraoperative near-infrared fluorescence identification of the extrahepatic bile ducts using clinically-available contrast agents. Surgery. 2010 doi: 10.1016/j.surg.2009.12.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka E, Choi HS, Humblet V, et al. Real-time intraoperative assessment of the extrahepatic bile ducts in rats and pigs using invisible near-infrared fluorescent light. Surgery. 2008;144:39–48. doi: 10.1016/j.surg.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alford R, Ogawa M, Choyke PL, Kobayashi H. Molecular probes for the in vivo imaging of cancer. Mol Biosyst. 2009;5:1279–91. doi: 10.1039/b911307j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alford R, Simpson HM, Duberman J, et al. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol Imaging. 2009;8:341–54. [PubMed] [Google Scholar]
- 45.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. Tumor-specific detection of an optically targeted antibody combined with a quencher-conjugated neutravidin “quencher-chaser”: a dual “quench and chase” strategy to improve target to nontarget ratios for molecular imaging of cancer. Bioconjug Chem. 2009;20:147–54. doi: 10.1021/bc8003765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogawa M, Regino CA, Choyke PL, Kobayashi H. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol Cancer Ther. 2009;8:232–9. doi: 10.1158/1535-7163.MCT-08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsui A, Lee BT, Winer JH, et al. Real-time intraoperative near-infrared fluorescence angiography for perforator identification and flap design. Plast Reconstr Surg. 2009;123:125e–7e. doi: 10.1097/PRS.0b013e31819a3617. [DOI] [PubMed] [Google Scholar]
- 48.Aoki T, Yasuda D, Shimizu Y, et al. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J Surg. 2008;32:1763–7. doi: 10.1007/s00268-008-9620-y. [DOI] [PubMed] [Google Scholar]
- 49.Nimura H, Narimiya N, Mitsumori N, et al. Infrared ray electronic endoscopy combined with indocyanine green injection for detection of sentinel nodes of patients with gastric cancer. Br J Surg. 2004;91:575–9. doi: 10.1002/bjs.4470. [DOI] [PubMed] [Google Scholar]
- 50.Miyashiro I, Miyoshi N, Hiratsuka M, et al. Detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging: comparison with infrared imaging. Ann Surg Oncol. 2008;15:1640–3. doi: 10.1245/s10434-008-9872-7. [DOI] [PubMed] [Google Scholar]
- 51.Kusano M, Tajima Y, Yamazaki K, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel node navigation surgery in gastrointestinal cancer. Dig Surg. 2008;25:103–8. doi: 10.1159/000121905. [DOI] [PubMed] [Google Scholar]
- 52.Fujiwara M, Mizukami T, Suzuki A, Fukamizu H. Sentinel lymph node detection in skin cancer patients using real-time fluorescence navigation with indocyanine green: preliminary experience. J Plast Reconstr Aesthet Surg. 2008 doi: 10.1016/j.bjps.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 53.Tsujino Y, Mizumoto K, Matsuzaka Y, et al. Fluorescence navigation with indocyanine green for detecting sentinel nodes in extramammary Paget’s disease and squamous cell carcinoma. J Dermatol. 2009;36:90–4. doi: 10.1111/j.1346-8138.2009.00595.x. [DOI] [PubMed] [Google Scholar]
- 54.Sharma R, Wang W, Rasmussen JC, et al. Quantitative imaging of lymph function. Am J Physiol Heart Circ Physiol. 2007;292:H3109–18. doi: 10.1152/ajpheart.01223.2006. [DOI] [PubMed] [Google Scholar]
- 55.Murawa D, Hirche C, Dresel S, Hunerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96:1289–94. doi: 10.1002/bjs.6721. [DOI] [PubMed] [Google Scholar]
