Abstract
The endogenous neurotransmitter noradrenaline exerts anti-inflammatory and neuroprotective effects in vitro and in vivo. Several studies report that noradrenaline levels are altered in the central nervous system of patients with multiple sclerosis and rodents with experimental autoimmune encephalomyelitis, which could contribute to pathology. Since the major source of noradrenaline are neurons in the locus coeruleus, we hypothesized that alterations in noradrenaline levels are a consequence of stress or damage to locus coeruleus neurons. In C57BL/6 mice immunized with myelin oligodendrocyte glycoprotein peptide 35–55 to develop chronic disease, cortical and spinal cord levels of noradrenaline were significantly reduced versus control mice. Immunohistochemical staining revealed increased astrocyte activation in the ventral portion of the locus coeruleus in immunized mice. The immunized mice showed neuronal damage in the locus coeruleus detected by a reduction of average cell size of tyrosine hydroxylase stained neurons. Analysis of the locus coeruleus of multiple sclerosis and control brains showed a significant increase in astrocyte activation, a reduction in noradrenaline levels, and neuronal stress indicated by hypertrophy of tyrosine hydroxylase stained cell bodies. However, the magnitude of these changes was not correlated with extent of demyelination or of cellular infiltrates. Together these findings demonstrate the presence of inflammation and neuronal stress in multiple sclerosis as well as in experimental autoimmune encephalomyelitis. Since reduced noradrenaline levels could be permissive for increased inflammation and neuronal damage, these results suggest that methods to raise noradrenaline levels or increase locus coeruleus function may be of benefit in treating multiple sclerosis.
Keywords: locus coeruleus, noradrenaline, tyrosine hydroxylase, GFAP, EAE
Introduction
The pathophysiological basis of multiple sclerosis is not entirely understood, however, several studies suggest that abnormalities of the noradrenergic system may be a contributing factor. While several studies reported changes (both increases and decreases) in peripheral levels of noradrenaline in multiple sclerosis (Cosentino et al., 2002; Rajda et al., 2006) and that peripheral lymphocytes from patients with multiple sclerosis show increased levels of adrenergic receptors (Zoukos et al., 1992; Karaszewski et al., 1993), little is known regarding noradrenaline levels in the CNS. In one study, noradrenaline levels were found to be higher in CSF samples from patients with multiple sclerosis (both relapsing remitting and progressive patients) compared with controls (Barkhatova et al., 1998). In contrast, in patients with relapsing remitting multiple sclerosis, CSF levels of the noradrenaline metabolite methoxyhydroxyphenylglycol were negatively correlated to the duration of illness and number of relapses (Markianos et al., 2009), which could reflect a diminished response of the noradrenergic system to ongoing disease. Alterations of CNS noradrenaline levels have also been described in experimental autoimmune encephalomyelitis (EAE), a T cell mediated demyelinating animal model of multiple sclerosis. In canine EAE, CSF and white matter noradrenaline levels were increased during early times before clinical signs were present, but were reduced after symptoms appeared (Khoruzhaia and Saakov, 1975). Other studies reported reduced levels of noradrenaline in brainstem and spinal cords of rats with EAE (White et al., 1983; Krenger et al., 1986). Taken together, these studies provide evidence that perturbations of the CNS noradrenergic system occur during multiple sclerosis, although it remains unclear how noradrenaline levels are changed, what the consequences are of those changes, and what the underlying causes of the observed perturbations are.
The primary source of noradrenaline in the CNS derives from the locus coeruleus, located on the lateral face of the fourth ventricle in the upper dorsolateral pontine tegmentum (Benarroch, 2009). Noradrenaline released from locus coeruleus neurons can act on adrenergic receptors present on neurons and glial cells via adenylate cyclase and phospholipase C signal transduction pathways. Noradrenaline can modulate synaptic transmission, membrane potential and excitability of neurons. In astrocytes, noradrenaline can activate glycogen metabolism and calcium signalling, and in blood vessels helps regulate blood flow and blood brain barrier permeability. The locus coeruleus has been well studied with respect to effects on arousal, stress, memory and attention (Samuels and Szabadi, 2008; Sara, 2009), and also with regard to regulation of neuro-inflammation, neuronal survival and neurogenesis (Marien et al., 2004).
It is well documented that locus coeruleus cell numbers are reduced during normal ageing, as are brain noradrenaline levels (Marien et al., 2004). Damage and loss of locus coeruleus noradrenergic neurons is accelerated in certain progressive neurodegenerative diseases including Alzheimer’s disease (Mann et al., 1983; Bondareff et al., 1987; German et al., 1992; Weinshenker, 2008) and Parkinson’s disease (Mann and Yates 1983; Rommelfanger and Weinshenker, 2007); and patients with Alzheimer’s disease have reduced levels of noradrenaline compared with controls (Adolfsson et al., 1979; Palmer and DeKosky 1993). Locus coeruleus neuronal loss is correlated with plaque and tangle numbers (Bondareff et al., 1987) and duration of illness (Zarow et al., 2003), and the greatest neuronal loss was observed in the locus coeruleus (83% loss in Alzheimer’s disease; 68% loss in Parkinson’s disease) compared with other subcortical nuclei (nucleus basalis, substantia nigra pars compacta) (Lyness et al., 2003; Zarow et al., 2003). In contrast, the characterization of possible locus coeruleus damage in multiple sclerosis is limited to a magnetic resonance study that showed impairment of selective attention that increased with axonal damage occurring at the right locus coeruleus (Gadea et al., 2004).
Locus coeruleus neuronal loss or damage has been reported to occur in certain transgenic mouse models of Alzheimer’s disease (TgAPP mice). In mice expressing the familial V717F mutation of human amyloid precursor protein, tyrosine hydroxylase positive neurons in the dorsal central portion of the locus coeruleus were reduced in size in 24-month-old mice compared with age matched wild-type mice (German et al., 2005), and the total number of locus coeruleus tyrosine hydroxylase positive neurons was reduced in aged female TgAPP mice compared with non-transgenic controls (O’Neil et al., 2007). Levels of noradrenaline were reduced in 11-month-old mice expressing human amyloid precursor protein Swedish mutation and presenilin-1 M146V mutation compared with controls (Pugh et al., 2007); as were levels of the noradrenaline transporter messenger RNA (Jardanhazi-Kurutz et al., 2010). That locus coeruleus loss can have pathological consequences is supported by findings that experimental lesion of the locus coeruleus exacerbates brain inflammation induced by endotoxin, cytokines or β-amyloid (Heneka et al., 2002; Pugh et al., 2007); and increases amyloid burden, neuronal damage and behavioural deficits in TgAPP mice (Heneka et al., 2006; Kalinin et al., 2007). Evidence for a functional role for locus coeruleus in EAE comes from our previous studies in which locus coeruleus noradrenergic neurons were lesioned using the locus coeruleus-selective neurotoxin DSP-4 (Simonini et al., 2010). Locus coeruleus lesion did not affect either the incidence of disease or the average day of disease onset, suggesting that initial peripheral events responsible for development of EAE, such as T-cell activation and migration into the CNS, were not modified by DSP-4 treatment. However, clinical severity was significantly increased in the DSP4-treated group beginning ∼2 weeks after immunization, consistent with the premise that locus coeruleus functional status influences EAE disease.
Studies showing that noradrenaline is neuroprotective and reduces inflammatory responses suggest that the effects of locus coeruleus damage are a consequence of changes in noradrenaline levels. Early studies showed that noradrenaline reduces class II antigen (Frohman et al., 1988) and cytokine expression in astrocytes (Benveniste et al., 1995; Szabo et al., 1997), and reduced expression of inducible nitric oxide synthase type 2 in astrocytes (Feinstein et al., 1993; Pahan et al., 1997; Galea et al., 2003), microglia (Carnevale et al., 2007) and neurons (Madrigal et al., 2006). In vivo, increasing noradrenaline using an alpha-2-adrenoceptor antagonist reduced inflammation due to aggregated amyloid beta (Kalinin et al., 2006b); selective noradrenaline reuptake inhibitors reduced CNS chemokine, cytokine, and cell adhesion expression following systemic endotoxin injection (O’Sullivan et al., 2009, 2010) and increased anti-inflammatory cytokines (McNamee et al., 2010); and a noradrenaline precursor reduced astrocyte activation in EAE (Simonini et al., 2010). Noradrenaline also reduces neurotoxicity due to inflammatory (Madrigal et al., 2005) or excitotoxic (Troadec et al., 2001) stimuli, or incubation with amyloid beta (Madrigal et al., 2007).
The above findings suggest that the consequences of locus coeruleus loss may be related to associated reductions in noradrenaline. In the current study we re-examined the question of whether loss of CNS noradrenaline occurs in EAE or in multiple sclerosis, and present data demonstrating the presence of neuronal damage and glial inflammation in the locus coeruleus.
