Abstract
Allyl isothiocyanate (AITC), which occurs in many common cruciferous vegetables, was recently shown to be selectively delivered to bladder cancer tissues through urinary excretion and to inhibit bladder cancer development in rats. The present investigation was designed to test the hypothesis that AITC-containing cruciferous vegetables also inhibit bladder cancer development. We focused on an AITC-rich mustard seed powder (MSP-1). AITC was stably stored as its glucosinolate precursor (sinigrin) in MSP-1. Upon addition of water, however, sinigrin was readily hydrolyzed by the accompanying endogenous myrosinase. This myrosinase was also required for full conversion of sinigrin to AITC in vivo, but the matrix of MSP-1 had no effect on AITC bioavailability. Sinigrin itself was not bioactive, whereas hydrated MSP-1 caused apoptosis and G2/M phase arrest in bladder cancer cell lines in vitro. Comparison between hydrated MSP-1 and pure sinigrin with added myrosinase suggested that the anticancer effect of MSP-1 was derived principally, if not entirely, from the AITC generated from sinigrin. In an orthotopic rat bladder cancer model, oral MSP-1 at 71.5 mg/kg (sinigrin dose of 9 μmol/kg) inhibited bladder cancer growth by 34.5% (P < 0.05) and blocked muscle invasion by 100%. Moreover, the anticancer activity was associated with significant modulation of key cancer therapeutic targets, including vascular endothelial growth factor, cyclin B1 and caspase 3. On an equimolar basis, the anticancer activity of AITC delivered as MSP-1 appears to be more robust than that of pure AITC. MSP-1 is thus an attractive delivery vehicle for AITC and it strongly inhibits bladder cancer development and progression.
Introduction
Allyl isothiocyanate (AITC; 3-isothiocyanato-1-propene or 2-propenyl isothiocyanate) belongs to a family of naturally occurring isothiocyanates (ITCs) and is a promising cancer preventive agent (1). AITC occurs in many common cruciferous vegetables and is particularly abundant in mustard, horseradish and wasabi. Indeed, it is mainly responsible for the pungent flavor of these vegetables. AITC is synthesized and stored as sinigrin (a glucosinolate) and is subsequently generated from the latter through myrosinase-catalyzed hydrolysis of sinigrin. Myrosinase coexists with sinigrin in vegetables, but glucosinolate hydrolysis does not normally occur until the vegetable is damaged, such as by insect chewing, fungal invasion, chopping and human mastication. Myrosinase activity also exists in the intestinal microflora in both animals and humans, and glucosinolates that escape the action of vegetable myrosinase may be hydrolyzed in vivo. However, it is not known to what extent sinigrin is converted to AITC in vivo.
We have recently shown that AITC selectively targets human bladder cancer cells, while sparing normal human bladder epithelial cells, is selectively delivered to bladder cancer tissues through urinary excretion and potently inhibits bladder cancer development and muscle invasion in an orthotopic rat bladder cancer model (2). Thus, AITC is a highly promising agent for bladder cancer prevention and treatment. AITC may be especially valuable for prevention of recurrence and progression of superficial bladder cancers. Most human bladder cancers present as superficial cancer (no muscle invasion) at initial diagnosis and are exposed to urine. Existing therapeutic agents against recurrence of superficial bladder cancer, including immunotherapy with Bacillus Calmette–Guerin bacteria and chemotherapeutic agents not only have limited utility and efficacy (3,4) but also require urethral catheterization for intravesical delivery, to take advantage of the superficial nature of the cancer and to reduce systemic toxicity. In contrast, intravesical delivery of AITC is achieved through oral administration.
In light of the promising anticancer activity of AITC against bladder cancer and its ability to reach bladder cancer tissue selectively via urinary excretion, as described above, questions arose as to whether plants that are rich sources of AITC/sinigrin can also inhibit bladder cancer development, whether sinigrin can be sufficiently converted to AITC in vivo and whether the plant matrix interferes with absorption, urinary excretion and/or the anticancer activity of AITC. Mustard seed powder (MSP) is a well-known rich source of AITC with thousands of years’ history of use in Chinese traditional medicine, Ayurvedic medicine and various other traditional folk medicines and cuisines. A previous study showed the chemopreventive effects of MSP on chemically induced turmorigenesis in forestomach and uterine cervix in mice (5). In the present report, we show that total ITC content varies greatly among commercial MSP preparations. Focusing on the MSP, which has the highest ITC content (MSP-1), we show that AITC is the predominant, if not the only ITC, and that all AITC is stored stably as sinigrin in the powder. Enough myrosinase is also present in MSP-1 to allow full conversion of sinigrin to AITC upon addition of water in vitro and after oral ingestion in vivo, but the myrosinase apparently has no catalytic effect on sinigrin in the original powder even though it may not be completely dry. We further show that MSP-1 possesses potent anticancer activity in both cultured bladder cancer cells and an orthotopic rat bladder cancer model in vivo and that the MSP matrix has no effect on absorption and urinary excretion of AITC. However, in animals given purified sinigrin, AITC yield in vivo is extremely low, indicating poor conversion from sinigrin to AITC by the myrosinase-like enzyme in the intestinal microflora, and if not hydrolyzed by myrosinase, sinigrin has no anticancer activity.
Materials and methods
Materials
Sinigrin and myrosinase were purchased from Sigma–Aldrich (St Louis, MO). The MSPs include hot oriental MSP (MSP-1) purchased from Spice House (Chicago, IL), MSP-2 from Frontier Natural Products (Norway, IA), MSP-3 from Raw Deal (Flanders, NJ) and MSP-4 from Viable Herbal Solutions (Langhome, PA). The powders have a particle size of ∼60 mesh and were prepared from the seeds of Sinapis alba or Brassica juncea, but the exact genotype and cultivar for each powder are not clear. Antibodies specific for cleaved caspase 3 (Cat. # 9661) and cleaved poly adenosine diphosphate ribose polymerase (PARP; Cat. # 9542) were purchased from Cell Signaling Technology (Beverly, MA). Antibodies specific for vascular endothelial growth factor (VEGF; Cat. # SC-152) and cyclin B1 (Cat. # SC-245) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody specific for glyceraldehyde 3-phosphate dehydrogenase (Cat. # MAB374) was purchased from Millipore (Billerica, MA).