- 56.Sevick-Muraca EM, Sharma R, Rasmussen JC, et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology. 2008;246:734–41. doi: 10.1148/radiol.2463070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tagaya N, Yamazaki R, Nakagawa A, et al. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg. 2008;195:850–3. doi: 10.1016/j.amjsurg.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 58.Zaheer A, Lenkinski RE, Mahmood A, et al. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat Biotechnol. 2001;19:1148–54. doi: 10.1038/nbt1201-1148. [DOI] [PubMed] [Google Scholar]
- 59.Figueiredo JL, Passerotti CC, Sponholtz T, et al. A novel method of imaging calcium urolithiasis using fluorescence. J Urol. 2008;179:1610–4. doi: 10.1016/j.juro.2007.11.100. [DOI] [PubMed] [Google Scholar]
- 60.Aoki T, Murakami M, Yasuda D, et al. Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J Hepatobiliary Pancreat Surg. 2009 doi: 10.1007/s00534-009-0197-0. [DOI] [PubMed] [Google Scholar]
- 61.Ishizawa T, Tamura S, Masuda K, et al. Intraoperative fluorescent cholangiography using indocyanine green: a biliary road map for safe surgery. J Am Coll Surg. 2009;208:e1–4. doi: 10.1016/j.jamcollsurg.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 62.Bhushan KR, Misra P, Liu F, et al. Detection of breast cancer microcalcifications using a dual-modality SPECT/NIR fluorescent probe. J Am Chem Soc. 2008;130:17648–9. doi: 10.1021/ja807099s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhushan KR, Tanaka E, Frangioni JV. Synthesis of conjugatable bisphosphonates for molecular imaging of large animals. Angew Chem Int Ed Engl. 2007;46:7969–71. doi: 10.1002/anie.200701216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu F, Bloch N, Bhushan KR, et al. Humoral bone morphogenetic protein 2 is sufficient for inducing breast cancer microcalcification. Mol Imaging. 2008;7:175–86. [PMC free article] [PubMed] [Google Scholar]
- 65.Liu F, Misra P, Lunsford EP, et al. A dose- and time-controllable syngeneic animal model of breast cancer microcalcification. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gotoh K, Yamada T, Ishikawa O, et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol. 2009;100:75–9. doi: 10.1002/jso.21272. [DOI] [PubMed] [Google Scholar]
- 67.Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115:2491–504. doi: 10.1002/cncr.24291. [DOI] [PubMed] [Google Scholar]
- 68.Humblet V, Lapidus R, Williams LR, et al. High-affinity near-infrared fluorescent small-molecule contrast agents for in vivo imaging of prostate-specific membrane antigen. Mol Imaging. 2005;4:448–62. doi: 10.2310/7290.2005.05163. [DOI] [PubMed] [Google Scholar]
- 69.Humblet V, Misra P, Bhushan KR, et al. Multivalent scaffolds for affinity maturation of small molecule cell surface binders and their application to prostate tumor targeting. J Med Chem. 2009;52:544–50. doi: 10.1021/jm801033c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li C, Wang W, Wu Q, et al. Dual optical and nuclear imaging in human melanoma xenografts using a single targeted imaging probe. Nucl Med Biol. 2006;33:349–58. doi: 10.1016/j.nucmedbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Kwon S, Ke S, Houston JP, et al. Imaging dose-dependent pharmacokinetics of an RGD-fluorescent dye conjugate targeted to alpha v beta 3 receptor expressed in Kaposi’s sarcoma. Mol Imaging. 2005;4:75–87. doi: 10.1162/15353500200505103. [DOI] [PubMed] [Google Scholar]