Materials and methods
Induction of experimental autoimmune encephalomyelitis
A chronic form of EAE disease was actively induced in 8-week-old female C57Bl6 mice using synthetic myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35–55; MEVGWYRSPFSRVVHLYRNGK purchased from Anaspec, San Jose, CA, USA). Mice were injected subcutaneously with an emulsion of 300 µg MOG35–55 dissolved in 100 µl phosphate buffered saline, mixed with 100 µl complete Freund’s adjuvant containing 500 μg of Mycobacterium tuberculosis (Difco, Detroit, MI, USA). The animals then received an intraperitoneal injection of 200 ng of pertussis toxin (List Biochemicals, Campbell, CA, USA) in 200 µl phosphate buffered saline. Two days later the mice received a second pertussis toxin injection and one week later, a booster MOG35–55 injection. Clinical signs were scored as: 0, no clinical signs; 1, limp tail; 2, impaired righting; 3, paresis of one hind limb; 4; paresis of two hind limbs; 5, death. Animals were sacrificed 60 days after initial immunization.
Multiple sclerosis and control samples
Tissue specimens containing the area of the locus coeruleus were obtained from autopsied brains of eight patients with multiple sclerosis and eight normal controls (Table 1). A coronal section was dissected from the brainstem beginning at approximately +23 mm rostral of the obex (the point at which the fourth ventricle narrows to become the central canal of the spinal cord) and extending rostrally ∼5 mm. This area contains the majority of the locus coeruleus neurons (Paxinos and Mai, 2004). Samples were stored at −80°C until use. Neuropathological review of haematoxylin and eosin, and proteolipid protein stained sections containing the dorsal pons, the fourth ventricle and the ventral cerebellum demonstrated no significant histopathological changes in the control specimens, apart from mild-to-moderate arteriosclerotic changes in one (C5). Sections from patients with multiple sclerosis revealed evidence of multiple sclerosis plaques involving the locus coeruleus region in two cases (MS1 and MS7). These plaques showed loss of myelin with relative preservation of axons and no-to-minimal inflammation consistent with inactive plaques. Samples from the other patients with multiple sclerosis showed no evidence of either active or inactive plaques.
Table 1.
Demographics of human brain samples analysed
| Patient ID | Age (years) | Sex | Disease | Autolysis time (h) |
|---|---|---|---|---|
| MS1 | 50 | F | SP | 15.0 |
| MS2 | 54 | M | SP | 15.0 |
| MS3 | 71 | M | SP | 23.0 |
| MS4 | 70 | F | CP | 9.0 |
| MS5 | 82 | F | CP | 20.8 |
| MS6 | 63 | M | PP | nd |
| MS7 | 52 | M | SP | nd |
| MS8 | 56 | M | SP | nd |
| C1 | 93 | F | 20.3 | |
| C2 | 77 | M | 12.3 | |
| C3 | 54 | M | 19.0 | |
| C4 | 81 | F | 11.3 | |
| C5 | 73 | F | 12.0 | |
| C6 | 70 | M | 12.0 | |
| C7 | 77 | M | nd | |
| C8 | 34 | F | nd |
C = control; CP = chronic progressive multiple sclerosis; MS = multiple sclerosis; nd = not determined; SP = secondary progressive multiple sclerosis, PP = primary progressive multiple sclerosis.
Measurements of noradrenaline levels
Cell lysates were prepared from samples of frontal cortex, spinal cord and locus coeruleus of EAE and non-EAE mice. From human samples, a combined cell lysate was prepared from 3 mm tissue punches taken from the area located immediately ventral and lateral to the locus coeruleus (primarily consisting of the central tegmental tract, the medial longitudinal fasciculus and the central grey of the pons). The locus coeruleus itself was not included since that was used for immunohistochemical studies. Tissues were homogenized on ice in 40 volumes of 0.01 Normal HCl, 1 mM EDTA and 4 mM sodium metabisulphite. Enzyme-linked immunosorbent assay for noradrenaline was performed according to the manufacturer’s instructions (Rocky Mountain Diagnostics Inc., Colorado Springs, CO, USA).
Messenger RNA analysis
Total RNA from the locus coeruleus and spinal cord of control and EAE mice was isolated using TRIzol® reagent (Invitrogen/GIBCO). Quantitative real time polymerase chain reaction was carried out as described (Kalinin et al., 2006b) to determine relative levels of the locus coeruleus-enriched receptor Ear2, and the brain derived neurotrophic factor (BDNF). The primers used for BDNF were forward 5′-AGAGCAGCTGCCTTGATGTT-3′ and reverse 5′-TCGTCAGACCTCTCGAACCT-3′; for Ear2 were 5′-GAAAGCATTACGGCGTGTTC-3′ and reverse 5′-TGGTGCTGATCAATCTGACAG-3′; and for α-actin were forward 5′-CCTGAAGTACCCCATTGAACA-3′ and reverse 5′-CACACGCAGCTCATTGTAGAA-3′. Relative messenger RNA levels were calculated from take-off cycle numbers, normalized to values measured for α-actin in the same samples and significant differences (P < 0.050) determined by unpaired t-tests.
Immunohistochemistry
Mouse and human brain samples were fixed overnight in 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.6, dehydrated through alcohol and xylene series, then embedded in paraffin. Serial sagittal sections (8 µm) were taken through the complete area of mouse locus coeruleus. Serial 8 µm coronal sections were collected from human tissue and organized such that a total of nine sections per brain were stained for each antibody, each section separated from the next by 280 µm and the total series spanning ∼2.24 mm.
Following paraffin removal, antigen retrieval was accomplished by boiling in 10 mM citrate buffer for 10 min, followed by a single wash in phosphate buffered saline containing Ca2+/Mg2+, and then blocked with 5% normal donkey serum. Sections were incubated at 4°C overnight with primary antibodies diluted in 1% normal donkey serum: rat monoclonal antibody anti-human glial fibrillary acidic protein (GFAP) B2.210 at 1:300 (Trojanowski et al., 1986), rabbit polyclonal anti-tyrosine hydroxylase at 1:300 (Pel-Freeze, Rogers, AK, USA), rabbit polycolonal anti-proteolipid protein at 1:800 (a gift from Dr Robert Skoff). After washing, sections were incubated 45 min at 37°C with appropriate secondary antibodies pre-absorbed to reduce cross reactivity (Jackson ImmunoResearch), conjugated to either RRX or FITC and used at a concentration of 1:200. Sections were washed, briefly post-fixed in 3.7% paraformaldehyde, quenched in 50 mM ammonium chloride and then the final five washes done in phosphate buffered saline with the first containing 800 ng/ml DAPI. Sections were cover-slipped using VECTASHIELD® mounting fluid (Vector Laboratories Inc., Burlingame, CA, USA).
Histological examination of human specimens was done by staining deparaffinized, washed sections with haematoxylin and eosin. Three serial sections through the pons of each specimen were examined for infiltrates and scored as follows: 1, no infiltrates; 2, few infiltrates within the locus coeruleus or around the fourth ventricle; 3, significant infiltrates within the locus coeruleus or near the fourth ventricle; 4, significant infiltrates throughout the section. Scoring was done by a blinded investigator.
Image analysis
Images were obtained on a Zeiss Axioplan 2 microscope with an MRm Axiocam for image acquisition and densitometric analysis conducted using Axiovision version 4.0 software (Carl Zeiss Inc., Thornwood, NY, USA). Image acquisition was conducted on sections stained simultaneously and exposed for identical amounts of time. For mouse locus coeruleus, one rectangular field of view (1050 × 1420 µm, total area 1.5 mm2) taken at ×100 magnification was analysed per section; this encompassed the entire locus coeruleus region and adjacent dorsal subcoeruleus area. For mouse substantia nigra, two fields of view were taken to include both substantia nigra pars compacta and substantia nigra pars reticulata. For human samples, two fields of view were imaged that encompassed the locus coeruleus and the region located medial to the dorsal locus coeruleus, which contains the dorsal tegmental nucleus. Quantitation of GFAP and proteolipid protein was done using an object area cut-off of 10 µm2 to include cell bodies and processes. The data were analysed to determine the total number of positively stained objects per field of view, and the total area covered by positively stained objects presented as a percentage of the total field of view. Quantitation of tyrosine hydroxylase stained cell bodies was accomplished using an object area cut-off of 60 µm2 to exclude counting of processes. Tyrosine hydroxylase data were analysed to determine the total number of positively stained cell bodies per field of view and the average cell body size.
Statistics
Group comparisons were done by unpaired t-tests; neuronal cell size distributions were analysed by two-way ANOVA and Bonferroni’s post hoc analyses. P < 0.05 were considered statistically significant. Relationships between tyrosine hydroxylase neuronal size and proteolipid protein or haematoxylin and eosin staining were done using Spearman non-parametric correlation analyses.
