Abstract
Pediatric obesity threatens the future health of a growing number of children worldwide. An added challenge in identifying the patients at greatest need for intervention due to their elevated risk for future disease is that pediatric obesity and the associated metabolic syndrome manifest differently among different ethnic groups. African–Americans and Hispanics are more likely to exhibit obesity and insulin resistance and are at a higher risk for developing Type 2 diabetes. Nevertheless, using current criteria, African–American adolescents are much less likely to be diagnosed with metabolic syndrome, largely owing to lower rates of dyslipidemia. Further development is needed in ethnicity-inclusive means of risk identification among adolescents to accurately target treatment toward children at highest risk for future disease and to motivate adolescent patients and their families towards lifestyle improvement. Effective targeting and intensive treatment efforts may help in avoiding future sequelae of obesity among all ethnicities.
Keywords: adolescent, cardiovascular disease, ethnicity, insulin resistance, metabolic syndrome, race, Type 2 diabetes mellitus
Pediatric obesity continues at a high prevalence in developed countries and constitutes a stormy cloud on the health horizon of children around the world [1]. This is because a subset of obese children are at high risk of developing significant chronic disease as a result of their obesity, including Type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) [2]. Long-term projections based on current trends suggest that childhood obesity will contribute to a threefold increase in the prevalence of coronary artery disease in the USA over the next 25 years [3]. Children born in the USA in the year 2000 are estimated to have a 35% chance of developing T2DM in their lifetime, and this chance approaches, or exceeds, 50% in the case of Hispanic and African–American females [4]. These contribute to a loss in life expectancy such that the current generation may not live as long as their parents [5].
The implications of pediatric obesity are complex because the risk for sequelae of obesity varies among individuals. Some obese adults are at relatively low risk of developing future disease [6,7]. Conversely, several ethnicities demonstrate a higher risk for developing chronic diseases related to obesity [8–10]. Given these variations in risk for sequelae of obesity and given that effective weight-loss efforts are time- and cost-intensive, it seems most prudent to select individuals, including children, for increased intervention based on their risk for future disease, keeping in mind ethnicity-based risks.
In this article, we will review ethnic differences in pediatric obesity, insulin resistance and the metabolic syndrome (MetS), and examine possible predictors that may assist in future disease prediction to better target weight-loss efforts in children of high risk.
Ethnic differences in obesity
While the obesity rate in developed countries is striking – representing 17% of children and adolescents in the USA and 6–10% in Europe and Australia [11–13] – what is perhaps more striking is that this rate is worse among certain ethnic groups. The most widely used estimates of obesity relate to BMI, with a BMI between the 85th and 95th percentile being categorized as ‘overweight’ and a BMI greater than 95% for age being categorized as ‘obese.’ Using these classifications, the latest data from the National Health and Nutrition Evaluation Survey 2007–2008 reveal rates of adolescent girls (12–19 years old) who are either overweight/obese or frankly obese are 46.3/29.2% in non-Hispanic blacks and 42.1/17.5% in Hispanic girls, compared with 29.9/14.5% in non-Hispanic white girls [12,14]. The ethnic differences in BMI elevations are not as striking among adolescent males, although there is a trend toward increased rates among Hispanic adolescent males, with overweight/obesity and frank obesity rates of 42.7/25.5% in Hispanics, compared with 33/19.8% among non-Hispanic blacks and 32.6/16.7% in non-Hispanic white adolescent males.
In many ways, BMI may not be the best measure of obesity, as BMI does not distinguish between elevations in bodyweight by fat tissue versus muscle mass (although BMI correlates increasingly well with body fat at higher BMIs [15]). Because of its strong association with insulin resistance and risk for disease, estimates of central fat tissue are often used as better determinants of risk for future disease. As discussed later regarding the pathophysiology of insulin resistance, central adiposity may produce these effects via an increase in release of inflammatory cytokines and a decrease in release of substances such as adiponectin, which are associated with increased insulin sensitivity [16]. The most commonly used estimate of central adiposity is waist circumference (WC). During adolescence, Mexican–American boys and girls have higher rates of increased WC, and these elevated rates persist through adulthood relative to non-Hispanic whites. Starting in adolescence (12–19 years of age), non-Hispanic black women have higher rates of elevated WC than do non-Hispanic whites, and this difference widens over time [17]. Waist-to-hip ratio, an overall excellent predictor of mortality in adults, is highest in Hispanic adolescent females [18] and higher in black women compared with whites [19]. Interestingly, waist-to-hip ratio does not appear to be as good a predictor of cardiovascular mortality among Hispanics [20].
Non-Hispanic black males have lower rates of elevated WC than do non-Hispanic white and Mexican–American males, both in adolescence and later adulthood [21,22]. In adolescence, Hispanic males have the highest waist-to-hip ratio and non- Hispanics have the lowest [18], and these findings continue among adult men [19,20]. Therefore, increased rates of obesity and elevated WC among African–Americans are limited to adolescent and adult women and are not seen among adolescent and adult men, while among Hispanics, elevations in BMI and WC are seen in both males and females in each age group.
Ethnic differences in sequelae of obesity
As mentioned previously, the majority of interest in worsening prevalence of obesity is primarily due to the increased risk for future sequelae of T2DM and CVD. Thus, this discussion would not be complete without considering ethnic variation in the develop ment of adult disease. As suggested by the aforementioned data on elevations in BMI and WC, it may be expected that there would also be increased rates of future disease among various ethnic subsets. As we will see, the prevalence of both T2DM and CVD exhibit ethnic variation, suggesting that not all of the population carries the same risk. Nevertheless, the data on future disease do not entirely match up with expectations from obesity estimates.
The disease most closely associated with obesity is T2DM, which is marked by insulin resistance leading to a point at which insulin needs exceed its supply and blood sugars are elevated [23]. T2DM is more common among both African–American and Hispanic adolescents and adults (Table 1) [8,9]. Among adolescents, African–Americans have an incidence of T2DM that is fourfold higher than that seen among non-Hispanic white children. The incidence of T2DM is approximately threefold higher among Hispanic children than non-Hispanic whites. In adulthood, this trend continues so that prevalence of T2DM among individuals >20 years of age is 12.8% among non-Hispanic blacks, 8.4% among Mexican–Americans and 6.6% among non-Hispanic whites [8]. These trends hold true for males as well as females, which is at odds with the lower rates of central obesity among African–American men. Death rates from T2DM are more than twofold higher among African–American adults than white adults in each age category and Hispanic adults have rates 20–30% higher than whites [24].
Table 1.
