Abstract
Aims
Pre-treatment with dietary ω3 polyunsaturated fatty acids (ω3-PUFA) has been reported to reduce the incidence of new-onset atrial fibrillation (AF) following cardiac surgery. In a canine cardiac surgery model, we evaluated the impact of dietary ω3-PUFA on atrial electrophysiological properties, inflammatory markers, the atrial endothelin-1 (ET-1) system, and the expression and distribution of connexin 43.
Methods and results
Adult mongrel dogs received either normal chow (NC, n = 11) or chow supplemented with fish oil (FO, 0.6 g ω3-PUFA/kg/day, n = 9) for 3 weeks before surgery. A left thoracotomy was performed, and the left atrial appendage (LAA) was excised. Atrial pacing/recording wires were placed, and the pericardium/chest was closed. The atrial ratio of ω6/ω3 lipids decreased from 15–20 in NC to 2–3 in FO. FO treatment lowered pre-surgical and stabilized post-surgical arachidonate levels. Peak neutrophil to lymphocyte ratio was lower and decayed faster in FO-treated animals. Extensive inflammatory cell infiltration was present in NC atria, but was reduced in FO-treated dogs. FO-treated animals had lower post-surgical atrial expression of inducible nitric oxide synthase (iNOS) and reduced plasma ET-1. Expression of ET-1 and inositol trisphosphate receptor type-2 proteins in the LAA was also reduced. FO treatment prolonged post-operative atrial effective refractory period, slowed heart rate, and enhanced heart rate variability. Importantly, AF (>30 s) was inducible in four of six NC dogs, but no FO dogs.
Conclusion
Dietary FO attenuated AF inducibility following cardiac surgery by modulating autonomic tone and heart rate. FO also reduced atrial inflammation, iNOS, and ET-1 expression.
Keywords: Post-operative atrial fibrillation, Omega-3 fatty acids, Heart rate, Endothelin-1, Myeloperoxidase
1. Introduction
Atrial fibrillation (AF) following cardiac surgery (post-operative atrial fibrillation, POAF) affects 20–50% of patients, with an incidence that depends on age and type of surgery. POAF is associated with increased risk of morbidity and mortality. Efforts to prevent POAF have been largely unsuccessful, perhaps owing to the complex aetiology of the disorder and fragile state of the at-risk patients. Factors thought to contribute to POAF include: systemic inflammatory activation;1 elevated leucocyte count,2 atrial fibrosis;3 inflammatory tachycardia and changes in atrial action potential morphology;4 and oxidant stress resulting from increased NADPH oxidase activity.5
Pre-clinical studies may help to elucidate the mechanisms underlying post-operative atrial arrhythmogenesis. Two models have been published: the canine sterile pericarditis model, in which talc and epicardial gauze are used as inflammatory stimuli,6 and a right atrial incision model.7 In both the models post-operative arrhythmogenesis was related to altered patterns of atrial conduction owing to changes in the expression and distribution of connexins resulting from inflammatory cell infiltration.7,8 Prednisone decreased neutrophil infiltration and reduced the duration of AF episodes induced with burst pacing.7,9 Concerns about the impact of steroids on wound healing and glucose homeostasis following surgery have limited enthusiasm for systemic steroid administration.
1.1. Omega-3 fatty acids for prevention of post-operative AF
The highly unsaturated fatty acid lipid pool (HUFA, composed of polyunsaturated fatty acids, PUFA, of 20–22 carbon length) includes both ω6-PUFA (arachidonic acid, AA, etc.) and ω3-PUFA (eicosapentaenoic acid, EPA; docosahexaenoic acid, DHA). As ω3- and ω6-PUFA are structurally similar, the enzymes that metabolize ω6-PUFA also metabolize ω3-PUFA. While ω6 metabolites tend to promote inflammation and vasospasm, ω3 metabolites typically have less inflammatory and vasoconstrictor activity.10 The ratio of ω6 to ω3 PUFA in tissue HUFA varies as a function of diet. Diets containing abundant vegetable oils and chicken have higher ω6/ω3 ratios than diets with a greater abundance of fish. This ratio varies regionally. While the American diet maintains ∼15% of ω3 in tissue HUFA, diets in Japan or Iceland maintain the ω3 fraction above 50%.11 Populations with the lowest percent ω3 in the HUFA tend to have the highest cardiovascular mortality.11
In 2005, a clinical trial was reported in which cardiac surgery patients treated with a supplement containing ω3-PUFA had less POAF (15.2%) than untreated patients (33%, P = 0.013).12 As side effects associated with dietary FO are minimal, this study generated significant clinical interest. Unfortunately, several recent efforts to replicate this study using a similar dose with oral delivery have been negative.13,14 Although baseline dietary differences may contribute, reasons for the lack of consistent results cannot be easily determined from clinical trials.