Results
Locus coeruleus damage in experimental autoimmune encephalomyelitis
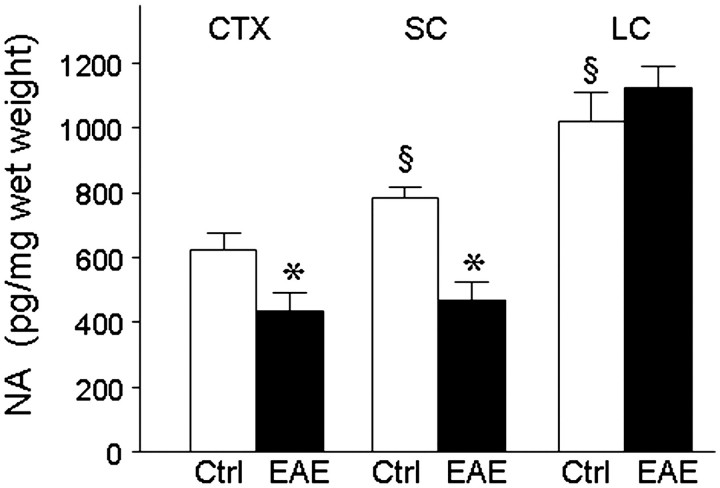
Noradrenaline levels were measured by specific enzyme-linked immunosorbent assay in homogenates prepared from the locus coeruleus, the frontal cortex and spinal cords of control and myelin oligodendrocyte glycoprotein-immunized EAE mice 60 days after immunization (Fig. 1), at which point the EAE mice had moderate to severe disease severity (clinical scores of 2.0–4.0). In control mice, noradrenaline levels were lowest in the frontal cortex, higher in spinal cord, and greatest in the locus coeruleus. A significant decrease was observed in both the frontal cortex and spinal cord of EAE mice as compared with age- and sex-matched controls. These results point to perturbations in CNS noradrenaline levels during EAE, as has been reported in other mouse models (Szot et al., 2009).
Figure 1.
Cortical and spinal cord noradrenaline levels are decreased in EAE. Tissue lysates were prepared from EAE mice (filled bars) at 60 days after the initial myelin oligodendrocyte glycoprotein (MOG) peptide immunization, at which point they had clinical scores of 2–4. Age- and sex-matched non-immunized mice served as controls (Ctrl, open bars). Noradrenaline (NA) levels were quantified by specific enzyme-linked immunosorbent assay in samples from frontal cortex (CTX: n = 10 controls, n = 9 EAE); spinal cord (SC: n = 15 controls, n = 12 EAE) and locus coeruleus (LC: n = 6 controls, n = 4 EAE). Data is pg noradrenaline per mg wet weight tissue and are means ± SEM; *P < 0.05 versus control; §P < 0.05 versus control CTX (unpaired t-test).
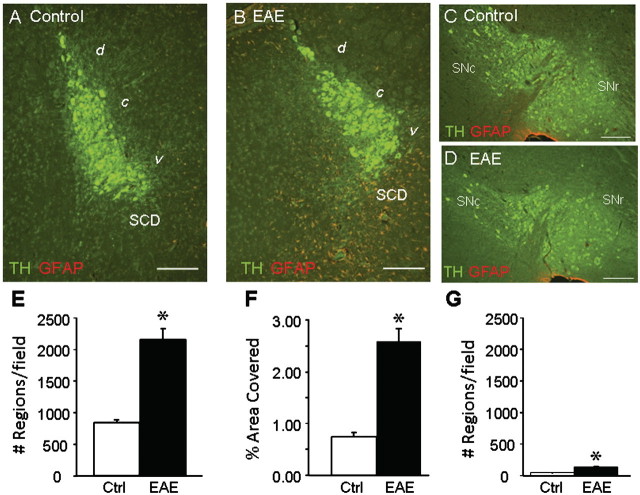
Immunohistochemical staining was carried out to assess glial activation and inflammation in the locus coeruleus and surrounding area (Fig. 2A and B). In the dorsal and central parts of the locus coeruleus, low levels of GFAP staining were observed in control and EAE mice. However, in the ventral locus coeruleus and the area immediately below, which contains noradrenergic subcoeruleus dorsal neurons and fibres, both the number of GFAP positive stained cells and processes and the total area covered by GFAP positive staining were significantly increased in the EAE mice (Fig. 2E and F). The fact that increased GFAP positive staining was primarily observed in the ventral locus coeruleus and dorsal subcoeruleus areas, both of which send projections to the spinal cord (Holstege and Bongers 1991; Proudfit and Clark 1991; Tanaka et al., 1997), and not in the central and dorsal portion of the locus coeruleus suggests that increased inflammation is associated with topographically defined tyrosine hydroxylase positive neurons and is not a general consequence of diffuse inflammation in EAE. Consistent with this, although GFAP staining within the substantia nigra (Fig. 2C and D) was increased in EAE versus control mice, the levels measured (Fig. 2G) were <10% of those detected in the locus coeruleus.
Figure 2.
GFAP staining is increased in locus coeruleus of EAE mice. Serial sagittal sections were prepared from four EAE and three control mice taken at Day 60, and stained with antibodies to tyrosine hydroxylase (TH, green) and GFAP (red). Representative images taken from the mid-central portion of the locus coeruleus and from the substantia nigra are shown from control (A and C) and EAE (B and D) mice. (A and B) The fourth ventricle is located above and to the left; the area containing dorsal subcoeruleus neurons (SCD) is indicated. GFAP staining was quantified in 8–12 serial sections per mouse. Data are means ± SEM for total area stained (per cent field of view) in the locus coeruleus; and for total number of stained objects (cell bodies and processes) per field of view in F the locus coeruleus and the substantia nigra. *P < 0.05 versus control (unpaired t-test). Scale bars are 200 µm. d = dorsal; c = central; v = ventral; SNc = substantia nigra pars compacta; SNr = substantia nigra pars reticulata.
Specific staining for the tyrosine hydroxylase positive neurons (Fig. 3) did not reveal any reduction in the number of tyrosine hydroxylase positive stained cells in EAE locus coeruleus (Fig. 3E); however, smaller cells were present in the EAE locus coeruleus (Fig. 3C and D) and quantitation showed the average cell body size was significantly reduced in EAE versus control mice (Fig. 3F). Analysis of tyrosine hydroxylase positive cell sizes showed a significant alteration in the frequency distribution between control and EAE mice (Fig. 3G). In control mice, most cells (∼60%) had sizes ranging from 200–450 µm2, with fewer cells in the lower and higher ranges. In EAE mice there were fewer cells in the range of 200–450 µm2, and an increased percentage of cells in the two smallest size ranges (between 0 and 100 µm2). In contrast to the locus coeruleus, we did not detect any significant differences in tyrosine hydroxylase staining (either number of cells or average size) in the substantia nigra of EAE versus control mice (Fig. 3E and F).
Figure 3.
Tyrosine hydroxylase positive stained neurons are smaller in locus coeruleus of EAE mice. Serial sagittal sections were prepared from four EAE and three control mice taken at Day 60, and stained to detect tyrosine hydroxylase in the locus coeruleus and the substantia nigra. Representative images from the mid-central portion of the locus coeruleus of control (A) and EAE (B) mice are shown which highlight the presence of smaller-sized tyrosine hydroxylase positive stained neurons (asterisk) in EAE (D) but not control (C) mice. (E) Quantitative analysis carried out of 8–12 serial sections per mouse through the locus coeruleus (LC) or through the substantia nigra (SN) (representative images are shown in Fig. 2) did not show a significant change in the total number of tyrosine hydroxylase (TH) positive stained neurons per section. (F) Quantitation of average cell size using data from three sections per mouse revealed a significant reduction of locus coeruleus, but not substantia nigra, neuronal cell body in EAE versus control (mean ± SEM; *P < 0.05). (G) The distribution of tyrosine hydroxylase positive stained cell sizes was determined from 804 control cells (open circles) and 1276 EAE cells (filled circles). Control mice have the greatest percentages of cells in the range of 200–400 µm2, with fewer cells in the lower and higher size ranges. In EAE mice a greater percentage of cells were present in the smaller (<150 µm2) size range. Data are means ± SD of distributions calculated for the three control and four EAE mice. *P < 0.05 versus control [two-way ANOVA F(15, 1) = 4.17; and Bonferroni’s post hoc]. Each cell size bin includes cells having areas of that size ±25 µm2. Scale bars are 200 µm in A and B.