Ethnic differences in incidence of Type 2 diabetes.
| Ethnic group | Incidence rate in each age group (95% CI) | |
|---|---|---|
| 10–14 years old | 15–19 years old | |
| Non-Hispanic white | 3.0 (2.3–4.0) | 5.6 (4.5–6.9) |
| African–American | 22.3 (18.1–27.5) | 19.4 (15.3–24.5) |
| Hispanic | 8.9 (6.4–12.3) | 17.0 (13.3–21.8) |
The table shows incidence rates (and confidence intervals) of diabetes (2002–2003) per 100,000 person-years by age group and race/ethnicity.
Adapted with permission from [9].
It is important to note that even in T2DM, the major cause of obesity-related death is CVD. African–Americans have been found to have significant differences in cardiovascular physiology, including an increase in left ventricular wall thickness compared with whites at similar levels of blood pressure [25]. This may be due to differences in peripheral vascular resistance, as demonstrated by multiple studies using ischemic occlusion, where the brachial artery is occluded by a blood pressure cuff and arterial measurements are made after blood flow is restored. When matched with whites for baseline blood pressure, African–Americans have less postocclusion dilation [26], increased resistance [25,27] and reduced overall blood flow [28]. These findings have been hypothesized to be related to greater insulin release in African–Americans, as will be discussed in greater detail later [28].
Death rates from CVD also exhibit ethnic discrepancies, with African–American men and women having higher rates of death from heart disease and cerebrovascular disease throughout adulthood, including twofold higher death rates than whites and Hispanics at each adult age category up to 65 years old [24]. The reason for this elevated death rate is not clear, although higher rates of hypertension among African–Americans (discussed further later) and less access to medical care have been suggested [10].
Ethnic differences in insulin resistance
There is a strong suggestion that much of the adult sequelae of obesity is driven by the association of obesity with insulin resistance. This is true of the MetS (discussed further later), as well as T2DM [23] and CVD [29,30]. Insulin resistance refers to an increase in the amount of insulin required to stimulate glucose uptake in a given individual. The underlying pathophysiology relating obesity and insulin resistance is at least in part related to dysfunction of excessive amounts of visceral adipose tissue [16,31]. The hypertrophied adipocytes in this setting increase their release of inflammatory mediators (eventually leading to an increase in C-reactive protein [CRP]) [32,33] and decrease their release of adiponectin [34–36], an adipokine that improves insulin sensitivity in laboratory animals [37,38].
Measures of insulin resistance
Insulin resistance is best measured using a standardized research procedure known as a euglycemic–hyperinsulinemic clamp, in which glucose is infused at a fixed rate and insulin is infused at a rate necessary to maintain normal serum glucose levels. Individuals with insulin resistance require more insulin infused to maintain stable levels of glucose. Using this technique, African–American children and adolescents have a higher degree of insulin resistance (by 20%) than non-Hispanic whites of similar weight and pubertal stage (Table 2) [39,40]. Similarly, Hispanic adults have a higher degree of insulin resistance by euglycemic clamp than whites (again by 20%), and this difference remains significant after adjusting for BMI [41].
Table 2.
Ethnic differences in insulin sensitivity and levels of adiponectin in adolescents.
| Parameter | African–Americans | Caucasians |
|---|---|---|
| n (male/female) | 41/42 | 41/37 |
| Age in years (range) | 12.2 (8.5–16.8) | 12.3 (8.0–17.2) |
| BMI (kg/m2, mean ± SEM) | 24.8 ± 0.9 | 24.6 ± 0.96 |
| Insulin sensitivity (mg × kg/ FFM × min, mean ± SEM) |
11.0 ± 0.65* | 13.2 ± 0.91 |
| Adiponectin (μg/ml, mean ± SEM) |
10.2 ± 0.6* | 12.1 ± 0.68 |
p < 0.05 versus Caucasians.
Adiponectin levels and insulin sensitivity are lower in African–American adolescents compared with whites who are well matched in age, gender and BMI. Data were obtained during a euglycermic clamp study in which glucose was infused at a fixed rate in each individual and insulin was infused at a rate necessary to maintain constant blood sugar levels.
FFM: Fat free mass; SEM: Standard error of the mean.
Adapted with permission from [40].
While the euglycemic clamp is the gold standard to assess insulin resistance, other standardized protocols have also been used to assess for ethnic differences in insulin action. Frequently sampled intravenous glucose tolerance tests involve infusing a known amount of glucose and measuring insulin and glucose values at multiple time points to assess both insulin release and glucose clearance. Using this technique, researchers have demonstrated lower insulin sensitivity in Hispanics and African–Americans as compared with non-Hispanic whites [42], although another group of researchers did not find a difference in insulin sensitivity between white and African–American adolescents [28]. Oral glucose tolerance tests, performed similarly to the intravenous glucose tolerance tests, have also been used to demonstrate lower insulin sensitivity in non-Hispanic blacks versus whites [43].
Finally, surrogate measures of insulin resistance are using fasting levels of insulin, including insulin itself and the homeostasis model of insulin resistance, which incorporates fasting glucose levels. Fasting insulin itself is used widely in clinical settings, but without clear implications because of a lack of standardized assay procedure. A commonly used physical examination finding associated with high insulin levels is acanthosis nigricans, a thickening and darkening of the skin. Acanthosis nigricans is noted more commonly in African–American versus white children [44]. Studies analyzing levels of fasting insulin report similar ethnic differences to those reported using the euglycemic clamp, with non-Hispanic blacks and Mexican–Americans exhibiting 10 and 21% higher insulin levels (respectively) than seen in non-Hispanic white adults [45]. Non-Hispanic blacks and Hispanics are more likely to have an elevated homeostasis model of insulin resistance than whites [46]. Together, studies of insulin resistance by ethnicity paint an overall consistent picture of increased insulin resistance among African–American adolescents as compared with whites. While there is not adequate space in this article to address all ethnic groups, it should be noted that Native American groups that have been evaluated also have higher rates of insulin resistance than whites, as well as higher rates of obesity and T2DM [47].
Factors underlying insulin resistance
Other factors known to be associated with insulin resistance and future disease sequelae also exhibit ethnic differences. As eluded to previously, elevated levels of CRP are in part reflective of the inflammation that contributes to insulin resistance. As such, levels of high-sensitivity CRP (hsCRP) have been shown to precede the development of T2DM [48] and CVD [49]. It is not surprising, therefore, that levels of hsCRP are higher among African–American and Mexican–American adolescents and adult women [50,51].