- 72.Matsui A, Lee BT, Winer JH, et al. Quantitative assessment of perfusion and vascular compromise in perforator flaps using a near-infrared fluorescence-guided imaging system. Plast Reconstr Surg. 2009;124:451–60. doi: 10.1097/PRS.0b013e3181adcf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsui A, Lee BT, Winer JH, et al. Image-guided perforator flap design using invisible near-infrared light and validation with x-ray angiography. Ann Plast Surg. 2009;63:327–30. doi: 10.1097/SAP.0b013e318193493d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee BT, Matsui A, Hutteman M, et al. Intraoperative Near-infrared Fluorescence Imaging in Perforator Flap Reconstruction: Current Research and Early Clinical Experience. J Reconstr Microsurg. 2009 doi: 10.1055/s-0029-1244805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsui A, Lee BT, Winer JH, et al. Submental perforator flap design with a near-infrared fluorescence imaging system: the relationship among number of perforators, flap perfusion, and venous drainage. Plast Reconstr Surg. 2009;124:1098–104. doi: 10.1097/PRS.0b013e3181b5a44c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Newman MI, Samson MC. The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J Reconstr Microsurg. 2009;25:21–6. doi: 10.1055/s-0028-1090617. [DOI] [PubMed] [Google Scholar]
- 77.Pestana IA, Coan B, Erdmann D, et al. Early experience with fluorescent angiography in free-tissue transfer reconstruction. Plast Reconstr Surg. 2009;123:1239–44. doi: 10.1097/PRS.0b013e31819e67c1. [DOI] [PubMed] [Google Scholar]
- 78.Unno N, Suzuki M, Yamamoto N, et al. Indocyanine green fluorescence angiography for intraoperative assessment of blood flow: a feasibility study. Eur J Vasc Endovasc Surg. 2008;35:205–7. doi: 10.1016/j.ejvs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Rangaraj AT, Ghanta RK, Umakanthan R, et al. Real-time visualization and quantification of retrograde cardioplegia delivery using near infrared fluorescent imaging. J Card Surg. 2008;23:701–8. doi: 10.1111/j.1540-8191.2008.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soltesz EG, Laurence RG, De Grand AM, et al. Image-guided quantification of cardioplegia delivery during cardiac surgery. Heart Surg Forum. 2007;10:E381–6. doi: 10.1532/HSF98.20071099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balacumaraswami L, Abu-Omar Y, Choudhary B, et al. A comparison of transit-time flowmetry and intraoperative fluorescence imaging for assessing coronary artery bypass graft patency. J Thorac Cardiovasc Surg. 2005;130:315–20. doi: 10.1016/j.jtcvs.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 82.Balacumaraswami L, Taggart DP. Digital tools to facilitate intraoperative coronary artery bypass graft patency assessment. Semin Thorac Cardiovasc Surg. 2004;16:266–71. doi: 10.1053/j.semtcvs.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 83.Balacumaraswami L, Taggart DP. Intraoperative imaging techniques to assess coronary artery bypass graft patency. Ann Thorac Surg. 2007;83:2251–7. doi: 10.1016/j.athoracsur.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 84.Chang K, Jaffer F. Advances in fluorescence imaging of the cardiovascular system. J Nucl Cardiol. 2008;15:417–28. doi: 10.1016/j.nuclcard.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 85.Desai ND, Miwa S, Kodama D, et al. Improving the quality of coronary bypass surgery with intraoperative angiography: validation of a new technique. J Am Coll Cardiol. 2005;46:1521–5. doi: 10.1016/j.jacc.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 86.Desai ND, Miwa S, Kodama D, et al. A randomized comparison of intraoperative indocyanine green angiography and transit-time flow measurement to detect technical errors in coronary bypass grafts. J Thorac Cardiovasc Surg. 2006;132:585–94. doi: 10.1016/j.jtcvs.2005.09.061. [DOI] [PubMed] [Google Scholar]
- 87.Detter C, Wipper S, Russ D, et al. Fluorescent cardiac imaging: a novel intraoperative method for quantitative assessment of myocardial perfusion during graded coronary artery stenosis. Circulation. 2007;116:1007–14. doi: 10.1161/CIRCULATIONAHA.106.655936. [DOI] [PubMed] [Google Scholar]
