Abstract
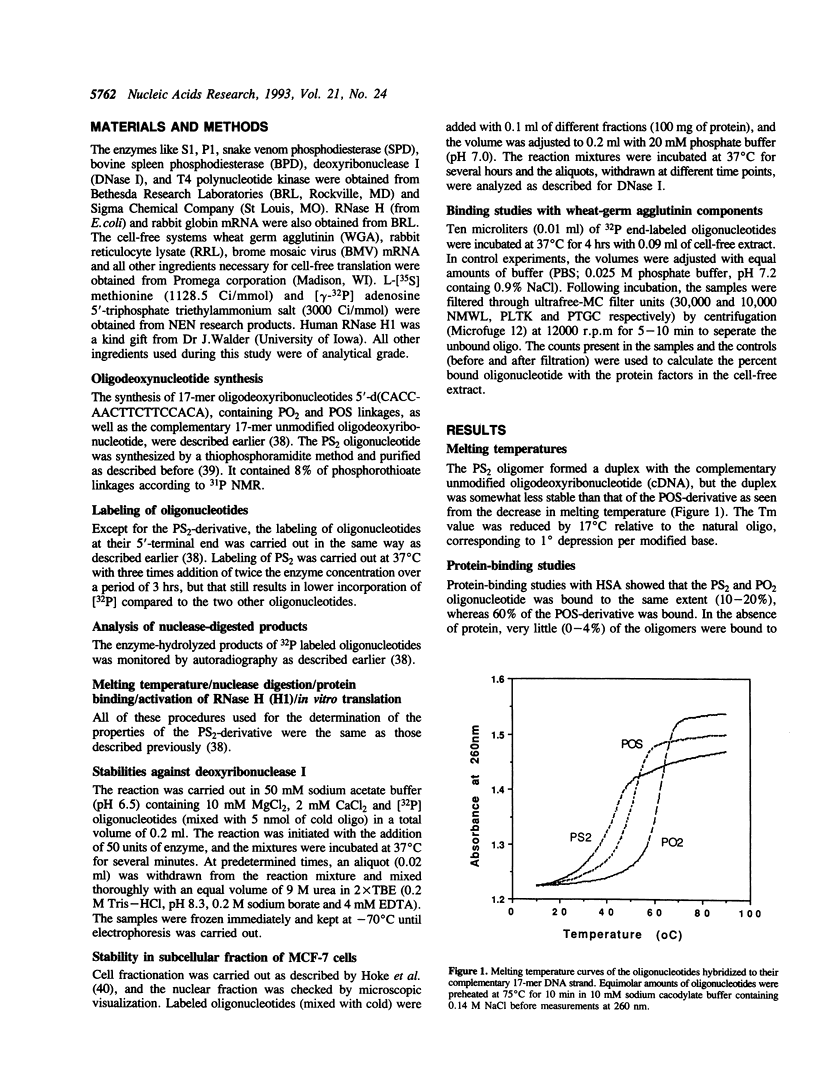
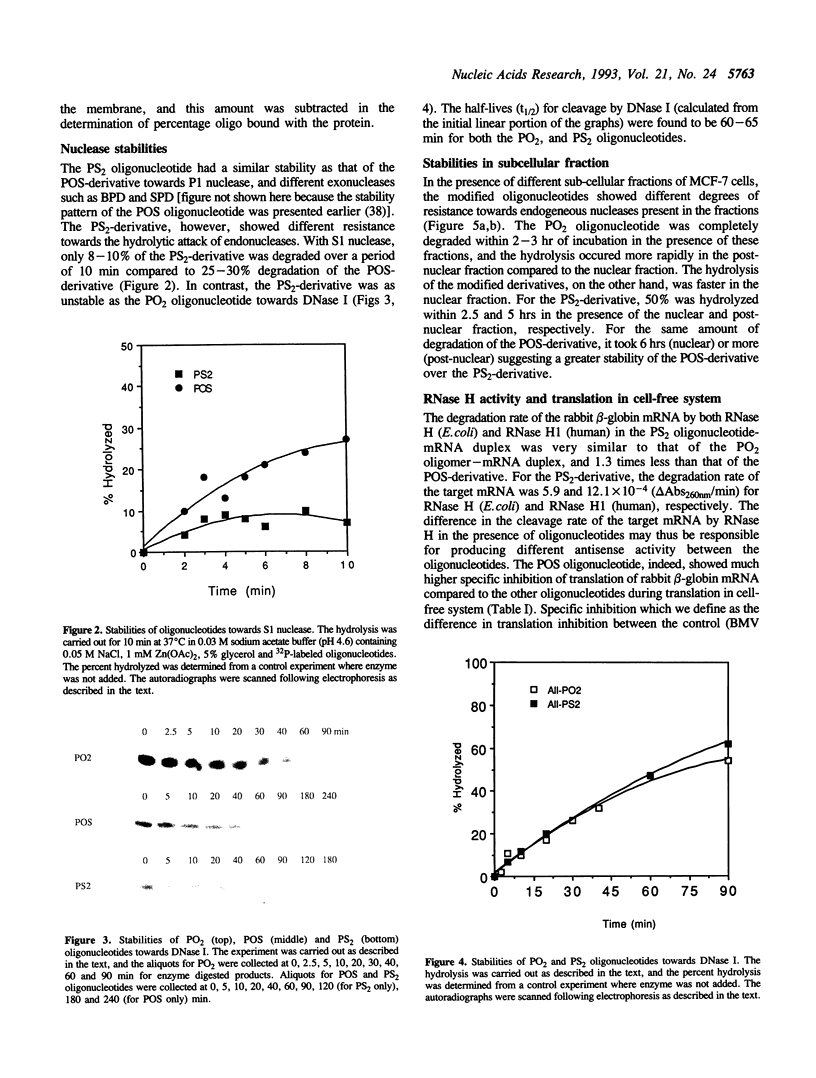
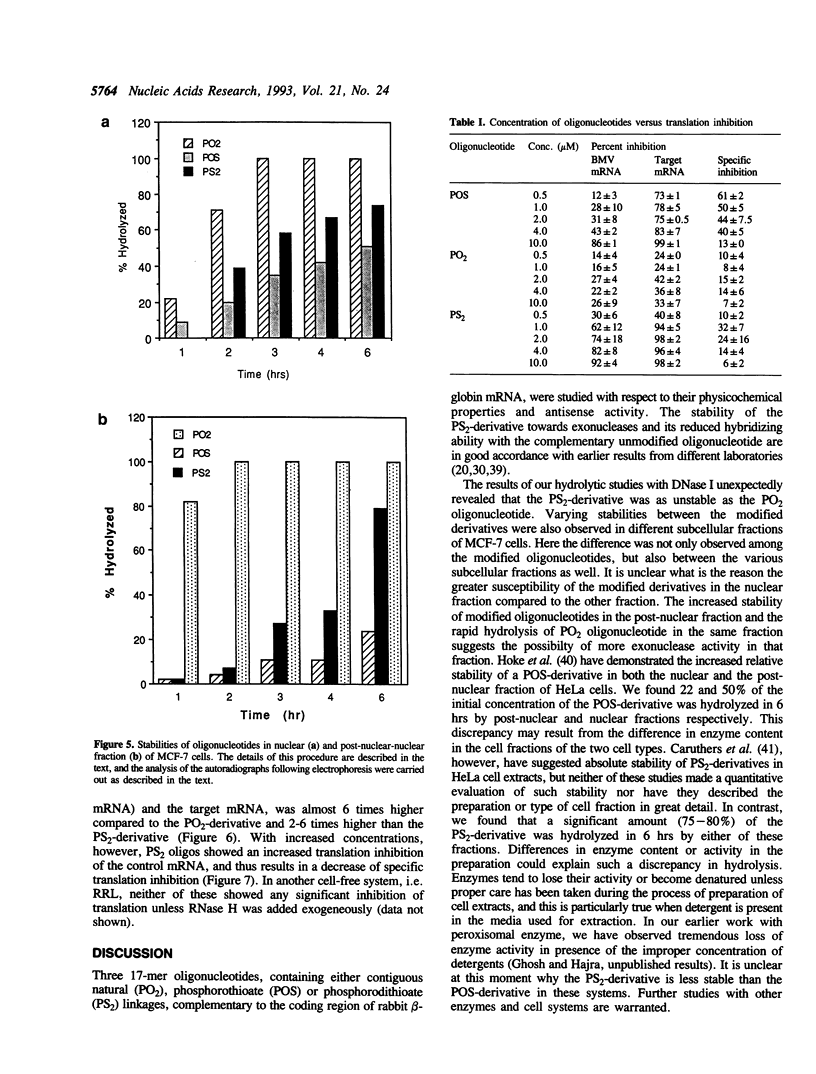
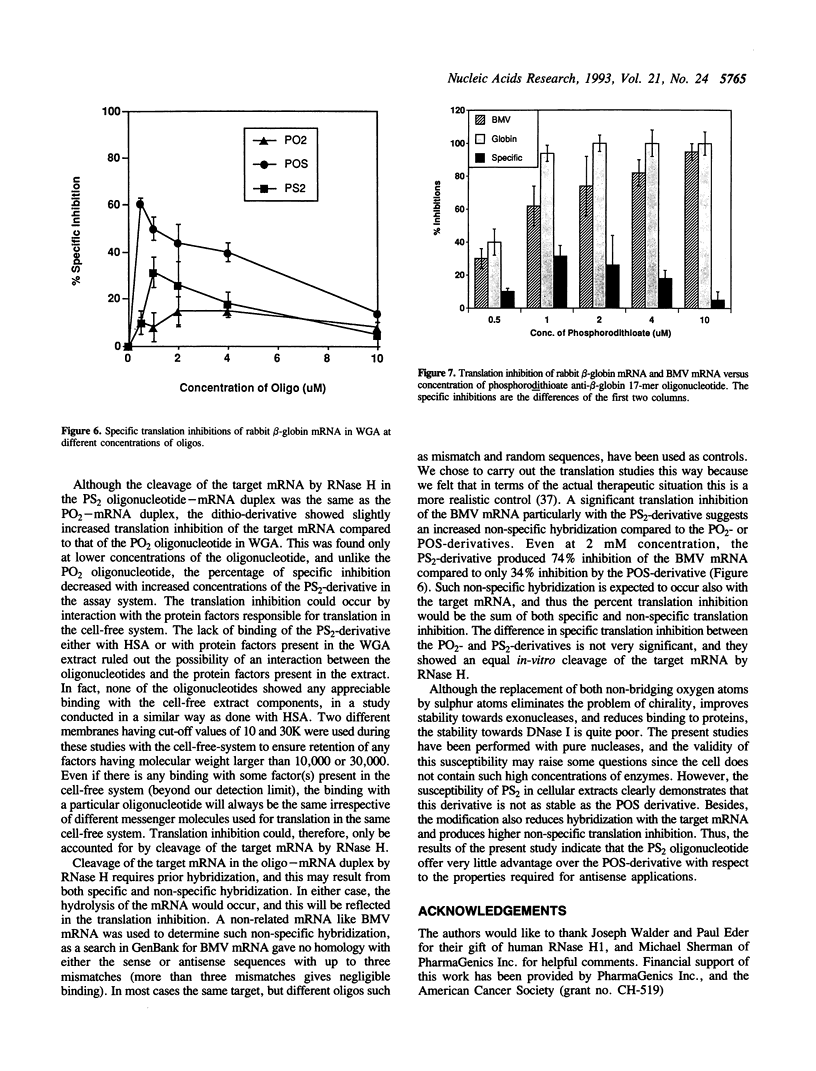
An all phosphorodithioate oligodeoxyribonucleotide (PS2; 17-mer) complementary to the coding region of the rabbit beta-globin mRNA was compared with the normal (PO2) and phosphorothioate (POS) oligonucleotide of the same size and sequence with respect to physicochemical properties and antisense activity in cell-free systems. The melting temperature (Tm) of the PS2-cDNA duplex was reduced by 17 degrees C relative to the PO2-cDNA duplex, compared to 11 degrees C for the POS-cDNA duplex, suggesting a decreased stability of the duplex with an increasing sulfur substitution. Like the POS-derivative, the PS2 oligonucleotide is quite stable against exonucleases, but these modified oligonucleotides showed different stability towards endonucleases and also towards different sub-cellular fractions of MCF-7 cells. During in vitro protein binding studies, the PS2 oligonucleotide showed similar binding (10-20%) to that of the PO2 oligonucleotide, while the POS oligonucleotide bound 60%. In cell-free translation, the PS2 oligonucleotide produced slightly higher specific translation inhibition of rabbit beta-globin mRNA compared to that of the PO2 oligonucleotide, and this was true only at concentration below 2 mM. The POS-derivative, except at 10 mM concentration, always showed higher translation arrest of the rabbit beta-globin mRNA compared to that of the other two oligonucleotides. The present study suggests that the PS2 oligonucleotide offers very little advantage over the POS oligonucleotide for use as an antisense analog.