Similarly, low levels of adiponectin appear to contribute to worsening insulin resistance [38,52] and precede worsening of MetS [53]. Levels of adiponectin are 12–16% lower among African–American and Hispanic adolescents compared with non-Hispanic whites, paralleling the higher rates of insulin resistance in these groups (Table 2) [40,54]. Thus, both the degree of insulin resistance and two important associated etiologic factors are worse among African–Americans and Hispanics, corresponding with the higher risk of T2DM in these groups.
Ethnic differences in the MetS
While validated measures of insulin resistance are widely used in research, measuring the degree of insulin resistance in individual patients is more difficult to assess in clinical settings. This is partly because of variability in laboratory testing of insulin levels. Nevertheless, there are a group of clinical findings associated with insulin resistance, CVD and T2DM as part of the MetS [55]. This cluster of individual cardiovascular risk factors include central obesity, hypertension, hypertriglyceridemia, low levels of high-density lipoprotein cholesterol (HDL-C) and elevated fasting glucose. MetS is diagnosed based on specific sets of criteria based on abnormalities in these components. While several sets of pediatric criteria have been proposed for use in children, there is no gold, universally accepted definition [56]. The most commonly used definitions of pediatric MetS are shown in Table 3, one of which is based on the Adult Treatment Panel III definition of MetS for adults [55,57] and the other was proposed by the International Federation of Diabetes (IDF) [58]. The utility of MetS has been demonstrated in that chil- dren with MetS have an odds ratio of 11.5 for developing T2DM within 25–30 years [59]. This makes this group of children a logical target for intensive weight-loss interventions [58].
Table 3.
Pediatric metabolic syndrome criteria.
| Criteria | Obesity | Hypertension | High TG (mg/dl) | HDL (mg/dl) | Elevated fasting glucose (mg/dl) |
Ref. |
|---|---|---|---|---|---|---|
| Adult Treatment Panel III adolescent adaptation |
WC ≥90% | SBP or DBP ≥90% for age, sex, height |
TG ≥110 mg/dl | HDL ≤40 | ≥100 | [57] |
| International Diabetes Federation |
6–10 years of age: WC ≥90% |
≥130/85 mmHg | TG ≥150 mg/dl | HDL <40 | ≥100 | [58] |
| 10–16 years of age: WC ≥90% |
≥130/85 mmHg | TG ≥150 mg/dl | HDL: Males <40 Females <50 |
≥100 |
In the pediatric metabolic syndrome criteria adaptated from the Adult Treatment Panel III definition in adults, individuals are diagnosed with metabolic syndrome (MetS) if they have three or more of the findings listed among the individual components of MetS. In the International Diabetes Federation Criteria individuals are required to have an elevated waist circumference (on ethnicity-specific basis) and two additional findings from the other components of MetS.
DBP: Diastolic blood pressure; HDL: High-density lipoprotein; SBP: Systolic blood pressure; TG: Triglyceride; WC: Waist circumference.
Nevertheless, there are barriers to using MetS to identify children that are in greatest need of weight loss as MetS exhibits a strong ethnicity-based bias. African–Americans are diagnosed with MetS at lower rates than non-Hispanic whites (Table 4), a difference that is apparent into adulthood and persists, even after adjusting for socioeconomic and lifestyle factors [22]. This lower prevalence of MetS in African–Americans occurs despite African–Americans having higher rates of several of the MetS-associated abnormalities discussed previously, including obesity, insulin resistance, systemic inflammation, low levels of adiponectin and future disease sequelae [31,60]. As we will see, the lower rate of MetS diagnosis among African–Americans probably has more to do with individual MetS components than a decrease in MetS-associated risks.
Table 4.
Ethnic differences in the diagnosis of metabolic syndrome.
| MetS criteria | Percentage with metabolic syndrome (%) | |||
|---|---|---|---|---|
|
Non-Hispanic white |
Hispanic |
Non-Hispanic black |
||
| Adult Treatment Panel III adolescent adaptation |
Males | 11.8 | 12.9 | 3.9 |
| Females | 5.8 | 9.4 | 4.2 | |
| International Diabetes Federation |
Males | 8.4 | 9.4 | 2.5 |
| Females | 4.4 | 6.4 | 4.2 | |
Prevalence of MetS by race/ethnicity using a pediatric adaptation of the Adult Treatment Panel III definition of MetS and the pediatric International Diabetes Federation definition of MetS and a pediatric adaptation (both 12–19-year-old adolescents from National Health and Nutrition Evaluation Survey 1999–2006).
MetS: Metabolic syndrome.
Waist circumference
As discussed previously, there are interethnicity differences in WC. The fact that African–American boys and men are less likely to develop elevated WC may be one source of decreased MetS diagnosis among African–American males. This difference is particularly true in diagnoses using the IDF criteria of MetS, which uses an elevated WC as a pre requisite for MetS diagnosis (Table 3). The IDF specifies the use of ethnicity-specific WC cut-off values [58], although in practice, WC cut-offs have been used based on percentiles for white adolescents in the absence of data, suggesting a need for different thresholds among blacks and Hispanics [61,62]. In Asians, who develop MetS at lower WC elevations than other groups, the IDF specifies lower cut-off values to determine elevated WC [61,63].
Elevated fasting glucose
Most pediatric and adult MetS criteria use a fasting glucose level of greater than or equal to 100 mg/dl as their cut-off to define an elevated fasting glucose, compared with a fasting glucose level above 125 mg/dl, which meets criteria for diabetes diagnosis [64]. Ethnic differences have been observed regarding elevations in fasting glucose. Although African–Americans are more likely to develop T2DM over time, African–American males are less likely to exhibit elevated fasting glucose throughout age categories from adolescence to later adulthood. In adolescence, the rate of elevated fasting glucose among non-Hispanic black males is 9.4%, compared with 14.8% for non-Hispanic whites and 15.2% for Hispanics [22].
The paradox between higher rates of diabetes and lower rates of elevated fasting glucose may reflect differences in hepatic insulin resistance, which is felt to be largely responsible for mild elevations in fasting glucose, while diabetes is due to a combination of hepatic insulin resistance, peripheral insulin resistance and β-cell failure [23,60]. The lower rate of elevated fasting glucose among African–Americans may indicate lower rates of hepatic insulin resistance, although this requires further investigation. During adolescence, the lower rates of elevated fasting glucose among African–Americans probably contributes to the lower rates of MetS diagnosis, although as we will see, ethnic differences are more marked among other MetS components.