As the impact of dietary ω3-PUFA on atrial pathophysiology in the context of cardiac surgery is poorly understood, this pre-clinical study was designed to evaluate the impact of supplemental FO on the systemic and cellular inflammatory response, and on the atrial substrate for POAF. Leukotriene B4 (LTB4) is an ω6-PUFA metabolite that acts as a potent neutrophil chemokine, critical for the recruitment of neutrophils to injured tissues.15 We hypothesized that by lowering ω6-PUFA abundance, a FO diet might limit atrial inflammation following surgery. Blood samples and atrial tissue specimens were obtained at defined times prior to, during, and following cardiac surgery. We assessed the impact of a dietary FO supplement on white cell distribution, plasma and atrial lipid composition, inflammatory cells and mediators, heart rate and AF inducibility, connexin 43 (Cx43) abundance, distribution and phosphorylation, and the expression of endothelin-1 (ET-1) and relevant related proteins.
2. Methods
2.1. Canine cardiac surgery model
Adult mongrel dogs of either sex (15–25 kg) were fed either a standard lab chow (normal chow, NC, Teklad no. 8653, containing 10% fat with negligible ω3 content, n = 11), or the same chow supplemented with fish oil (FO, containing 0.6 g/kg/day EPA/DHA, n = 9) for 3 weeks prior to an initial cardiac surgery. A similar FO diet decreased infarct size and reduced ventricular arrhythmias in dogs with experimental myocardial infarction16 (detailed methods are described in the Supplementary material online, Methods).
A left thoracotomy was performed under surgical anaesthesia, the pericardium was opened, and the left atrial appendage (LAA) was excised using a surgical cutting stapler. The excised LAA was used as an internal control from the initial surgery (post-operative day 0, POD-0). Pacing and recording wires were placed in the left atrium (LA) to evaluate effective refractory period (ERP) and AF inducibility. Animals received antibiotics and were allowed to recover until terminal study at POD-2 (n = 9) or POD-4 (n = 11). Animals in the FO and NC groups received the same diet after surgery that they did prior to surgery.
Blood samples were collected for analysis of white cell count (Advia 120 analyzer), C-reactive protein (CRP), and ET-1 before dietary treatment (baseline), on the day of initial surgery (prior to anaesthesia, POD-0) and daily thereafter until the terminal study.
In 13 animals, Holter recordings were obtained from the day of the initial surgery until the terminal surgery. In these animals, AF inducibility (>30 s duration) and LA ERP were evaluated daily in the conscious state. After conscious testing on the day of the terminal surgery (POD-2 or POD-4), a midline thoracotomy was performed under surgical anaesthesia and the animal was euthanized by induction of ventricular fibrillation. The heart was removed and the LA and right atria (RA) were excised and rapidly frozen for lipid analysis, immunostaining, and biochemical studies. All study protocols were approved by the Cleveland Clinic Institutional Animal Care and Use Committee and were performed in accord with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
2.2. Biomarker assays
CRP was measured in plasma using the Kamiya KT-093 canine ELISA. Plasma and atrial ET-1 levels were assessed using the Biomedica BI-20052 ELISA.
2.3. Western blot, immunostaining, and histology
Western blot17 was used to assess the expression of several proteins from LAA at the time of initial surgery and the LA and RA at terminal surgery. Antibodies for western blot were obtained from: inducible nitric oxide synthase (iNOS; Abcam); Cx43 (Sigma); non-phosphorylated Cx43 (Zymed); IP3R-I (Santa Cruz). Immunostaining was performed as described17 using specific antibodies for: ET-1 (Abcam), endothelin receptors type A and B (ETAR and ETBR, Alomone Labs), inositol trisphosphate (IP3) receptors I and II (IP3R-I and IP3R-II, Santa Cruz), and Cx43 (Sigma). Confocal microscopy was used to image the distribution of these proteins. Myeloperoxidase (MPO) staining was performed on formalin-fixed tissue sections using an MPO-specific antibody (Dako) with a haematoxylin counterstain.
