Abstract
Diamond Blackfan anemia is a genetic syndrome characterized by red blood cell aplasiain association with developmental abnormalities such as growth retardation, orofacial, hand or limb malformations, urogenital anomalies and heart defects. The only known cause is heterozygosity for mutations in genes encoding ribosomal proteins. Understanding how defective ribosome biogenesis and function, important for all cells, causes defects in erythropoiesis and tissue-specific phenotypes during development is paramount to the evolution of effective treatment protocols. Here, we discuss how animal models based on mammals, insects and fish replicate genetic or developmental aspects of DBA and have led to the identification of pathways and candidate molecules that are important in the pathogenesis of the disease. A recurring theme in many of these models suggests that defective ribosome biogenesis induces a p53-dependent cell cycle checkpoint in cells that require high levels of ribosome production and leads to cell type-specific, whole animal phenotypes.
Introduction
Animal models of human disease are used to investigate questions, test hypotheses and examine therapeutic outcomes that are difficult to address in the clinical setting. A good animal model should (1) accurately reproduce the disease phenotype, response to current therapies and overall prognosis, (2) recapitulate the genetic abnormalities observed in human patients and (3) utilize the same molecular pathways exploited by the disease1. Although it may be difficult to meet all of these criteria, scientists frequently choose a model to study human disease while recognizing its implicit caveats and limitations. Fortunately, our understanding of Diamond Blackfan anemia (DBA) and other inherited bone marrow failure syndromes has matured in recent years with the help of robust, genetically-tractable animal models.
DBA is human syndrome characterized by erythrocyte aplasia in association with growth retardation, limb, cardiac and/or craniofacial malformations. Most patients exhibit anemia, macrocytosis, reticulocytopenia, and a paucity of red blood cell precursor cells within a normocellular marrow2,3. Other features of the disease include elevated erythrocyte adenosine deaminase activity and an elevated fetal hemoglobin concentration. Current therapies include supportive red blood cell transfusion and corticosteroids; stem cell transplantation is the only definitive treatment for the hematologic features of DBA, yet can be complicated by donor suitability and the toxicity of myeloablative regimens4. With the current therapeutic armamentarium, the overall survival of DBA patients is 75% at 40 years of age, a number that is negatively affected by a patient’s transfusion requirements4, 5.
Ribosomal protein mutations have been identified in individuals with DBA6. In most patients, ribosomal protein mutations are heterozygous and sporadic, however, inherited forms of DBA exist in which ribosomal protein mutations segregate in an autosomal dominant manner2. Mutations in Ribosomal Protein S19 (RPS19), RPS24, RPS17, RPS10, RPS26, RPS7, RPL5,RPL11 and RPL35A account for approximately 50% of the known genetic alterations in affected individuals3,6. Many mutations result in ribosomal protein deficiency and, thus, implicate ribosomal protein haploinsufficiency in the pathogenesis of DBA3. Recent work in mice suggests, however, that a portion of ribosomal protein mutations may act through a dominant negative mechanism7.
Spontaneous and induced ribosomal protein mutations have been identified or created in a number of model organisms7–16. Studies based on these models suggest that ribosomal protein mutations compromise ribosome biogenesis, protein synthesis, cell division and cell survival12,15,17,18. While some DBA phenotypes (such as growth retardation) may be the direct result of impaired cell cycle dynamics, it is not known how mutations that impair translation and cell cycle kinetics in every cell of an organism yield tissue-specific phenotypes, such as limb abnormalities and anemia. Here, we will focus on novel mouse, fly and fish models, and will highlight how these tools have shed light on the pathogenesis of DBA and identified new targets for therapeutic intervention.
Mouse models to study the effects of RPS19 mutations
Rps19 knockout mice
In 1999, Draptchinskaia and colleagues6 identified mutations in RPS19, a gene encoding a ribosomal protein, in a proportion of DBA cases. Not surprisingly, their observations posed more questions than they answered: how do mutations in a ubiquitously distributed protein with a well understood role in protein translation lead to a disease with tissue-specific phenotypes such as anemia? Did RPS19 have a specific role in translation in the red blood cell or did it have an extra ribosomal function? A mouse model might allow these questions to be approached and in 2004, a Rps19 knockout mouse was reported13 (Table 1).
Table 1.
Ribosomal protein mouse mutants model DBA.
| Gene | Molecular approach | Whole animal and cellular phenotype | Implications | Ref(s) |
|---|---|---|---|---|
| Rps19 | Knockout allele | Homozygote-early embryonic lethality; Heterozygote-no hematologic or developmental phenotype; normal Rps19 expression | Compensation mechanisms in mice | 13, 14 |
| Rps19 | Xenograft immunodeficient mice with human DBA cells | Improved engraftment and erythropoiesis in RPS19 rescued marrow | Invokes RPS19 haploinsufficiency in DBA; possible role for gene therapy | 22, 23 |
| Rps19 | Transgene carrying a pathogenic missense mutation (β-actin promoter) | Ubiquitous expression-embryonic lethal; Inducible expression-growth retardation, anemia, reduced erythroid progenitors and red blood cells, rRNA processing defects | RPS19 missense mutations may act through dominant negative mechanism | 7 |
| Rps19 | Knockdown in erythroid progenitors (ex vivo) | Impaired proliferation, G1 arrest, induction of p53, decreased Myb and Kit | p53-induced cell cycle arrest results in decreased Kit and impaired erythropoiesis | 26 |
| Rps19 | ENU-induced missense mutation | Homozygote-early embryonic lethality; Heterozygote-dark skin, white belly spot, growth retardation, mild anemia | Activation of p53 mediates tissue specific phenotypes | 16 |
| Rps6 | Conditional allele | Liver deletion (2 alleles)-proliferation defect after partial hepatectomy; Ubiquitous deletion (one allele)-embryonic lethality, prolonged cell cycle, increased apoptosis; T-cell deletion (2 alleles)-impaired T-cell development; T-cell deletion (one allele)-decreased T-cell survival, proliferation defect in activated T-cells; Keratinocyte deletion (one allele)-dark skin; Melanocyte deletion (one allele)-light skin | Cell cycle checkpoint responds to defects in ribosome biogenesis; activation of p53 mediates tissue specific phenotypes | 15,16, 30, 31 |
| Rpl24 | Spontaneous mutation | White spotting, retinal defects, kinked tail, growth retardation; mutant cells have a prolonged cell cycle and a growth disadvantage | Phenotypes are mediated by p53 | 12, 32 |
| 5q deletion (Rps14) | Conditional deletion of ~500kb on 5qsyntenic region (Rps14 and 8 other genes) | Macrocytic anemia, megakaryocyte dysplasia, bone marrow apoptosis, progenitor cell defects | Hematopoietic phenotypes are mediated by p53 | 33 |
A null Rps19 allele (Rps19ko) was created by replacement of coding exons with a selectable neomycin cassette13. As would be expected for an essential gene, no Rps19ko/ko animals were recovered from Rps19ko/+ intercrosses (even when 3.5 day old blastocysts were examined), suggesting that homozygosity for Rps19 leads to early embryonic lethality. Unfortunately, however, Rps19ko/+ mice did not recapitulate the DBA phenotype: heterozygous animals did not exhibit developmental defects, differences in hematological parameters, bone marrow composition or hematopoietic cell in vitro colony forming ability compared to Rps19+/+ animals. In addition, Rps19 hemizygosity had no effect on erythrocyte ADA activity or response to stimulation by erythropoietin14. Curiously, Rps19 mRNA and protein levels from spleens of control and mutant animals were similar. Breeding the Rps19ko allele into a second genetic background did not alter the phenotype and lead investigators to conclude that Rps19 mutant mice were able to compensate for the loss of one Rps19 allele in a way that DBA patients were not.
