Abstract
Acetic acid produces an irritating sensation that can be attributed to activation of nociceptors within the trigeminal ganglion that innervate the nasal or oral cavities. These sensory neurons sense a diverse array of noxious agents in the environment, allowing animals to actively avoid tissue damage. Although receptor mechanisms have been identified for many noxious chemicals, the mechanisms by which animals detect weak acids, such as acetic acid, are less well understood. Weak acids are only partially dissociated at neutral pH and, as such, some can cross the cell membrane, acidifying the cell cytosol. The nociceptor ion channel TRPA1 is activated by CO2, through gating of the channel by intracellular protons, making it a candidate to more generally mediate sensory responses to weak acids. To test this possibility, we measured responses to weak acids from heterologously expressed TRPA1 channels and trigeminal neurons with patch clamp recording and Ca2+ microfluorometry. Our results show that heterologously expressed TRPA1 currents can be induced by a series of weak organic acids, including acetic, propionic, formic, and lactic acid, but not by strong acids. Notably, the degree of channel activation was predicted by the degree of intracellular acidification produced by each acid, suggesting that intracellular protons are the proximate stimulus that gates the channel. Responses to weak acids produced a Ca2+-independent inactivation that precluded further activation by weak acids or reactive chemicals, whereas preactivation by reactive electrophiles sensitized TRPA1 channels to weak acids. Importantly, responses of trigeminal neurons to weak acids were highly overrepresented in the subpopulation of TRPA1-expressing neurons and were severely reduced in neurons from TRPA1 knockout mice. We conclude that TRPA1 is a general sensor for weak acids that produce intracellular acidification and suggest that it functions within the pain pathway to mediate sensitivity to cellular acidosis.
INTRODUCTION
Acetic acid (also known as ethanoic acid) consumed as vinegar produces both sour and irritating sensations and has been prized as a condiment since ancient times. Recent evidence suggests that the taste of acetic acid is mediated by a subset of taste cells, defined by expression of the channel protein PKD2L1, that reside in taste buds on the tongue and palate epithelium (Huang et al., 2006; Ishimaru et al., 2006; LopezJimenez et al., 2006). In contrast, the irritating sensation produced by weak acids, such as acetic acid, is likely mediated by sensory neurons with cell bodies in the trigeminal ganglion (TG) and whose processes terminate in the nasal and oral cavity (Brand, 2006). Consistent with activation of nociceptors, exposure to acetic acid at concentrations as low as 10 ppm produces a feeling of irritation in humans (Ernstgård et al., 2006), and in rats intraperitoneal injection of acetic acid produces a writhing that has been the basis for screening of putative analgesic agents (Ogawa and Kotani, 1987). Direct responses of nociceptors to weak acids have been measured using nerve recording, which showed increases in activity in response to a variety of acids, including carbonic acid, formed when CO2 is dissolved, and propanoic acid (also called propionic acid [PA]), a food preservative (Silver and Moulton, 1982).
The defining characteristic of weak acids is that they do not fully dissociate in solution. In the undissociated form, weak acids can more easily penetrate the epithelium to reach free nerve endings and can cross the cell membrane of nociceptive afferents to acidify the cell cytosol. Thus, the nociceptive effects of weak acids could be a result of receptors that detect extracellular protons or ones that detects intracellular protons. Within nociceptors, ion channels that are sensitive to extracellular protons and might mediate responses to weak acids include the acid-sensing ion channels (ASICs) (Waldmann and Lazdunski, 1998) and the capsaicin (Cap) receptor, TRPV1. TRPV1 is activated by extracellular protons (Caterina et al., 1997; Tominaga et al., 1998), but acetic acid does not specifically activate TRPV1 in heterologous cells (Silver et al., 2006), and knockout of TRPV1 does not diminish respiratory changes in response to inspired acetic acid (Symanowicz et al., 2004). ASIC3, which is the predominant ASIC isoform expressed in nociceptors, has been proposed to mediate the response to tissue acidosis that accompanies cardiac ischemia (Immke and McCleskey, 2001; Sutherland et al., 2001). However, on the acetic acid–induced writhing assay ASIC3−/−, animals show an increase in writhing rather than a decrease (Chen et al., 2002).
Another candidate to mediate the irritant sensation of weak acids is the ion channel TRPA1 (Story et al., 2003). TRPA1 is coexpressed with TRPV1 (Story et al., 2003) in nociceptors that innervate the nasal and respiratory epithelia, and it serves, along with TRPV1, as one of the principle detectors for environmental irritants that cause pain and inflammation (Bandell et al., 2004; Jordt et al., 2004; Bautista et al., 2006; Kwan et al., 2006). Agents that activate TRPA1 include plant products, such as mustard oil (MO), cinnamaldehyde (Cin), industrial products such as formaldehyde and acrolein, and products of oxidative stress (Patapoutian et al., 2009). These TRPA1 agonists are reactive electrophiles that bind covalently to amino terminal cysteines (Hinman et al., 2006; Macpherson et al., 2007). Recently, we found that CO2, which is not a reactive electrophile, could activate TRPA1 and that this activation could be attributed to direct gating of the channel by intracellular protons. Because carboxylic acids such as acetic acid can also diffuse into the cell and produce intracellular acidification, TRPA1 may also serve as a sensor for this class of compounds.
Here, we show that TRPA1 is both necessary and sufficient to mediate the response of nociceptors to weak acids. When expressed in heterologous cells, TRPA1 could be activated by acetic acid and other weak acids, and the magnitude of current activation was strongly correlated with the degree of intracellular acidification. In trigeminal sensory neurons, responses to weak acids were correlated with responses to TRPA1 agonists and were dependent on a functional TRPA1 gene. We conclude that activation of TRPA1 in response to carboxylic acids and CO2 shares a common mechanism based on gating of the channel by intracellular protons, and suggest that TRPA1 functions within the trigeminal system to mediate aversive responses to ingested or inspired acids.
MATERIALS AND METHODS
Nerve recording
24 adult male Sprague-Dawley rats (250–400 g) were used in this study (Harlan). All experimental procedures were approved by Wake Forest University’s Animal Care and Use Committee. The procedure for recording from the rat ethmoid nerve has been described previously (Silver et al., 2006). In brief, animals were anesthetized with an intraperitoneal injection of urethane (1.0 g/kg ethyl carbamate), and a tracheal tube was inserted toward the lungs to allow the rat to breathe room air. A second tube was inserted up through the trachea to the nasopharynx. Rat Ringer’s solution (5.4 mM KCl, 5 mM HEPES, 135 mM NaCl, and 1.8 mM CaCl2, pH 7.2) was pumped through this tube and allowed to drip from the nose. The rat was secured in a head holder, and the ethmoid nerve was exposed and placed on two platinum iridium wire hook electrodes. The cavity was then filled with halocarbon oil (Sigma-Aldrich) to prevent the nerve from drying out and to provide electrical insulation.
Multiunit neural activity was amplified (P-511; Grass Technologies) and monitored using an audio monitor (AM-8; Grass Technologies). The raw neural output from the ethmoid nerve bundle was digitized (MP100; Biopac Systems, Inc.) and recorded using the AcqKnowledge data acquisition program (v3.7.1; Biopac Systems, Inc.) and integrated using a short time-averaging circuit with a time constant of 0.5 s.