Animals
Female F344 rats (8 weeks of age) were purchased from Harlan (Indianapolis, IN) and were acclimatized for ∼1 week before use. The rats were maintained at 21–23°C and a 12 h light/dark cycle with free access of food (Harlan Teklad LM-485 mouse/rat sterilizable diet) and water. All animal protocols and procedures were approved by the Roswell Park Cancer Institute Animal Care and Use Committee.
Measurement of total ITC, glucosinolates and myrosinase in MSP
Total ITC level in each MSP was measured by the 1,2-benzenedithiol-based cyclocondensation assay (6). Each MSP was incubated with myrosinase in phosphate-buffered saline (PBS) (1 mg/ml) in the presence of exogenous myrosinase (0.1 IU/mg MSP) for 30 min at room temperature prior to ITC measurement. Pure sinigrin was used as a control to confirm full hydrolysis of glucosinolates in MSP. In a parallel experiment, an MSP-1 was incubated in PBS (1 mg/ml) without exogenous myrosinase at room temperature for specific times before ITC measurement to assess the effect of endogenous myrosinase. To measure the stability of MSP-1 as an ITC source, the total ITC level was rechecked after storage of this substance at room temperature for 10 months.
To measure sinigrin content, each MSP was thoroughly mixed at ∼61 mg/ml in a mixture of four solvents, containing an equal volume of dimethyl sulfoxide, acetonitrile, dimethylformamide and water (DADW). This solvent mixture irreversibly inactivates myrosinase and efficiently extracts glucosinolates (7). The mixture was then cleared of insoluble materials by low-speed centrifugation and analyzed for sinigrin content by high-performance liquid chromatography, using a ZIC-HILIC hydrophilic interaction chromatography column from Sequant (Umea, Sweden; 150 mm × 4.6 mm, 5 μm, 200 Å) (8). Pure sinigrin was used as a chromatographic standard. In a parallel experiment, MSP or sinigrin was treated with myrosinase for 0.5 h at 30°C in an aqueous 1 ml solution, containing 1 μmol sinigrin or ∼6 mg MSP, 0.2 ml of 100 mM sodium phosphate (pH 6.0), 0.01 ml of 50 mM ascorbic acid (an activator of myrosinase) (9), 0.02 IU myrosinase and 0.79 ml water. The digests were analyzed for disappearance of glucosinolates by ZIC-HILIC, as described above.
To measure the endogenous myrosinase activity, MSP was thoroughly suspended in water (∼10 mg/ml) and cleared of insoluble materials by filtration through a Millipore Millex-HV filter. Myrosinase activity was measured in a 1 ml reaction solution by monitoring the initial rate of hydrolysis of sinigrin spectrophotometrically at 227 nm at room temperature (molar extinction coefficient of 6784 M−1 cm−1). Each 1 ml reaction contained 50 μl sample, which contained 6.3 nmol of sinigrin in the case of MSP-1, 10 μl of 50 mM ascorbic acid in water, 200 μl of 100 mM sodium phosphate (pH 6.0) and 735 μl water, and 5 μl of 10 mM sinigrin in water was finally added to initiate the reaction. One unit of myrosinase activity equates to the hydrolysis of 1 μmol sinigrin/min.
Cell culture, proliferation assay, cell death enzyme-linked immunosorbent assay and flow cytometry
Human bladder cancer UM-UC-3 cells and rat bladder cancer AY-27 cells were used in the study. The sources of the cell lines as well as their culture conditions have been recently reported (2).
To determine the antiproliferative activity of MSP or sinigrin, cells were grown in 96-well plates (5 × 103 cells per well with 0.15 ml medium) for 24 h and then grown for 72 h in fresh medium (200 μl/well) containing MSP, sinigrin or vehicle. In the case of sinigrin, the culture medium was added with or without myrosinase (0.1 IU/ml). Cell growth was measured at the end of treatment using the 3-(4,6-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (10), from which the half maximal inhibitory concentration (IC50) of each substance was calculated.
Induction of apoptosis by MSP was measured using a Cell Death Detection ELISAplus kit purchased from Roche Diagnostics (Indianapolis, IN), following the manufacturer’s instruction. Briefly, cells were grown in 96-well plates and treated with MSP, as described above. At the end of treatment, the cells were lysed with lysis buffer, and after a low-speed centrifugation, a portion of the supernatant fraction was used for measurement of cytoplasmic levels of histone-associated mononucleosomes or oligonucleosomes.
Cell cycle arrest by MSP was analyzed by flow cytometry. Briefly, 1.5 × 106 cells were grown in a 10 cm plate with 10 ml medium for 24 h and then treated with MSP for 24 h before analysis by flow cytometry as described previously (11). Both MSP and sinigrin were freshly dissolved in a small volume of PBS.
Measurement of AITC bioavailability
Groups of five female F344 rats (10–12 weeks of age) were administered a single oral dose of MSP or sinigrin in ∼0.5 ml water. The solutions were prepared fresh and given to the animals within 30 min of preparation. A control group of rats were given only water. The rats were immediately transferred to metabolism cages (Tecniplast, Exton, PA; 1 rat per cage) and were given free access to food and water for 24 h during which all urine was collected. Total urinary AITC equivalents were determined using the high-performance liquid chromatography-based cyclocondensation assay, as described previously (12).