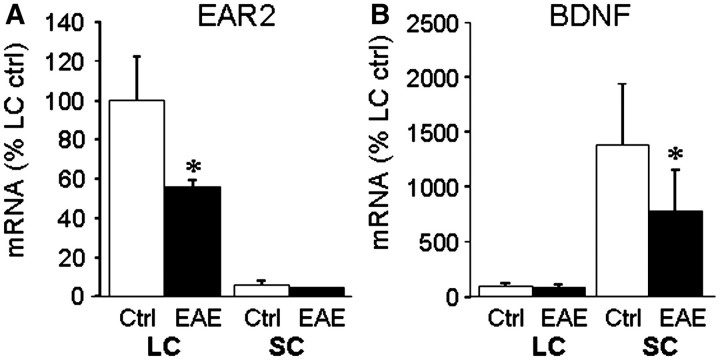
An additional indication of selective damage occurring in the locus coeruleus is suggested by quantitative polymerase chain reaction analysis (Fig. 4), which shows that messenger RNA levels of Ear2, a nuclear receptor involved in the early stages of locus coeruleus maturation (Warnecke et al., 2005), were significantly reduced in the locus coeruleus of EAE mice versus controls. Ear2 levels in the spinal cord were <10% of those measured in the locus coeruleus and were not significantly altered in EAE. In contrast, analysis for the neurotrophic factor BDNF, required for locus coeruleus survival (Holm et al., 2003), showed low levels in the locus coeruleus that were not affected by EAE, and much higher levels in the spinal cord that were significantly reduced in EAE compared with control samples.
Figure 4.
Levels of EAR2 and BDNF messenger RNAs are reduced in EAE. Total cytosolic RNA was prepared from (A) locus coeruleus (LC) and (B) spinal cord (SC) of three EAE and three control mice at Day 60, converted to complementary DNA, and relative levels of the messenger RNAs for EAR2 and BDNF measured by real time quantitative polymerase chain reaction, and normalized to values for α-actin measured in the same samples. The data are the mean ± SD of messenger RNA levels relative to those measured in the control locus coeruleus samples. *P < 0.05; EAE versus control.
Locus coeruleus damage in multiple sclerosis
To determine if comparable inflammation occurred in patients with multiple sclerosis, we carried out immunochemical staining of brain samples for GFAP (Fig. 5A and B). Increased GFAP positive staining was observed both in the locus coeruleus itself as well as in the medially located dorsal tegmental nucleus of patients with multiple sclerosis compared with controls. GFAP positive staining was detected around tyrosine hydroxylase positive stained neurons in the locus coeruleus but not outside of the locus coeruleus (Fig. 5C and D). Quantitative analysis showed a significant increase in both the locus coeruleus and dorsal tegmental nucleus in the number of GFAP positive stained objects (Fig. 5E) and the percentage area stained (Fig. 5F).
Figure 5.
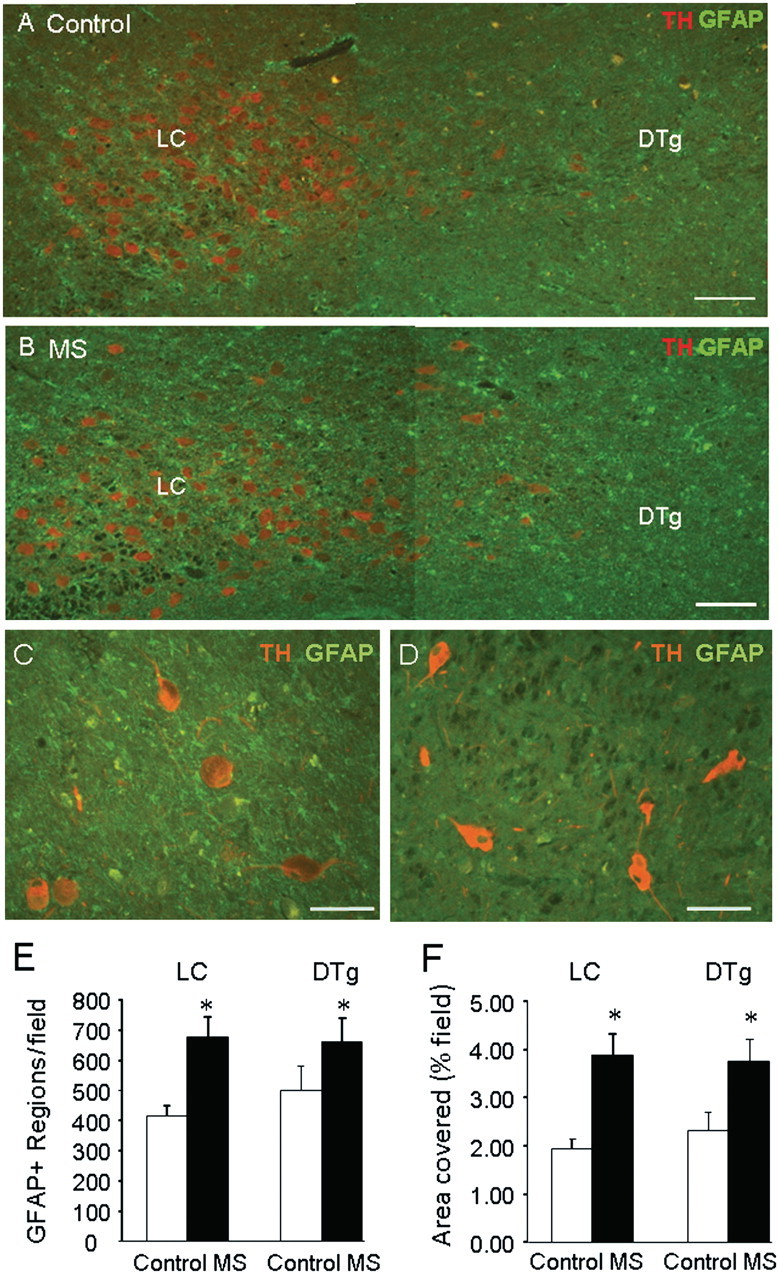
GFAP staining is increased in the locus coeruleus region of multiple sclerosis brains. Serial coronal sections through the locus coeruleus (LC) were prepared from five patients with multiple sclerosis (MS) and six controls, and stained for tyrosine hydroxylase (TH) and GFAP. The fourth ventricle is located above and to the right. Representative images from control (A) and patients (B) with multiple sclerosis show increased GFAP positive staining in the locus coeruleus and adjacent area (containing the dorsal tegmental nuclei, DTg). Representative images from one multiple sclerosis sample showing presence of GFAP staining around tyrosine hydroxylase positive stained neurons in locus coeruleus (C) but not in adjacent central pons (D). Quantitation of staining showed a significant increase in (E) the number of GFAP positive stained objects (cell bodies and processes) and (F) the total area stained (per cent field of view) in both the locus ceruleus and the dorsal tegmental nuclei of multiple sclerosis samples versus controls. Data are means ± SEM of nine sections per brain; *P < 0.05 versus controls. Scale bars are 200 µm in A and B and 100 µm in C and D.
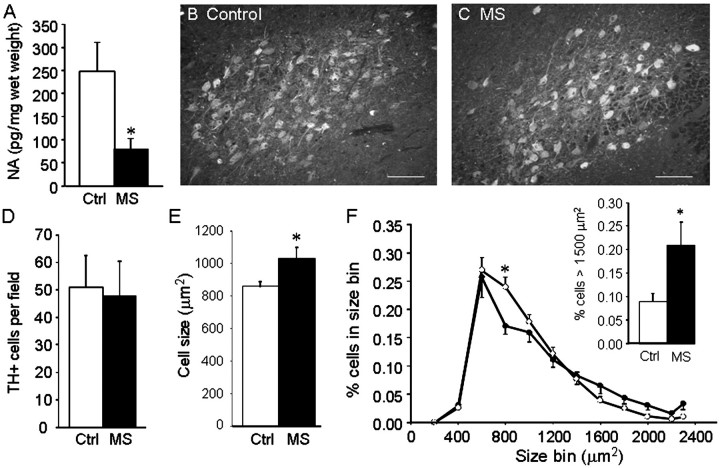
Measurement of noradrenaline levels in samples from the adjacent ventral and lateral portions of the pons (the locus coeruleus was used for immunostaining) from five multiple sclerosis samples and seven controls (other samples did not provide sufficient tissue for enzyme-linked immunosorbent assay) showed a significant reduction in multiple sclerosis samples (Fig. 6A). Immunostaining (Fig. 6B and C) did not reveal any change in the average number of tyrosine hydroxylase positive stained cell bodies per field (Fig. 6D). However the average cell size (873 ± 33 µm2) in control samples, which is similar to values reported by others for human locus coeruleus neurons (German et al., 1988), was significantly increased by ∼30% in the multiple sclerosis samples (Fig. 6E). This increase was due to a larger percentage of tyrosine hydroxylase positive neurons having cell body areas in the size range of 1500–2300 µm2 (Fig. 6F) and a decrease in the percentage of cells in the smaller range from 700 to 900 µm2.