Rat Ringer’s solution, at room temperature, was delivered continuously by a pump through the nasophayngeal tube into the rat’s nasal cavity at a rate of 10 ml/min. Compounds added to rat Ringer’s solution were delivered in 0.5-mL aliquots injected into the solution flow, with an 8-min washout period between each test stimuli. Test stimuli were HCl, formic acid, acetic acid, and PA. The responses to the acid test stimuli were standardized to the response to 100 mM cyclohexanone.
cDNAs and expression in HEK-293 cells
For most experiments, we used a yellow fluorescent protein (YFP) fusion of rat TRPA1 (rTRPA1; Wang et al., 2008). Where stated explicitly, the following constructs were used (cotransfected at 20:1 with green fluorescent protein): native rTRPA1, human TRPA1, mouse TRPA1, and rat TRPV1. Point mutations were generated by using the Quick-Change Mutagenesis kit (Agilent Technologies) and verified by sequencing (Macrogen, Inc.). All constructs were transiently transfected into HEK-293 cells using TransIT-LT1 Transfection Reagent (Mirus Bio LLC). Recordings were performed ∼24–48 h after transfection at room temperature.
Culture of TG neurons
Experimental procedures were approved by the Institutional Animal Care and Use committee of the University of Southern California. Mice were derived from crosses of TRPA1−/− (B6;129P-Trpa1tm1Kykw/J) and wild-type (B6129PF2/J) mice (The Jackson Laboratory) and were genotyped by PCR. TG neurons were dissociated from postnatal 1–8-wk-old mice as described previously (Wang et al., 2010). In brief, the isolated trigeminal ganglia were sequentially incubated in 0.25% collagenase type I for 40∼60 min at 37°C and in 0.05% trypsin for 1 min at 37°C. The isolated cells were plated onto glass coverslips coated with matrigel and incubated at 37°C for 24–48 h in culture media supplemented with 100 ng/ml nerve growth factor (NGF). Data shown represent the results from three to five cultures from 6–10 mice.
Patch clamp recording
Patch clamp electrophysiology was performed as described previously (Liu and Liman, 2003; Zhang et al., 2007). For whole cell recordings, the membrane potential was ramped from −80 to +80 mV (1 V/s). Extracellular solution was exchanged either by moving a linear array of microperfusion pipes (Warner Instruments) or by gravity flow through the chamber. For most experiments, except where otherwise noted, responses were quantified as the change in the magnitude of the current as compared with current before the agonist delivery.
Intracellular pH and Ca2+ imaging
Imaging of changes in pH and in intracellular Ca2+ was essentially as described previously (Zhang et al., 2007). To measure changes in intracellular pH, Oregon Green 488 carboxylic acid diacetate (carboxy-DFFDA) was loaded into cells, and changes in pH were measured as the decrease in fluorescent emission at 520 nm upon excitation at 488 nm. Carboxy-DFFDA was chosen based on its acidic pKa (4.7), which allowed us to detect the responses to the strongest stimuli without saturation of the indicator. To measure changes in intracellular Ca2+, cells were loaded with Fura-2 AM, and Ca2+ levels were measured from the ratio of emission in response to excitation at 340 and 380 nM. All cells showed nonspecific responses to PA (see Fig. S1), which may be attributed to effects of intracellular acidification on emission by the dye or on Ca2+ handling (Lattanzio and Bartschat, 1991; Martínez-Zaguilán et al., 1996). Responses were, therefore, categorized as positive if the change in F340/F380 after the application of agonist was more than four standard deviations greater than the average nonspecific response (0.365 AU). The same threshold was applied to all agonists and all conditions for consistency.
Solutions
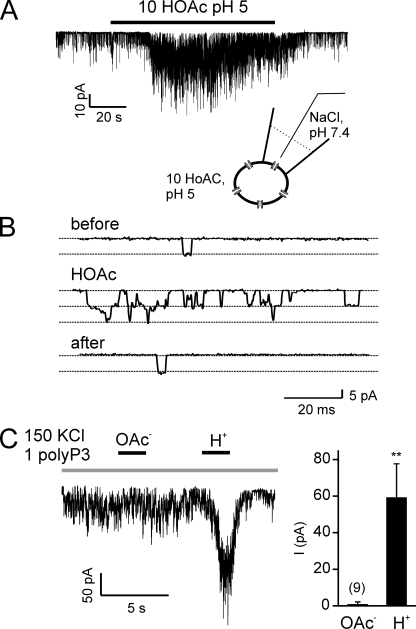
For Ca2+-imaging experiments, the bath solution was: 150 mM NaCl, 10 mM HEPES, and 2 mM CaCl2, pH 7.4. HEPES-buffered acidic solution was bath solution adjusted to pH 6.5. PA solution contained: 100 mM PA, 60 mM NaCl, and 2 mM CaCl2, pH 6.5. High K+ solution was 150 mM KCl, 10 mM HEPES, and 2 mM CaCl2, pH 7.4. For intracellular pH imaging and whole cell and cell-attached recordings, the bath solution was (unless otherwise stated): 150 mM NaCl, 0.5 mM EGTA, and 10 mM HEPES, pH 7.4. Acidic solutions contained: 150 mM NaCl, 0.5 mM EGTA, and either 10 mM HEPES, pH 5, 10 mM MES, pH 5, or 10 mM acetic acid, pH 5, 6, or 7. Other solutions containing weak acids were similarly composed, and where the concentration of the acid was >10 mM, the concentration of NaCl was decreased accordingly. Internal solution contained: 145 mM CsCl, 5 mM EGTA, 3 mM CaCl2 (100 nM free Ca2+), 2 mM MgATP, and 10 mM HEPES, pH 7.4 with CsOH. To exclude the possibility that the observed effects were a result of release of Ca2+ from the chelator in response to intracellular acidification, in some experiments, Ca2+ and MgATP were excluded from the internal solution (as stated and Fig. 6). For cell-attached and excised patch experiments, the pipette solution contained: 150 mM NaCl, 10 mM HEPES, and 0.5 mM EGTA, pH 7.4. For excised patch experiments, solution applied to the cytoplasmic side of the cell contained: 150 mM KCl, 0.5 mM EGTA, and 1 mM pentasodium tripolyphosphate hexahydrate (polyP3) (Kim and Cavanaugh, 2007) buffered with either 10 mM HEPES, pH 7.4, or 10 mM MES, pH 5.5, or 10 mM acetic acid, pH 7.3.
Figure 6.
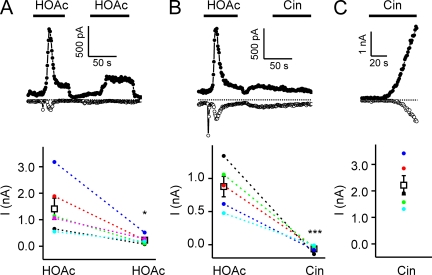
Responses of TRPA1 to weak acids self-desensitize and cross-desensitize responses to Cin. Whole cell currents evoked in HEK-293 cells expressing TRPA1. (A) TRPA1 currents evoked in response to 10 mM of acetic acid, pH 5, decayed after activation and could not be evoked again by acetic acid, indicating that the channels had entered an inactivated state. (B) After inactivation by 10 mM of acetic acid, pH 5, TRPA1 currents could not be activated by 100 µM Cin, indicating that acetic acid could cross-desensitize responses to Cin. (C) 100 µM Cin elicited large currents in TRPA1-expressing cells that were not preexposed to acetic acid. Currents were recorded in the absence of extracellular Ca2+. Average data are shown below each representative trace. Data are represented by the mean ± SEM. Significance was determined with the two-tailed paired (A; comparison between responses to the first and second application of acetic acid) or unpaired (B and C; comparison between responses to Cin with or without preexposure to acetic acid) Student’s t test. *, P < 0.05; ***, P < 0.001.