An orthotopic rat bladder cancer model
The anticancer activity of MSP was evaluated in an orthotopic rat bladder cancer model. The details of this model have been recently reported (2). Briefly, female F344 rats (8–10 weeks of age) were inoculated orthotopically via a urethra catheter with AY-27 cells (1 × 106 cells in 0.5 ml serum-free medium per rat). One day after the inoculation, the rats were randomly assigned to receive by gavage vehicle control (3.3 ml water per kg or ∼0.5 ml per rat) or MSP that was freshly prepared in an equal volume of water, once daily for 3 weeks. The MSP solution was given to the animals within 30 min of preparation. The animals were monitored and weighed daily and were euthanized 24 h after the last dose, and the bladders were quickly removed and weighed. Approximately half of each bladder was fixed in formalin for histological analysis and the other half was frozen in liquid nitrogen for western blot analysis.
Western blot analysis
Cells were grown in 10 cm plates for 24 h (1.5 × 106 cells per plate in 10 ml medium), treated with MSP (dissolved in culture medium) for 24 h and then harvested for analysis. Cells after harvest were washed with ice-cold PBS and lysed in radioimmunoprecipitation assay buffer supplemented with a protease inhibitor cocktail (Sigma–Aldrich). Bladder tumor samples were thoroughly washed in ice-cold PBS, frozen with liquid nitrogen, reduced to powder with a biopulverizer and finally homogenized in radioimmunoprecipitation assay buffer supplemented with a protease inhibitor cocktail mentioned above in glass homogenizers. Cell lysates and tissue homogenates were cleared of debris by low-speed centrifugation and measured for protein contents using the bicinchoninic acid kit (Pierce, Rockford, IL). The samples were then resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes, which were probed by specific antibodies and visualized using SuperSignal West Pico Chemiluminescence Detection System (Thermo Scientific, Rockford, IL) or ECL plus (GE Healthcare, Piscataway, NJ).
Histological analysis
Rat bladders fixed in formalin were paraffin embedded, cut to ∼4 μm thickness and stained with standard hematoxylin and eosin. The slides were examined for bladder and tumor histology using a Nikon 50i light microscope. Tumor muscle invasion was assessed at high magnification (greater than or equal to ×200).
Statistical analysis
All results are expressed as mean ± standard error of the mean. Difference between the means of two groups was analyzed for statistical significance using unpaired two-tailed Student’s t-test with P < 0.05 being considered significant (GraphPad Version 5.00; GraphPad Software, San Diego, CA).
Results and discussion
MSP as a vehicle for AITC delivery
In a preliminary experiment, MSP purchased from four different commercial sources were compared for total ITC content. Each MSP was suspended in PBS and treated with exogenous myrosinase for 30 min to fully hydrolyze glucosinolates to ITCs, which was followed by measurement of total ITC content by the high-performance liquid chromatography-based cyclocondensation assay. Longer incubation time with myrosinase did not increase ITC yield. Pure sinigrin was used to confirm full hydrolysis of glucosinolate by myrosinase. MSP-1 showed the highest ITC level (125.1 μmol/g powder), followed by MSP-2 (91.8 μmol/g), MSP-3 (5.1 μmol/g) and MSP-4 (3.9 μmol/g) (Figure 1A). Thus, total ITC levels in these powders vary by as much as 32-fold. Our subsequent experiments were restricted to MSP-1. Upon re-assay of this substance after storage at room temperature for 10 months, there was no decrease in total ITC level, indicating remarkable stability. Further analysis showed that a significant amount of myrosinase activity was also present in MSP-1 (29.2 ± 3.6 IU/g powder). Thus, incubation of the powder in PBS without exogenous myrosinase at room temperature yielded 91% (114 μmol/g) of total ITCs, although a longer incubation time (1.5 h) was needed (Figure 1A). Further incubation of MSP-1 in PBS, however, led to a decrease in total ITC levels (Figure 1A), suggesting that some of the ITCs formed during the incubation might subsequently be further metabolized or degraded in the aqueous environment or lost due to evaporation (AITC is quite volatile).
Fig. 1.
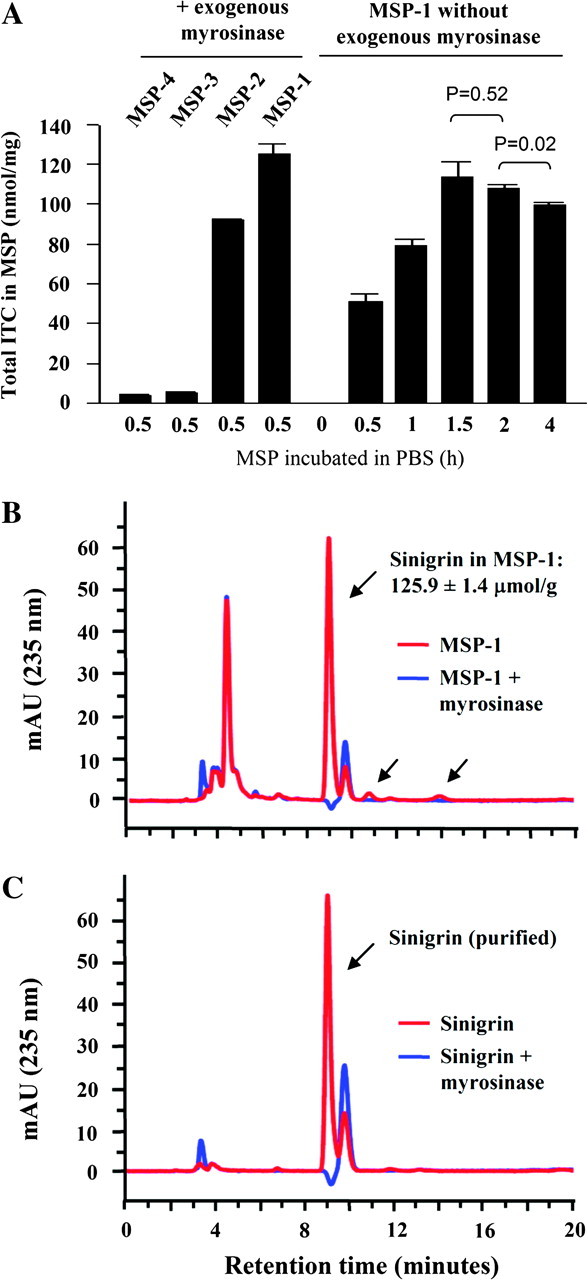
Characterization of MSP. (A) Four different preparations (from four commercial sources) of MSP, including MSP-4, MSP-3, MSP-2 and MSP-1, were each incubated with exogenous myrosinase in PBS (1 mg powder per ml with 0.1 U myrosinase) for 30 min at room temperature (longer incubation time did not lead to further increase in ITC yield). MSP-1 was also incubated in PBS at room temperature for 0.5–4 h without exogenous myrosinase. At the end of incubation, total ITC levels in each solution were measured by the cyclocondensation assay. Each value is a mean ± SEM (n = 3). The result at the 0 time point was obtained by mixing MSP-1 with DADW, so that the endogenous myrosinase was inactivated and potential conversion from sinigrin to AITC was blocked. (B) MSP-1 was either mixed with DADW or incubated with exogenous myrosinase in phosphate buffer for 30 min before high-performance liquid chromatography. The arrows point to sinigrin and two potential minor glucosinolates of unknown identity. The compound or compounds representing the peak around 4 min has not been characterized, but it is not a glucosinolate, because myrosinase treatment had no effect on the peak. (C) Sinigrin was mixed in water with or without myrosinase in phosphate buffer for 30 min before high-performance liquid chromatography. The results are representative of at least three experiments.
