In the current issue of Cancer Cell, Loizou et al. report that deletion of ATM Interactor (ATMIN) in developing B cells leads to profound genomic instability and clonal B cell leukemia, revealing a novel tumor suppressor function for ATMIN.
Lymphocyte development requires ordered assembly of the antigen receptor loci through V(D)J recombination. B lymphocytes also undergo additional gene modifications - somatic hypermutation (SHM) and class switch recombination (CSR) - to achieve high affinity and diverse effector functions (Nussenzweig and Nussenzweig, 2010). V(D)J recombination and CSR both entail the formation of DNA double strand breaks (DSBs) as an obligate intermediate, invoke strong DNA damage responses and require efficient DSB repair for completion (Gostissa et al., 2011). In repair deficient cells, unrepaired DSBs generated during V(D)J recombination and CSR often participate in oncogenic chromosomal translocations that eventually lead to malignant transformation, especially in the absence of an appropriate checkpoint response (Gostissa et al., 2011). Indeed, aberrant resolution of these DSBs is likely responsible for many of the recurrent chromosomal translocations characteristic of human lymphoid malignancies (Nussenzweig and Nussenzweig, 2010).
The ATM kinase is a master regulator of the DNA damage response. Physiological DSBs generated during V(D)J recombination and CSR signal through the MRN (MRE11-RAD50-NBS1) complex to activate ATM, which then phosphorylates substrates involved in both DNA repair and cell cycle checkpoint control (Figure 1)(Lavin, 2008). Recent studies also indicate that the ATM kinase can also be activated independent of DSBs, such as under hypotonic conditions or with chloroquine, although the molecular mechanism and physiological significance of DSB-independent ATM activation remain unclear (Lavin, 2008). In human patients, homozygous inactivation of ATM causes Ataxia Telangiectasia, a rare genetic syndrome associated with genomic instability and predisposition to lymphoid malignancies. Somatic inactivation of ATM has been reported in many human cancers, including a subsets of B and T cell malignancies (Boultwood, 2001).
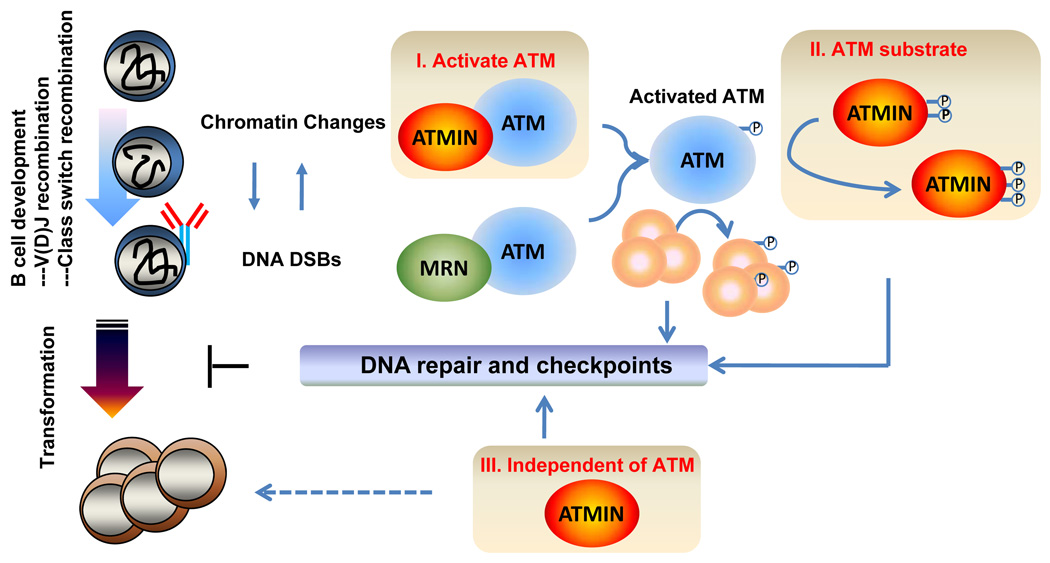
Figure 1. ATMIN is a tumor suppressor in developing B cells.