Chemicals
Cin, MO, formic acid, PA, lactic acid, tartaric aid, and citric acid were purchased from Sigma-Aldrich. Acetic acid was purchased from EMD. Carboxy-DFFDA and Fura-2 were from Invitrogen. Collagenase type I, DMEM, F-12, trypsin, gentamicin, and NGF were from Invitrogen. Matrigel was from BD.
Online supplemental material
Fig. S1 shows the distribution of response magnitudes measured in calcium imaging from trigeminal neurons and transfected HEK-293 cells. All cells showed nonspecific responses to PA, which may be attributed to effects of intracellular acidification on emission by the dye or on Ca2+ handling (Lattanzio and Bartschat, 1991; Martínez-Zaguilán et al., 1996). Responses were, therefore, categorized as positive if the change in F340/F380 after the application of agonist was more than four standard deviations greater than the average nonspecific response (0.365 AU). The same threshold was applied to all agonists and all conditions for consistency. Fig. S1 is available at http://www.jgp.org/cgi/content/full/jgp.201110615/DC1.
RESULTS
Nerve responses to carboxylic acids
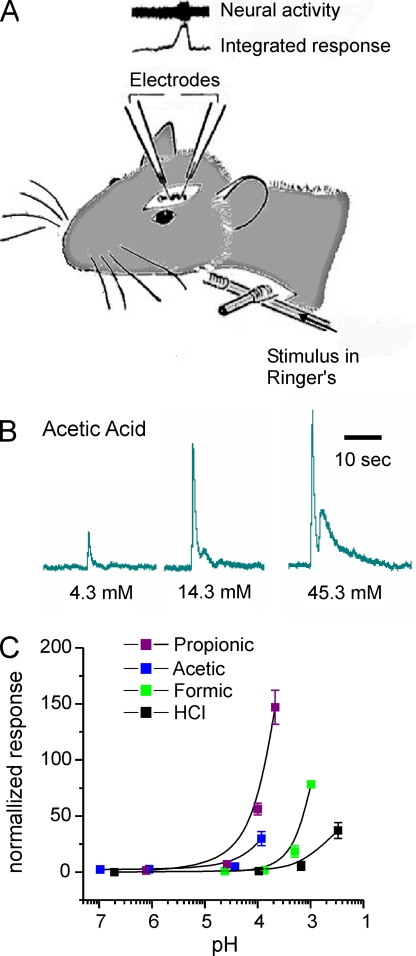
Previous experiments have showed that nerve fibers that innervate the nasal and oral cavities can be activated by weak acids (Silver and Moulton, 1982; Bryant and Moore, 1995), but whether this activity can be attributed to changes in extracellular pH or to the specific properties of weak acids has not been well defined. To investigate the receptor mechanisms that contribute to the irritant sensation produced by weak acids, we measured responses from the ethmoid nerve of the rat, a branch of the ophthalmic division of the trigeminal nerve, which innervates the anterior nasal cavity (Fig. 1 A). Test stimuli were delivered to the rat’s nasal cavity through a nasopharyngeal tube. The stimuli chosen were a series of carboxylic acids that differed in chain length: formic acid, acetic acid, and PA. HCl was used to test the effect of extracellular protons alone. Each acid was tested at increasing concentration (decreasing pH). Representative data shown in Fig. 1 B demonstrate that the response to acetic acid is robust and dose dependent. To compare responses across stimuli, we normalized the data to the response evoked by cyclohexanone and plotted the normalized response as a function of pH. As can be seen in Fig. 1 C, the response magnitude increased as the pH of the solution decreased for each acid tested. However, the relationship between pH and response magnitude was not invariant across acids; propionic and acetic acid elicited much larger responses than did formic acid or HCl at the same pH (approximately pH 4). In addition, we noted a trend for the response magnitude to increase as the chain length increased, suggesting that degree of membrane permeability may be a partial determinant of the response magnitude. Collectively, these data indicate that the irritating sensation produced by weak acids cannot be solely attributed to extracellular acidification.
Figure 1.
Responses of the trigeminal nerve to acetic acid cannot be attributed to changes in extracellular pH. (A) Diagram showing the method of stimulus delivery and position of the recording electrode. (B) Integrated nerve responses to acetic acid applied at increasing concentrations as indicated. (C) Relationship between the magnitude of the nerve response and the extracellular pH for each acid tested. Responses were normalized to the response to cyclohexanone applied immediately before and after exposure to each acid. Data represent the mean ± SEM.
TRPA1 is activated by carboxylic acids
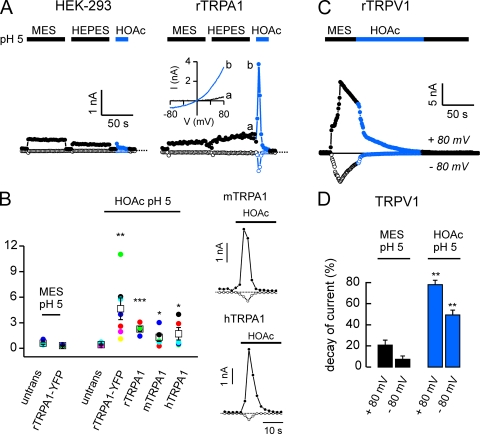
Many weak acids, in the protonated form, can diffuse across cell membrane and cause intracellular acidification. Our previous results showed that the ion channel TRPA1, which is expressed in a subset of trigeminal neurons, is activated by intracellular protons (Wang et al., 2010), making it a possible sensor for weak acids in the nociceptive pathway. To determine whether TRPA1 could be activated by weak acids, we expressed rTRPA1 in HEK-293 cells and measured responses to applied acids with whole cell patch clamp electrophysiology. TRPA1 currents were strongly and rapidly activated by acetic acid (HOAc), pH 5.0 (Fig. 2, A and B; I+80 = 4,642 pA ± 1,276; n = 7). The response to acetic acid, pH 5.0, was significantly larger than the response to a solution of pH 5.0 that did not contain acetic acid, indicating that it could not be attributed to the effects of protons binding on the outside of the cell. This current was not observed in untransfected HEK-293 cells, where instead acetic acid evoked a rapidly inactivating Na+-selective ASIC current and a small noninactivating Cl− current (Fig. 2, A and B). The acetic acid–activated current in TRPA1-expressing HEK-293 cells reversed at −3.6 ± 1.0 mV (n = 7), as expected for a nonselective cation channel, and showed mild outward rectification (−I+80/I−80 = 3.3 ± 0.4; n = 7; Fig. 2 A). As these experiments were performed with rTRPA1 fused to YFP, we confirmed that acetic acid also activated an rTRPA1 construct not fused to YFP (Fig. 2 B). Acetic acid also activated large currents in a subset of HEK cells transfected with human TRPA1 (hTRPA1) or mouse TRPA1 (mTRPA1; Fig. 2 B), indicating that activation of the channel by acetic acid is a conserved property of the channel and may underlie irritant effects of weak acids in humans as well as in rodents.
Figure 2.