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris C. H., Blake K. R., Miller P. S., Reddy M. P., Ts'o P. O. Inhibition of vesicular stomatitis virus protein synthesis and infection by sequence-specific oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1986 Oct 7;25(20):6268–6275. doi: 10.1021/bi00368a065. [DOI] [PubMed] [Google Scholar]
- Bielinska A., Shivdasani R. A., Zhang L. Q., Nabel G. J. Regulation of gene expression with double-stranded phosphorothioate oligonucleotides. Science. 1990 Nov 16;250(4983):997–1000. doi: 10.1126/science.2237444. [DOI] [PubMed] [Google Scholar]
- Bjergårde K., Dahl O. Solid phase synthesis of oligodeoxyribonucleoside phosphorodithioates from thiophosphoramidites. Nucleic Acids Res. 1991 Nov 11;19(21):5843–5850. doi: 10.1093/nar/19.21.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Thuong N. T., Toulmé J. J., Hélène C. Enzymatic amplification of translation inhibition of rabbit beta-globin mRNA mediated by anti-messenger oligodeoxynucleotides covalently linked to intercalating agents. Nucleic Acids Res. 1987 Jun 25;15(12):4717–4736. doi: 10.1093/nar/15.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Stein C. A., Loreau N., Thuong N. T., Neckers L. M., Subasinghe C., Hélène C., Cohen J. S., Toulmé J. J. Comparative inhibition of rabbit globin mRNA translation by modified antisense oligodeoxynucleotides. Nucleic Acids Res. 1989 Jun 12;17(11):4255–4273. doi: 10.1093/nar/17.11.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Yu Z., Shinozuka K., Zon G., Wilson W. D., Strekowska A. Comparative inhibition of ras p21 protein synthesis with phosphorus-modified antisense oligonucleotides. Anticancer Drug Des. 1989 Oct;4(3):221–232. [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Froehler B., Ng P., Matteucci M. Phosphoramidate analogues of DNA: synthesis and thermal stability of heteroduplexes. Nucleic Acids Res. 1988 Jun 10;16(11):4831–4839. doi: 10.1093/nar/16.11.