Hypertension
Hypertension has been long known to be more prevalent among African–Americans. This increased prevalence is thought to be due to both genetic and environmental factors [65], although during adulthood the increased prevalence continues even after adjustment for lifestyle factors, suggesting that genetic factors may predominate [22]. Children and adults of Hispanic heritage are less likely to have hypertension, a tendency that may suggest underlying genetic causes, as it also persists even after adjustment for lifestyle factors [22]. However, the clinical significance of these ethnic differences and their relationship with MetS is unclear, given that blood pressure has been frequently shown to have the weakest association with insulin resistance among MetS components [65,66].
Low HDL-C
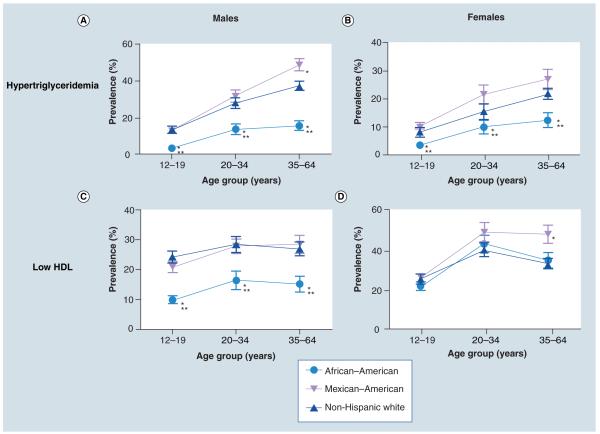
High-density lipoprotein cholesterol serves as a scavenger of excess cholesterol in circulation and in the periphery, and thus low levels of HDL-C confer an increased risk for atherosclerosis. The removal of HDL-C from the circulation is mediated in large part by hepatic lipase, an enzyme that exhibits increased activity with an increasing degree of insulin resistance [67]. There are ethnic differences in HDL-C levels in that non-Hispanic black males have rates of low HLD-C that are nearly half the rates seen in non-Hispanic whites and Hispanics (Figure 1) [22]. While adolescent and adult women have a higher prevalence of low HDL-C than men, they do not exhibit the same ethnic differences, demonstrating that HDL-C levels are modulated by gender as well as ethnicity. The normal levels of HDL-C in non-Hispanic black males, despite higher degrees of insulin resistance, are potentially due to lower activity of hepatic lipase among African–Americans, even in the presence of insulin resistance [68,69]. These lower rates of HDL-C among African–Americans do not appear to confer significant cardio protection but probably contribute to the lower rates of MetS diagnosis among African–American adolescent and adult males.
Figure 1. Ethnic differences in elevated triglycerides and low high-density lipoprotein.
Prevalence of elevated triglyceride level and low high-density lipoprotein (per International Federation of Diabetes metabolic syndrome criteria) are shown for non-Hispanic whites, Mexican–Americans and non-Hispanic blacks across the age range from childhood through later adulthood.
*p < 0.05 versus non-Hispanic whites.
**p < 0.01 versus non-Hispanic whites.
HDL: High-density lipoprotein.
Adapted with permission from [22].
Triglycerides
The most marked ethnic difference regarding MetS components is observed in triglyceride levels. African–Americans have sharply lower rates of hypertriglyceridemia than seen in non-Hispanic whites or Hispanics. Using the triglyceride cut-off value of 110 mg/dl from the MetS criteria of Ford et al. (Table 3) [57], non-Hispanic black adolescents have a prevalence of hypertriglyceride- mia of 9.1% compared with 28.9% for non-Hispanic whites and 26.1% for Mexican–American adolescents [70]. These differences are even more striking when using the cut-off value of 150 mg/dl from the IDF criteria in which only 3.5% of non-Hispanic black adolescents exhibit elevations, compared with 8.1–13.5% of non-Hispanic whites and 10.4–12.8% of Mexican–American adolescents (Figure 1) [22]. These drastic differences are seen in both males and females and exist across the age spectrum [21,22]. The reason for these differences may be similar to that seen for HDL-C and its clearance by hepatic lipase. Triglycerides are cleared from the circulation by lipoprotein lipase, an enzyme whose activity is decreased in the presence of insulin resistance, leading to higher levels of triglycerides. Compared with non-Hispanic whites, African– Americans have higher activity of lipoprotein lipase, presumably contributing to the lower rates of hypertriglyceridemia [71–73]. Nevertheless, increases in the degree of insulin resistance are associated with increasing levels of triglycerides in African–Americans as seen in other ethnicities [74]. Thus, it appears that non-Hispanic black individuals start with lower baseline levels of triglyceride and are less likely to exceed cut-off values specified in different MetS criteria. These discrepancies in triglyceride levels are the major cause of ethnic differences in the diagnosis of MetS.
Summary of ethnic differences in MetS components
In summary, there are multiple differences in individual components of MetS that contribute to the discrepancies in MetS diagnosis between ethnicities. The major cause of lower rates of MetS diagnosis among African–Americans is the lower tendency for dyslipidemia, although African–American males also have lower rates of central obesity and elevated fasting glucose [21,22]. In this sense, our attempts at using the construct of MetS to identify increased future risk appear to fail among African–Americans, given the lower rates of MetS diagnosis but higher rates of insulin resistance and MetS-associated disease.
These ethnic differences in MetS do not appear to hold true for Hispanic individuals. While Hispanics have a higher rate of elevated WC than other groups (and a lower rate of hypertension), for the most part, Hispanics have similar rates of these abnormalities as seen in non-Hispanic whites. While this has not been demonstrated in prospective trials, it suggests that MetS may perform well in identifying Hispanic adolescents at higher risk for development of future disease. Nevertheless, the lower rate of MetS diagnosis among African–Americans raises clear problems in using MetS to identify future risk among all ethnicities.
Ethnicity & targeting children for weight loss
Despite a growing emphasis on weight-loss treatments, it should be noted that most efforts at weight loss are not successful. A randomized study of pediatric weight-loss efforts that included non-Hispanic white, non-Hispanic black and Hispanic children 8–16 years of age revealed that the standard clinical care in weight-loss promotion resulted in an increase in BMI over the course of the 12-month study period [75]. This control group received counseling with a physician, nutritionist and social worker at study visits, with extensive recommendations regarding dietary, exercise and other lifestyle changes. If anything negative could be said about these efforts, it is that the visits occurred only every 6 months. Most physicians with experience in counseling children regarding weight-loss efforts would probably agree that successful improvement is difficult at best [76].
Interestingly, as opposed to the 1.5 kg/m2 increase in BMI in the control group, the intensive treatment group in this study experienced a 1.8 kg/m2 decrease in BMI over 12 months, co-incident with decreases in body fat and insulin resistance. This treatment group received twice-weekly counseling for the first 6 months, followed by biweekly counseling for the next 6 months. The children met with exercise physiologists, participating in organized game activities on a regular basis. Clearly, these investigators and their subjects had access to many more resources than do most treating clinicians. Still, the improvement in outcomes through this program argues that with increased efforts, successful weight loss can be achieved. Because these efforts are likely to be expensive, it may become increasingly important to choose patients judiciously for increased intervention.