Acetic acid activates TRPA1 but not TRPV1. Whole cell currents evoked in response to acids from untransfected HEK-293 cells or HEK-293 cells transfected with YFP-rTRPA1 or rTRPV1 as indicated. (A) 10 mM of acetic acid (HOAc) titrated to pH 5, but not 10 mM MES, pH 5, or 10 mM HEPES, pH 5, activated a large, outwardly rectifying current in TRPA1-expressing cells (right). Current–voltage relationship obtained from ramp depolarization (1V/s) at the time indicated shows reversal of the current near 0 mV and mild outward rectification. Small currents were evoked in untransfected cells in response to pH 5.0 solution buffered with MES, HEPES, or acetic acid (left). (B) Average data from experiments as in A show that the activation of TRPA1 by acetic acid is conserved in humans and rodents. Significance (compared with activation of untransfected cells by acetic acid) was determined with the one-tailed Student’s t test (n = 4–7). Representative traces for responses from mouse or human transfected cells are shown on the right. (C) 10 mM MES, pH 5.0, activated a large TRPV1 current, which was inhibited by subsequent application of 10 mM of acetic acid, pH 5. (D) Average data (n = 10) from experiments as in C, where current decay was measured during two 10-s windows immediately before and after acetic acid application. Significance was determined by the Wilcoxon signed-rank test. Average data are represented by the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In contrast and as reported previously (Caterina et al., 1997; Tominaga et al., 1998), TRPV1 channels expressed in HEK-293 cells were strongly activated by extracellular saline buffered to pH 5.0 (Fig. 2 C). The addition of acetic acid at pH 5.0 produced no further activation of the currents; instead, we observed an inhibition of the outward and inward currents (Fig. 2, C and D). The inhibition of TRPV1 was poorly reversible, indicating that acetic acid promoted entry into an inactivated state.
Because intracellular Ca2+ can be released from the chelator EGTA at acidic pH and potentially activate or inactivate TRPA1 (Wang et al., 2008), we tested whether the activation of TRPA1 by weak acids could be attributed to an increase in intracellular Ca2+ levels. The magnitude of the currents elicited by 10 mM acetic acid, pH 5, was no different when Ca2+ was excluded from the pipette as when it was included (2.4 ± 0.5 nA and n = 6 compared with 2.3 ± 0.6 nA and n = 5 at +80 mV). Thus, the activation of TRPA1 by intracellular acidification in response to weak acids is not secondary to an effect of intracellular acidification on intracellular Ca2+ levels.
TRPA1 is activated by conditions that produce intracellular acidification
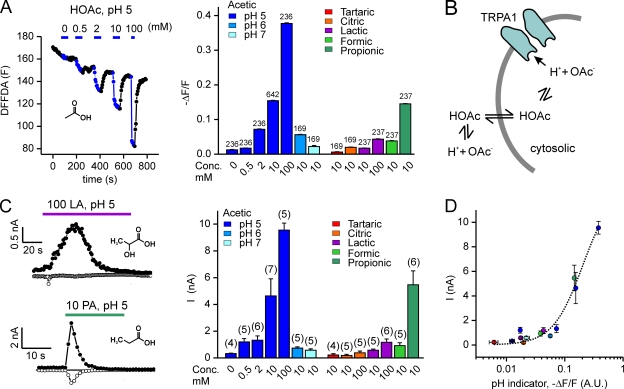
We previously reported that CO2 activates TRPA1 by acidifying the cell cytosol (Wang et al., 2010). Acetic acid and other carboxylic acids are well known to produce intracellular acidification and thus might activate TRPA1 through the same mechanism. Acetic acid has a pKa of 4.88, and at pH 5.0, a substantial portion of the acid (∼37%) is in the protonated membrane-permeable form. In contrast, the standard pH buffers HEPES and MES are both zwitterionic, not membrane permeable at pH 5.0, and not expected to produce intracellular acidification. We confirmed that under the conditions of our experiments, acetic acid produces intracellular acidification by measuring the response of HEK cells loaded with a pH-sensitive fluorescent probe, carboxy-DFFDA. Acidic extracellular solution containing acetic acid and not HEPES or MES produced robust intracellular acidification. Moreover, as expected, increasing the concentration of acetic acid, while holding the extracellular pH constant, produced a more robust and rapid acidification of the cell cytosol. Also, as expected, at more alkaline pH (pH 6 or pH 7), the same concentration of acetic acid produced weaker intracellular acidification (Fig. 3 A).
Figure 3.
TRPA1 is activated by weak acids that acidify the cell cytosol. (A) Changes in emission from the pH-sensitive fluorescent probe carboxy-DFFDA in HEK-293 cells in response to acetic acid at varying concentrations (0, 0.5, 2, 10, and 100 mM at pH 5) and pH (10 mM; pH 5, 6, and 7) and to a panel of carboxylic acids (10 or 100 mM; pH 5). A representative experiment is shown on the left. (B) Proposed model for how acetic acid might activate TRPA1. (C) Currents evoked in HEK-293 cells expressing TRPA1 (peak magnitude at + 80 mV) in response to acetic acid at varying concentrations and pH, and to other carboxylic acids as indicated. Same color scheme as in A. Data for 0 and 10 mM of acetic acid, pH 5, were reproduced from Fig. 2 B. Representative traces of current activation by 100 mM of lactic acid (LA), pH 5, and 10 mM PA, pH 5, are shown on the left. Inset shows the structures of the acids. (D) The magnitude of the TRPA1 current plotted as a function of the change in fluorescence of the pH-sensitive dye carboxy-DFFDA. Colors correspond to the scheme in A and B. The correlation between the change in fluorescence and the magnitude of the TRPA1 current suggests that intracellular pH is the proximate stimulus that gates TRPA1 in response to extracellularly applied weak acids. Data are represented by the mean ± SEM.
To assess whether intracellular acidification accounts for the activation of TRPA1 by acetic acid (Fig. 3 B), we measured responses of TRPA1 to acetic acid at varying pHs and concentrations. At a constant pH (pH 5), increasing the concentration of acetic acid from 10 to 100 mM produced both a larger and quicker activation of TRPA1 (9,560 ± 520 pA; time to peak = 0.9 ± 0 s; n = 5; Fig. 3 C). Conversely, decreasing the concentration of acetic acid from 10 to 2 or 0.5 mM while holding the pH constant produced a smaller and slower activation of TRPA1 (1,324 ± 325 pA, time to peak = 11 ± 1 s, and n = 6; and 1,203 ± 240 pA, time to peak 48 ± 6 s, and n = 5 at +80 mV, respectively). Moreover, when the concentration of acetic acid was held constant and the pH was made more basic (pH 6 and pH 7), we observed more modest activation of TRPA1 (739 ± 92 pA, time to peak = 20 ± 2 s, and n = 5; and 575 ± 105 pA, time to peak 127 ± 22 s, and n = 5, respectively; Fig. 3 C). Thus, conditions that produced the strongest intracellular acidification activated the largest TRPA1 currents.
To extend our results, we tested a panel of carboxylic acids that varied in chain length and differed in their ability to acidify the cell cytosol. As measured with fluorescent pH indicator DFFDA, PA produced strong intracellular acidification, whereas lactic acid and formic acid produced modest intracellular acidification, and tartaric and citric acid produced virtually no intracellular acidification (Fig. 3 A). Consistently, we observed strong activation of TRPA1 by 10 mM PA (pH 5; 5,466 ± 1,047 pA; n = 6), modest activation by 10 mM of formic acid (pH 5; 926 ± 212 pA; n = 5) and lactic acid (10 and 100 mM, pH 5; 573 ± 85 pA; n = 5; and 1,170 ± 251 pA; n = 6, respectively), and no activation by tartaric acid and citric acid (Fig. 3 C). PA also activated TRPA1 at pH 6.0 (10 mM) and pH 7.0 (10 mM; 1,146 ± 93 pA and n = 5; and 654 ± 152 pA, respectively; n = 6).
To determine whether the degree of intracellular acidification correlated with the extent of TRPA1 activation, we plotted the magnitude of the TRPA1 currents against the relative change in the fluorescent pH indicator DFFDA for all acids under all conditions (Fig. 3 D). The strong correlation (r = 0.88 and P < 0.005) suggests that intracellular acidification is the proximate stimulus that gates TRPA1 in response to weak acids.