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M. K., Ghosh K., Cohen J. S. Phosphorothioate-phosphodiester oligonucleotide co-polymers: assessment for antisense application. Anticancer Drug Des. 1993 Feb;8(1):15–32. [PubMed] [Google Scholar]
- Ghosh M. K., Ghosh K., Cohen J. S. Translation inhibition by phosphorothioate oligodeoxynucleotides in cell-free systems. Antisense Res Dev. 1992 Summer;2(2):111–118. doi: 10.1089/ard.1992.2.111. [DOI] [PubMed] [Google Scholar]
- Hoke G. D., Draper K., Freier S. M., Gonzalez C., Driver V. B., Zounes M. C., Ecker D. J. Effects of phosphorothioate capping on antisense oligonucleotide stability, hybridization and antiviral efficacy versus herpes simplex virus infection. Nucleic Acids Res. 1991 Oct 25;19(20):5743–5748. doi: 10.1093/nar/19.20.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Comparative hybrid arrest by tandem antisense oligodeoxyribonucleotides or oligodeoxyribonucleoside methylphosphonates in a cell-free system. Nucleic Acids Res. 1988 Apr 25;16(8):3341–3358. doi: 10.1093/nar/16.8.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus-Sekura C. J., Woerner A. M., Shinozuka K., Zon G., Quinnan G. V., Jr Comparative inhibition of chloramphenicol acetyltransferase gene expression by antisense oligonucleotide analogues having alkyl phosphotriester, methylphosphonate and phosphorothioate linkages. Nucleic Acids Res. 1987 Jul 24;15(14):5749–5763. doi: 10.1093/nar/15.14.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. S., Beaton G., Stein C. A., Matsukura M., Caruthers M. H. Inhibition of human immunodeficiency virus activity by phosphorodithioate oligodeoxycytidine. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6265–6269. doi: 10.1073/pnas.89.14.