2.4. Lipid analysis
Plasma and atrial lipid fatty acid composition was assessed using capillary gas chromatography by Lipid Technologies (Austin, MN, USA).
2.5. Statistical analysis
Two-group analyses of normally distributed variables were assessed using t-tests. Paired t-tests were used to assess differences between matched cardiac chambers in the same animal, while unpaired t-tests were used to assess differences between two independent groups. ANOVA was used to assess differences across multiple groups followed by Bonferroni post-test for individual pair comparisons. Values of P < 0.05 were considered statistically significant. All values are expressed as mean ± SEM.
3. Results
3.1. FO/plasma/atrial lipid content
A diet highly enriched in ω3-PUFA was used to increase atrial ω3 content. Figure 1B shows that the FO diet increased atrial ω3 in HUFA from 7.5 to 35%, and plasma ω3 in HUFA increased from 9 to 55% (Figure 1A). As the ω3-PUFA content increased, the AA% in HUFA decreased (Figure 1C). In the LAA on POD 0, the AA% was 78% in NC compared with 58% in the FO group (Figure 1D). The ω6/ω3 ratio dropped from 18 to 1.3 in plasma, and from 21 to 3 in LAA (Figure 1E and F). LTB4 attracts neutrophils into injured tissues. LTB5, the homologous ω3 metabolite, has ∼1/1000x the chemotactic activity of LTB4. LTB4 formation is attenuated when the abundance of EPA (20:5 ω3) is increased; FO treatment profoundly reduced the atrial 20:4/20:5 ratio from 134:1 to 4:1 (Figure 1H).
Figure 1.
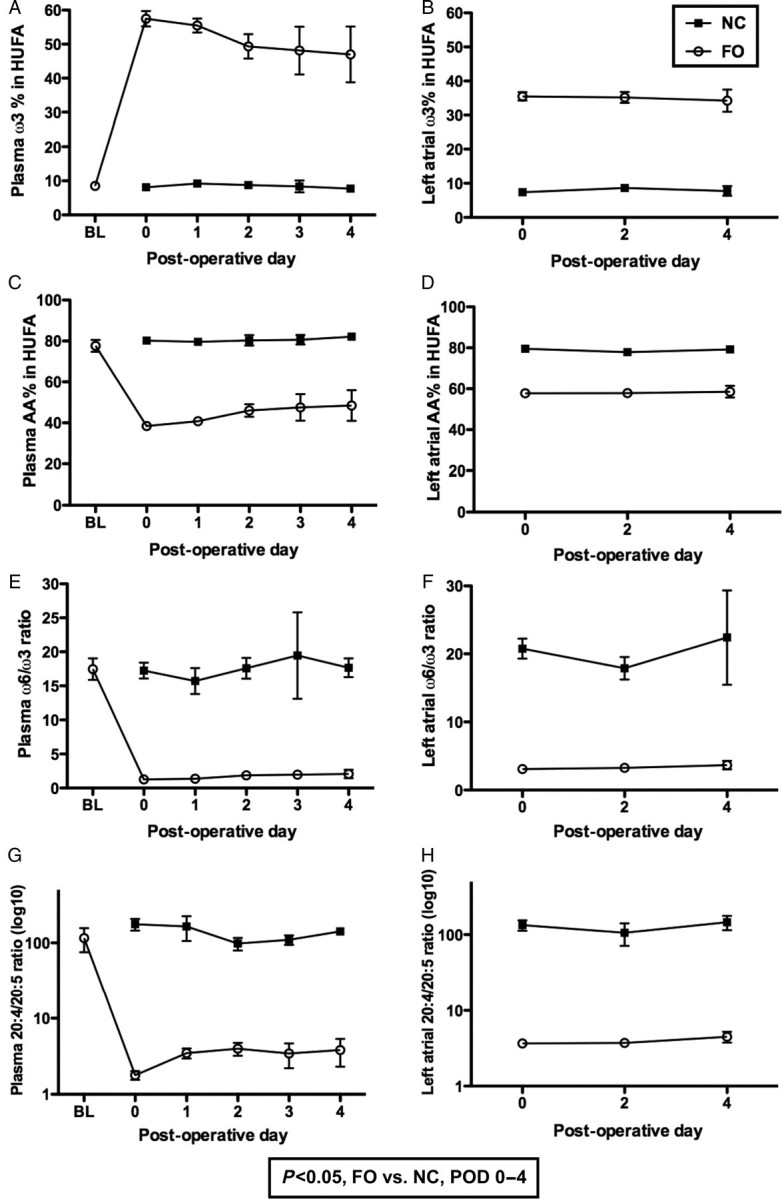
Plasma and tissue lipid analysis plots show plasma (A) and atrial ω3% (B), arachidonic acid % (C, D), ω6/ω3 ratio (E, F), and 20:4/20:5 (AA/EPA) ratio; and AA/EPA ratio (G, H) in the highly unsaturated fatty acid lipid pool (HUFA) of NC-treated controls and FO-treated animals.