A prediction of the previous experiments is that it should be possible to activate TRPA1 channels without direct exposure of the extracellular surface of the cell to acids, simply by acidifying the cell cytosol. To test this prediction, we measured activity of TRPA1 channels in cell-attached patches, where the pipette solution was held at neutral pH (7.4) and acetic acid at pH 5.0 was applied outside the area of the patch. Under these conditions, we observed robust channel activity (Fig. 4, A and B; similar results were obtained in six out of six patches). The amplitude of the channels (−11.52 ± 0.65 pA; n = 6) at −80 mV was as expected based on the previously reported unitary conductance of 140 pS (Wang et al., 2008). No channel openings were observed in untransfected cells under the same conditions. Note that we omitted Ca2+ from the extracellular solution, so that activation of the channel could not be attributed secondarily to influx of Ca2+ through TRPA1 channels in the area outside the patch.
Figure 4.
TRPA1 channels are activated by intracellular protons. (A) Channel activity from a TRPA1-expressing HEK-293 cell in cell-attached patch clamp (Vm = −80 mV) in response to the addition of 10 mM of acetic acid at pH 5.0 outside the patch. (B) Openings of single channels are shown on an expanded time scale from before, during, and after the application of acetic acid as indicated. Similar results were obtained in six out of six patches. (C) Channel activity in an inside-out patch from a HEK-293 cell transfected with TRPA1 (Vm = −80 mV) in response to cytoplasmic delivery of acetate anions or protons (pH 5.5; 1 mM polyP3 was added to all solutions to retain channel activity; Kim and Cavanaugh, 2007). Summary data represent the mean ± SEM. **, P < 0.01 (two-tailed Student’s t test).
Finally, to directly test whether the acetate moiety or the proton is responsible for the activation of TRPA1, we tested responses to each in excised inside-out patches from TRPA1-expressing HEK cells. These experiments confirmed our previous observation that TRPA1 channels could be activated by intracellular protons (Wang et al., 2010). No activation by acetate was observed (n = 9; Fig. 4 C).
Relationship between activation and inactivation of TRPA1 by weak acids and reactive compounds
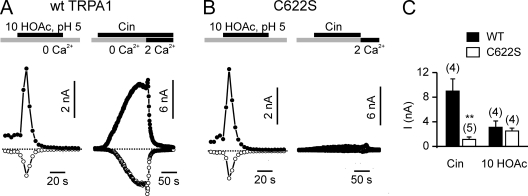
Previous work has shown that the activation of TRPA1 by reactive compounds is mediated by intracellular N-terminal cysteines (Hinman et al., 2006; Macpherson et al., 2007). To determine whether intracellular protons and reactive compounds act on the same site of TRPA1, we tested responses to acids of TRPA1-mutant channels that were unresponsive to reactive compounds because of a mutation in a critical cysteine residue, C622S. As shown in Fig. 5, this mutation strongly attenuated activation of TRPA1 channels by Cin. Interestingly, responses to acetic acid were preserved in the mutant, suggesting that activation by weak acids is mechanistically distinct from activation by reactive compounds.
Figure 5.
Acid and reactive compounds act on different sites of TRPA1. Whole cell currents evoked in HEK-293 cells transfected with wild-type TRPA1 or cysteine mutant C622S. (A and B) 100 µM Cin strongly activated wild-type TRPA1 but only weakly activated the TRPA1 mutant C622S. The subsequent addition of 2 mM Ca2+ induced no further activation and promoted rapid inactivation of both wild-type and C622S currents. In contrast, 10 mM of acetic acid, pH 5.0, strongly activated both wild-type TRPA1- and C622S-mutant channels. (C) Average peak current amplitude measured at +80 mV from experiments as in A and B. Comparison between wild type and C622S was with the two-tailed Student’s t test. Data are represented by the mean ± SEM. **, P < 0.01.
TRPA1 channels inactivate in response to reactive compounds, a process that is dependent on Ca2+ entry into the cell (Wang et al., 2008). We also observed a rapid decay of the current followed by activation by 10 mM of acetic acid, pH 5.0 (Fig. 6 A; T1/2 = 5.0 ± 0.6 s; n = 6). This decay of the TRPA1 current represents entry of the channels into an inactivated state, as additional responses to acetic acid could not be evoked during the time period of the recording (Fig. 6 A). In contrast to inactivation of TRPA1 by reactive electrophiles, inactivation in response to weak acids occurred in the absence of extracellular Ca2+. Inactivation was also not a consequence of release of Ca2+ from intracellular buffers, as the rate of inactivation in response to 10 mM of acetic acid, pH 5.0, was no different when Ca2+ was excluded from the pipette as when it was included (T1/2 = 4.0 ± 0.5 s and n = 6 compared with 3.2 ± 0.8 s and n = 5).
Given the differences between activation and inactivation of TRPA1 in response to acids and reactive compounds, we wondered whether inactivation in response to weak acids would preclude activation in response to reactive electrophiles. When TRPA1 was inactivated by 10 mM of acetic acid, pH 5, subsequent application of Cin produced little activation (Fig. 6 B). Control cells not exposed to acetic acid responded robustly to Cin (Fig. 6 B). Thus, responses to weak acids can cross-desensitize responses to reactive electrophiles.
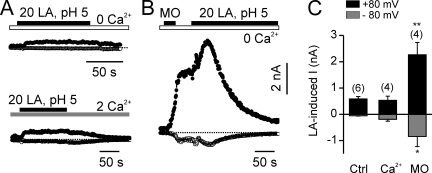
Previous experiments have shown that exposure to reactive electrophiles sensitizes TRPA1 channel to activation by cold temperature (del Camino et al., 2010). TRPA1 was only modestly activated by some physiologically relevant weak acids, including lactic acid, even in the presence of extracellular Ca2+ (Fig. 7, A and C), raising the possibility that responses to these acids might be unmasked by preactivation with reactive electrophiles. To test this possibility, we activated cells either with 20 mM of lactic acid or with 20 mM of lactic acid after preactivation with 25 µM MO in the absence of extracellular Ca2+. Responses to lactic acid were significantly larger after preactivation with MO (Fig. 7, B and C), suggesting that reactive electrophiles sensitize TRPA1 to activation by weak acids.
Figure 7.
Lactic acid and MO act in synergy to activate TRPA1. (A) 20 mM lactic acid, pH 5, activated small TRPA1 currents in TRPA1-expressing HEK-293 cells (top). The addition of extracellular Ca2+ produced no enhancement of activation by lactic acid (bottom). (B) Preexposure to MO greatly increased TRPA1 current activation in response to lactic acid. (C) Average data from experiments as in A and B. Data are represented by the mean ± SEM. Significance was determined with the two-tailed Student’s t test. *, P < 0.05; **, P < 0.01.