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. S., Caruthers M. H. Phosphorodithioate DNA as a potential therapeutic drug. Science. 1993 Mar 12;259(5101):1564–1570. doi: 10.1126/science.7681216. [DOI] [PubMed] [Google Scholar]
- Miller P. S., McParland K. B., Jayaraman K., Ts'o P. O. Biochemical and biological effects of nonionic nucleic acid methylphosphonates. Biochemistry. 1981 Mar 31;20(7):1874–1880. doi: 10.1021/bi00510a024. [DOI] [PubMed] [Google Scholar]
- Murata T., Iwai S., Ohtsuka E. Synthesis and characterization of a substrate for T4 endonuclease V containing a phosphorodithioate linkage at the thymine dimer site. Nucleic Acids Res. 1990 Dec 25;18(24):7279–7286. doi: 10.1093/nar/18.24.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin P. S., Agrawal S., Civeira M. P., Goodchild J., Ikeuchi T., Zamecnik P. C. Inhibition of acquired immunodeficiency syndrome virus by oligodeoxynucleoside methylphosphonates. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7448–7451. doi: 10.1073/pnas.85.20.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer S., Eckstein F. Inhibition of deoxyribonucleases by phosphorothioate groups in oligodeoxyribonucleotides. Nucleic Acids Res. 1988 Dec 23;16(24):11691–11704. doi: 10.1093/nar/16.24.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986 Sep;13(2):97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Rossi J. J., Murakawa G. J., Spallone P. A., Stephens D. A., Kaplan B. E., Eritja R., Wallace R. B., Cantin E. M. Inhibition of human immunodeficiency virus by using an oligonucleoside methylphosphonate targeted to the tat-3 gene. J Virol. 1988 Oct;62(10):3914–3917. doi: 10.1128/jvi.62.10.3914-3917.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