3.2. C-reactive protein/inflammatory cells
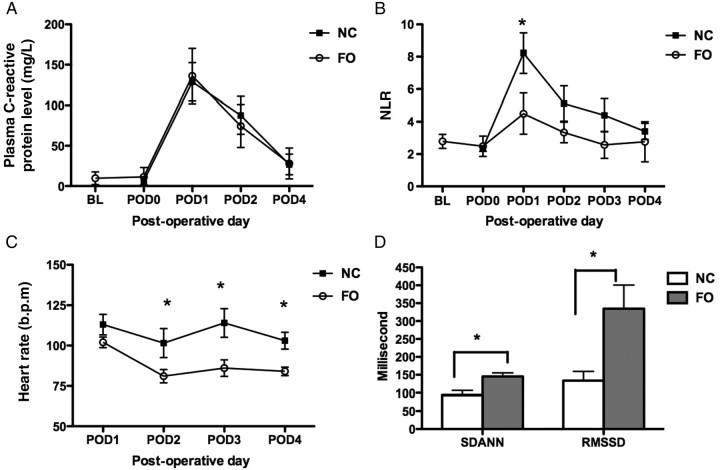
Plasma C-reactive protein is a commonly used marker of systemic inflammation. Figure 2A shows that plasma C-reactive protein levels were similar in both the NC- and FO-treated dogs. Surgery also evoked a profound cellular inflammatory response, dominated by an acute increase in neutrophil count. Figure 2B shows that although the neutrophil to lymphocyte ratio (NLR) peaked at the same time in NC- and FO-treated animals. Further, the peak NLR was lower and recovered more quickly in the FO-treated animals.
Figure 2.
Biomarkers, HRV, and atrial arrhythmia inducibility. (A) Plot shows plasma C-reactive protein at baseline (BL, 3 weeks before FO treatment), at POD-0 (day of surgery and prior to LAA excision), POD-1, -2, and -4 in both NC- and FO-treated animal (n = 8–9 per group). (B) Plot shows neutrophil-lymphocyte ratio (NLR) at BL, and POD 0–4. (C) Plot shows heart rate following surgery (POD 1–4). (D) Column plot showing mean ± SEM of RMSSD and SDANN RR intervals for all 5 min segments. For NC, n = 6; FO, n = 7. *P < 0.05 for FO vs. NC.
3.3. Heart rate/heart rate variability/arrhythmia inducibility
Cardiac surgery is often accompanied by an inflammatory tachycardia. Holter and arrhythmia inducibility data were obtained from six NC- and seven FO-treated animals following the initial surgery. Figure 2C shows that post-operative heart rate was more elevated in NC than in FO animals (P = 0.0003). In addition to differences in mean heart rate, Figure 2D shows that the root mean square of the successive normal sinus RR interval differences (RMSSD) and the standard deviation of the averaged normal sinus (SDANN) RR intervals for all 5 -min segments, heart rate variability (HRV) parameters associated with vagal tone,18 were enhanced by FO treatment. Left atrial ERP (LAERP) was shorter in NC- than in FO-treated animals; this difference was significant on POD-2 (cycle length 400 ms, NC:70 ± 22.8 ms; FO: 122 ± 34.5 ms, P = 0.009).
In cardiac surgery patients, AF is most common on POD-2. AF inducibility was evaluated in six NC- and seven FO-treated dogs on POD-2. Atrial arrhythmias (>30 s duration) were induced by burst pacing in four of six control, but in none (0/7) of the FO-treated animals tested (P = 0.02).