Figure 6.
Locus coeruleus neuronal stress is present in multiple sclerosis. (A) Noradrenaline (NA) levels were measured in 10 areas located near the locus coeruleus as described in ‘Methods’ section, in samples from five patients with multiple sclerosis (MS) and seven controls (Ctrl), and values from the 10 areas averaged. Data are means ± SEM of pg noradrenaline/mg wet weight; *P < 0.05 versus controls. Representative images of tyrosine hydroxylase staining from control (B) and patient with multiple sclerosis (C) encompassing most of the locus coeruleus. Quantitative image analysis carried out on nine serial sections from eight multiple sclerosis and eight control samples did not show any significant difference in the total number of tyrosine hydroxylase positive stained neurons per field of view (D); however, the average cell size was significantly increased in patients with multiple sclerosis (E). The distribution of tyrosine hydroxylase positive cell sizes (F) calculated from 3408 cells in the patients with multiple sclerosis (filled circles) and 3660 cells from the controls (open circles) shows fewer cells of size range 700–900 µm2 in patients with multiple sclerosis compared with controls [F(11, 1) = 2.131, P = 0.021], with a significantly greater percentage of cells having areas >1500 µm2 in the patients with multiple sclerosis (F, inset). Data are means ± SEM, *P < 0.01 versus controls (Bonferroni’s post hoc). Each cell size bin includes cells with areas that size ± 100 µm2. Scale bars are 200 µm.
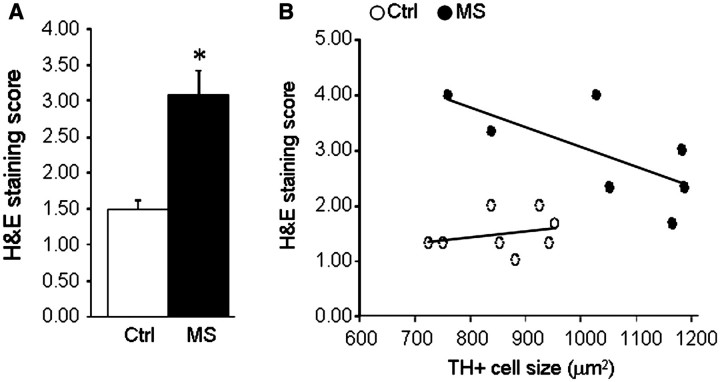
To determine if locus coeruleus stress is related to the extent of multiple sclerosis pathology, we examined haematoxylin and eosin stained sections for cellular infiltrates, and proteolipid protein-stained sections for myelin content. In the locus coeruleus, proteolipid protein staining was strong in most control specimens (Fig. 7A) although some had weaker staining (Fig. 7C); similarly some multiple sclerosis specimens showed strong staining (Fig. 7B) while others had much weaker staining (Fig. 7D). Quantitative image analysis revealed a non-statistically significant decrease in the multiple sclerosis samples of the total area covered by proteolipid protein staining (Fig. 7E). Comparison of proteolipid protein staining to the average tyrosine hydroxylase positive neuronal cell size (considered as an index of stress) revealed a direct correlation between increased neuronal size and increased proteolipid protein staining in the multiple sclerosis samples, but not in the control samples (Fig. 7F). Measurements of the extent of cellular infiltrates staining in the locus coeruleus and surrounding tissue (Fig. 8) revealed a significant increase in the multiple sclerosis samples versus controls, and an inverse correlation between average tyrosine hydroxylase positive neuronal size and the magnitude of haematoxylin and eosin staining in the multiple sclerosis samples.
Figure 7.
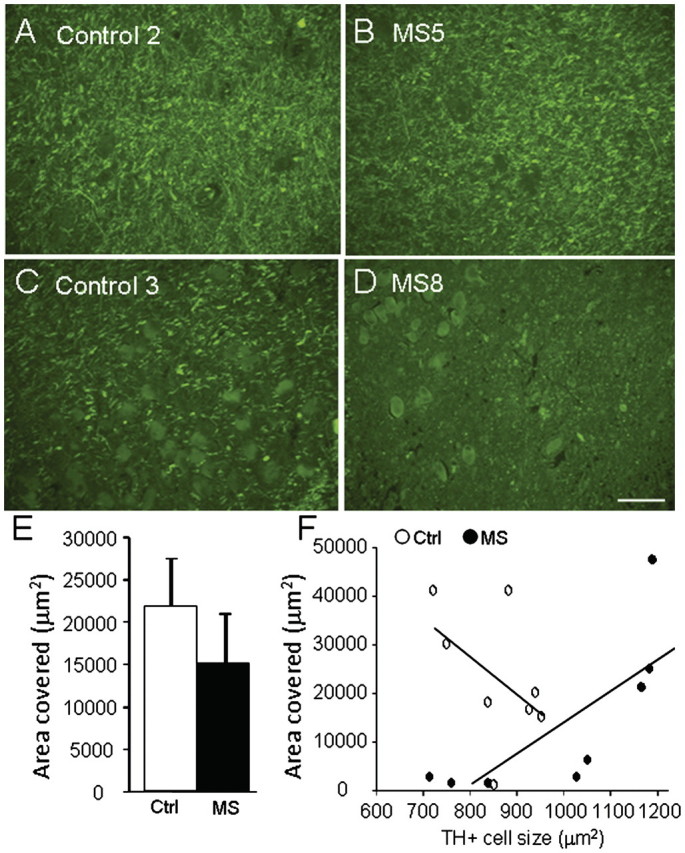
Relationship between proteolipid protein staining and locus coeruleus stress. Serial coronal sections through the locus coeruleus (LC) were prepared from eight patients with multiple sclerosis (MS) and eight controls, and stained for proteolipid protein. Representative images showing staining from two controls (A and C) and two patients with multiple sclerosis (B and D), show large variation in extent of proteolipid protein staining. (E) Quantitation of total proteolipid protein area stained (mean ± SE of 2–3 sections per brain) shows a non-significant (P = 0.32) decrease in multiple sclerosis samples versus controls. (F) Comparison of proteolipid protein area stained versus average tyrosine hydroxylase positive cell size in control (open circles) and multiple sclerosis (closed circles) sections reveals a significant correlation in the multiple sclerosis samples (Spearman r = 0.93, P = 0.0022). Scale bars are 200 µm.
Figure 8.
Relationship between haematoxylin and eosin staining and locus coeruleus stress. Serial coronal sections through the locus coeruleus were prepared from eight patients with multiple sclerosis and eight controls, stained with haematoxylin and eosin (H&S) for cell infiltrates, and sections (three per brain) scored from 1–4 as described in the ‘Methods’ section. (A) Data are mean ± SE of average haematoxylin and eosin score in controls and multiple sclerosis samples (*P < 0.01). (B) Comparison of average haematoxylin and eosin score versus average tyrosine hydroxylase positive cell size in control (open circles) and multiple sclerosis (closed circles) sections reveals a significant correlation in the multiple sclerosis samples (Spearman r = −0.773, P = 0.028).
Discussion
To date, limited studies have examined locus coeruleus pathophysiology or the role of central noradrenaline in multiple sclerosis or its animal model EAE. Our data confirm that there are significant reductions in central levels of noradrenaline in both the brains and spinal cords of EAE mice, and that locus coeruleus neuronal damage is present as indicated by tyrosine hydroxylase positive neuronal cell shrinkage. Our data provide evidence for inflammation occurring in and near to the locus coeruleus in human multiple sclerosis samples, for reduced noradrenaline levels in the tissue surrounding the locus coeruleus and for hypertrophy of tyrosine hydroxylase positive stained neurons. Although several possible explanations could account for reduced noradrenaline levels (decreased synthesis, increased metabolism, increased re-uptake) our findings of inflammation in the locus coeruleus, as well as stress in tyrosine hydroxylase positive neurons argues for loss of noradrenaline synthesis as a contributing cause.
Additional evidence for neuronal damage comes from findings that in EAE, there is a significant reduction in expression of Ear2 in the locus coeruleus, but not the spinal cord. Ear2 is an orphan nuclear receptor expressed during early development in the area where the locus coeruleus develops, and in Ear2 null mice over 70% of locus coeruleus neurons are absent in the adult (Warnecke et al., 2005). While the role of Ear2 in the adult is largely unknown, its ability to repress lymphocyte expression of IL-17 (Hermann-Kleiter et al., 2008), a proinflammatory cytokine implicated in EAE and multiple sclerosis disease pathogenesis (Segal, 2010), suggests that decreased Ear2 could allow for increased IL-17 expression and increased inflammation.