We next measured sinigrin level in MSP-1. The powder was mixed with the DADW solvent mixture, which was shown previously to engender full extraction of glucosinolates and ITCs from vegetable powders while simultaneously inhibiting myrosinase-mediated hydrolysis of glucosinolates. Sinigrin was measured by an analytical ZIC-HILIC, and pure sinigrin was used as a positive control. For comparison, the powder was also incubated with exogenous myrosinase in an aqueous solution prior to sinigrin analysis. The sinigrin level was measured at 125.9 μmol/g powder but hydrolyzed completely after myrosinase treatment (Figure 1B). No ITC was detected in the DADW extracts (Figure 1A), indicating that sinigrin was maintained intact in MSP-1 despite the presence of myrosinase. Moreover, sinigrin content in MSP-1 was almost identical to the total ITC level measured after myrosinase treatment, as described above. Comparison of the HILIC chromatograms of MSP-1 with and without myrosinase treatment showed potential presence of two very minor glucosinolates (Figure 1, peaks indicated by the arrows), as would be expected based on previous work (13). Although the identities of these glucosinolates are not known, their small peak area (∼3% the area of the sinigrin peak; peak areas of glucosinolates roughly corresponds to molar concentrations), and the fact that sinigrin content in MSP-1 matches closely with total ITC generated after myrosinase treatment suggest that these glucosinolates may generate little if any ITC.
Sinigrin hydrolysis in vivo
Previous studies have shown that up to 40% of certain ingested glucosinolates may be hydrolyzed to ITCs by a myrosinase-like enzyme present in the intestinal microflora (14–16). ITCs are primarily metabolized in vivo through the mercapturic acid pathway, giving rise to dithiocarbamates (mainly N-acetylcysteine conjugates) that are excreted and concentrated in urine (1). To determine the extent to which sinigrin is hydrolyzed to AITC in vivo, groups of five rats were administered a single oral dose of either sinigrin at 5 and 50 μmol/kg or MSP-1 at the sinigrin dose of 9 and 90 μmol/kg. Higher doses of sinigrin from MSP-1 were used unintentionally due to initial underdetection of its level in the powder. The rats were immediately moved to metabolism cages (one rat per cage) for 24 h urine collection. For comparison, another group of rats were administered AITC at 10 μmol/kg before 24 h urine collection. Our previous rat study showed that >90% of urinary excretion of AITC equivalents (AITC plus its dithiocarbamate metabolites) occurred within 24 h of AITC dosing (17). AITC equivalents in urine were measured by the cyclocondensation assay. This assay does not detect sinigrin but detects both ITC and its dithiocarbamate metabolites. All measurements were adjusted by the basal urinary level of total ITC and dithiocarbamate (average concentration of 8.6 ± 2.3 μM or 0.05 ± 0.01 μmol in 24 h urine), which was measured in a group of rats that were administered only the vehicle. Urinary recovery as AITC equivalent in 24 h urine represented only 3–5% of the administered dose when sinigrin was used, whereas it increased to 53–56% of the administered sinigrin when MSP-1 was used, which was virtually identical to the 55% recovery detected in rats administered AITC (Figure 2A). MSP-1 was freshly suspended in water and given to the rats within 30 min. These results show that the conversion of sinigrin to AITC in vivo by the myrosinase-like enzyme in the gastrointestinal microflora is almost negligible, but the myrosinase carried by MSP-1 could fully hydrolyze sinigrin in vivo. These results also suggest that the MSP-1 matrix does not affect the absorption and urinary excretion of AITC generated from sinigrin. It is noteworthy that our previous study in female Sprague–Dawley rats showed that 70% of the AITC dose (25 μmol/kg) was recovered in 24 h urine (17), which is significantly higher than that in female F344 rats described above, suggesting potential species difference in absorption and/or urinary excretion of AITC.
Fig. 2.