Ethnic differences in response to weight-loss efforts
There appear to be ethnic differences in the response to clinical weight-loss efforts. While studies targeting both Mexican–American [77] and African–American children [78] have demonstrated effective weight loss in treatment arms compared with control arms, other studies have demonstrated that compared with non-Hispanic whites, Hispanic and African–American individuals are more likely to drop out of weight treatment efforts [79–82] or to lose less weight during the intervention [83,84]. Some of these differences may be due to ethnic differences in physical activity. The National Physical Activity and Weight Loss Survey revealed that non-Hispanic blacks and Hispanics were less likely to engage in physical activities during leisure time than non-Hispanic whites [85].
A major difference between ethnicities lies in the perception of overweight status. African–American and Hispanic adults who are overweight are less likely than non-Hispanic whites to perceive themselves as overweight [86,87], as is true of African–American adolescents [88]. Among US adolescents who are overweight (as assessed by BMI percentile), only 55% of African–American adolescents correctly identified their weight status compared with 70% of Hispanic and 72% of white overweight adolescents [88]. These relatively low rates of accurate identification of overweight status are paralleled by parents of overweight children, of whom only 61% correctly perceive their children’s weight status [89]. However, such parental misperceptions do not appear to vary by ethnicity [90].
These low rates of perceived obesity are important given that perception of being overweight has been frequently shown as an important motivator to achieve weight reduction among par- ents and adolescents [91,92]. Altering these perceptions, as well as other means of communicating risk, may increase adolescents’ motivation to lose weight.
Need for risk-stratifying tools among all ethnicities
An important direction in pediatric weight loss will be the formulation and validation of new tools to assess future disease risk and motivate children and adolescents toward lifestyle change [93,94]. As stated by Goetz, such tools may be able to “get [people] off the couch in a way that their doctor tut-tutting about their pounds cannot” [95]. Owing to ethnic differences in clinical features of obesity and MetS, these new tools will probably have to be demonstrated to be accurate among all ethnicities before they are accepted for widespread use.
MetS diagnosis
As discussed previously, ethnic variation in the components of MetS result in what is probably an underdiagnosis among African–Americans. Still, a diagnosis of MetS serves as an example of the potential for predictive tools, in that a longitudinal study demonstrated that individuals diagnosed with MetS during childhood (compared with those without MetS) had an odds ratio of 11.5 for developing diabetes by 25 years later [59]. Such an impressive increase in risk could be used to motivate children, parents and medical professionals to encourage weight loss in at-risk children. Potential variations on MetS diagnosis include use of a continuous set of criteria [96,97] or a risk score that is specific for each ethnic- ity [31]. My research collaborator, Matthew Gurka, and I are in the process of developing the latter.
Fasting insulin
Although progression to T2DM also requires a decrease in function at the level of the β-cell, measures of the degree of insulin resistance represent a potential means of accurate risk prediction. From a clinical perspective, fasting insulin levels are the most commonly means of estimating risk, although assay variability and a lack of prospective data make it currently impossible to assign a cut-off value or relative risk to elevated fasting insulin levels.
Glucose measurements
Fasting glucose levels have been shown to have predictive power in assessing risk for diabetes, as children with a fasting insulin level of >100 mg/dl have a relative risk of 4.7 for developing T2DM by 39 years of age [98]. Given the lower likelihood of African–Americans to have elevated fasting glucose despite their increased for T2DM, however, it is uncertain how sensitive this test would prove among African–Americans. Other glucose-based markers include oral glucose-tolerance tests (OGTTs), which assess the ability to clear a glucose load in a timely manner. In this test, glucola is administered at 1.5 g/kg up to a maximum of 75 g, and a 2-h blood sugar of >140 mg/dl represents impaired glucose tolerance [64]. In an obesity referral clinic, eight out of 33 children with impaired glucose tolerance progressed to T2DM within an average of 20 months, compared with none of 84 children with normal glucose tolerance, suggesting that OGTTs could prove to be a good estimate of future risk [99]. Nevertheless, at this point, OGTTs are not currently recommended as screening tests by the American Diabetes Association due to their variable results and the increased difficulty in performing them [100].
HbA1c
Recently, the American Diabetes Association has placed increased emphasis on the use of hemoglobin A1c (HbA1c) as a tool to screen for diabetes risk [101]. In adults, mild elevations in HbA1c (5.5–5.9) are clear indicators of increased risk for the development of future diabetes [102]. However, it is not known how well mild elevations in HbA1c will function in pediatrics, nor is it known whether this will function equally well among all ethnicities.
Adiponectin
As discussed previously, adiponectin appears to be in the causative pathway for the production of insulin resistance. In one study, low levels of adiponectin predicted worsening of MetS within 6 years, although it should be noted that this was performed in an all-white cohort [53]. Adiponectin levels are lower in African–Americans, congruent with their increase in insulin resistance [40], suggesting its potential to predict risk in an ethnicity-inclusive manner.
Markers of inflammation
Elevated levels of hsCRP have been demonstrated in numerous studies in adults to predict future development of CVD and diabetes [48,103]. It is not certain whether this predictive ability will hold true in children. Levels of hsCRP appear to be less tightly associated with MetS in children than in adults [56], potentially because of fluctuation with minor illnesses [104]. It remains to be seen whether other markers of inflammation could prove to be specific markers of increased risk.
Prevention
While the identification of methods of risk prediction among currently obese children is important, a far more likely aim in the future will be the prevention of obesity among all ethnic groups. Although weight-loss interventions are likely to decrease future risks, once children are obese, there is a high likelihood that they will remain obese as adults. An important avenue for improved clinical care will be in targeting all children (and especially those with a family history of obesity and T2DM) for ongoing anticipatory guidance counseling on healthy lifestyle choices to avoid becoming overweight. There is a high likelihood that preventing a worsening in the degree of overweight is likely to also decrease the incidence of these long-term sequelae of obesity. This is supported by the results of the Diabetes Prevention Trial in adults. Adults randomized to lose weight via dietary changes and intensive exercise decreased their risk of progressing to T2DM by 58% below the control group and 31% below a group randomized to receive metformin therapy [105].