B cell development requires sequential modifications of the antigen receptor loci through V(D)J recombination and CSR. Both V(D)J recombination and CSR generate DNA DSBs and involve chromatin changes, which activates ATM and lead to the phosphorylation of ATM substrates. ATMIN potentially contributes to DNA repair and checkpoint control in developing B cells [I] as an activator of ATM in response to chromatin changes; [II] as a substrate of ATM and [III] independent of ATM. Together ATMIN deficiency leads to genomic instability and B cell lymphomas.
The ATM Interactor (ATMIN, also called ASCIZ) is a nuclear Zn2+ finger protein with a C-terminal cluster of SQ/TQ motifs that can be phosphorylated by ATM and related kinases (Kanu and Behrens, 2007; McNees et al., 2005). ATMIN binds to ATM under basal conditions and has been proposed to regulate ATM activity upon hypotonic stress (Kanu and Behrens, 2007). In the current issue of Cancer Cell, Loizou et al. report that inactivation of ATMIN in developing B cells leads to a 50% reduction in CSR. The CSR defects in ATMIN-deficient B cells are accompanied by general genomic instability as well as antigen receptor locus specific breaks (Loizou et al., 2011). This phenotype is very similar, though less severe than, that described for ATM-deficient B cells (Lavin, 2008). Moreover, up to 40% of mice with B cell specific deletion of ATMIN developed B lymphoma within six months (Loizou et al., 2011), revealing a strong tumor suppressor function of ATMIN. What do these observations reveal about the functions of ATMIN and its relationship to ATM?
Loizou et al observed that focus formation by activated ATM (p-S1987) is reduced in ATMIN-deficient B cells, suggesting sub-optimal ATM activation. While DSBs generated during CSR were thought to be the primary signal for ATM activation, Loizou et al showed that ATM is activated normally in ATMIN-deficient B cells by DSBs, but not by hypotonic stress (Loizou et al., 2011). Therefore they proposed that DSB-independent activation of ATM via ATMIN ensures robust ATM activity for efficient DNA repair in switching B cells (Figure 1). Although hypotonic stress was proposed to activate ATM by altering chromatin structure, the physiological stimulus that triggers ATMIN-dependent ATM activation in switching B cells remains unclear. During both V(D)J recombination and CSR, the formation of DSBs by lymphocyte-specific factors (i.e., RAG or AID) is accompanied by well-documented chromatin movements (e.g. looping, pairing) and histone modifications, which could potentially stimulate ATMIN-dependent activation of ATM independent of DSBs (Gostissa et al., 2011). In this context, ATMIN-deficient B cells should provide a unique platform to address the molecular mechanisms of DSB-independent ATM activation in DNA repair and tumor suppression. Curiously, while ATMIN is required for ATM activation by hypotonic stress in B cells (Loizou et al., 2011), studies on ATMIN-deficient fibroblasts have yielded conflicting results, with one group observing profound defects in hypotonic stress-induced ATM activation (Kanu and Behrens, 2007) and another group reporting no defects (Jurado et al., 2010). Identifying the physiological signal that triggers ATMIN-dependent activation of ATM in B cells may also help resolve this disparity.
Since ATMIN has multiple SQ/TQ motifs (15–18 depending on species) and is phosphorylated by ATM in vivo (McNees et al., 2005), it could also function downstream of ATM in DNA repair and checkpoint control (Figure 1). ATMIN is recruited to nuclear foci after methyl methanesulfonate (MMS) treatment and ATMIN-deficient cells display hypersensitivity to H2O2 and MMS, reduced RAD51 focus formation, and defects in base excision repair (Jurado et al., 2010; Kanu and Behrens, 2007; Kanu et al., 2010; McNees et al., 2005). Nonetheless, it is still unclear whether ATM phosphorylation of ATMIN plays a role in these processes. Interestingly, unlike other ATM substrates (e.g., H2AX), ATMIN is significantly phosphorylated under basal conditions (McNees et al., 2005), though the functions of basal ATMIN phosphorylation are not known. In addition, ATMIN may also participate in the DNA damage response indirectly through interaction with other ATM substrates –such as CHK2 (McNees et al., 2005).