As well as sounding a warning to those embarking on genetic engineering projects in the mouse, these results suggested that compensation mechanisms observed in the mouse may be at play in DBA patients. Could these pathways account for the variable penetrance observed in DBA pedigrees where the same genetic lesion can have different phenotypic outcomes? In this light, efforts to understand the compensatory mechanism would be instructive: is Rps19 mRNA synthesized at a faster rate or is it more stable in these animals?
Xenotransplantation in DBA
Although the genetic data implicating RPS19 in DBA were compelling, the molecular mechanism underlying the specific phenotypes in affected patients remained puzzling. Definitive proof for a role of RPS19 in the pathogenesis of DBA was achieved using in vitro and ex vivo approaches. Knocking down RPS19 expression in human bone marrow cells (CD34+) impairederythropoiesis in vitro19, 20 and could be rescued by ectopic expression of RPS19 or dexamethasone treatment. Similarly, erythropoietic defects observed in DBA patient cells (CD34+) could be partially corrected by RPS19 overexpression21. Conclusive evidence connecting RPS19 and DBA came from experiments in which CD34+ cells mobilized from patients with known RPS19 haploinsufficiency were infected with a RPS19 retrovirus and transplanted into NOD/SCID immunodeficient mice (a strain of mice that permits the engraftment and proliferation of human blood cells) (Table 1). Infected cells expressing high levels of RPS19 showed improved engraftment and erythropoiesis compared with uninfected patient cells22 or those expressing low levels of RPS1923. Thus, mounting evidence linked RPS19 haploinsufficiency and DBA, and proposed a role for gene therapy in the treatment of DBA.
A dominant negative Rps19 mouse model
Pathogenic deletions, nonsense or frame shift mutations in ribosomal protein genes often lead to reduced protein expression in DBA patients24 and implicate haploinsufficiency in the disease. Intriguingly, however, many DBA mutations are missense25 and posit a role for a dominant negative mechanism. In these patients, mutant proteins are predicted to disrupt ribosome biogenesis and/or the function of mature ribosomes to bring about the phenotype.
To explore a role for a dominant negative mechanism in DBA, a transgenic approach was employed by Devlin and colleagues: a pathogenic RPS19 mutation, R62W, was introduced into mice7. Ubiquitous expression of the transgene was lethal in early embryogenesis, but inducible expression at later time points during development phenocopied DBA. Growth retardation, mild anemia, and reduced erythroid progenitors and circulating red blood cells characterized transgenic animals. In addition, ribosomal RNA processing defects were detected in bone marrow cells. Gene expression analysis showed, among other changes, a significant decrease in Kit mRNA. Remarkably, the expression of the Rps19R62W was only 10% of the expression of the endogenous Rps19 mRNA, and thus a more robust phenotype may be observed if expression of the transgene is titrated. These results suggest the R62W mutation may produce its effect by a dominant negative mechanism, acting to inhibit or delay ribosome biogenesis.
Knocking down Rps19 in mouse erythropoiesis
A number of investigators have proposed that erythrocyte development is particularly sensitive to mutations affecting ribosomes because developing red blood cell progenitors produce substantial amounts of hemoglobin, a process that requires a large pool of mature ribosomes and biosynthetic capacity. Sieff and colleagues explored these ideas using a culture system in which embryonic mouse liver cells are harvested at day 14.5 (E14.5, a time point in which the mouse liver is enriched for erythroid progenitors) and cultured under conditions which support erythroid development26. During the first 24 hours of culture, erythroid cells divide rapidly and RNA production exceeds the increase in cell number. Knocking down Rps19 in these cells impairs proliferation, but does not affect differentiation. Cells also accumulate in G1 and are characterized by increased p53 (mouse protein, also known as TRP53) and, as previously observed in DBA patient cells27, decreased Myb and Kit expression compared to control cells. This system recapitulates several features of DBA and suggests that ribosomal protein mutations impair erythropoiesis via p53-induced cell cycle arrest. In addition, these studies point to a role for KIT signaling in DBA pathogenesis.
An Rps19 missense mutation in mice with dark skin
During the course of a forward genetic screen in mice for defects in skin color, McGowan and colleagues16 identified a dark skin mutant (Dsk) that harbored a missense mutation in Rps19. The Rps19Dsk3/+ animal is characterized by a dark tail, ears and feet, a white belly spot, growth retardation and mild, macrocytic anemia (a ~5–10% reduction in red blood cell count). Based on transcriptional changes in the skin, p53 activation was identified as a critical event linking ribosomal protein mutations to changes in skin color: p53 is both necessary and sufficient for dark skin. Intriguingly, deletion of p53 from the bone marrow of Rps19Dsk3/+ (by crossing to the Trp53ko allele) also mitigated the anemia phenotype. Thus, stabilization of p53 (human protein, also known as TP53) is a plausible mechanism to account for anemia in DBA and highlights a new pathway and set of molecules that could be the target for therapeutic intervention in affected individuals.
Other mouse strains relevant to DBA pathogenesis
Since the original description of RPS19 mutations6, genetic alterations in at least eight other ribosomal proteins have been identified in DBA patients affecting both the 40S and 60S ribosomal subunits3. In addition, RPS14 has been implicated in the pathogenesis of 5q-myelodysplastic syndrome (MDS), an adult onset disorder characterized by interstitial deletion of the long arm of chromosome 5, macrocytic anemia and a low rate of progression to acute myeloid leukemia28, 29. Thus, a spectrum of ribosomal protein mutations brings about similar phenotypes and underscores the rationale for studying mice that carry ribosomal protein mutations other than Rps19.
Genetically engineered and spontaneous ribosomal protein mouse mutants have been particularly useful for dissecting the molecular pathways leading to the tissue-specific phenotypes observed in DBA patients. Mouse models with conditional deletion of Rps615, 16,30,31, heterozygous deletion of Rpl2412, 32 or conditional deletion of a set of genes found on human 5q (including Rps14)33 have been generated (Table 1). Work based on these animal models has characterized a new cell cycle checkpoint and also highlight a role for activation of the transcription factor p53 in effecting in vivo phenotypes in individuals that carry ribosomal protein mutations.