- 88.Di Giammarco G, Pano M, Cirmeni S, et al. Predictive value of intraoperative transit-time flow measurement for short-term graft patency in coronary surgery. J Thorac Cardiovasc Surg. 2006;132:468–74. doi: 10.1016/j.jtcvs.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 89.Sekijima M, Tojimbara T, Sato S, et al. An intraoperative fluorescent imaging system in organ transplantation. Transplant Proc. 2004;36:2188–90. doi: 10.1016/j.transproceed.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 90.Taggart DP, Balacumaraswami L, Venkatapathy A. Radial artery jump graft from anterior to posterior descending coronary artery. Asian Cardiovasc Thorac Ann. 2009;17:143–6. doi: 10.1177/0218492309103294. [DOI] [PubMed] [Google Scholar]
- 91.Taggart DP, Choudhary B, Anastasiadis K, et al. Preliminary experience with a novel intraoperative fluorescence imaging technique to evaluate the patency of bypass grafts in total arterial revascularization. Ann Thorac Surg. 2003;75:870–3. doi: 10.1016/s0003-4975(02)04669-6. [DOI] [PubMed] [Google Scholar]
- 92.Veerman T, Haussler A, Genoni M, et al. Novadaq SPY: intraoperative quality assessment in off-pump coronary artery bypass grafting. Chest. 2004;125:418–24. doi: 10.1378/chest.125.2.418. [DOI] [PubMed] [Google Scholar]
- 93.Waseda K, Ako J, Hasegawa T, et al. Intraoperative fluorescence imaging system for on-site assessment of off-pump coronary artery bypass graft. JACC Cardiovasc Imaging. 2009;2:604–12. doi: 10.1016/j.jcmg.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 94.Yasuda T, Watanabe G, Tomita S. Transaortic injection technique in fluorescence imaging: novel intraoperative assessment of anastomosis in off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2005;130:560–1. doi: 10.1016/j.jtcvs.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 95.Zaheer A, Murshed M, De Grand AM, et al. Optical imaging of hydroxyapatite in the calcified vasculature of transgenic animals. Arterioscler Thromb Vasc Biol. 2006;26:1132–6. doi: 10.1161/01.ATV.0000210016.89991.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flaumenhaft R, Tanaka E, Graham GJ, et al. Localization and quantification of platelet-rich thrombi in large blood vessels with near-infrared fluorescence imaging. Circulation. 2007;115:84–93. doi: 10.1161/CIRCULATIONAHA.106.643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bruneau M, Sauvageau E, Nakaji P, et al. Preliminary personal experiences with the application of near-infrared indocyanine green videoangiography in extracranial vertebral artery surgery. Neurosurgery. 2010;66:305–11. doi: 10.1227/01.NEU.0000363596.52283.65. discussion 311. [DOI] [PubMed] [Google Scholar]
- 98.Imizu S, Kato Y, Sangli A, et al. Assessment of incomplete clipping of aneurysms intraoperatively by a near-infrared indocyanine green-video angiography (Niicg-Va) integrated microscope. Minim Invasive Neurosurg. 2008;51:199–203. doi: 10.1055/s-2008-1080916. [DOI] [PubMed] [Google Scholar]
- 99.Uluc K, Kujoth GC, Baskaya MK. Operating microscopes: past, present, and future. Neurosurg Focus. 2009;27:E4. doi: 10.3171/2009.6.FOCUS09120. [DOI] [PubMed] [Google Scholar]
- 100.Gibbs-Strauss SL, Nasr KA, Fish KM, et al. Nerve-highlighting fluorescent contrast agents for image-guided surgery. Mol Imaging. 2010 In Press. [PMC free article] [PubMed] [Google Scholar]
- 101.Gibbs-Strauss SL, Rosenberg M, Clough BL, et al. First-in-human clinical trials of imaging devices: an example from optical imaging; Conf Proc IEEE Eng Med Biol Soc.; 2009; pp. 2001–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guidance for Industry, Investigators, and Reviewers: Exploratory IND Studies. 2006 www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM078933.pdf.