Although ATM-null mice are viable (Lavin, 2008), ATMIN-null mice are embryonic lethal with severe pulmonary developmental defects (Jurado et al., 2010; Kanu et al., 2010). As such, ATMIN clearly has ATM-independent functions (Figure 1), some of which may potentially contribute to tumor suppression. Despite less severe CSR defects, more than 40% of mice with B cell specific deletion of ATMIN succumbed to B cell lymphomas within a year (Loizou et al., 2011), while germline ATM-null mice succumb to T cell lymphomas between 3 and 9 months with no documented B cell tumors (Lavin, 2008). Mice carrying a hypomorphic ATM mutation (equivalent to7636del9 in human) do develop B cell malignancies but with a long latency (>40 weeks) and low penetrance (3 out of 40). Why ATMIN deficiency seemly promotes B cell lymphomas in mice more efficient than does ATM deficiency is unknown and of considerable interest. In particular, human patients with germline ATM mutations do developed B cell lymphomas. Notably ATMIN deficient B cell lymphomas display diverse surface phenotypes, ranging from those of early native B cell to those of plasma cells, and have various chromosomal translocations, most of which do not involve immunoglobulin loci. Molecular characterization of the translocation junctions and the development stages (e.g. light chain rearrangement, somatic hypermutation status) would shed some lights on how ATMIN executes tumor suppressor function and how it is related to ATM function in B cells.
Somatic mutations of ATM are found in a broad spectrum of human cancers whereas tumor-specific lesions of ATMIN have not been reported in human cancers so far. Nevertheless, given its role in DNA repair and tumor suppression reported by Loizou et al, ATMIN may also serve as a suppressor of human tumor development and as such a potential target for therapeutic intervention. The study by Loizou et al provides a valuable framework for future studies aiming at the molecular mechanism by which ATMIN and ATM promote DNA repair and suppress tumor formation.
Acknowledgement
We thank Drs. Richard Baer and Frederick W. Alt for their comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boultwood J. Ataxia telangiectasia gene mutations in leukaemia and lymphoma. J Clin Pathol. 2001;54:512–516. doi: 10.1136/jcp.54.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostissa M, Alt FW, Chiarle R. Mechanisms that Promote and Suppress Chromosomal Translocations in Lymphocytes. Annu Rev Immunol. 2011;29:319–350. doi: 10.1146/annurev-immunol-031210-101329. [DOI] [PubMed] [Google Scholar]
- Jurado S, Smyth I, van Denderen B, Tenis N, Hammet A, Hewitt K, Ng JL, McNees CJ, Kozlov SV, Oka H, et al. Dual functions of ASCIZ in the DNA base damage response and pulmonary organogenesis. PLoS Genet. 2010;6:e1001170. doi: 10.1371/journal.pgen.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanu N, Behrens A. ATMIN defines an NBS1-independent pathway of ATM signalling. EMBO J. 2007;26:2933–2941. doi: 10.1038/sj.emboj.7601733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanu N, Penicud K, Hristova M, Wong B, Irvine E, Plattner F, Raivich G, Behrens A. The ATM cofactor ATMIN protects against oxidative stress and accumulation of DNA damage in the aging brain. J Biol Chem. 2010;285:38534–38542. doi: 10.1074/jbc.M110.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- Loizou JI, Sancho R, Kanu N, Bolland DJ, Yang F, Rada C, Corcoran AE, Behrens A. ATMIN is required for maintenance of genomic stability and suppression of B cell lymphoma. Cancer Cell. 2011 doi: 10.1016/j.ccr.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNees CJ, Conlan LA, Tenis N, Heierhorst J. ASCIZ regulates lesionspecific Rad51 focus formation and apoptosis after methylating DNA damage. EMBO J. 2005;24:2447–2457. doi: 10.1038/sj.emboj.7600704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig A, Nussenzweig MC. Origin of chromosomal translocations in lymphoid cancer. Cell. 2010;141:27–38. doi: 10.1016/j.cell.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]