Rps6 inducible knockout
Studies using a conditional Rps6 knockout allele in mice have provided considerable insight into ribosome biology and have defined a cell cycle checkpoint that responds to the state of ribosome biogenesis. Under in vivo conditions that require cell proliferation, heterozygosity for Rps6 leads to cell cycle arrest: conditional deletion of Rps6 in the mouse liver prevents hepatocytes from dividing after partial hepatectomy, and hemizygosity for Rps6 during gastrulation induces a proliferative defect that contributes to early embryonic lethality15, 30. In addition, T-cells lacking one copy of Rps6 fail to proliferate when activated31. Thus, cells with reduced ribosomal protein gene dosage may generate sufficient ribosomes under homeostatic conditions, but exhibit defects in ribosome biogenesis in a setting that requires rapid cell division. Checkpoint control mechanisms that sense defects in ribosome synthesis may prevent cell cycle progression and contribute to the tissue-restricted phenotypes observed in ribosomal protein mutants.
The emerging role of p53
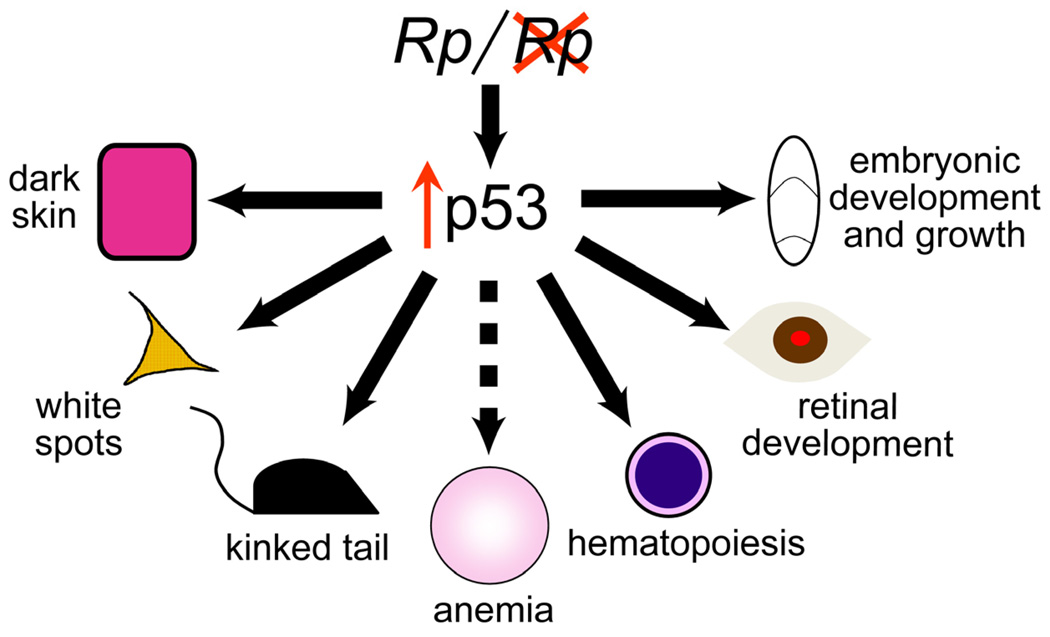
p53, the extensively studied transcription factor, is known for its ability to stem cancer development in response to genotoxic stress by regulating genes involved in processes such as cell cycle progression, senescence and apoptosis. Evidence from a number of model systems suggests that p53 is also poised to respond to stress signals induced by ribosomal protein mutations and phenotypic outcomes depend upon the underlying cell type in which p53 activation occurs16, 30, 31. For example, haploinsufficiency for Rps6 in mouse skin induces melanocytosis and dark skin, a phenotype that is completely abrogated in the absence of Trp5316. In addition, coat color, skin and retinal defects that characterize the spontaneous, semidominant mouse mutant, Belly spot and tail (Bst, Rpl24 mutation) are mitigated by the loss of p5312, 32(Figure 1).
Figure 1.
Activation of p53 links ribosomal protein haploinsufficiency to pleiotropic whole animal phenotypes in the mouse. p53 stabilization in keratinocytes (pink) leads to dark skin, in melanocytes (orange) to white coat color and in embryonic tissues to early lethality. Studies based on ribosomal protein mutants posit a mechanism by which activation of p53 induces red cell hypoplasia and anemia in DBA (hashed arrow).
Activation of p53 has also been linked to hematopoietic phenotypes in the mouse33 (Figure 1). Recently, Barlow and colleagues generated mice with conditional deletion of the syntenic region in 5q- MDS (including Rps14). Like human patients, mutant mice exhibit macrocytic anemia, megakaryocyte dysplasia, increased apoptosis of bone marrow cells and progenitor cell defects. To evaluate p53-dependence, mutant mice were crossed into the Trp53ko/ko background and hematologic parameters evaluated: an absence of p53 ameliorated many of the hematologic phenotypes. Thus, ribosomal protein haploinsufficiency triggers p53-dependent phenotypes in a number of organ systems including the bone marrow, thus providing a molecular connection between mutations that lead to a generalized impairment of ribosome biogenesis and tissue-specific manifestations observed in DBA (and MDS) patients.
Mutation in the ribosomal protein binding motif of MDM2
p53 is maintained at low levels by MDM2 (human protein, also known as HDM2)-mediated ubiquitylation in normal cells. Accumulating evidence suggests that certain ribosomal proteins (including RPL11, RPL5 and RPL23) are bound and sequestered by MDM2 in cells with defective ribosome biogenesis and inhibit its activity towards p53. Thus, p53 accumulates and downstream pathways are activated. Macias and colleagues34 introduced a base pair substitution into the zinc finger domain of the mouse Mdm2 locus (knock in allele, Mdm2C305F) that disrupts ribosomal protein binding (Table 1). While Mdm2C305F/C305F mice exhibit a normal p53 response to DNA damage, they fail to activate p53 after perturbations in ribosome biogenesis. Homozygous mutant mice also develop c-MYC induced lymphomas more rapidly than nonmutant animals, suggesting that the ribosomal protein-MDM2 interaction serves as a barrier against tumor formation. Interestingly, p19Arf was not required for the Mdm2-mediated response to ribosomal stress. The Mdm2C305F knock in mice provide in vivo evidence that interaction between ribosomal proteins and the p53-MDM2 pathway is one mechanism by which p53 surveys the integrity of ribosome biogenesis and function.
Flvcr mutant mice
Despite extensive efforts to sequence all ribosomal protein genes in affected individuals, molecular lesions remain elusive in fifty percent of DBA cases. Thus, mutations in genes other than ribosomal proteins should be considered in the pathogenesis of DBA, either as the primary cause of the disease or as genetic factors that influence the penetrance and expressivity of the disease.