A subset of trigeminal neurons responds to carboxylic acids
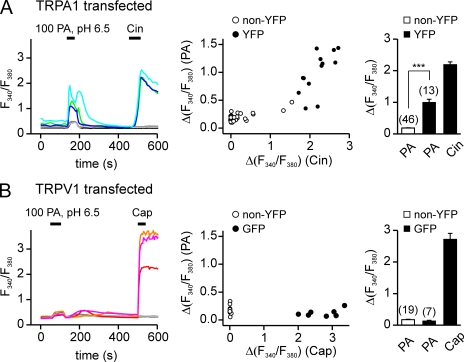
The preceding experiments show that TRPA1 is activated by acetic acid and PA, but not by HCl, and, therefore, has the pharmacological properties expected for a sensor of weak acids in the trigeminal system. But is it required for the sensory response? To test this possibility, we measured responses of sensory neurons to acids with Ca2+ imaging. To avoid confounding our data with well-described effects of external pH acting through TRPV1 or ASIC channels that are coexpressed with TRPA1 in the nociceptors, the stimulus that we used was 100 mM PA at pH 6.5, which strongly activates sensory nerves in an intact preparation (Fig. 1 and Silver and Moulton, 1982; Bryant and Moore, 1995). This stimulus produced a large Ca2+ elevation in HEK-293 cells expressing TRPA1 (12/13 cells responded; Fig. 8 A), and the response had a characteristic biphasic time course. In contrast, only a small monophasic response was detected in cells transfected with TRPV1 (Fig. 8 B; 0/7 GFP+ cells). Because this small response was detected in all cells tested, we attributed it to previously described effects of intracellular pH on the emission of the Ca2+ indicator, rather than to a true elevation of intracellular Ca2+ (Lattanzio and Bartschat, 1991). Thus, 100 mM PA, pH 6.5, can be used to specifically test the effects of intracellular acidification under conditions that minimize extracellular acidification, in the context of native sensory neurons.
Figure 8.
PA activates TRPA1 in Ca2+ imaging. Agonist-induced elevation of intracellular Ca2+ in HEK-293 cells transfected with TRPV1 or TRPA1. (A) TRPA1-expressing HEK cells responded to both 100 mM PA, pH 6.5, and 100 µM Cin. (B) TRPV1-expressing HEK cells responded only to 1 µM Cap and showed nonspecific responses to PA. Scatter plot shows the amplitude of the responses to PA as a function of the responses to Cin (A) or Cap (B). Average responses to PA and Cin (A) or PA and Cap (B) are shown in the bar graph. Data are represented by the mean ± SEM. Significance was determined with the two-tailed Student’s t test. ***, P < 0.001.
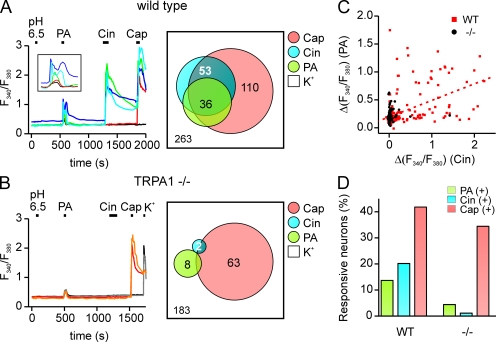
To determine whether there is a specific population of nociceptors that is sensitive to carboxylic acids, we measured agonist responses in cultured trigeminal sensory neurons using ratiometric Ca2+ imaging. Each culture was tested sequentially with 10 mM of HEPES-buffered solution, pH 6.5, 100 mM PA, pH 6.5, 100 µM Cin, 1 µM Cap, and 150 mM KCl, in that order, with a recovery period of at least 5 min between exposure to each stimulus. As shown in Fig. 9 A, a subset of cells (14%; 36/263) responded to PA but not to HEPES-buffered solution adjusted with HCl to the same pH (6.5). Interestingly, the response of these cells to PA showed a biphasic time course (Fig. 9 A), reminiscent of the nerve response to PA and the responses of TRPA1-expressing HEK cells to PA (Figs. 1 B and 8 A).
Figure 9.
Responses of TG neurons to weak acids are TRPA1 depedent. (A) Elevation of intracellular Ca2+ in sensory neurons isolated from the TG of wild-type mice in response to weak acids and other agonists. Traces illustrate the different types of responses observed: cells responsive to PA, Cin, and Cap (but not to 10 mM HEPES, pH 6.5) are shown with green, blue, and cyan traces; a cell responsive to Cap, but not to Cin or PA, is shown in red; and a cell that responded to none of the agonists is shown in black. (B) TG neurons from TRPA1 knockout mice showed only reduced sensitivity to PA and Cin but retained normal responses to Cap (orange and red traces). Venn diagrams show the aggregate results from all experiments. Numbers represent a total count of the responsive cells to each agonist, and overlap and size of each circle are drawn to scale. (C) Summary data from experiments as in A and B showing the magnitude of the PA response as a function of the Cin response in the same cell. Note that the PA response was positively correlated with the magnitude of the Cin response in cells from wild-type mice (r = 0.52 and P < 0.0001). (D) The percentage of different populations of TG neurons in cultures from wild-type and TRPA1 knockout mice.
Many of the PA-responsive cells responded subsequently to the TRPA1 agonist Cin and the TRPV1 agonist Cap (Fig. 9 A). To determine whether PA responses were enriched in the population of cells that express either TRPA1 or TRPV1, we measured the degree to which responses to Cin or Cap were correlated with responses to PA. Using data from all cells, we found a significant correlation between the magnitude of the response to PA and both the magnitude of the response to Cin and the magnitude of the response to Cap (r = 0.52 and P < 0.0001, and r = 0.44 and P < 0.0001, respectively; Fig. 9 C). To determine whether responses were enriched in all TRPV1-expressing cells or only cells that coexpressed TRPV1 and TRPA1, we categorized cells as responsive or nonresponsive and performed a χ2 analysis of the data (see Materials and methods and Fig. S1). Overall, most PA-sensitive cells responded to a later application of both Cin and Cap, which defines the TRPA1/TRPV1-expressing populations (25/36), whereas only a minority of PA-responsive cells responded to a later application of Cap but not Cin (6/36; TRPV1-only population). A χ2 analysis shows that among the Cap-sensitive cells, PA-responsive cells were significantly enriched in the population that was also Cin sensitive as compared with the population that was Cin insensitive (25/42 compared with 6/68; P < 0.0001; two-tailed χ2). Collectively, these data provide evidence for the presence of a subset of trigeminal neurons that are sensitive to PA but not to HCl at the same pH, and that are enriched within the population of cells that are sensitive to TRPA1 agonists. Thus, TRPA1 may mediate responses of native sensory neurons to weak acids.
TRPA1 is required for the response to weak acids in trigeminal neurons
To determine whether TRPA1 is required for the trigeminal response to weak acids, we measured responses with ratiometric Ca2+ imaging from trigeminal neurons isolated from mice carrying a targeted deletion of the TRPA1 gene (Kwan et al., 2006). Cultures were challenged with 100 mM PA, pH 6.5, and subsequently with Cin, Cap, and KCl (150 mM) as described above. In these cultures, responses to the TRPA1 agonist Cin were nearly eliminated (2/183; Fig. 9 B), whereas responses to the TRPV1 agonist Cap were completely intact (63/183; Fig. 9 B) as reported previously (Bautista et al., 2006; Kwan et al., 2006). Remarkably, responses to PA were also largely eliminated with just 4% of electrically responsive cells responding to PA (8/183) as compared with 14% (36/263) in cultures from wild-type mice (Fig. 9, B and D; P < 0.005 and two-tailed χ2). The residual PA responses from cultures of knockout mice were on average smaller than responses from wild-type cultures (0.48 ± 0.03 vs. 0.86 ± 0.06 AU; P < 0.01 and two-tailed Mann-Whitney U test); they may represent nonspecific responses that escaped our threshold or responses that were TRPA1 independent. Thus, under the conditions of our experiments, the TRPA1 gene is essential for the response of trigeminal neurons to carboxylic acids.