3.4. MPO staining
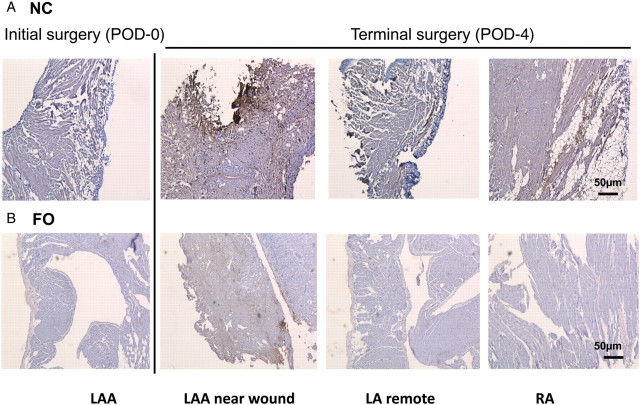
MPO is abundantly expressed in neutrophils and macrophages. Tissues from LAA (POD-0), LA, and RA (POD-2, -4) were evaluated for evidence of injury, and MPO staining (as a measure of macrophage/neutrophil infiltration). Figure 3 shows tissue sections from representative NC- and FO-treated animals that had terminal surgery on POD-4. Extensive inflammatory cell infiltration (and MPO staining, brown/black) was evident in the LA near the wound of the NC animal, but staining was also apparent in the LA away from the wound and in the RA. In contrast, in FO animals, MPO staining was markedly lower, particularly in the LA remote from the wound and in the RA.
Figure 3.
MPO staining. Bright field images show MPO staining (brown/black) recorded at 5× magnification from LAA obtained at the time of initial surgery, terminal LA near the wound and remote from the wound, and terminal RA at POD-4 from (A) a single representative NC animal, and (B) a representative FO-treated animal.
3.5. Cx43 distribution/phosphorylation
LAA Cx43 expression and phosphorylation were compared between groups (Supplementary material online, Figure S1). FO treatment did not affect either baseline LAA total Cx43 expression (Supplementary material online, Figure S1B) or the ratio of non-phosphorylated Cx43 (at serine 368; S368) to total Cx43 (npCx43/t-Cx43; Supplementary material online, Figure S1C).
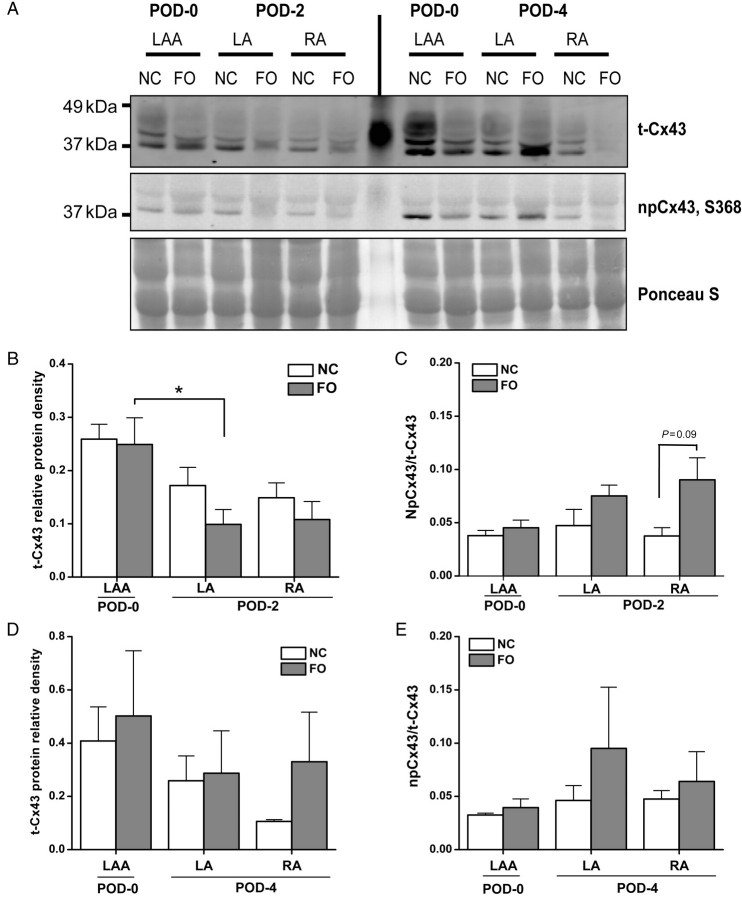
In the canine sterile pericarditis model, epicardial but not endocardial Cx43 and Cx40 expression was downregulated.8 Here, although epicardial inflammation was apparent, no transmural gradient of Cx43 expression was detected in the LA (Supplementary material online, Figure S2). Immunostaining revealed that the area around the surgical wound of NC atria had an increased abundance of lateralized Cx43, with a heterogeneous but higher expression of Cx43 (Supplementary material online, Figure S2A) than was present in the FO-treated atria (Supplementary material online, Figure S2B). Western blotting suggested a trend for lower Cx43 abundance in both groups following surgery (Figure 4B and D). On POD-2, expression of Cx43 was lower in the LA than in the POD-0 LAA; this difference was significant only in the FO animals (Figure 4B). FO treatment did not significantly affect the npCx43/t-Cx43 ratio on POD-2 (Figure 4C) or POD-4 (Figure 4E).