- 103.Mills G. The exploratory IND. J Nucl Med. 2008;49:45N–47N. [PubMed] [Google Scholar]
- 104.Skovronsky D. Use of eINDs for evaluation of multiple related PET amyloid plaque imaging agents. J Nucl Med. 2008;49:47N–48N. [PubMed] [Google Scholar]
- 105.Lakowicz JR, Berndt KW. Lifetime-selective fluorescence imaging using an rf phase-sensitive camera. Rev. Sci. Instrum. 1991;62:1727–34. [Google Scholar]
- 106.Lakowicz JR, Szmacinski H, Nowaczyk K, et al. Fluorescence lifetime imaging. Anal Biochem. 1992;202:316–30. doi: 10.1016/0003-2697(92)90112-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hutchinson CL, Lakowicz JR, Sevick-Muraca EM. Fluorescence lifetime-based sensing in tissues: a computational study. Biophys J. 1995;68:1574–82. doi: 10.1016/S0006-3495(95)80330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Leary MA, Boas DA, Li XD, et al. Fluorescence lifetime imaging in turbid media. Opt Lett. 1996;21 doi: 10.1364/ol.21.000158. [DOI] [PubMed] [Google Scholar]
- 109.Gioux S, Lomnes SJ, Choi HS, Frangioni JV. Low-frequency wide-field fluorescence lifetime imaging using a high-power near-infrared LED light source. J Biomed Opt. 2010 doi: 10.1117/1.3368997. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.May A, Bhaumik S, Gambhir SS, et al. Whole-body, real-time preclinical imaging of quantum dot fluorescence with time-gated detection. J Biomed Opt Let. 2009;14:060504-1–3. doi: 10.1117/1.3269675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Munro I, McGinty J, Galletly N, et al. Toward the clinical application of time-domain fluorescence lifetime imaging. J Biomed Opt. 2005;10:051403. doi: 10.1117/1.2102807. [DOI] [PubMed] [Google Scholar]
- 112.Requejo-Isidro J, McGinty J, Munro I, et al. High-speed wide-field time-gated endoscopic fluorescence-lifetime imaging. Opt Lett. 2004;29:2249–51. doi: 10.1364/ol.29.002249. [DOI] [PubMed] [Google Scholar]
- 113.Patterson MS, Chance B, Wilson B. Time resolved reflectance and transmittance for the noninvasive measurement of tissue optical properties. Appl Opt. 1989;28:2331–2336. doi: 10.1364/AO.28.002331. [DOI] [PubMed] [Google Scholar]
- 114.Farrell TJ, Patterson MS, Wilson B. A diffusion theory model of spatially resolved, steady-state diffuse reflectance for the noninvasive determination of tissue optical properties in vivo. Med Phys. 1992;19:879–88. doi: 10.1118/1.596777. [DOI] [PubMed] [Google Scholar]
- 115.Patterson MS, Pogue BW. Mathematical model for time-resolved and frequency-domain fluorescence spectroscopy in biological tissues. Appl Opt. 1994;33:1963–1974. doi: 10.1364/AO.33.001963. [DOI] [PubMed] [Google Scholar]
- 116.Kienle A, Lothar L, Patterson MS, et al. Spatially resolved absolute diffuse reflectance measurements for noninvasive determination of the optical scattering and absorption coefficients of biological tissues. Appl Opt. 1996;35:2304–2314. doi: 10.1364/AO.35.002304. [DOI] [PubMed] [Google Scholar]
- 117.Cuccia DJ, Bevilacqua F, Durkin AJ, et al. Quantitation and mapping of tissue optical properties using modulated imaging. J Biomed Opt. 2009;14:024012. doi: 10.1117/1.3088140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cuccia DJ, Bevilacqua F, Durkin AJ, Tromberg BJ. Modulated imaging: quantitative analysis and tomography of turbid media in the spatial-frequency domain. Opt Lett. 2005;30:1354–6. doi: 10.1364/ol.30.001354. [DOI] [PubMed] [Google Scholar]
- 119.Dognitz N, Wagnieres G. Determination of tissue optical properties by steady-state spatial frequency-domain reflectometry. Lasers Med Sci. 1998;13:55–65. [Google Scholar]