Our data demonstrate locus coeruleus tyrosine hydroxylase positive neuronal atrophy in EAE but not neuronal loss, suggesting that focal inflammatory lesions, which might be expected to cause neuronal death, are relatively sparse in the locus coeruleus in this model. An alternative explanation for increased locus coeruleus neuronal stress are reduced levels of necessary trophic factors or receptors, as suggested by our findings that messenger RNA levels of BDNF are decreased in EAE spinal cord. Locus coeruleus neurons express high levels of the BDNF receptor TrkB during normal development, and a localization, synthesis and anterograde transport of BDNF within noradrenergic neurons has been described (Fawcett et al., 1998). TrkB deficient mice have 30% fewer locus coeruleus neurons (Holm et al., 2003), and more recently, BDNF and NT4 were shown to be potent co-inducers of noradrenergic phenotype in primary locus coeruleus cultures (Traver et al., 2006). Since noradrenaline increases expression of several neurotrophins including BDNF (Zafra et al., 1992), diminished locus coeruleus function and lower noradrenaline levels could contribute to damage by reducing neurotrophic support from glial cells as well as from locus coeruleus neurons themselves (Fawcett et al., 1998).
The above changes are consistent with our findings of reduced cortical and spinal cord levels of noradrenaline, which derive from locus coeruleus afferent fibres. This does not appear to be due to loss of noradrenaline neurons, since locus coeruleus tyrosine hydroxylase positive cell numbers were not reduced. However, the decrease in average cell size, similar to that reported for TgAPP mice (German et al., 2005), suggests that the locus coeruleus neurons may be compromised in their ability to synthesize or store noradrenaline. Further studies to examine other structural or functional markers of noradrenaline neuronal integrity in the cortex (e.g. fibre density, noradrenaline release or transporter expression) could address that question. Consistent with the decrease of spinal cord noradrenaline levels, we observed increased astrocyte activation in the ventral portion of the locus coeruleus as well as in the area immediately beneath, which contains the dorsal portion of the subcoeruleus neurons, two areas that send noradrenergic afferents primarily to the spinal cord (Holstege and Bongers 1991; Proudfit and Clark 1991; Tanaka et al., 1997). Since spinal cord pathology is a hallmark of myelin oligodendrocyte glycoprotein peptide induced EAE, this raises the possibility that locus coeruleus inflammation or damage may be due in part to retrograde signals originating in the cord, as postulated to occur to cholinergic (Pearson et al., 1983) and adrenergic (German et al., 1987) neurons in Alzheimer’s disease. It is not clear why a similar loss was detected in the frontal cortex, since there are few ventral projections to this area. However, this could suggest the presence of more subtle perturbations of dorsally located tyrosine hydroxylase positive neurons that were not detected by our assays. Alternatively, this could be due to increased noradrenaline metabolism in the cortex, rather than reduced noradrenaline production, for example by the enzyme COMT1, whose expression is significantly increased in the brain under inflammatory conditions (Helkamaa et al., 2007).
Our findings regarding locus coeruleus damage in patients with multiple sclerosis also point to locus coeruleus neuronal stress or damage. We observed a statistically significant decrease in noradrenaline levels in the central pons area immediately adjacent to the locus coeruleus through which locus coeruleus neurons send projections; to our knowledge this is the first direct demonstration of reduced noradrenaline levels in multiple sclerosis brain. We also measured a statistically significant increase in GFAP staining in the locus coeruleus and the adjacent dorsal tegmental nucleus, similar to the increase observed in the EAE mice. As for EAE, GFAP staining was not always associated with tyrosine hydroxylase positive stained neurons suggesting selective stress or damage to locus coeruleus and dorsal tegmental nucleus neurons.
Our results show that the average size of locus coeruleus tyrosine hydroxylase positive neurons was increased in the multiple sclerosis samples versus controls, in contrast to neuronal atrophy observed in EAE. The discrepancy may reflect important differences in EAE versus multiple sclerosis disease, or could be due to species differences, or to relative ages and the duration of the disease. In EAE, mice were 4-months old at the end of the study and had clinical signs for 2 months. In contrast, the patients with multiple sclerosis ranged in age from 49 to 82, and their disease was an ongoing condition over several years, during which time locus coeruleus neurons may have undergone changes not present in an acute animal model. There is conflicting evidence as to how locus coeruleus neuronal morphology is effected in other neurodegenerative diseases, with swollen cell bodies described in both Alzheimer’s disease and Parkinson’s disease brains (Chan-Palay, 1991), but cell atrophy (Mann, 1983) and selective loss of large neurons (Hoogendijk et al., 1995) observed in some patients with Alzheimer’s disease and locus coeruleus neuronal shrinkage in TgAPP mice (German et al., 2005). The basis for these differences remains unclear, but may reflect differences in the proportion of surviving locus coeruleus neurons versus those that are undergoing cell death.
An important contributor to disease progression in multiple sclerosis and EAE is leukocyte infiltration through the blood brain barrier. It is well known that noradrenergic innervation of cerebral vasculature preserves the integrity of the blood brain barrier (Harik and McGunigal, 1984). Correspondingly, we have shown that locus coeruleus lesion leads to disorganization of tight junctions in cerebral endothelial cells (Kalinin et al., 2006a). Locus coeruleus damage could therefore increase infiltration of activated lymphocytes and exacerbation of disease.
The precise cause(s) of locus coeruleus stress remain to be determined. In both EAE and multiple sclerosis, diffuse axonal damage or inflammation throughout the CNS could account for our findings in the locus coeruleus. However, this does not appear to be the direct cause since two measurements of multiple sclerosis pathology (increased infiltrates, reduced proteolipid protein staining) were inversely correlated to locus coeruleus neuronal stress. Furthermore, findings that show increased GFAP staining is not diffusely spread throughout the locus coeruleus but is primarily in the dorsal portion suggest a topographically defined location for inflammation and axonal damage. Since this area of the locus coeruleus, as well as the dorsal subcoeruleus, sends projections to the lumbar spinal cord, we propose that focal axonal damage and inflammation occurring in the spinal cord results in loss of necessary trophic support or damage to noradrenergic fibre terminals.
Demonstration of perturbations of noradrenaline levels in both EAE and multiple sclerosis provides a rationale for proposing therapeutic strategies to activate, replace or supplement locus coeruleus-noradrenaline transmission. Such an approach has been validated to some extent by preclinical and clinical investigations. Antidepressants that inhibit noradrenaline reuptake can increase BDNF expression in the hippocampus (Russo-Neustadt et al., 1999), and similarly increasing CNS noradrenaline levels using the selective noradrenaline reuptake inhibitor atomoxetine reduced chemokine and cell adhesion molecule expression following systemic inflammation (O’Sullivan et al., 2010). In EAE studies we showed that raising CNS noradrenaline levels with l-threo-3,4-dihydroxyphenylserine (a direct precursor of noradrenaline) stabilized or improved clinical severity (Simonini et al., 2010). Suggestions of benefit from raising CNS noradrenaline levels in humans come from a limited number of clinical trials. In a small clinical trial (69 patients with multiple sclerosis per arm), treatment with l-phenylalanine (required for noradrenaline synthesis) together with the noradrenaline reuptake inhibitor lofepramine reduced total cumulative disability over 24 weeks as compared with the control group (Wade et al., 2002). In a subset of 15 of those patients, effects of treatment on MRI were observed including a significantly reduced T1 lesion number (Puri et al., 2001). Together, the preclinical and clinical outcomes of these noradrenaline-based approaches suggest that other pharmacological strategies to increase synaptic noradrenaline transmission may hold promise as alternative or additional therapies in multiple sclerosis.
Funding
This work was supported in part by grants from the Department of Veteran Affairs and from Partners for Cures (Chicago, IL). The human brain and spinal fluid resource center, Veterans Affairs West Los Angeles Healthcare Center, is sponsored by the National Institute for Neurological Disorders and Stroke, the National Institute for Mental Health, the National Multiple Sclerosis Society and the Department of Veteran Affairs.
Acknowledgements
We thank Anthony Sharp for assistance with EAE studies; Dr Conwell Anderson for assistance with LC anatomy; and Dr Tibor Valyi-Nagy for neuropathological review of brain sections. Human specimens were provided by Dr Bruce Trapp and the human brain and spinal fluid resource center, Veterans Affairs West Los Angeles Healthcare Center. We thank Dr Marc Marien for helpful discussions throughout the progress of this work and for critical reading of the article.