Feline leukemia virus induces a DBA-like red cell aplasia in cats by interfering with the activity of its cellular receptor Flvcr, a heme exporter. Although efforts to identify causative mutations in the FLVCR1 gene in DBA families have been unrevealing thus far (FLVCR1 maps to 1q31.3 near a putative DBA locus)35, Flvcr mutant mice are characterized by a striking, DBA-like phenotype36 (Table 2). Flvcr-null (Flvcrko/ko) mice die by E16.5 and have impaired definitive erythropoiesis. Embryos also exhibit abnormal limb, hand, and digit development, flattened faces and hypertelorism. Conditional inactivation of Flvcr in postnatal mice phenocopies DBA: mutant mice feature severe hyperchromic, macrocytic anemia and a block in erythroid development (at the pro-erythroblast stage). Investigators hypothesize that the failure in red blood cell development is due to accumulation of free heme, which leads to apoptosis and anemia. Intriguingly, alternatively spliced forms of FLVCR1 have been found in primary cells from DBA patients and lead to altered protein function and/or stability37. Although a causal role for FLVCR alterations in DBA is uncertain, the phenotypic overlap is striking and suggests that pathways that control heme accumulation, transport and/or degradation may contribute to the pathogenesis of the disease36.
Table 2.
Other mouse models of DBA.
| Gene | Molecular approach | Whole animal and cellular phenotype | Implications | Ref(s) |
|---|---|---|---|---|
| Flvcr | Knockout and conditional allele | Homozygote knockout-early embryonic lethality, impaired erythropoiesis, dysmorphicfacies and limbs; Postnatal deletion-anemia, block in erythropoiesis | Phenocopies many DBA features; role for excess heme in DBA | 36 |
| Tcof1 | Knockout | Heterozygote-craniofacial hypoplasia, neural crest apoptosis | Phenotypes are mediated by p53 | 40 |
| Sbds | Knockout | Homozygote-early embryonic lethality; Heterozygote-no phenotype | Gene dosage influences disease penetrance | 45 |
| Dkc1 | Hypomorphic allele | rRNA modification defects occur early, telomere shortening occurs later | Impaired ribosome function is important for disease initiation, telomere shortening modifies the phenotype | 49 |
| Dkc1 | Knockin pathogenic mutations | Mutant cells have a growth disadvantage | Growth disadvantage is independent of telomere length | 52 |
| Kit | Spontaneous and engineered | White belly spot, macrocytic anemia | Abnormal Kit signaling may contribute to DBA | 54, 56 |
| Kitl | Spontaneous and engineered | White belly spot, macrocytic anemia | Abnormal Kit signaling may contribute to DBA | 54, 55 |
| Mdm2 | Knockin (base pair substitution, C305F) | Mutation impairs mdm2-ribosomal protein binding; perturbations in ribosome biogenesis do not lead to activation of p53 | May correct phenotypes in ribosomal protein mutants and DBA patients | 34 |
Mouse models of other ribosomopathies
Treacher-Collins syndrome
Treacher-Collins syndrome is a rare, autosomal dominant disorder caused by haploinsufficiency for TCOF1 (the gene that encodes TCOF1 or Treacle). Treacher-Collins syndrome is considered a ribosomopathy since TCOF1 is a nucleolar protein present in pre-ribosomal complexes38 and is necessary for ribosome biogenesis39. Although Treacher-Collins patients have normal hematopoiesis, affected individuals exhibit DBA-like craniofacial abnormalities, which reflect abnormal neural crest development.
The Tcof1 knockout mouse (Tcof1ko/+) accurately reproduces the human phenotype: mutant mice exhibit severe craniofacial hypoplasia and dysplasia40, a phenotype that is profoundly influenced by genetic background (Table 2)41. Tcof1ko/+ embryos have fewer migrating neural crest cells than wild type embryos due to extensive neuroepithelial cell apoptosis, and also exhibit defects in ribosome biogenesis. Blocking p53 function in mutant animals (by crossing into a Trp53ko background or by treating with the p53 inhibitor, pifithrin-α) mitigates neuroepithelial cell apoptosis and results in normal craniofacial development, but does not correct abnormalities in ribosome function. Thus, current hypotheses suggest that TCOF1 mutations trigger defects in ribosome biogenesis that bring about p53 stabilization in Treacher Collins patients, and like DBA, phenotypic features are dependent on the extent of p53 activation and the cell type in which it occurs.
Shwachman-Diamond syndrome (SDS)
SDS is an autosomal recessive disorder characterized by bone marrow failure, neutropenia, exocrine pancreatic dysfunction and a predisposition to leukemia42. It is caused by mutations in SBDS43 which encodes a nucleolar protein that facilitates assembly of the 60S and 40S ribosomal subunits44, and is, thus, classified as a ribosomopathy.
Sbds heterozygous mice (Sbdsko/+) mice are indistinguishable from wild type, but homozygotes (Sbdsko/ko) die in early embryogenesis (Table 2)45. These observations are relevant to the human disease and underscore the essential role of SBDS: most patients carry one null allele and one hypomorphic allele (an allele that encodes a protein with some residual function). Two null alleles have never been found in the same patient.
Interestingly, a recent study points to a role for SBDS in the bone marrow stem cell niche in MDS and bone marrow failure46. Tissue specific deletion of Sbds in mouse osteoprogenitor cells (thought to be a component of the stem cell niche in the bone marrow) induces marrow dysfunction and MDS, and raises the possibility that mutations that disrupt ribosome biogenesis act in the marrow stroma and contribute to the pathogenesis of ribosomopathies like SDS and DBA.
Dyskeratosis congenita (DC)
Dyskeratosis congenita presents during the first decade of life with hyperpigmentation and nail dystrophy. Most patients develop peripheral cytopenias of one or more lineages, and many progress to bone marrow failure. DC is a genetically heterogeneous disease caused by mutations in components of the telomerase complex47: DKC1 (X-linked DC, protein product is DKC1 or dyskerin), TERT (autosomal recessive, telomerase reverse transcriptase), TERC (autosomal dominant DC, telomerase RNA), TINF2 (autosomal dominant DC), NOP10 (autosomal recessive DC) and NHP2 (autosomal recessive DC). Several DC genes exhibit extra-telomeric functions as well. The most poignant example is DKC1; dyskerin is responsible for conversion of uridines to pseudouridines on newly synthesized rRNA molecules, a process that is conserved across species and is central to ribosome assembly. Thus, teasing apart the contributions of telomere biology and ribosome biogenesis to the DC phenotype has been challenging.