Conclusion
In conclusion, pediatric obesity has greatly affected all ethnicities and socioeconomic strata, but is most prevalent among lower socioeconomic status groups, Hispanics and African–Americans. Because effective treatment is likely to require significant resources, it will be important to stratify risk to target children with the greatest need for weight loss. Our current means of risk identification probably underestimate risk among African–American adolescents. New tools for accurate risk prediction are needed both to identify children at highest risk for sequelae of obesity and to motivate patients and their families. Finally, prevention efforts targeting all ethnicities may be the most effective means of avoiding future disease in the coming generations.
Acknowledgments
Work on this manuscript was funded by a career development award from the NIH (5K08HD060739–02).
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Crocker MK, Yanovski JA. Pediatric obesity: etiology and treatment. Endocrinol. Metab. Clin. North Am. 2009;38:525–548. doi: 10.1016/j.ecl.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K, Coxson P, Pletcher MJ, et al. Adolescent overweight and future adult coronary heart disease. N. Engl. J. Med. 2007;357:2371–2379. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 4.Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 5.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st Century. N. Engl. J. Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 6.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. • Demonstrates that a large minority of obese adults do not demonstrate multiple other risk factors for cardiovascular disease. They used BMI to categorize obesity and may have found a lower prevalence of ‘healthy obesity’ had they used elevated waist circumference or waist-to-hip ratio.
- 7.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch. Intern. Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 8.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33:562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dabelea D, Bell RA, D’Agostino RB, Jr, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 10.Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 11.Commonwealth Scientific Industrial Research Organisation . Australian National Children’s Nutrition and Physical Activity Survey – Main Findings. Department of Health and Ageing; Canberra, Australia: 2008. [Google Scholar]
- 12.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int. J. Pediatr. Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 14.Lieb DC, Snow RE, DeBoer MD. Socioeconomic factors in the development of childhood obesity and diabetes. Clin. Sports Med. 2009;28:349–378. doi: 10.1016/j.csm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124(Suppl. 1):S23–S34. doi: 10.1542/peds.2008-3586E. [DOI] [PubMed] [Google Scholar]
- 16.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. •• Reports on the pathophysiology behind insulin resistance due to visceral adiposity.
- 17.Kimm SY, Barton BA, Obarzanek E, et al. Racial divergence in adiposity during adolescence: The NHLBI Growth and Health Study. Pediatrics. 2001;107:E34. doi: 10.1542/peds.107.3.e34. [DOI] [PubMed] [Google Scholar]
- 18.Gillum RF. Distribution of waist-to-hip ratio, other indices of body fat distribution and obesity and associations with HDL cholesterol in children and young adults aged 4–19 years: The Third National Health and Nutrition Examination Survey. Int. J. Obes. Relat. Metab. Disord. 1999;23:556–563. doi: 10.1038/sj.ijo.0800866. [DOI] [PubMed] [Google Scholar]
- 19.Reis JP, Araneta MR, Wingard DL, et al. Overall obesity and abdominal adiposity as predictors of mortality in U.S. White and black adults. Ann. Epidemiol. 2009;19:134–142. doi: 10.1016/j.annepidem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Herrera VM, Casas JP, Miranda JJ, et al. Interethnic differences in the accuracy of anthropometric indicators of obesity in screening for high risk of coronary heart disease. Int. J. Obes. (Lond.) 2009;33:568–576. doi: 10.1038/ijo.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker SE, Gurka MJ, Oliver MN, et al. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr. Metab. Cardiovasc. Dis. 2010 doi: 10.1016/j.numecd.2010.05.006. DOI: 10.1016/j.numecd.2010.05.006. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of Type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. •• Reports in incredible detail the basic and clinical research describing the progression to Type 2 diabetes mellitus.
- 24.Heron M. National Vital Statistics Reports. 14. Vol. 58. National Center for Health Statistics; MD, USA: 2010. [Google Scholar]
- 25.Hinderliter AL, Blumenthal JA, Waugh R, et al. Ethnic differences in left ventricular structure: relations to hemodynamics and diurnal blood pressure variation. Am. J. Hypertens. 2004;17:43–49. doi: 10.1016/j.amjhyper.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Perregaux D, Chaudhuri A, Rao S, et al. Brachial vascular reactivity in blacks. Hypertension. 2000;36:866–871. doi: 10.1161/01.hyp.36.5.866. [DOI] [PubMed] [Google Scholar]
- 27.Hinderliter AL, Sager AR, Sherwood A, et al. Ethnic differences in forearm vasodilator capacity. Am. J. Cardiol. 1996;78:208–211. doi: 10.1016/s0002-9149(96)90397-5. [DOI] [PubMed] [Google Scholar]
- 28.Duck MM, Hoffman RP. Impaired endothelial function in healthy African–American adolescents compared with Caucasians. J. Pediatr. 2007;150:400–406. doi: 10.1016/j.jpeds.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard G, O’Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93:1809–1817. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 30.Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005;112:32–38. doi: 10.1161/CIRCULATIONAHA.104.520130. [DOI] [PubMed] [Google Scholar]
- 31.DeBoer MD. Underdiagnosis of the metabolic syndrome in non-Hispanic black adolescents: a call for ethnic-specific criteria. Curr. Cardiovasc. Risk Rep. 2010;4:302–310. doi: 10.1007/s12170-010-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin LJ, Woo JG, Daniels SR, et al. The relationships of adiponectin with insulin and lipids are strengthened with increasing adiposity. J. Clin. Endocrinol. Metab. 2005;90:4255–4259. doi: 10.1210/jc.2005-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefan N, Bunt JC, Salbe AD, et al. Plasma adiponectin concentrations in children: relationships with obesity and insulinemia. J. Clin. Endocrinol. Metab. 2002;87:4652–4656. doi: 10.1210/jc.2002-020694. [DOI] [PubMed] [Google Scholar]
- 36.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and Type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 37.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 38.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 39.Arslanian S, Suprasongsin C. Differences in the in vivo insulin secretion and sensitivity of healthy black versus white adolescents. J. Pediatr. 1996;129:440–443. doi: 10.1016/s0022-3476(96)70078-1. [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Bacha F, Gungor N, Arslanian SA. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes Care. 2006;29:51–56. doi: 10.2337/diacare.29.1.51. •• Reports the results of insulin clamp studies on cohorts of white and African–American subjects, offering new insights into differences in insulin sensitivity.