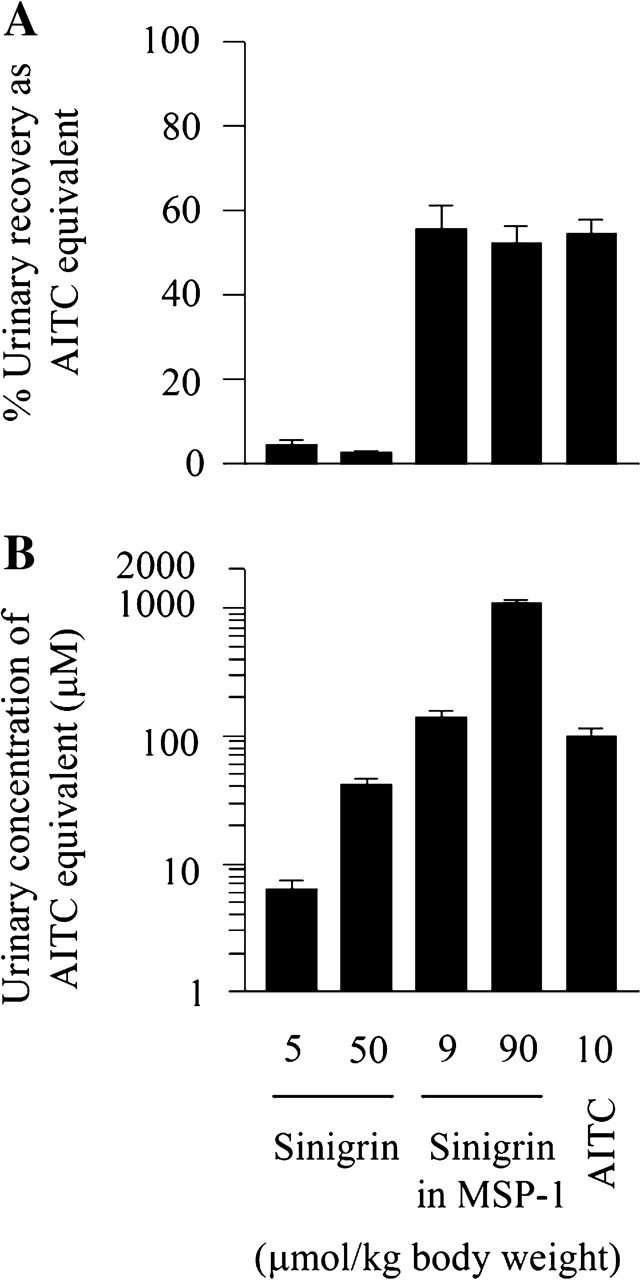
Sinigrin hydrolysis and urinary excretion of AITC. Groups of five female F344 rats were administered a single oral dose of sinigrin, MSP-1 or AITC. Both sinigrin and MSP-1 were mixed in water, whereas AITC was mixed in soy oil, which were given to rats within 30 min of preparation. The rats were kept in metabolism cage for 24 h urine collection (1 rat per cage). Urinary levels of ITC equivalents were measured by the cyclocondensation assay. All values were adjusted by background urinary levels of ITC equivalents, which were 8.6 ± 2.3 μM (average 24 h urinary concentration) and 0.05 ± 0.01 μmol (24 h urine), determined in another group of rats. Each value is mean ± SEM (n = 5).
Consistent with the marked difference in urinary AITC recovery rates described above between pure sinigrin and sinigrin carried in MSP-1, average 24 h urinary concentrations of AITC equivalents were 6.3 and 41.5 μM in rats given pure sinigrin at 5 and 50 μmol/kg, respectively, whereas the corresponding urinary concentrations were 140.5 and 1084.5 μM in rats given MSP-1 at the sinigrin doses of 9 and 90 μmol/kg (Figure 2B). The average 24 h urinary concentration of AITC equivalents was 100.7 μM in rats given AITC at 10 μmol/kg, which is not statistically different from that in rats given MSP at the sinigrin dose of 9 μmol/kg.
The anticancer effect of MSP-1 on bladder cancer cells in vitro
The anticancer activity of MSP-1 was first assessed in vitro using human bladder cancer UM-UC-3 cells and rat bladder cancer AY-27 cells, the latter of which were also used in the animal studies described later. The proliferation of both UM-UC-3 cells and AY-27 cells was inhibited by MSP-1 in a dose-dependent manner, with IC50 values of 10.8 and 8.6 μM of sinigrin (85.8 and 68.3 μg MSP-1 per ml culture medium), respectively (Figure 3A). Pure sinigrin was ineffective, but in the presence of myrosinase, its IC50 values of 13.3 μM (UM-UC-3) and 8.5 μM (AY-27) were comparable with that of MSP-1 calculated in sinigrin concentration (Figure 3A). As shown in Figures 1A and 2, adequate myrosinase is present in MSP-1 for full hydrolysis of its sinigrin. Collectively, these results suggest that AITC formed from sinigrin may account principally if not entirely for the anticancer activity of MSP-1.
Fig. 3.
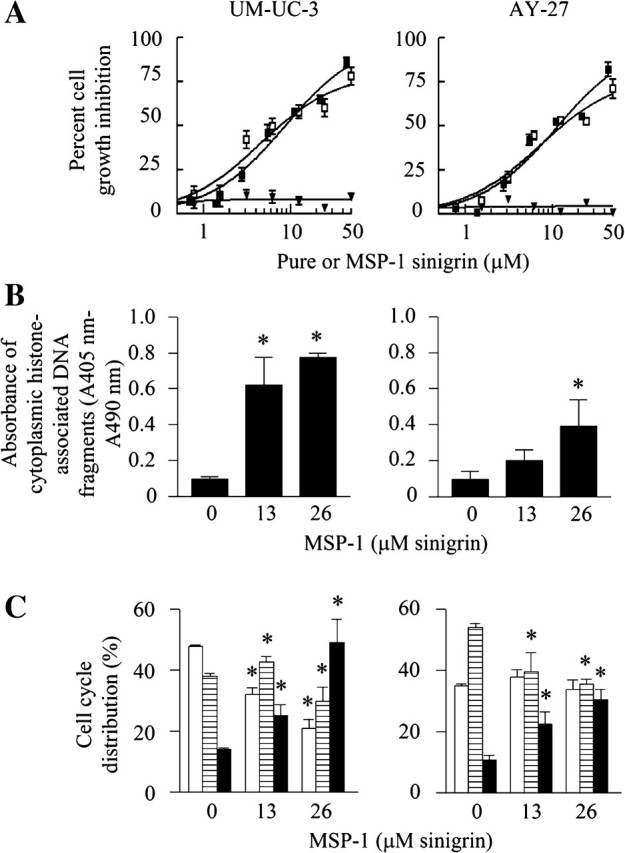
The effect of sinigrin and MSP-1 on survival and proliferation of bladder cancer cells. (A) UM-UC-3 cells and AY-27 cells were grown in 96-well plates and treated with MSP-1 (filled sqaures), sinigrin (inverted filled triangles) and sinigrin plus myrosinase (open squares) for 72 h, followed by measurement of cell density by 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. (B) Cells were grown in 96-well plates and treated with MSP for 24 h. Apoptosis was measured by an enzyme-linked immunosorbent assay. (C) Cells were grown in 10 cm dishes and treated with MSP-1 for 24 h. Cell cycle distribution was measured by flow cytometry (open bars, G1; striped bars, S; filled bars, G2/M). Each value is mean ± SEM (n = 3–8). *P < 0.05 compared with the control.