Mouse models for DC do not phenocopy the human disease accurately due in part to species specific differences in telomere length48. Nonetheless, Ruggero and colleagues49 generated a hypomorphic Dkc1 allele that exhibits impaired rRNA pseudouridylation in early generations, prior to the onset of clinical disease (Table 2). Reduction of telomere length becomes evident in later generations, and thus points to a mechanism in which impaired ribosome function is important for initiation of DC, while telomere shortening modifies the phenotype further. Additional data suggests that defects in pseudouridylation lead to impaired translation of mRNAs containing IRES sequences and posits a mechanism by which defective ribosome activity leads to the DC phenotype50, 51. Other mouse models highlight a role for telomere length in the pathogenesis of DC52, 53 (Table 2). Experiments based on mice that carry pathogenic human mutations find that mutant cells have a growth disadvantage compared to wild type cells, a phenotype that occurs in the context of normal telomere length. In the absence of telomerase, the differences in growth are mitigated.
Although it is clear that telomere dysfunction plays a role in the pathogenesis of DC, defects in ribosome biosynthesis and function may contribute to the disease phenotype. Thus, akin to hypotheses for extra-ribosomal functions for ribosomal proteins in DBA, DC genes may be pleiotropic in function and action.
White spotting mutants: an old model revisited
In mice, mutations at either the Dominant White Spotting (W, encoded by c-Kit) or the Steel (Sl, encoded by Kit ligand, Kitl) locus result in a clinical syndrome that is similar to DBA (Table 2)54,55. Animals are characterized by macrocytic anemia, reticulocytopenia and impaired erythrocyte development56. In addition, hematopoietic stem cell self-renewal, survival and differentiation programs are abnormal27, 57. Akin to human DBA patients, hematologic phenotypes observed in W mice can be rescued by stem cell transplantation: mutations in DBA patients and c-Kit mutants have a cell autonomous effect on bone marrow stem cells54. (Kitl acts in the bone marrow environment to produce a non-cell autonomous phenotype)54. Thus, investigators have postulated a role for KIT signaling in DBA26, 58, 59.
Several lines of evidence suggest that white spotting mutants may not accurately model DBA, however. First, the anemia in W and Sl mice does not improve with traditional corticosteroid therapy60. Second, W and Sl mice manifest phenotypes in other organs that are not apparent in DBA patients: cutaneous abnormalities, mast cell defects and infertility are characteristic of W and Sl mice since Kit signaling is involved in common biologic processes used by cell types such as skin, mast and germ cells. Finally, extensive efforts to identify molecular alterations in KIT or KITLG in DBA patients have been unsuccessful61–63.
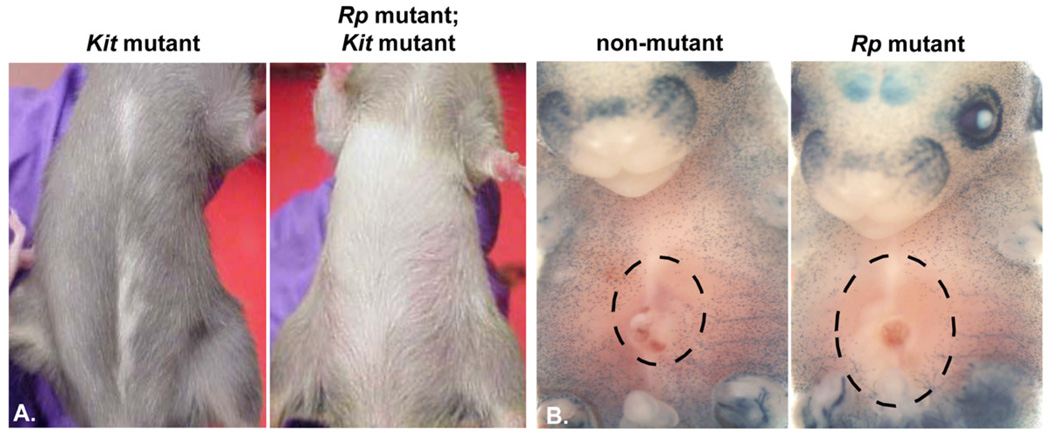
Despite these observations, KIT signaling may contribute to the pleiotropic phenotypes observed in individuals that carry ribosomal protein mutations. In fact, ribosomal protein mutant mice share non-hematopoietic phenotypes with spotting mutants: white spots, an anatomic location in which pigment cells fail to migrate during development, are observed in adult animals (Figure 2A, B)12, 16, 32. These observations are bolstered by in vitro studies showing that erythroid progenitors with reduced Rps19 gene dosage express lower levels of Kit protein than control cells (see above)26. Thus, c-KIT may be an excellent target for new drug development in DBA since the signaling pathway may contribute to erythrocyte development, as well as non-hematopoietic phenotypes in affected individuals.
Figure 2.
Ribosomal protein (Rp) mouse mutants share cutaneous phenotypes with white spotting mutants. A. An adult mouse carrying a Kit mutation is characterized by a white belly spot (left). The phenotype is enhanced when placed on a ribosomal protein mutant background (right). B. A histochemical marker for pigment cells (a LacZ transgene driven by regulatory elements from a pigment cell gene) stains developing melanocytes (blue) in E15.5 mouse embryos. In nonmutant animals, pigment cell precursors have moved to the developing skin in a dorsal to ventral expansion. Ribosomal protein mutant embryos have fewer X-gal positive cells in the skin (denoted by the large hashed oval devoid of blue staining) compared to nonmutant animals.
Minute flies and fish
Drosophila
Dominantly inherited Minute mutants, first described in Drosophila more than 80 years ago64, represent more than 50 different genetic loci and share phenotypic features including short, thin bristles, delayed larval development and recessive lethality. The genetic basis for this class of mutants puzzled generations of geneticists, but evidence now suggest that all mutations affect ribosomal proteins10, 18.
Studies based on Minute flies have been instructive for scientists and clinicians whose focus is DBA. Early on, investigators focused on shared phenotypes: ribosomal protein mutations in both species are associated with developmental delay and small size. In addition, phenotypes are apparent in tissues and cells that require high rates of protein synthesis – bone marrow cells in human patients and sensory bristles in flies. Thus, current hypotheses suggest that reduced ribosomal protein gene dosage does not compromise cellular process under normal homeostatic conditions, but evokes tissue specific phenotypes in cells that require high protein synthesis (e.g. cell proliferation during development or hematopoiesis).
Phenotypes in Minute flies are, for the most part, due to ribosomal protein haploinsufficiency. Intriguingly, however, the converse is not true; haploinsufficiency for each ribosomal protein does not bring about a Minute phenotype18. This observation is paralleled in DBA, where RPS19 is mutated in 25% of cases and RPL5 is mutated in 7% of cases, but mutations in other ribosomal proteins do not bring about the disease phenotype2, 3. One hypothesis to explain the apparent disparity suggests that ribosomal proteins are expressed at different levels, and phenotypes become evident when the quantity of any one ribosomal protein falls below a critical amount. Thus, variation in expression of different ribosomal proteins (which could be influenced by the rate of transcription, translation and/or transcript/protein stability) may contribute to variable penetrance and expressivity observed in Minute flies and DBA patients.