Figure 4.
Cx43 distribution/phosphorylation. (A) Representative western blots of t-Cx43 expression, npCx43, and total protein loading control from POD-0 LAA, terminal LA, terminal RA after surgery on POD-2 and POD-4 in NC and FO groups. (B, D) Column plots of mean ± SEM of t-Cx43 protein band relative density relative to total protein abundance at both POD-2 (B) and POD-4 (D). (C, E) Column plots of mean ± SEM of npCx43/t-Cx43 ratio protein at POD-2 (C) and POD-4 (E); n = 6–8 per group (three to four per day). Asterisk (*) indicates P < 0.05.
3.6. iNOS protein expression
Supplementary material online, Figure S3A shows a representative western blot of iNOS protein expression in atrial tissues. FO treatment decreased iNOS protein abundance in POD-0 LAA (P = 0.046; Supplementary material online, Figure S3B). Relative to baseline LAA, iNOS protein tended to be higher following cardiac surgery in the LA of NC animals at POD-2 (P = 0.09; Supplementary material online, Figure S3C). Compared with NC, FO treatment significantly decreased iNOS in terminal LA on POD-2 (Supplementary material online, Figures S3C and D).
3.7. Plasma and atrial ET-1 measurements
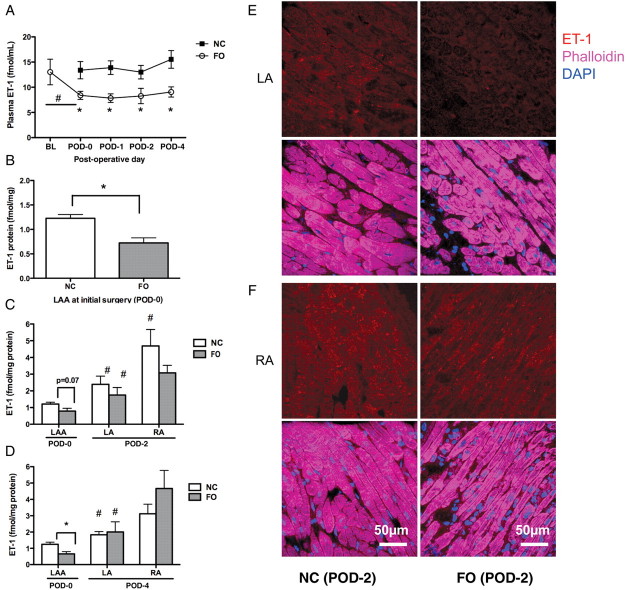
Plasma ET-1 was evaluated at baseline, prior to surgery (POD-0), and daily thereafter (Figure 5A). The FO diet significantly lowered the plasma ET-1 levels at all times. Although plasma ET-1 tended to increase after cardiac surgery, the change was not significant in either group. ET-1 levels were also measured in LAA (POD-0), and from terminal LA and RA tissues (POD-2, -4; Figure 5B–D). Analogous to the plasma studies, the FO diet significantly lowered LAA ET-1 levels at the initial surgery (P = 0.002; Figure 5B). Relative to POD-0 LAA expression, ET-1 protein expression was higher in the LA in both study groups on POD-2 (P < 0.05, NC vs. FO groups, respectively; Figure 5C). On POD-2, ET-1 content of terminal RA tissues was approximately twice that of the LA (Figure 5C). To determine whether the greater abundance of ET-1 in the RA was owing to cardiac surgery or to intrinsic regional variation, LA and RA specimens were evaluated from control chow non-surgical dogs (n = 7). In all groups, RA ET-1 content was higher than in the LA, suggesting that there are baseline regional differences in ET-1 expression (data not shown). Although FO treatment tended to lower RA and LA ET-1 expression at POD-2, this difference was not statistically significant (Figure 5C).
Figure 5.