- 41.Ferrannini E, Gastaldelli A, Matsuda M, et al. Influence of ethnicity and familial diabetes on glucose tolerance and insulin action: a physiological analysis. J. Clin. Endocrinol. Metab. 2003;88:3251–3257. doi: 10.1210/jc.2002-021864. [DOI] [PubMed] [Google Scholar]
- 42.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African–American and Hispanic children. Diabetes Care. 2002;25:2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 43.Preeyasombat C, Bacchetti P, Lazar AA, et al. Racial and etiopathologic dichotomies in insulin hypersecretion and resistance in obese children. J. Pediatr. 2005;146:474–481. doi: 10.1016/j.jpeds.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen TT, Keil MF, Russell DL, et al. Relation of acanthosis nigricans to hyperinsulinemia and insulin sensitivity in overweight African American and white children. J. Pediatr. 2001;138:474–480. doi: 10.1067/mpd.2001.112657. [DOI] [PubMed] [Google Scholar]
- 45.Meng YX, Ford ES, Li C, et al. Association of C-reactive protein with surrogate measures of insulin resistance among nondiabetic US from National Health and Nutrition Examination Survey 1999–2002. Clin. Chem. 2007;53:2152–2159. doi: 10.1373/clinchem.2007.088930. [DOI] [PubMed] [Google Scholar]
- 46.Rodden AM, Diaz VA, Mainous AG, et al. Insulin resistance in adolescents. J. Pediatr. 2007;151:275–279. doi: 10.1016/j.jpeds.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Stefan N, Stumvoll M, Weyer C, et al. Exaggerated insulin secretion in Pima Indians and African–Americans but higher insulin resistance in Pima Indians compared to African–Americans and Caucasians. Diabet. Med. 2004;21:1090–1095. doi: 10.1111/j.1464-5491.2004.01290.x. [DOI] [PubMed] [Google Scholar]
- 48.Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing Type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 49.Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 50.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the metabolic syndrome is associated with disproportionately high levels of high-sensitivity C-reactive protein in non-Hispanic black adolescents: an analysis of NHANES 1999–2006. Diabetes Care. 2011;34 doi: 10.2337/dc10-1877. DOI: 10.2337/dc2310–1877. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wee CC, Mukamal KJ, Huang A, et al. Obesity and C-reactive protein levels among white, black, and hispanic US adults. Obesity (Silver Spring) 2008;16:875–880. doi: 10.1038/oby.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazzer S, Vermorel M, Montaurier C, et al. Changes in adipocyte hormones and lipid oxidation associated with weight loss and regain in severely obese adolescents. Int. J. Obes. (Lond.) 2005;29:1184–1191. doi: 10.1038/sj.ijo.0802977. [DOI] [PubMed] [Google Scholar]
- 53.Kynde I, Heitmann BL, Bygbjerg IC, et al. Hypoadiponectinemia in overweight children contributes to a negative metabolic risk profile 6 years later. Metabolism. 2009;58:1817–1824. doi: 10.1016/j.metabol.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 54.Winer JC, Zern TL, Taksali SE, et al. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2006;91:4415–4423. doi: 10.1210/jc.2006-0733. [DOI] [PubMed] [Google Scholar]
- 55.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 56.DeBoer MD, Gurka MJ. Ability among adolescents for the metabolic syndrome to predict elevations in factors associated with Type 2 diabetes and cardiovascular disease: data from the National Health and Nutrition Examination Survey 1999–2006. Metab. Syndr. Relat. Disord. 2010;8:343–353. doi: 10.1089/met.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ford ES, Li C, Cook S, et al. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 58.Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 59.Morrison JA, Friedman LA, Wang P, et al. Metabolic syndrome in childhood predicts adult metabolic syndrome and Type 2 diabetes mellitus 25 to 30 years later. J. Pediatr. 2008;152:201–206. doi: 10.1016/j.jpeds.2007.09.010. • These investigators used data from a prospectively followed cohort to demonstrate that children with metabolic syndrome were more likely to develop Type 2 diabetes mellitus as young adults.
- 60.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J. Pediatr. 2009;155:e7–e11. doi: 10.1016/j.jpeds.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet. Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 62.Ford ES, Li C, Zhao G, et al. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care. 2008;31:587–589. doi: 10.2337/dc07-1030. [DOI] [PubMed] [Google Scholar]
- 63.Zimmet P, Alberti KG, Kaufman F, et al. The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatr. Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 64.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 65.Gaillard T. Insulin resistance and cardiovascular disease risk in black people of the African diaspora. Curr. Cardio. Risk Rep. 2010;4:186–194. [Google Scholar]
- 66.Martinez-Vizcaino V, Martinez MS, Aguilar FS, et al. Validity of a single-factor model underlying the metabolic syndrome in children: a confirmatory factor analysis. Diabetes Care. 2010;33:1370–1372. doi: 10.2337/dc09-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blades B, Vega GL, Grundy SM. Activities of lipoprotein lipase and hepatic triglyceride lipase in postheparin plasma of patients with low concentrations of HDL cholesterol. Arterioscler. Thromb. 1993;13:1227–1235. doi: 10.1161/01.atv.13.8.1227. [DOI] [PubMed] [Google Scholar]
- 68.Clarenbach JJ, Vega GL, Adams-Huet B, et al. Variability in postheparin hepatic lipase activity is associated with plasma adiponectin levels in African Americans. J. Investig. Med. 2007;55:187–194. doi: 10.2310/6650.2007.07001. [DOI] [PubMed] [Google Scholar]
- 69.Vega GL, Clark LT, Tang A, et al. Hepatic lipase activity is lower in African American men than in white American men: effects of 5′ flanking polymorphism in the hepatic lipase gene (LIPC) J. Lipid Res. 1998;39:228–232. [PubMed] [Google Scholar]
- 70.Johnson WD, Kroon JJ, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents, National Health and Nutrition Examination Survey (NHANES), 2001–2006. Arch. Pediatr. Adolesc. Med. 2009;163:371–377. doi: 10.1001/archpediatrics.2009.3. [DOI] [PubMed] [Google Scholar]
- 71.Bower JF, Deshaies Y, Pfeifer M, et al. Ethnic differences in postprandial triglyceride response to a fatty meal and lipoprotein lipase in lean and obese African American and Caucasian women. Metabolism. 2002;51:211–217. doi: 10.1053/meta.2002.29991. [DOI] [PubMed] [Google Scholar]
- 72.Despres JP, Couillard C, Gagnon J, et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler. Thromb. Vasc. Biol. 2000;20:1932–1938. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 73.Friday KE, Srinivasan SR, Elkasabany A, et al. Black–white differences in postprandial triglyceride response and postheparin lipoprotein lipase and hepatic triglyceride lipase among young men. Metabolism. 1999;48:749–754. doi: 10.1016/s0026-0495(99)90175-0. [DOI] [PubMed] [Google Scholar]
- 74.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. • Demonstrated that despite starting with lower baseline levels of triglycerides, non-Hispanic black adults exhibited a similar increase in triglycerides with increasing insulin resistance, as was observed for non-Hispanic whites and Mexican–Americans.