Zebrafish
Two groups have used antisense morpholino approaches to knockdown rps19 in zebrafish11, 65. Results from these studies yield similar conclusions: rps19-deficiency leads to hematopoietic and developmental abnormalities akin to those observed in DBA patients. Morphants are characterized by defective erythropoiesis and lower hemoglobin concentrations, features that are rescued by rps19 overexpression. In addition, many animals exhibit small size, as well as other morphologic abnormalities. Akin to mice that carry ribosomal protein mutations, knockdown of other ribosomal proteins in fish brings about a phenotype similar to the rps19 morphant65 and phenotypes are rescued in the absence of p5311, 66.
rps and rpl mutations also cause tumors in zebrafish67, 68, a finding that parallels observations suggesting that DBA is a cancer predisposition syndrome. From a forward genetic screen, investigators identified several fish lines that develop malignant peripheral nerve sheath tumors (MPNST) and are heterozygous for different ribosomal proteins. Intriguingly, the presence of tumors correlates with growth impairment (i.e. a “Minute”-like phenotype) and raises the possibility that additional tumorigenic mutations might confer a growth advantage among cells that are growth impaired. These studies also raise the possibility that the cancer predisposition observed in humans that carry ribosomal protein mutations might be the result of a translational defect.
Conclusions
Animal models for DBA have been pivotal in advancing our understanding of the disease. Approaches based on xenotransplantation and genetic engineering in the mouse have delineated potential genetic mechanisms: ribosomal protein mutations can act through haploinsufficient or dominant negative mechanisms to bring about red blood cell aplasia, an idea consistent with observations that the structure of the genetic mutation (e.g. deletion vs. missense) does not predict clinical outcome in DBA patients69. Thus, additional efforts to delineate the pathogenesis of DBA can focus on molecular pathways common to all DBA patients.
Although hemizygosity for different ribosomal proteins has been recognized for decades to cause developmental delay and reduced organismal growth, ribosomal protein mutations also cause remarkably specific phenotypes, including white spotting in mice, short bristles in flies and anemia in humans. Hypotheses based on extra ribosomal functions for certain ribosomal proteins have been put forward since it is unclear how mutations in ubiquitously expressed genes with a well-known role in protein translation would manifest as tissue-restricted phenotypes. Two additional, complementary (and not mutually exclusive) mechanisms linking ribosomal protein mutations and bone marrow failure in DBA have emerged from studies based on animal models.
First, defective ribosome biogenesis may activate p53-dependent pathways in many (if not all) cells, and distinct phenotypic outcomes depend upon variation in the type of response. For example, p53 may activate a distinct set of genes in the skin to effect changes in skin or hair color and an alternate transcriptome in maturing erythrocytes to bring about anemia. Intriguingly, most phenotypes associated with ribosomal protein mutations are apparent in tissues characterized by rapid cellular proliferation – skin, bone marrow, developing embryos – thus linking ribosome synthesis and function with cell cycling, p53 and whole animal phenotypes.
A second hypothesis suggests that the balance of heme and globin synthesis is fundamental in DBA. During erythropoiesis, immature red blood cells synthesize a massive amount of globin, a process that requires a large pool of mature ribosomes. Erythroid cells compromised for ribosome function may not be able to maintain the level of globin synthesis required for maturation, leading to a relative excess of free heme, apoptosis and anemia. Additional studies using model organisms and built on these ideas would be instructive: assays to measure free heme in developing red blood cell from ribosomal protein mutants would support these ideas, and therapeutic approaches to titrate heme or increase globin production may be helpful for treating affected patients. Thus, animal models have and will continue to aid efforts to generate new hypotheses, identify pathogenic pathways and pinpoint new targets for therapeutic intervention in DBA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
K.A.M. and P.J.M. have no financial disclosures or conflicts of interest.
References
- 1.Chow P. The rationale for the use of animals models in biomedical research. In: Chow P, Ng R, Ogden B, editors. Using animal models in biomedical research: a primer for the investigator. World Scientific Publishing Company; 2008. [Google Scholar]
- 2.Lipton JM, Ellis SR. Diamond Blackfan anemia 2008–2009: broadening the scope of ribosome biogenesis disorders. Curr Opin Pediatr. 2010;22:12–19. doi: 10.1097/MOP.0b013e328334573b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010 doi: 10.1182/blood-2009-10-178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlachos A, Muir E. How I treat Diamond Blackfan anemia. Blood. 2010 doi: 10.1182/blood-2010-02-251090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150:179–188. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draptchinskaia N, Gustavsson P, Andersson B, Pettersson M, Willig TN, Dianzani I, et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet. 1999;21:169–175. doi: 10.1038/5951. [DOI] [PubMed] [Google Scholar]
- 7.Devlin EE, Dacosta L, Mohandas N, Elliott G, Bodine DM. A transgenic mouse model demonstrates a dominant negative effect of a point mutation in the RPS19 gene associated with Diamond-Blackfan anemia. Blood. 2010 doi: 10.1182/blood-2010-03-275776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leger-Silvestre I, Caffrey JM, Dawaliby R, Alvarez-Arias DA, Gas N, Bertolone SJ, et al. Specific Role for Yeast Homologs of the Diamond Blackfan Anemia-associated Rps19 Protein in Ribosome Synthesis. J Biol Chem. 2005;280:38177–38185. doi: 10.1074/jbc.M506916200. [DOI] [PubMed] [Google Scholar]
- 9.Weijers D, Franke-van Dijk M, Vencken RJ, Quint A, Hooykaas P, Offringa R. An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development. 2001;128:4289–4299. doi: 10.1242/dev.128.21.4289. [DOI] [PubMed] [Google Scholar]
- 10.Kongsuwan K, Yu Q, Vincent A, Frisardi MC, Rosbash M, Lengyel JA, et al. A Drosophila Minute gene encodes a ribosomal protein. Nature. 1985;317:555–558. doi: 10.1038/317555a0. [DOI] [PubMed] [Google Scholar]