Inhibition of cell proliferation by MSP-1 was associated with marked induction of apoptosis and cell cycle arrest. Thus, treatment of UM-UC-3 cells and AY-27 cells with MSP-1 at the sinigrin concentrations of 13 and 26 μM for 24 h resulted in up to 8.2- and 4.2-fold increases in apoptosis activity, respectively, for the two cell lines (Figure 3B). Up to 49.1% UM-UC-3 cells and 30.4% AY-27 cells were in G2/M phase after MSP-1 treatment compared with 10.9–14.0% of control cells present in G2/M phase (Figure 3C). Similar results were shown previously with AITC (2). These results show that the antiproliferative effect of MSP-1 on the bladder cancer cells resulted at least in part from activation of apoptosis and cell cycle arrest.
The effect of MSP-1 on bladder cancer growth and muscle invasion in vivo
MSP-1 was assessed for inhibition of bladder cancer in vivo in an orthotopic rat bladder cancer model, which closely resembles human bladder cancer development. Orthotopic bladder cancer growth was initiated by intravesical inoculation of bladder cancer AY27 cells (1 × 106 cells per rat) in female F344 rats. MSP-1 was administered by gavage once daily for 3 weeks, which was started 1 day after cancer cell inoculation. The animals were monitored closely, weighed daily and euthanized at the end of the treatment. The bladders were removed promptly for assessment of potential impact of the test substance on tumor growth. The animals in all groups behaved normally and there was no significant difference in body weight gain over the experimental period among the groups (Figure 4A). Bladder tumors formed in nearly all rats. The tumors in the control group weighed 337.1 ± 48.6 mg (Figure 4A), which is five times the normal bladder weight (67.7 ± 1.8 mg), showing the explosive cancer growth rate. Moreover, tumors invaded the musculature in 71% of the tumor-bearing bladders (Figure 4B). Similar tumor growth and muscle invasion rates were previously seen (2). Treatment with MSP-1 at the sinigrin doses of 9 or 90 μmol/kg body wt (71.5 or 715 mg MSP-1 per kg body wt) reduced tumor weight by 35% (P < 0.05) and 23%, respectively. More interestingly, none of the tumor-bearing bladders of rats treated with the low-dose MSP-1 showed muscle invasion, whereas the muscle invasion rate in the high-dose MSP-1 group was 62%, which is only slightly lower than that in the control group (Figure 4C). Thus, MSP-1 at the low dose is more effective than the high dose. Although the reason is not known, we recently showed that AITC at 10 μmol/kg was also more effective than at higher doses in inhibiting bladder tumor development in the same model (2). However, compared with AITC, which at 10 μmol/kg inhibited bladder cancer growth rate by 30% and muscle invasion rate by 73% (2), the anticancer efficacy of MSP-1 appears to be more robust, particularly in blocking muscle invasion.
Fig. 4.
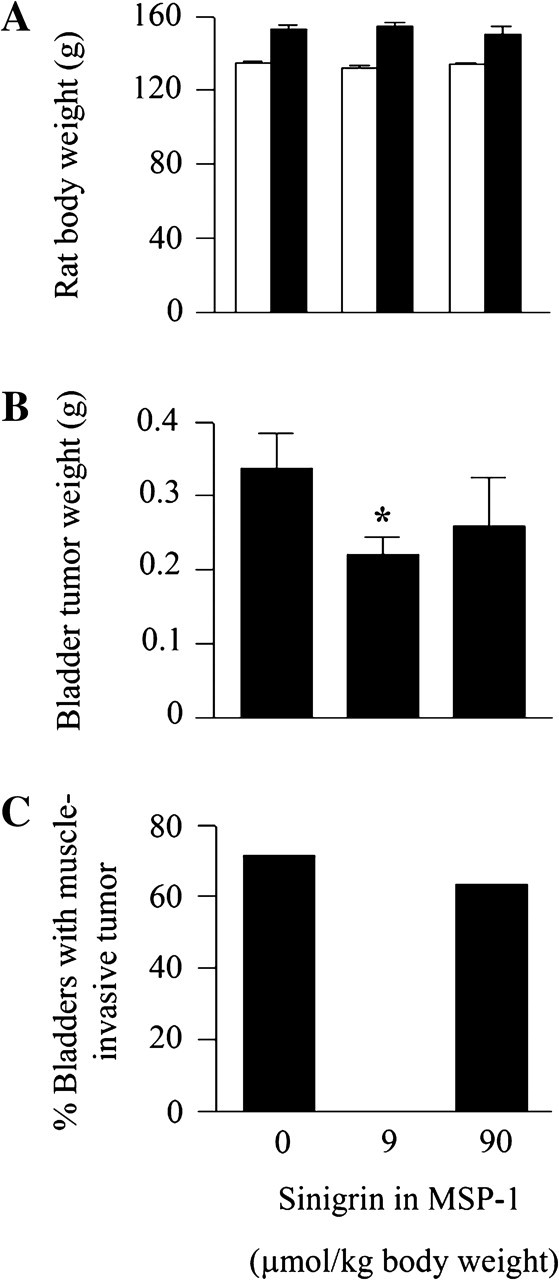
Inhibition of bladder cancer development by MSP-1. Female F344 rats were inoculated with AY-27 cells intravesically via a urethra catheter to initiate development of orthotopic bladder cancer. Oral administration of MSP-1 or vehicle (water) once daily was started 1 day after cancer cell inoculation and ended 3 weeks later. The number of rats per group varied from 11–29. (A) Initial (open bars) and final (filled bars) body weights. (B) Tumor weight was calculated by subtracting the average normal bladder weight from tumor-bearing bladder weight. *P < 0.05. Each value in A and B is mean ± SEM. (C) Percentage of bladder where the tumor invaded the muscle tissue.