Plasma and cardiac ET-1 measurements. (A) Plot shows plasma ET-1 at BL (3 weeks before FO treatment), POD-0 (day of surgery and prior to LAA excision), POD-1, -2, and -4 in both NC and FO groups. *P < 0.05 for FO vs. NC. #P < 0.05 for BL vs. POD-0 (FO group); n = 9 per group. (B) Column plot shows mean ± SEM of ET-1 protein in LAA obtained at the time of initial surgery. (C, D) Column plots show mean ± SEM of ET-1 expression in POD-0 LAA, terminal LA, and RA on POD-2 (C) and POD-4 (D) in NC and FO animals; n = 7–9 per group. Hash symbol (#) indicates a significant difference within group; LA vs. LAA, or RA vs. LA, P < 0.05. (E, F) Representative confocal images of ET-1 staining (red) from LA (E) and RA (F) in NC and FO groups at POD-2. Overlay images are stained with phalloidin (purple, myocytes) and DAPI (blue, nuclei).
Immunostaining confirmed that ET-1 (red) is more abundant in the LA than in the LAA (not shown) and is present in cardiac myocytes (phalloidin, purple, Figure 5E). FO treatment lowered ET-1 protein expression in LA cardiac myocytes, but had less effect in the RA (Figure 5F).
3.8. Endothelin receptor intensity and distribution
ET-1 acts on two receptors, ETAR and ETBR. Immunostaining revealed that ETAR and ETBR expression was abundant in atrial myocytes (α-actinin, red). No marked differences in receptor localization were observed between study groups (Supplementary material online, Figures S4 and S5).
Relative to POD-0 LAA, ETAR expression was lower in NC terminal LA at POD-2 (P < 0.05), but not at POD-4 (Supplementary material online, Figure S4C). In the FO-treated group, LA ETAR expression was unchanged at POD-2 but higher at POD-4 (P < 0.05; Supplementary material online, Figure S4). FO treatment had no impact on ETAR expression at POD-2, but showed more abundant ETAR expression at POD-4 in terminal LA (P = 0.018). These data suggest that ETAR expression is reduced after cardiac surgery, especially at POD-2, and that this effect was attenuated or reversed by FO treatment. ETAR expression in the RA tended to be similar to POD-0 LAA expression, suggesting that LA injury is associated with greater changes of ETAR expression in the LA than in the RA.
ETBR expression was similar between FO and NC groups at POD-2 and POD-4 (Supplementary material online, Figure S5). Relative to POD-0 LAA, ETBR expression in terminal LA was lower at POD-2 and POD4 in NC animals; the trend was similar but not significant in the FO-treated group.
3.9. IP3 receptor expression and distribution
ET-1 can modulate intracellular Ca2+ via generation of IP3 that interacts with IP3R-I and IP3R-II. Like ET-1 receptors, IP3Rs were distributed in atrial myocytes (Figure 6 and Supplementary material online, Figure S6). No changes in receptor localization were observed between study groups.
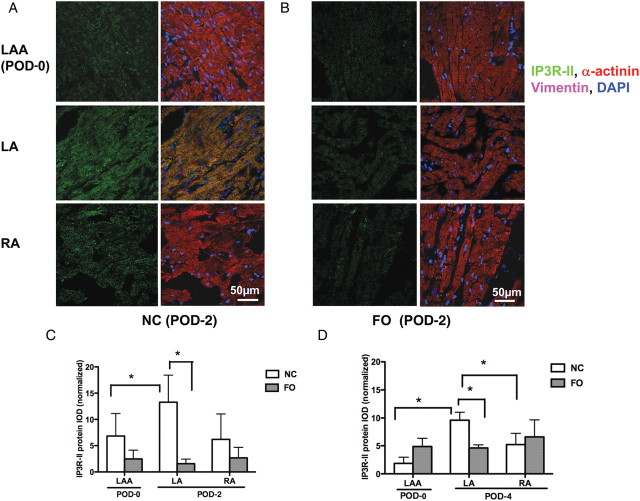
Figure 6.
IP3R-II protein expression following surgery. Representative confocal images of IP3R-II staining (green) in NC (A) and FO (B) groups at POD-2, with the corresponding overlay images with α-actinin (red) to stain cardiac myocytes, vimentin to stain fibroblasts (purple), and DAPI to stain nuclei (blue). Note that IP3R-II is highly colocalized with α-actinin, with a striated distribution pattern. (C, D) Column plots show mean ± SEM IP3R-II-integrated optical density normalized to α-actinin area in atrial myocytes on POD-2 (C) and POD-4 (D). *P < 0.05, n = 6 per group.