- 11.Danilova N, Sakamoto KM, Lin S. Ribosomal protein S19 deficiency in zebrafish leads to developmental abnormalities and defective erythropoiesis through activation of p53 protein family. Blood. 2008;112:5228–5237. doi: 10.1182/blood-2008-01-132290. [DOI] [PubMed] [Google Scholar]
- 12.Oliver ER, Saunders TL, Tarle SA, Glaser T. Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development. 2004;131:3907–3920. doi: 10.1242/dev.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsson H, Davey EJ, Draptchinskaia N, Hamaguchi I, Ooka A, Leveen P, et al. Targeted disruption of the ribosomal protein S19 gene is lethal prior to implantation. Mol Cell Biol. 2004;24:4032–4037. doi: 10.1128/MCB.24.9.4032-4037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsson H, Davey EJ, Frojmark AS, Miyake K, Utsugisawa T, Flygare J, et al. Erythropoiesis in the Rps19 disrupted mouse: Analysis of erythropoietin response and biochemical markers for Diamond-Blackfan anemia. Blood Cells Mol Dis. 2006;36:259–264. doi: 10.1016/j.bcmd.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Volarevic S, Stewart MJ, Ledermann B, Zilberman F, Terracciano L, Montini E, et al. Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science. 2000;288:2045–2047. doi: 10.1126/science.288.5473.2045. [DOI] [PubMed] [Google Scholar]
- 16.McGowan KA, Li JZ, Park CY, Beaudry V, Tabor HK, Sabnis AJ, et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet. 2008;40:963–970. doi: 10.1038/ng.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 18.Marygold SJ, Roote J, Reuter G, Lambertsson A, Ashburner M, Millburn GH, et al. The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 2007;8:R216. doi: 10.1186/gb-2007-8-10-r216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flygare J, Kiefer T, Miyake K, Utsugisawa T, Hamaguchi I, Da Costa L, et al. Deficiency of ribosomal protein S19 in CD34+ cells generated by siRNA blocks erythroid development and mimics defects seen in Diamond-Blackfan anemia. Blood. 2005;105:4627–4634. doi: 10.1182/blood-2004-08-3115. [DOI] [PubMed] [Google Scholar]
- 20.Ebert BL, Lee MM, Pretz JL, Subramanian A, Mak R, Golub TR, et al. An RNA interference model of RPS19 deficiency in Diamond-Blackfan anemia recapitulates defective hematopoiesis and rescue by dexamethasone: identification of dexamethasone-responsive genes by microarray. Blood. 2005;105:4620–4626. doi: 10.1182/blood-2004-08-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamaguchi I, Flygare J, Nishiura H, Brun AC, Ooka A, Kiefer T, et al. Proliferation deficiency of multipotent hematopoietic progenitors in ribosomal protein S19 (RPS19)-deficient diamond-Blackfan anemia improves following RPS19 gene transfer. Mol Ther. 2003;7:613–622. doi: 10.1016/s1525-0016(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 22.Zivny J, Jelinek J, Pospisilova D, Plasilova M, Necas E, Stopka T. Diamond blackfan anemia stem cells fail to repopulate erythropoiesis in NOD/SCID mice. Blood Cells Mol Dis. 2003;31:93–97. doi: 10.1016/s1079-9796(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 23.Flygare J, Olsson K, Richter J, Karlsson S. Gene therapy of Diamond Blackfan anemia CD34(+) cells leads to improved erythroid development and engraftment following transplantation. Exp Hematol. 2008;36:1428–1435. doi: 10.1016/j.exphem.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Gazda HT, Zhong R, Long L, Niewiadomska E, Lipton JM, Ploszynska A, et al. RNA and protein evidence for haplo-insufficiency in Diamond-Blackfan anaemia patients with RPS19 mutations. Br J Haematol. 2004;127:105–113. doi: 10.1111/j.1365-2141.2004.05152.x. [DOI] [PubMed] [Google Scholar]
- 25.Boria I, Quarello P, Avondo F, Garelli E, Aspesi A, Carando A, et al. A new database for ribosomal protein genes which are mutated in Diamond-Blackfan Anemia. Hum Mutat. 2008;29:E263–E270. doi: 10.1002/humu.20864. [DOI] [PubMed] [Google Scholar]
- 26.Sieff CA, Yang J, Merida-Long LB, Lodish HF. Pathogenesis of the erythroid failure in Diamond Blackfan anaemia. Br J Haematol. 2009;148:611–622. doi: 10.1111/j.1365-2141.2009.07993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gazda HT, Kho AT, Sanoudou D, Zaucha JM, Kohane IS, Sieff CA, et al. Defective ribosomal protein gene expression alters transcription, translation, apoptosis, and oncogenic pathways in Diamond-Blackfan anemia. Stem Cells. 2006;24:2034–2044. doi: 10.1634/stemcells.2005-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Berghe H, Cassiman JJ, David G, Fryns JP, Michaux JL, Sokal G. Distinct haematological disorder with deletion of long arm of no. 5 chromosome. Nature. 1974;251:437–438. doi: 10.1038/251437a0. [DOI] [PubMed] [Google Scholar]
- 29.Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, Galili N, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451:335–339. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulic S, Panic L, Barkic M, Mercep M, Uzelac M, Volarevic S. Inactivation of S6 ribosomal protein gene in T lymphocytes activates a p53-dependent checkpoint response. Genes Dev. 2005;19:3070–3082. doi: 10.1101/gad.359305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panic L, Tamarut S, Sticker-Jantscheff M, Barkic M, Solter D, Uzelac M, et al. Ribosomal protein S6 gene haploinsufficiency is associated with activation of a p53-dependent checkpoint during gastrulation. Mol Cell Biol. 2006;26:8880–8891. doi: 10.1128/MCB.00751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barkic M, Crnomarkovic S, Grabusic K, Bogetic I, Panic L, Tamarut S, et al. The p53 tumor suppressor causes congenital malformations in Rpl24-deficient mice and promotes their survival. Mol Cell Biol. 2009;29:2489–2504. doi: 10.1128/MCB.01588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barlow JL, Drynan LF, Hewett DR, Holmes LR, Lorenzo-Abalde S, Lane AL, et al. A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q- syndrome. Nat Med. 2009;16:59–66. doi: 10.1038/nm.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macias E, Jin A, Deisenroth C, Bhat K, Mao H, Lindstrom MS, et al. An ARF-independent c-MYC-activated tumor suppression pathway mediated by ribosomal protein-Mdm2 Interaction. Cancer Cell. 2010;18:231–243. doi: 10.1016/j.ccr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quigley JG, Gazda H, Yang Z, Ball S, Sieff CA, Abkowitz JL. Investigation of a putative role for FLVCR, a cytoplasmic heme exporter, in Diamond-Blackfan anemia. Blood Cells Mol Dis. 2005;35:189–192. doi: 10.1016/j.bcmd.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 37.Rey MA, Duffy SP, Brown JK, Kennedy JA, Dick JE, Dror Y, et al. Enhanced alternative splicing of the FLVCR1 gene in Diamond Blackfan anemia disrupts FLVCR1 expression and function that are critical for erythropoiesis. Haematologica. 2008;93:1617–1626. doi: 10.3324/haematol.13359. [DOI] [PubMed] [Google Scholar]