Glossary
Abbreviations
- BDNF
brain derived neurotrophic factor
- EAE
experimental autoimmune encephalomyelitis
- GFAP
glial fibrillary acidic protein
References
- Adolfsson R, Gottfries CG, Roos BE, Winblad B. Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br J Psychiatry. 1979;135:216–23. doi: 10.1192/bjp.135.3.216. [DOI] [PubMed] [Google Scholar]
- Barkhatova VP, Zavalishin IA, Askarova LS, Shavratskii VK, Demina EG. Changes in neurotransmitters in multiple sclerosis. Neurosci Behav Physiol. 1998;28:341–4. doi: 10.1007/BF02464784. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology. 2009;73:1699–704. doi: 10.1212/WNL.0b013e3181c2937c. [DOI] [PubMed] [Google Scholar]
- Benveniste EN, Huneycutt BS, Shrikant P, Ballestas ME. Second messenger systems in the regulation of cytokines and adhesion molecules in the central nervous system. Brain Behav Immun. 1995;9:304–14. doi: 10.1006/brbi.1995.1029. [DOI] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M, Rossor MN, Iversen LL, Reynolds GP, et al. Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease. Alzheimer Dis Assoc Disord. 1987;1:256–62. doi: 10.1097/00002093-198701040-00005. [DOI] [PubMed] [Google Scholar]
- Carnevale D, De SR, Minghetti L. Microglia-neuron interaction in inflammatory and degenerative diseases: role of cholinergic and noradrenergic systems. CNS Neurol Disord Drug Targets. 2007;6:388–97. doi: 10.2174/187152707783399193. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Alterations in the locus coeruleus in dementias of Alzheimer's and Parkinson's disease. Prog Brain Res. 1991;88:625–30. doi: 10.1016/s0079-6123(08)63839-x. [DOI] [PubMed] [Google Scholar]
- Cosentino M, Zaffaroni M, Marino F, Bombelli R, Ferrari M, Rasini E, et al. Catecholamine production and tyrosine hydroxylase expression in peripheral blood mononuclear cells from multiple sclerosis patients: effect of cell stimulation and possible relevance for activation-induced apoptosis. J Neuroimmunol. 2002;133:233–40. doi: 10.1016/s0165-5728(02)00372-7. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, et al. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci. 1998;18:2808–21. doi: 10.1523/JNEUROSCI.18-08-02808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Reis DJ. Norepinephrine suppresses inducible nitric oxide synthase activity in rat astroglial cultures. J Neurochem. 1993;60:1945–8. doi: 10.1111/j.1471-4159.1993.tb13425.x. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Vayuvegula B, Gupta S, van den Noort S. Norepinephrine inhibits gamma-interferon-induced major histocompatibility class II (Ia) antigen expression on cultured astrocytes via beta-2-adrenergic signal transduction mechanisms. Proc Natl Acad Sci USA. 1988;85:1292–6. doi: 10.1073/pnas.85.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea M, Martinez-Bisbal MC, Marti-Bonmati L, Espert R, Casanova B, Coret F, et al. Spectroscopic axonal damage of the right locus coeruleus relates to selective attention impairment in early stage relapsing-remitting multiple sclerosis. Brain. 2004;127:89–98. doi: 10.1093/brain/awh002. [DOI] [PubMed] [Google Scholar]
- Galea E, Heneka MT, Dello RC, Feinstein DL. Intrinsic regulation of brain inflammatory responses. Cell Mol Neurobiol. 2003;23:625–35. doi: 10.1023/A:1025084415833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL, III, Woodward DJ, McIntire DD, Smith WK, et al. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–76. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- German DC, Nelson O, Liang F, Liang CL, Games D. The PDAPP mouse model of Alzheimer's disease: locus coeruleus neuronal shrinkage. J Comp Neurol. 2005;492:469–76. doi: 10.1002/cne.20744. [DOI] [PubMed] [Google Scholar]
- German DC, Walker BS, Manaye K, Smith WK, Woodward DJ, North AJ. The human locus coeruleus: computer reconstruction of cellular distribution. J Neurosci. 1988;8:1776–88. doi: 10.1523/JNEUROSCI.08-05-01776.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, White CL, III, Sparkman DR. Alzheimer's disease: neurofibrillary tangles in nuclei that project to the cerebral cortex. Neuroscience. 1987;21:305–12. doi: 10.1016/0306-4522(87)90123-0. [DOI] [PubMed] [Google Scholar]
- Harik SI, McGunigal T., Jr The protective influence of the locus ceruleus on the blood-brain barrier. Ann Neurol. 1984;15:568–74. doi: 10.1002/ana.410150609. [DOI] [PubMed] [Google Scholar]
- Helkamaa T, Reenilä I, Tuominen RK, Soinila S, Väänänen A, Tilgmann C, et al. Increased catechol-O-methyltransferase activity and protein expression in OX-42-positive cells in the substantia nigra after lipopolysaccharide microinfusion. Neurochem Int. 2007;51:412–23. doi: 10.1016/j.neuint.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O'Banion MK, et al. Noradrenergic depletion potentiates beta -amyloid-induced cortical inflammation: implications for Alzheimer's disease. J Neurosci. 2002;22:2434–42. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Ramanathan M, Jacobs AH, Dumitrescu-Ozimek L, Bilkei-Gorzo A, Debeir T, et al. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci. 2006;26:1343–54. doi: 10.1523/JNEUROSCI.4236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann-Kleiter N, Gruber T, Lutz-Nicoladoni C, Thuille N, Fresser F, Labi V, et al. The nuclear orphan receptor NR2F6 suppresses lymphocyte activation and T helper 17-dependent autoimmunity. Immunity. 2008;29:205–16. doi: 10.1016/j.immuni.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm PC, Rodriguez FJ, Kresse A, Canals JM, Silos-Santiago I, Arenas E. Crucial role of TrkB ligands in the survival and phenotypic differentiation of developing locus coeruleus noradrenergic neurons. Development. 2003;130:3535–45. doi: 10.1242/dev.00565. [DOI] [PubMed] [Google Scholar]
- Holstege JC, Bongers CM. Ultrastructural aspects of the coeruleo-spinal projection. Prog Brain Res. 1991;88:143–56. doi: 10.1016/s0079-6123(08)63804-2. [DOI] [PubMed] [Google Scholar]
- Hoogendijk WJ, Pool CW, Troost D, van ZE, Swaab DF. Image analyser-assisted morphometry of the locus coeruleus in Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis. Brain. 1995;118(Pt 1):131–43. doi: 10.1093/brain/118.1.131. [DOI] [PubMed] [Google Scholar]
- Jardanhazi-Kurutz D, Kummer MP, Terwel D, Vogel K, Dyrks T, Thiele A, et al. Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem Int. 2010;57:375–82. doi: 10.1016/j.neuint.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Kalinin S, Feinstein DL, Xu HL, Huesa G, Pelligrino DA, Galea E. Degeneration of noradrenergic fibres from the locus coeruleus causes tight-junction disorganisation in the rat brain. Eur J Neurosci. 2006a;24:3393–400. doi: 10.1111/j.1460-9568.2006.05223.x. [DOI] [PubMed] [Google Scholar]
- Kalinin S, Gavrilyuk V, Polak PE, Vasser R, Zhao J, Heneka MT, et al. Noradrenaline deficiency in brain increases beta-amyloid plaque burden in an animal model of Alzheimer's disease. Neurobiol Aging. 2007;28:1206–14. doi: 10.1016/j.neurobiolaging.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kalinin S, Polak PE, Madrigal JL, Gavrilyuk V, Sharp A, Chauhan N, et al. Beta-amyloid-dependent expression of NOS2 in neurons: prevention by an alpha2-adrenergic antagonist. Antioxid Redox Signal. 2006b;8:873–83. doi: 10.1089/ars.2006.8.873. [DOI] [PubMed] [Google Scholar]
- Karaszewski JW, Reder AT, Anlar B, Arnason GW. Increased high affinity beta-adrenergic receptor densities and cyclic AMP responses of CD8 cells in multiple sclerosis. J Neuroimmunol. 1993;43:1–7. doi: 10.1016/0165-5728(93)90068-a. [DOI] [PubMed] [Google Scholar]
- Khoruzhaia TA, Saakov BA. (Change in monoamine content and monoamine oxidase activity in brain structures during experimental allergic encephalomyelitis) Biull Eksp Biol Med. 1975;79:80–2. [PubMed] [Google Scholar]
- Krenger W, Honegger CG, Feurer C, Cammisuli S. Changes of neurotransmitter systems in chronic relapsing experimental allergic encephalomyelitis in rat brain and spinal cord. J Neurochem. 1986;47:1247–54. doi: 10.1111/j.1471-4159.1986.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Lyness SA, Zarow C, Chui HC. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: a meta-analysis. Neurobiol Aging. 2003;24:1–23. doi: 10.1016/s0197-4580(02)00057-x. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Feinstein DL, Dello RC. Norepinephrine protects cortical neurons against microglial-induced cell death. J Neurosci Res. 2005;81:390–6. doi: 10.1002/jnr.20481. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Kalinin S, Richardson JC, Feinstein DL. Neuroprotective actions of noradrenaline: effects on glutathione synthesis and activation of peroxisome proliferator activated receptor delta. J Neurochem. 2007;103:2092–101. doi: 10.1111/j.1471-4159.2007.04888.x. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Russo CD, Gavrilyuk V, Feinstein DL. Effects of noradrenaline on neuronal NOS2 expression and viability. Antioxid Redox Signal. 2006;8:885–92. doi: 10.1089/ars.2006.8.885. [DOI] [PubMed] [Google Scholar]