DISCUSSION
Sensory neurons that innervate skin and viscera provide important information concerning the presence of damaging substances of external or internal origin. Among the conditions that are detrimental to cell survival is severe intracellular acidification, which has been shown to cause cell death in mitochondria-dependent apoptosis (Matsuyama et al., 2000). Previous work showed that TRPA1 functions within nociceptors as a sensor for intracellular acidification in response to CO2 (Wang et al., 2010). Our data now provide evidence that TRPA1 is a general sensor for weak acids that cause intracellular acidification. Specifically, we show that when expressed in HEK-293 cells, TRPA1 is activated by acetic acid and other weak acids, and that the magnitude of the current activation is well correlated with the extent of intracellular acidification produced by each acid. That TRPA1 contributes to the response of sensory neurons to weak acids was confirmed in cultures of trigeminal neurons, where responses to weak acids were significantly reduced in the absence of a functional TRPA1 gene.
Our data confirm that responses of nociceptors to weak acids are different from responses to strong acids and provide a framework with which to understand how this difference arises. Weak acids, by definition, do not dissociate completely in solution, and the conjugate bases of many organic acids are membrane permeable. Thus, the most parsimonious explanation for the strong nociceptive effects of these disparate molecules is that they act by crossing cell membranes, shuttling protons in the process. This process may deliver protons across epithelial layers to free nerve endings where they can act on extracellular proton sensors such as TRPV1 or ASICs, or it may shuttle protons into the cell cytosol where they act on intracellular proton sensors. A recent report showed that responses of nerve fibers from the superior laryngeal nerve to acetic acid were sensitive to the TRPV1 blocker iodo-resineratoxin, suggesting that delivery of protons to extracellular receptors contributes to the heightened sensory response evoked by acetic acid (Arai et al., 2010). Our data now indicate that another component of the response to weak acids is mediated by cytosolic acidification, which gates TRPA1 channels. TRPA1 may therefore provide an attractive target for the development of analgesic drugs to relieve acidotic pain (Julius and Basbaum, 2001).
Lactic acid generated as a result of increased anaerobic glycolysis during oxygen deprivation (hypoxia/ischemia) contributes to ischemic pain (Cohen and Woods, 1983). Previous work has shown the involvement of ASIC3 channels (Sutherland et al., 2001), which are expressed by dorsal root ganglion neurons that innervate the heart and which are potentiated in the presence of extracellular lactate (Immke and McCleskey, 2001). TRPA1 may also contribute to the sustained response of sensory neurons during ischemia, as it can be modestly activated by lactic acid, which can reach a concentration of 20 mM during hypoxia (Cohen and Woods, 1983). Moreover, responses to lactic acid were strongly potentiated by preactivation with reactive electrophiles, an experimental paradigm that may more closely mimic the physiological situation where cells are exposed to multiple signals. TRPA1 may therefore be a promising pharmaceutical target for the relief of ischemic pain.
Numerous agonists of TRPA1 have been identified that represent different classes of chemicals. Reactive electrophiles, such as MO and Cin activate TRPA1 by binding covalently to N-terminal cysteines (Hinman et al., 2006; Macpherson et al., 2007). These substances produce a long-lasting, poorly reversible activation of the channel, which is terminated by Ca2+-dependent inactivation (Jordt et al., 2004; Nagata et al., 2005; Zurborg et al., 2007; Wang et al., 2008). Our results show that weak acids represent a new class of TRPA1 activators. Activation by weak acids is independent of cysteine residues that mediate activation in response to reactive electrophiles and thus is likely to be mechanistically distinct. Interestingly, it was recently reported that the irritant component of extra virgin olive oil is a potent TRPA1 agonist; despite the fact that this chemical can form adducts with TRPA1, activation of TRPA1 was found to be independent of cysteines that mediate the effects of reactive electrophiles (Peyrot des Gachons et al., 2011).
We also report that TRPA1 is inactivated by weak acids. Inactivation of TRPA1 by weak acids attenuated further activation of the channel by both weak acids and by reactive electrophiles. This effect was observed in whole cell patch clamp recording and not in calcium imaging, suggesting either that recovery from inactivation may require an inter stimulus interval >60 s (which was typical for patch clamp experiments) but <300 s, which was used for calcium-imaging experiments, or that inactivation may require a cellular component that is dialyzed out during whole cell recording. Unlike inactivation in response to reactive electrophiles, which is dependent on entry of Ca2+ through the channel (Wang et al., 2008), inactivation in response to weak acids is Ca2+ independent. Because protons can compete with Ca2+ for binding to acidic residues, it is possible that the activating and inactivating effects of both Ca2+ and protons on TRPA1 could be ascribed to the same structural element (Immke and McCleskey, 2003). The structural domains that mediate Ca2+ activation or inactivation of TRPA1 channels are not known, and mutation of a putative EF hand in the N terminus of the channel has yielded conflicting results (Doerner et al., 2007; Zurborg et al., 2007; Wang et al., 2008). Identifying the binding site for protons and Ca2+ that induce activation or inactivation of TRPA1 among the ∼100 intracellular acidic residues is a challenge for the future.
The activation of TRPA1 by weak acids was conserved across several mammalian species, including humans, consistent with the observation that in people, propionic and other carboxylic acids elicit sensations described as irritating (Ernstgård et al., 2006). Paradoxically, humans choose to ingest acetic acid, as well as many other TRPA1 agonists, despite their irritant effects, a characteristic that is specific to our species (Bryant and Silver, 2000). A recent report showed that activation of TRPA1 by reactive electrophiles is conserved in the insect isoforms of TRPA1, indicating that this mode of activation existed in the ancestral channel (Kang et al., 2010). Whether activation by intracellular acidification is conserved across nonmammalian species is not known. Given that organic acids are abundant in the environment and have wide-spread detrimental effects, this mode of activation may well have an early evolutionary origin.
Acknowledgments
We thank H. Waters and E. Rohman for technical support and D. Arnold for critical review of the manuscript.
This work is supported by National Institutes of Health (grant DC004564 to E.R. Liman).