- 38.Hayano T, Yanagida M, Yamauchi Y, Shinkawa T, Isobe T, Takahashi N. Proteomic analysis of human Nop56p-associated pre-ribosomal ribonucleoprotein complexes. Possible link between Nop56p and the nucleolar protein treacle responsible for Treacher Collins syndrome. J Biol Chem. 2003;278:34309–34319. doi: 10.1074/jbc.M304304200. [DOI] [PubMed] [Google Scholar]
- 39.Trainor PA, Dixon J, Dixon MJ. Treacher Collins syndrome: etiology, pathogenesis and prevention. Eur J Hum Genet. 2009;17:275–283. doi: 10.1038/ejhg.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med. 2008;14:125–133. doi: 10.1038/nm1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon J, Dixon MJ. Genetic background has a major effect on the penetrance and severity of craniofacial defects in mice heterozygous for the gene encoding the nucleolar protein Treacle. Dev Dyn. 2004;229:907–914. doi: 10.1002/dvdy.20004. [DOI] [PubMed] [Google Scholar]
- 42.Shimamura A. Shwachman-Diamond syndrome. Semin Hematol. 2006;43:178–188. doi: 10.1053/j.seminhematol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Boocock GR, Morrison JA, Popovic M, Richards N, Ellis L, Durie PR, et al. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat Genet. 2003;33:97–101. doi: 10.1038/ng1062. [DOI] [PubMed] [Google Scholar]
- 44.Menne TF, Goyenechea B, Sanchez-Puig N, Wong CC, Tonkin LM, Ancliff PJ, et al. The Shwachman-Bodian-Diamond syndrome protein mediates translational activation of ribosomes in yeast. Nat Genet. 2007;39:486–495. doi: 10.1038/ng1994. [DOI] [PubMed] [Google Scholar]
- 45.Zhang S, Shi M, Hui CC, Rommens JM. Loss of the mouse ortholog of the shwachman-diamond syndrome gene (Sbds) results in early embryonic lethality. Mol Cell Biol. 2006;26:6656–6663. doi: 10.1128/MCB.00091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirwan M, Dokal I. Dyskeratosis congenita, stem cells and telomeres. Biochim Biophys Acta. 2009;1792:371–379. doi: 10.1016/j.bbadis.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He J, Gu BW, Ge J, Mochizuki Y, Bessler M, Mason PJ. Variable expression of Dkc1 mutations in mice. Genesis. 2009;47:366–373. doi: 10.1002/dvg.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruggero D, Grisendi S, Piazza F, Rego E, Mari F, Rao PH, et al. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- 50.Yoon A, Peng G, Brandenburger Y, Zollo O, Xu W, Rego E, et al. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312:902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- 51.Bellodi C, Kopmar N, Ruggero D. Deregulation of oncogene-induced senescence and p53 translational control in X-linked dyskeratosis congenita. EMBO J. 2010;29:1865–1876. doi: 10.1038/emboj.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu BW, Bessler M, Mason PJ. A pathogenic dyskerin mutation impairs proliferation and activates a DNA damage response independent of telomere length in mice. Proc Natl Acad Sci U S A. 2008;105:10173–10178. doi: 10.1073/pnas.0803559105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mochizuki Y, He J, Kulkarni S, Bessler M, Mason PJ. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc Natl Acad Sci U S A. 2004;101:10756–10761. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- 55.Wehrle-Haller B. The role of Kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Res. 2003;16:287–296. doi: 10.1034/j.1600-0749.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 56.Munugalavadla V, Kapur R. Role of c-Kit and erythropoietin receptor in erythropoiesis. Crit Rev Oncol Hematol. 2005;54:63–75. doi: 10.1016/j.critrevonc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Thoren LA, Liuba K, Bryder D, Nygren JM, Jensen CT, Qian H, et al. Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol. 2008;180:2045–2053. doi: 10.4049/jimmunol.180.4.2045. [DOI] [PubMed] [Google Scholar]
- 58.Abkowitz JL, Sabo KM, Nakamoto B, Blau CA, Martin FH, Zsebo KM, et al. Diamond-blackfan anemia: in vitro response of erythroid progenitors to the ligand for c-kit. Blood. 1991;78:2198–2202. [PubMed] [Google Scholar]
- 59.Olivieri NF, Grunberger T, Ben-David Y, Ng J, Williams DE, Lyman S, et al. Diamond-Blackfan anemia: heterogenous response of hematopoietic progenitor cells in vitro to the protein product of the steel locus. Blood. 1991;78:2211–2215. [PubMed] [Google Scholar]
- 60.Alter BP, Gaston T, Lipton JM. Lack of effect of corticosteroids in W/Wv and S1/S1d mice: these strains are not a model for steroid-responsive Diamond-Blackfan anemia. Eur J Haematol. 1993;50:275–278. doi: 10.1111/j.1600-0609.1993.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 61.Abkowitz JL, Broudy VC, Bennett LG, Zsebo KM, Martin FH. Absence of abnormalities of c-kit or its ligand in two patients with Diamond-Blackfan anemia. Blood. 1992;79:25–28. [PubMed] [Google Scholar]
- 62.Spritz RA, Freedman MH. Lack of mutations of the MGF and KIT genes in Diamond-Blackfan anemia. Blood. 1993;81:3165. [PubMed] [Google Scholar]
- 63.Drachtman RA, Geissler EN, Alter BP. The SCF and c-kit genes in Diamond-Blackfan anemia. Blood. 1992;79:2177–2178. [PubMed] [Google Scholar]
- 64.Schultz J. The Minute Reaction in the Development of DROSOPHILA MELANOGASTER. Genetics. 1929;14:366–419. doi: 10.1093/genetics/14.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uechi T, Nakajima Y, Chakraborty A, Torihara H, Higa S, Kenmochi N. Deficiency of ribosomal protein S19 during early embryogenesis leads to reduction of erythrocytes in a zebrafish model of Diamond-Blackfan anemia. Hum Mol Genet. 2008;17:3204–3211. doi: 10.1093/hmg/ddn216. [DOI] [PubMed] [Google Scholar]
- 66.Chakraborty A, Uechi T, Higa S, Torihara H, Kenmochi N. Loss of ribosomal protein L11 affects zebrafish embryonic development through a p53-dependent apoptotic response. PLoS One. 2009;4:e4152. doi: 10.1371/journal.pone.0004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amsterdam A, Sadler KC, Lai K, Farrington S, Bronson RT, Lees JA, et al. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol. 2004;2:E139. doi: 10.1371/journal.pbio.0020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai K, Amsterdam A, Farrington S, Bronson RT, Hopkins N, Lees JA. Many ribosomal protein mutations are associated with growth impairment and tumor predisposition in zebrafish. Dev Dyn. 2009;238:76–85. doi: 10.1002/dvdy.21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vlachos A, Ball S, Dahl N, Alter BP, Sheth S, Ramenghi U, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol. 2008;142:859–876. doi: 10.1111/j.1365-2141.2008.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]