- 120.Gioux S, Mazhar A, Cuccia DJ, et al. Three-dimensional surface profile intensity correction for spatially modulated imaging. J Biomed Opt. 2009;14:034045. doi: 10.1117/1.3156840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Konecky SD, Mazhar A, Cuccia D, et al. Quantitative optical tomography of sub-surface heterogeneities using spatially modulated structured light. Opt Express. 2009;17:14780–90. doi: 10.1364/oe.17.014780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mazhar A, Cuccia DJ, Gioux S, et al. Structured illumination enhances resolution and contrast in thick tissue fluorescence imaging. J Biomed Opt. 2010 doi: 10.1117/1.3299321. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gioux S, Ashitate Y, Hutteman M, Frangioni JV. Motion-gated acquisition for in vivo optical imaging. J Biomed Opt. 2009:14. doi: 10.1117/1.3275473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cerussi A, Shah N, Hsiang D, et al. In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy. J Biomed Opt. 2006;11:044005. doi: 10.1117/1.2337546. [DOI] [PubMed] [Google Scholar]
- 125.Fishkin JB, Coquoz O, Anderson ER, et al. Frequency-domain photon migration measurements of normal and malignant tissue optical properties in a human subject. Appl Opt. 1997;36:10–20. doi: 10.1364/ao.36.000010. [DOI] [PubMed] [Google Scholar]
- 126.Tromberg BJ, Shah N, Lanning R, et al. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia. 2000;2:26–40. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boyer JC, Manseau MP, Murray JI, van Veggel FC. Surface modification of upconverting NaYF(4) nanoparticles with PEG-phosphate ligands for NIR (800 nm) biolabeling within the biological window. Langmuir. 2009 doi: 10.1021/la902260j. [DOI] [PubMed] [Google Scholar]
- 128.Asanuma D, Kobayashi H, Nagano T, Urano Y. Fluorescence imaging of tumors with “smart” pH-activatable targeted probes. Methods Mol Biol. 2009;574:47–62. doi: 10.1007/978-1-60327-321-3_5. [DOI] [PubMed] [Google Scholar]
- 129.Lee H, Mason JC, Achilefu S. Heptamethine cyanine dyes with a robust C-C bond at the central position of the chromophore. J Org Chem. 2006;71:7862–5. doi: 10.1021/jo061284u. [DOI] [PubMed] [Google Scholar]
- 130.Lebeuf R, Ferezou I, Rossier J, et al. Straightforward synthesis of the near-infrared fluorescent voltage-sensitive dye RH1691 and analogues thereof. Org Lett. 2009;11:4822–5. doi: 10.1021/ol901846g. [DOI] [PubMed] [Google Scholar]
- 131.Matiukas A, Mitrea BG, Pertsov AM, et al. New near-infrared optical probes of cardiac electrical activity. Am J Physiol Heart Circ Physiol. 2006;290:H2633–43. doi: 10.1152/ajpheart.00884.2005. [DOI] [PubMed] [Google Scholar]
- 132.Walton RD, Mitrea BG, Pertsov AM, Bernus O. A novel near-infrared voltage-sensitive dye reveals the action potential wavefront orientation at increased depths of cardiac tissue; Conf Proc IEEE Eng Med Biol Soc.; 2009; pp. 4523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ntziachristos V, Yodh AG, Schnall M, Chance B. Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement. Proc Natl Acad Sci U S A. 2000;97:2767–72. doi: 10.1073/pnas.040570597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Choi HS, Liu W, Liu F, et al. Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol. 5:42–7. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu W, Choi HS, Zimmer JP, et al. Compact cysteine-coated CdSe(ZnCdS) quantum dots for in vivo applications. J Am Chem Soc. 2007;129:14530–1. doi: 10.1021/ja073790m. [DOI] [PMC free article] [PubMed] [Google Scholar]