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- ASIC
- acid-sensing ion channel
- Cap
- capsaicin
- Cin
- cinnamaldehyde
- MO
- mustard oil
- PA
- propionic acid
- rTRPA1
- rat TRPA1
- TG
- trigeminal ganglion
- YFP
- yellow fluorescent protein
References
- Arai T., Ohkuri T., Yasumatsu K., Kaga T., Ninomiya Y. 2010. The role of transient receptor potential vanilloid-1 on neural responses to acids by the chorda tympani, glossopharyngeal and superior laryngeal nerves in mice. Neuroscience. 165:1476–1489 10.1016/j.neuroscience.2009.11.051 [DOI] [PubMed] [Google Scholar]
- Bandell M., Story G.M., Hwang S.W., Viswanath V., Eid S.R., Petrus M.J., Earley T.J., Patapoutian A. 2004. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 41:849–857 10.1016/S0896-6273(04)00150-3 [DOI] [PubMed] [Google Scholar]
- Bautista D.M., Jordt S.E., Nikai T., Tsuruda P.R., Read A.J., Poblete J., Yamoah E.N., Basbaum A.I., Julius D. 2006. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 124:1269–1282 10.1016/j.cell.2006.02.023 [DOI] [PubMed] [Google Scholar]
- Brand G. 2006. Olfactory/trigeminal interactions in nasal chemoreception. Neurosci. Biobehav. Rev. 30:908–917 10.1016/j.neubiorev.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Bryant B.P., Moore P.A. 1995. Factors affecting the sensitivity of the lingual trigeminal nerve to acids. Am. J. Physiol. 268:R58–R65 [DOI] [PubMed] [Google Scholar]
- Bryant B., Silver W. 2000. Chemesthesis: the common chemical sense. The Neurobiology of Taste and Smell. Second edition Finger T.E., Silver W.L., Restrepo D., John Wiley and Sons, Inc, New York: 73–100 pp [Google Scholar]
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 389:816–824 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- Chen C.C., Zimmer A., Sun W.H., Hall J., Brownstein M.J., Zimmer A. 2002. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc. Natl. Acad. Sci. USA. 99:8992–8997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R.D., Woods H.F. 1983. Lactic acidosis revisited. Diabetes. 32:181–191 [DOI] [PubMed] [Google Scholar]
- del Camino D., Murphy S., Heiry M., Barrett L.B., Earley T.J., Cook C.A., Petrus M.J., Zhao M., D’Amours M., Deering N., et al. 2010. TRPA1 contributes to cold hypersensitivity. J. Neurosci. 30:15165–15174 10.1523/JNEUROSCI.2580-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner J.F., Gisselmann G., Hatt H., Wetzel C.H. 2007. Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 282:13180–13189 10.1074/jbc.M607849200 [DOI] [PubMed] [Google Scholar]
- Ernstgård L., Iregren A., Sjögren B., Johanson G. 2006. Acute effects of exposure to vapours of acetic acid in humans. Toxicol. Lett. 165:22–30 10.1016/j.toxlet.2006.01.010 [DOI] [PubMed] [Google Scholar]
- Hinman A., Chuang H.H., Bautista D.M., Julius D. 2006. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA. 103:19564–19568 10.1073/pnas.0609598103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A.L., Chen X., Hoon M.A., Chandrashekar J., Guo W., Tränkner D., Ryba N.J., Zuker C.S. 2006. The cells and logic for mammalian sour taste detection. Nature. 442:934–938 10.1038/nature05084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke D.C., McCleskey E.W. 2001. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat. Neurosci. 4:869–870 10.1038/nn0901-869 [DOI] [PubMed] [Google Scholar]
- Immke D.C., McCleskey E.W. 2003. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 37:75–84 10.1016/S0896-6273(02)01130-3 [DOI] [PubMed] [Google Scholar]
- Ishimaru Y., Inada H., Kubota M., Zhuang H., Tominaga M., Matsunami H. 2006. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA. 103:12569–12574 10.1073/pnas.0602702103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt S.E., Bautista D.M., Chuang H.H., McKemy D.D., Zygmunt P.M., Högestätt E.D., Meng I.D., Julius D. 2004. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 427:260–265 10.1038/nature02282 [DOI] [PubMed] [Google Scholar]
- Julius D., Basbaum A.I. 2001. Molecular mechanisms of nociception. Nature. 413:203–210 10.1038/35093019 [DOI] [PubMed] [Google Scholar]
- Kang K., Pulver S.R., Panzano V.C., Chang E.C., Griffith L.C., Theobald D.L., Garrity P.A. 2010. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 464:597–600 10.1038/nature08848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Cavanaugh E.J. 2007. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J. Neurosci. 27:6500–6509 10.1523/JNEUROSCI.0623-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan K.Y., Allchorne A.J., Vollrath M.A., Christensen A.P., Zhang D.S., Woolf C.J., Corey D.P. 2006. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 50:277–289 10.1016/j.neuron.2006.03.042 [DOI] [PubMed] [Google Scholar]
- Lattanzio F.A., Jr, Bartschat D.K. 1991. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem. Biophys. Res. Commun. 177:184–191 10.1016/0006-291X(91)91966-G [DOI] [PubMed] [Google Scholar]
- Liu D., Liman E.R. 2003. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. USA. 100:15160–15165 10.1073/pnas.2334159100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LopezJimenez N.D., Cavenagh M.M., Sainz E., Cruz-Ithier M.A., Battey J.F., Sullivan S.L. 2006. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J. Neurochem. 98:68–77 10.1111/j.1471-4159.2006.03842.x [DOI] [PubMed] [Google Scholar]
- Macpherson L.J., Dubin A.E., Evans M.J., Marr F., Schultz P.G., Cravatt B.F., Patapoutian A. 2007. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 445:541–545 10.1038/nature05544 [DOI] [PubMed] [Google Scholar]
- Martínez-Zaguilán R., Gurulé M.W., Lynch R.M. 1996. Simultaneous measurement of intracellular pH and Ca2+ in insulin-secreting cells by spectral imaging microscopy. Am. J. Physiol. 270:C1438–C1446 [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Llopis J., Deveraux Q.L., Tsien R.Y., Reed J.C. 2000. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2:318–325 10.1038/35014006 [DOI] [PubMed] [Google Scholar]
- Nagata K., Duggan A., Kumar G., García-Añoveros J. 2005. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 25:4052–4061 10.1523/JNEUROSCI.0013-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kotani S. 1987. Analgesic effects of N-acetylmuramyl-L-alanyl-D-isoglutamine in decreasing the acetic acid-induced abdominal-writhing response. Infect. Immun. 55:494–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A., Tate S., Woolf C.J. 2009. Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug Discov. 8:55–68 10.1038/nrd2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot des Gachons C., Uchida K., Bryant B., Shima A., Sperry J.B., Dankulich-Nagrudny L., Tominaga M., Smith A.B., III, Beauchamp G.K., Breslin P.A. 2011. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J. Neurosci. 31:999–1009 10.1523/JNEUROSCI.1374-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver W.L., Moulton D.G. 1982. Chemosensitivity of rat nasal trigeminal receptors. Physiol. Behav. 28:927–931 10.1016/0031-9384(82)90216-5 [DOI] [PubMed] [Google Scholar]
- Silver W.L., Clapp T.R., Stone L.M., Kinnamon S.C. 2006. TRPV1 receptors and nasal trigeminal chemesthesis. Chem. Senses. 31:807–812 10.1093/chemse/bjl022 [DOI] [PubMed] [Google Scholar]
- Story G.M., Peier A.M., Reeve A.J., Eid S.R., Mosbacher J., Hricik T.R., Earley T.J., Hergarden A.C., Andersson D.A., Hwang S.W., et al. 2003. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 112:819–829 10.1016/S0092-8674(03)00158-2 [DOI] [PubMed] [Google Scholar]
- Sutherland S.P., Benson C.J., Adelman J.P., McCleskey E.W. 2001. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc. Natl. Acad. Sci. USA. 98:711–716 10.1073/pnas.011404498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symanowicz P.T., Gianutsos G., Morris J.B. 2004. Lack of role for the vanilloid receptor in response to several inspired irritant air pollutants in the C57Bl/6J mouse. Neurosci. Lett. 362:150–153 10.1016/j.neulet.2004.03.016 [DOI] [PubMed] [Google Scholar]
- Tominaga M., Caterina M.J., Malmberg A.B., Rosen T.A., Gilbert H., Skinner K., Raumann B.E., Basbaum A.I., Julius D. 1998. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 21:531–543 10.1016/S0896-6273(00)80564-4 [DOI] [PubMed] [Google Scholar]
- Waldmann R., Lazdunski M. 1998. H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr. Opin. Neurobiol. 8:418–424 10.1016/S0959-4388(98)80070-6 [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Chang R.B., Waters H.N., McKemy D.D., Liman E.R. 2008. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol. Chem. 283:32691–32703 10.1074/jbc.M803568200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.Y., Chang R.B., Liman E.R. 2010. TRPA1 is a component of the nociceptive response to CO2. J. Neurosci. 30:12958–12963 10.1523/JNEUROSCI.2715-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhao Z., Margolskee R., Liman E. 2007. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J. Neurosci. 27:5777–5786 10.1523/JNEUROSCI.4973-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurborg S., Yurgionas B., Jira J.A., Caspani O., Heppenstall P.A. 2007. Direct activation of the ion channel TRPA1 by Ca2+. Nat. Neurosci. 10:277–279 10.1038/nn1843 [DOI] [PubMed] [Google Scholar]