- Mann DM. The locus coeruleus and its possible role in ageing and degenerative disease of the human central nervous system. Mech Ageing Dev. 1983;23:73–94. doi: 10.1016/0047-6374(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Mann DM, Yates PO. Pathological basis for neurotransmitter changes in Parkinson's disease. Neuropathol Appl Neurobiol. 1983;9:3–19. doi: 10.1111/j.1365-2990.1983.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Mann DM, Yates PO, Hawkes J. The pathology of the human locus ceruleus. Clin Neuropathol. 1983;2:1–7. [PubMed] [Google Scholar]
- Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004;45:38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Markianos M, Koutsis G, Evangelopoulos ME, Mandellos D, Karahalios G, Sfagos C. Relationship of CSF neurotransmitter metabolite levels to disease severity and disability in multiple sclerosis. J Neurochem. 2009;108:158–64. doi: 10.1111/j.1471-4159.2008.05750.x. [DOI] [PubMed] [Google Scholar]
- McNamee EN, Ryan KM, Griffin EW, González-Reyes RE, Ryan KJ, Harkin A, et al. Noradrenaline acting at central beta-adrenoceptors induces interleukin-10 and suppressor of cytokine signaling-3 expression in rat brain: Implications for neurodegeneration. Brain Behav Immun. 2010;24:660–71. doi: 10.1016/j.bbi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- O’Neil JN, Mouton PR, Tizabi Y, Ottinger MA, Lei DL, Ingram DK, et al. Catecholaminergic neuronal loss in locus coeruleus of aged female dtg APP/PS1 mice. J Chem Neuroanat. 2007;34:102–7. doi: 10.1016/j.jchemneu.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan JB, Ryan KM, Curtin NM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int J Neuropsychopharmacol. 2009;12:687–99. doi: 10.1017/S146114570800967X. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JB, Ryan KM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors inhibit expression of chemokines IP-10 and RANTES and cell adhesion molecules VCAM-1 and ICAM-1 in the CNS following a systemic inflammatory challenge. J Neuroimmunol. 2010;220:34–42. doi: 10.1016/j.jneuroim.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Pahan K, Namboodiri AM, Sheikh FG, Smith BT, Singh I. Increasing cAMP attenuates induction of inducible nitric-oxide synthase in rat primary astrocytes. J Biol Chem. 1997;272:7786–91. doi: 10.1074/jbc.272.12.7786. [DOI] [PubMed] [Google Scholar]
- Palmer AM, DeKosky ST. Monoamine neurons in aging and Alzheimer's disease. J Neural Transm Gen Sect. 1993;91:135–59. doi: 10.1007/BF01245229. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Mai JK. The Human Nervous System. San Diego, California, USA: Elsevier Academic Press; 2004. [Google Scholar]
- Pearson RC, Sofroniew MV, Cuello AC, Powell TP, Eckenstein F, Esiri MM, et al. Persistence of cholinergic neurons in the basal nucleus in a brain with senile dementia of the Alzheimer's type demonstrated by immunohistochemical staining for choline acetyltransferase. Brain Res. 1983;289:375–9. doi: 10.1016/0006-8993(83)90046-x. [DOI] [PubMed] [Google Scholar]
- Proudfit HK, Clark FM. The projections of locus coeruleus neurons to the spinal cord. Prog Brain Res. 1991;88:123–41. doi: 10.1016/s0079-6123(08)63803-0. [DOI] [PubMed] [Google Scholar]
- Pugh PL, Vidgeon-Hart MP, Ashmeade T, Culbert AA, Seymour Z, Perren MJ, et al. Repeated administration of the noradrenergic neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) modulates neuroinflammation and amyloid plaque load in mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. J Neuroinflammation. 2007;4:8. doi: 10.1186/1742-2094-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri BK, Bydder GM, Chaudhuri KR, Al Saffar BY, Curati WL, White SJ, et al. MRI changes in multiple sclerosis following treatment with lofepramine and l-phenylalanine. Neuroreport. 2001;12:1821–4. doi: 10.1097/00001756-200107030-00012. [DOI] [PubMed] [Google Scholar]
- Rajda C, Bencsik K, Fuvesi J, Seres E, Vecsei L, Bergquist J. The norepinephrine level is decreased in the lymphocytes of long-term interferon-beta-treated multiple sclerosis patients. Mult Scler. 2006;12:265–70. doi: 10.1191/135248506ms1269oa. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson's disease. Biochem Pharmacol. 2007;74:177–90. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679–82. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008;6:254–85. doi: 10.2174/157015908785777193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–23. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Segal BM. Th17 cells in autoimmune demyelinating disease. Semin Immunopathol. 2010;32:71–7. doi: 10.1007/s00281-009-0186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonini MV, Polak PE, Sharp A, McGuire S, Galea E, Feinstein DL. Increasing CNS noradrenaline reduces EAE severity. J Neuroimmune Pharmacol. 2010;5:252–9. doi: 10.1007/s11481-009-9182-2. [DOI] [PubMed] [Google Scholar]
- Szabo C, Hasko G, Zingarelli B, Németh ZH, Salzman AL, Kvetan V, et al. Isoproterenol regulates tumour necrosis factor, interleukin-10, interleukin-6 and nitric oxide produciton and protects against the development of vascular hyporeactivity in endotoxaemia. Immunology. 1997;90:95–100. doi: 10.1046/j.1365-2567.1997.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, Van DD, White SS, Franklin A, Staufenbiel M, De Deyn PP. Age-dependent changes in noradrenergic locus coeruleus system in wild-type and APP23 transgenic mice. Neurosci Lett. 2009;463:93–7. doi: 10.1016/j.neulet.2009.07.055. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Takahashi S, Oki J. Developmental regulation of spinal motoneurons by monoaminergic nerve fibers. J Peripher Nerv Syst. 1997;2:323–32. [PubMed] [Google Scholar]
- Traver S, Marien M, Martin E, Hirsch EC, Michel PP. The phenotypic differentiation of locus coeruleus noradrenergic neurons mediated by BDNF is enhanced by corticotropin releasing factor through the activation of a cAMP-dependent signaling pathway. Mol Pharmacol. 2006;70:30–40. doi: 10.1124/mol.106.022715. [DOI] [PubMed] [Google Scholar]
- Troadec JD, Marien M, Darios F, Hartmann A, Ruberg M, Colpaert F, et al. Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. J Neurochem. 2001;79:200–10. doi: 10.1046/j.1471-4159.2001.00556.x. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Atkinson B, Lee VM. An immunocytochemical study of normal and abnormal human cerebrospinal fluid with monoclonal antibodies to glial fibrillary acidic protein. Acta Cytol. 1986;30:235–9. [PubMed] [Google Scholar]
- Wade DT, Young CA, Chaudhuri KR, Davidson DL. A randomised placebo controlled exploratory study of vitamin B-12, lofepramine, and L-phenylalanine (the ‘Cari Loder regime’) in the treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;73:246–9. doi: 10.1136/jnnp.73.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke M, Oster H, Revelli JP, Alvarez-Bolado G, Eichele G. Abnormal development of the locus coeruleus in Ear2(Nr2f6)-deficient mice impairs the functionality of the forebrain clock and affects nociception. Genes Dev. 2005;19:614–25. doi: 10.1101/gad.317905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D. Functional consequences of locus coeruleus degeneration in Alzheimer's disease. Curr Alzheimer Res. 2008;5:342–5. doi: 10.2174/156720508784533286. [DOI] [PubMed] [Google Scholar]
- White SR, Bhatnagar RK, Bardo MT. Norepinephrine depletion in the spinal cord gray matter of rats with experimental allergic encephalomyelitis. J Neurochem. 1983;40:1771–3. doi: 10.1111/j.1471-4159.1983.tb08156.x. [DOI] [PubMed] [Google Scholar]
- Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci. 1992;12:4793–9. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–41. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- Zoukos Y, Leonard JP, Thomaides T, Thompson AJ, Cuzner ML. beta-Adrenergic receptor density and function of peripheral blood mononuclear cells are increased in multiple sclerosis: a regulatory role for cortisol and interleukin-1. Ann Neurol. 1992;31:657–62. doi: 10.1002/ana.410310614. [DOI] [PubMed] [Google Scholar]