The ability of MSP-1 to completely block muscle invasion of bladder cancer is especially exciting because muscle invasion is the event that separates relatively benign superficial bladder cancer that is generally not life-threatening from more advanced cancer that requires aggressive therapy and has poor prognosis. Approximately a third of bladder cancers at diagnosis show muscle invasion, and 15–30% of high-grade superficial bladder cancers progress to muscle invasion within 5 years. Muscle invasive bladder cancer remains a therapeutic challenge. Patients with muscle invasive bladder cancer not only require debilitating radical cystectomy but also face a poor survival outlook (18)
Potential anticancer mechanism of MSP-1
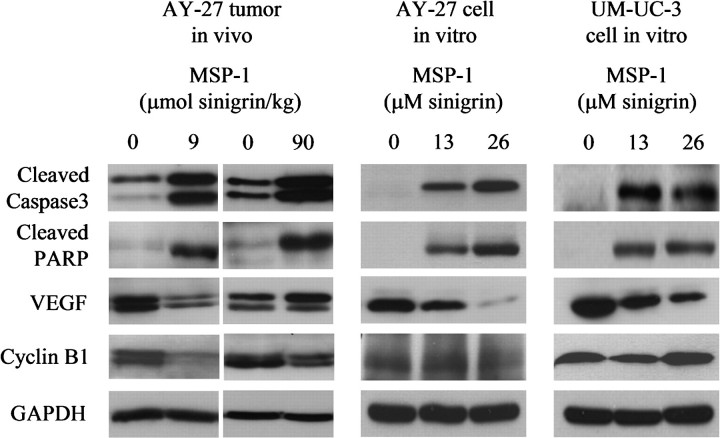
In view of the complete inhibition of muscle invasion of bladder cancers in rats treated with MSP-1, several proteins that are known to promote cancer invasion and metastasis were examined in the bladder cancer tissues and cultured bladder cancer cells. Both matrix metalloproteinase (MMP)-2 and MMP-9 were readily detected in the tumor tissues, but treatment with MSP-1 had no effect on their expression (results not shown). Likewise, MMP-2 and MMP-9 were readily detected in both UM-UC-3 cells and AY-27 cells in culture but not affected by MSP-1 (result not shown). The potential effect of MSP-1 on other MMPs has not been examined. However, MSP-1 treatment caused a significant decrease in VEGF (VEGF-A) in both cultured bladder cancers and bladder cancer tissues (Figure 5). Interestingly, the inhibitory effect of MSP-1 on VEGF in vivo was detected only at the low dose (9 μmol/kg sinigrin) but not the high dose (90 μmol/kg sinigrin), which correlates with the markedly more effective inhibition of tumor invasion into the muscle by MSP-1 at the low dose as described above. However, the reason for the lack of effect at the high MSP-1 dose is not known. Other members of VEGF have not been examined. VEGF is well known to promote cancer angiogenesis, invasion and metastasis and is a widely recognized anticancer target, although VEGF-C was shown previously to promote invasion and metastasis of certain cancer cells (19). A previous study showed that VEGF expression level was higher in more advanced tumors and invasive tumors in human bladder cancer (20).
Fig. 5.
The effect of MSP-1 on selected anticancer targets. UM-UC-3 cells and AY-27 cells in culture were treated with MSP-1 at the sinigrin concentrations of 13 or 26 μM for 24 h. The results are representative of at least two experiments. The bladder tumors were removed from rats, which were treated with vehicle or MSP-1 at the sinigrin doses of 9 or 90 μmol/kg once daily for 3 weeks, starting 1 day after cancer cell inoculation. The results are representative of tumors from other rats. Cell lysates and tumor homogenates were analyzed by western blotting, using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a loading control.
Moreover, MSP-1 also strongly activated caspase-3 and cleaved PARP in both UM-UC-3 cells and AY-27 cells (Figure 5), which is consistent with induction of apoptosis by MSP-1 in these cells (Figure 3). Caspase-3 and PARP were also uniformly activated or cleaved in bladder cancer tissues of rats treated with MSP-1 (Figure 5). Interestingly, unlike VEGF, the low and high doses of MSP-1 showed similar effects on caspase-3 and PARP in vivo. There was a varying degree of activation of caspase-3 in ∼10% of bladder tumors in the control group. The reason for this phenomenon is not clear, but there was no apparent difference in morphology (both gross morphology and hematoxylin/eosin-stained tissue slides) between these control tumors and control tumors showing no caspase-3 activation (results not shown). AITC showed similar effects on caspase-3 in bladder cancer cells in vitro and bladder cancer tissues in vivo, as previously reported (2).