- 75.Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697–2704. doi: 10.1001/jama.297.24.2697. • Demonstrated that a high degree of intervention could produce significant weight loss in obese adolescents.
- 76.Wickham EP, Stern M, Evans RK, et al. Prevalence of the metabolic syndrome among obese adolescents enrolled in a multidisciplinary weight management program: clinical correlates and response to treatment. Metab. Syndr. Relat. Disord. 2009;7:179–186. doi: 10.1089/met.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnston CA, Tyler C, McFarlin BK, et al. Weight loss in overweight Mexican American children: a randomized, controlled trial. Pediatrics. 2007;120:e1450–e1457. doi: 10.1542/peds.2006-3321. [DOI] [PubMed] [Google Scholar]
- 78.Naar-King S, Ellis D, Kolmodin K, et al. A randomized pilot study of multisystemic therapy targeting obesity in African–American adolescents. J. Adolesc. Health. 2009;45:417–419. doi: 10.1016/j.jadohealth.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 79.Jelalian E, Hart CN, Mehlenbeck RS, et al. Predictors of attrition and weight loss in an adolescent weight control program. Obesity (Silver Spring) 2008;16:1318–1323. doi: 10.1038/oby.2008.51. [DOI] [PubMed] [Google Scholar]
- 80.Tershakovec AM, Kuppler K. Ethnicity, insurance type, and follow-up in a pediatric weight management program. Obes. Res. 2003;11:17–20. doi: 10.1038/oby.2003.4. [DOI] [PubMed] [Google Scholar]
- 81.Wing RR, Anglin K. Effectiveness of a behavioral weight control program for blacks and whites with NIDDM. Diabetes Care. 1996;19:409–413. doi: 10.2337/diacare.19.5.409. [DOI] [PubMed] [Google Scholar]
- 82.Zeller M, Kirk S, Claytor R, et al. Predictors of attrition from a pediatric weight management program. J. Pediatr. 2004;144:466–470. doi: 10.1016/j.jpeds.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 83.Kumanyika SK, Obarzanek E, Stevens VJ, et al. Weight-loss experience of black and white participants in NHLBI-sponsored clinical trials. Am. J. Clin. Nutr. 1991;53:1631S–1638S. doi: 10.1093/ajcn/53.6.1631S. [DOI] [PubMed] [Google Scholar]
- 84.West DS, Prewitt T Elaine, Bursac Z, et al. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16:1413–1420. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 85.Marshall SJ, Jones DA, Ainsworth BE, et al. Race/ethnicity, social class, and leisure- time physical inactivity. Med. Sci. Sports Exerc. 2007;39:44–51. doi: 10.1249/01.mss.0000239401.16381.37. [DOI] [PubMed] [Google Scholar]
- 86.Johnson-Taylor WL, Fisher RA, Hubbard VS, et al. The change in weight perception of weight status among the overweight: comparison of NHANES III (1988–1994) and 1999–2004 NHANES. Int. J. Behav. Nutr. Phys. Act. 2008;5:9. doi: 10.1186/1479-5868-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yancey AK, Simon PA, McCarthy WJ, et al. Ethnic and sex variations in overweight self-perception: relationship to sedentariness. Obesity (Silver Spring) 2006;14:980–988. doi: 10.1038/oby.2006.112. [DOI] [PubMed] [Google Scholar]
- 88.Foti K, Lowry R. Trends in perceived overweight status among overweight and nonoverweight adolescents. Arch. Pediatr. Adolesc. Med. 2010;164:636–642. doi: 10.1001/archpediatrics.2010.90. [DOI] [PubMed] [Google Scholar]
- 89.Huang JS, Becerra K, Oda T, et al. Parental ability to discriminate the weight status of children: results of a survey. Pediatrics. 2007;120:e112–e119. doi: 10.1542/peds.2006-2143. [DOI] [PubMed] [Google Scholar]
- 90.Maynard LM, Galuska DA, Blanck HM, et al. Maternal perceptions of weight status of children. Pediatrics. 2003;111:1226–1231. [PubMed] [Google Scholar]
- 91.McCabe MP, Ricciardelli LA. Sociocultural influences on body image and body changes among adolescent boys and girls. J. Soc. Psychol. 2003;143:5–26. doi: 10.1080/00224540309598428. [DOI] [PubMed] [Google Scholar]
- 92.Rhee KE, De Lago CW, Arscott-Mills T, et al. Factors associated with parental readiness to make changes for overweight children. Pediatrics. 2005;116:e94–e101. doi: 10.1542/peds.2004-2479. [DOI] [PubMed] [Google Scholar]
- 93.Butterworth SW. Influencing patient adherence to treatment guidelines. J. Manag. Care Pharm. 2008;14:21–24. doi: 10.18553/jmcp.2008.14.S6-B.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suarez M, Mullins S. Motivational interviewing and pediatric health behavior interventions. J. Dev. Behav. Pediatr. 2008;29:417–428. doi: 10.1097/DBP.0b013e31818888b4. [DOI] [PubMed] [Google Scholar]
- 95.Goetz T. 75 million Americans may have something called metabolic syndrome. How Big Pharma turned obesity into a disease – then invented the drugs to cure it. Wired. 2006;10:152–157. [Google Scholar]
- 96.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc. Diabetol. 2008;7:17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kahn R, Buse J, Ferrannini E, et al. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 98.Morrison JA, Glueck CJ, Horn PS, et al. Childhood predictors of adult Type 2 diabetes at 9- and 26-year follow-ups. Arch. Pediatr. Adolesc. Med. 2010;164:53–60. doi: 10.1001/archpediatrics.2009.228. [DOI] [PubMed] [Google Scholar]
- 99.Weiss R, Taksali SE, Tamborlane WV, et al. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care. 2005;28:902–909. doi: 10.2337/diacare.28.4.902. [DOI] [PubMed] [Google Scholar]
- 100.Executive summary: standards of medical care in diabetes – 2009. Diabetes Care. 2009;32(Suppl. 1):S6–S12. doi: 10.2337/dc09-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pradhan AD, Rifai N, Buring JE, et al. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am. J. Med. 2007;120:720–727. doi: 10.1016/j.amjmed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 104.Melbye H, Hvidsten D, Holm A, et al. The course of C-reactive protein response in untreated upper respiratory tract infection. Br. J. Gen. Pract. 2004;54:653–658. [PMC free article] [PubMed] [Google Scholar]
- 105.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of Type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]