MSP-1 also caused significant downregulation of cyclin B1, a key regulator of G2/M phase, in cultured AY-27 bladder cancer cells and 67% bladder cancers (derived from AY-27 cells) in vivo (Figure 5). As in the case of caspase-3 and PARP, the low and high doses of MSP-1 showed similar effects on cyclin B1. Interestingly, the level of cyclin B1 in MSP-1-treated UM-UC-3 cells was not significantly different from that in control UM-UC-3 cells (Figure 5). But the result in UM-UC-3 cells should still be interpreted as cyclin B1 downregulation because cyclin B1 is selectively expressed in G2/M phase and the proportion of cells in G2/M phase after MSP-1 treatment increased up to 3.5-fold (Figure 3C). It is worth noting that AITC was shown previously to consistently downregulate cyclin B1 in both UM-UC-3 cells as well as in AY-27 cells in vitro and bladder tumors derived from AY-27 cells in vivo (2).
In summary, there is a considerable variation in AITC/sinigrin levels among commercial preparations of MSP. Restricting our experiments to MSP-1, which possesses the highest level of AITC/sinigrin, we show that all AITC is stored as sinigrin in the powder and that sinigrin is the predominant if not the only ITC-generating glucosinolate. Sinigrin is highly stable in MSP-1. However, significant myrosinase activity is also present, which catalyzes full conversion of sinigrin to AITC when the powder is mixed in water in vitro and after it is ingested in vivo. Moreover, the matrix of MSP-1 did not interfere with AITC absorption and urinary excretion. Thus, MSP-1 is a highly attractive delivery vehicle for AITC. While sinigrin itself is not bioactive, MSP-1 inhibits the growth of bladder cancer cells in vitro, which was associated with strong induction of apoptosis and G2/M phase arrest. It also significantly inhibits bladder cancer growth and completely blocks muscle invasion in vivo. The anticancer effect of MSP-1 was accompanied by modulation of multiple well-known cancer therapeutic targets, including activation of caspase-3, cleavage of PARP and downregulation of both cyclin B1 and VEGF. Comparison of dose response in vivo between inhibition of tumor growth and muscle invasion by MSP-1 and its modulation of the above-mentioned proteins suggests that downregulation of VEGF may be critical for MSP-1 to inhibit tumor invasion into the muscle. Taken together, MSP-1 is a highly promising substance for prevention and treatment of bladder cancer. Further preclinical and clinical evaluation of this substance is warranted.
Funding
This work was supported in part by National Cancer Institute (R01CA124627).
Acknowledgments
We thank Drs Geng Feng and W.David Holtzclaw for technical support and Ms Kitty K.Stephenson for critical reading of the manuscript.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AITC
allyl isothiocyanate
- DADW
an equal volume mixture of dimethyl sulfoxide, acetonitrile, dimethylformamide and water
- IC50
half maximal inhibitory concentration
- ITC
isothiocyanate
- MMP
matrix metalloproteinase
- MSP
mustard seed powder
- PARP
poly adenosine diphosphate ribose polymerase
- PBS
phosphate-buffered saline
- VEGF
vascular endothelial growth factor
References
- 1.Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010;54:127–135. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya A, et al. Inhibition of bladder cancer development by allyl isothiocyanate. Carcinogenesis. 2010;31:281–286. doi: 10.1093/carcin/bgp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pow-Sang JM, et al. Contemporary management of superficial bladder cancer. Cancer Control. 2000;7:335–339. doi: 10.1177/107327480000700402. [DOI] [PubMed] [Google Scholar]
- 4.Amling CL. Diagnosis and management of superficial bladder cancer. Curr. Probl. Cancer. 2001;25:219–278. doi: 10.1067/mcn.2001.117539. [DOI] [PubMed] [Google Scholar]
- 5.Gagandeep, et al. Chemopreventive effects of mustard (Brassica compestris) on chemically induced tumorigenesis in murine forestomach and uterine cervix. Hum. Exp. Toxicol. 2005;24:303–312. doi: 10.1191/0960327105ht526oa. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, et al. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal. Biochem. 1996;239:160–167. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 7.Doerr B, et al. Cultivar effect on Moringa oleifera glucosinolate content and taste: a pilot study. Ecol. Food Nutr. 2009;48:199–211. doi: 10.1080/03670240902794630. [DOI] [PubMed] [Google Scholar]
- 8.Wade KL, et al. Improved hydrophilic interaction chromatography method for the identification and quantification of glucosinolates. J. Chromatogr. A. 2007;1154:469–472. doi: 10.1016/j.chroma.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shikita M, et al. An unusual case of 'uncompetitive activation' by ascorbic acid: purification and kinetic properties of a myrosinase from Raphanus sativus seedlings. Biochem. J. 1999;341:725–732. doi: 10.1042/0264-6021:3410725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Tang L, et al. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J. Nutr. 2004;134:2004–2010. doi: 10.1093/jn/134.8.2004. [DOI] [PubMed] [Google Scholar]
- 12.Munday R, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68:1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 13.Fahey JW, et al. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro TA, et al. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol. Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 15.Getahun SM, et al. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol. Biomarkers Prev. 1999;8:447–451. [PubMed] [Google Scholar]
- 16.Kensler TW, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol. Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 17.Munday R, et al. Evaluation of isothiocyanates as potent inducers of carcinogen-detoxifying enzymes in the urinary bladder: critical nature of in vivo bioassay. Nutr. Cancer. 2006;54:223–231. doi: 10.1207/s15327914nc5402_9. [DOI] [PubMed] [Google Scholar]
- 18.Herr HW, et al. Defining optimal therapy for muscle invasive bladder cancer. J. Urol. 2007;177:437–443. doi: 10.1016/j.juro.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Su JL, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9:209–223. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Donmez G, et al. Vascular endothelial growth factor (VEGF), matrix metalloproteinase-9 (MMP-9), and thrombospondin-1 (TSP-1) expression in urothelial carcinomas. Pathol. Res. Pract. 2009;205:854–857. doi: 10.1016/j.prp.2009.07.015. [DOI] [PubMed] [Google Scholar]