Image analysis revealed no differences in IP3R-I expression between FO and NC dogs. Relative to POD-0 LAA, IP3R-I expression was higher in the LA (Supplementary material online, Figure S6A–D) at both POD-2 and POD-4. Western blot analysis confirmed the changes observed by immunostaining (Supplementary material online, Figure S6E).
Relative to POD-0 LAA, IP3R-II expression was higher in terminal LA of NC but not FO-treated animals (Figure 6A–D). Unlike IP3R-I, FO treatment significantly reduced IP3R-II expression in terminal LA at both POD-2 and POD-4 (Figure 6C and D). In both study groups, RA IP3R expression was similar to that in the LAA.
4. Discussion
POAF increases the duration of hospital stay and hospital costs, and is associated with increased morbidity and mortality. Calo et al.12 reported that a dietary FO supplement (2 g/day, beginning 5–7 days before surgery) was effective in decreasing the incidence of POAF. Changes in plasma and atrial lipid composition in response to dietary supplement are both time- and dose-dependent. Although plasma levels can change quickly, steady-state tissue changes require about 3 weeks.19 Clinically, the impact of modifying atrial vs. plasma ω3-content has not been carefully evaluated, with respect to determining the degree of change needed to impact arrhythmogenesis following cardiac surgery. Here, we sought to address this issue using a pre-clinical surgical model in which excision of the LAA during initial surgery served as the primary trauma, rather than the epicardial application of talc and gauze,8 or creation of an RA incision.7 With our approach, LAA tissue from the initial surgery provided a baseline specimen for within-animal paired analyses. The previously reported epicardial–endocardial gradient in connexins may be owing to epicardial injury caused by the talc and gauze.8 Our model avoids this pro-arrhythmic but artefactual response.
The impact of dietary ω3-PUFA depends both on the level of competing ω6-PUFA in the diet, and on the amount stored in tissue lipids. The composition of tissue lipids varies globally by region, reflecting variations in dietary preference and availability. Figure 1F shows that canine atrial tissues have a very low fraction of ω3-PUFA, with an ω6/ω3 ratio of 21. The plasma and atrial ω3 in the NC HUFA pool is 8%, similar to that of the American population. In contrast, individuals consuming a Mediterranean diet have ∼35% ω3 in the HUFA, and those consuming a traditional Japanese diet have more than 50% ω3 in HUFA.11 Our experimental diet shifted the ω3-HUFA content of the atria from a level similar to that of individuals regularly consuming an American diet to one closer to that of individuals consuming a Mediterranean diet. This change lowered post-operative heart rate, improved HRV, decreased inflammatory cell infiltration, modulated the expression and distribution of Cx43, and attenuated atrial ET-1 expression.
4.1. Impact of FO on post-operative tachycardia and HRV
FO treatment slowed heart rate and enhanced HRV after surgery. Tachycardia following cardiac surgery is common, and is associated with increased risk of POAF.4 Tachycardia might result from enhanced sympathetic tone, loss of vagal tone, or modulation of pacemaker activity. HRV analysis suggests that vagal tone was preserved and/or enhanced by FO treatment. Vagal stimulation is associated with anti-inflammatory activity.20 As AA metabolites prostaglandin F2a (PGF2α) and thromboxane A2 (TxA2) are implicated in the genesis of inflammatory tachycardia,21 decreased AA abundance in the FO-treated animals may contribute to heart rate slowing. Dietary ω3-PUFA have also been shown to suppress pacemaker currents (If).22 Each of these pathways may contribute to the ω3-PUFA-mediated heart rate slowing following surgery.
4.2. Impact of FO on post-operative C-reactive protein and inflammatory cells
Previous studies in canine surgical models suggested a critical role for inflammatory cell infiltration as a mediator of POAF,7,9 and showed that interventions that limit this infiltration can limit conduction heterogeneity, AF inducibility, and AF episode duration. Here, post-surgical plasma CRP levels were not affected by FO treatment (Figure 2A), and were not different between animals in which AF was vs. was not inducible. That the CRP response did not vary by treatment group demonstrates that plasma CRP is not strongly modulated by dietary lipids, and may not be linked to the arrhythmogenic substrate for POAF. In contrast, atrial MPO staining was strongly modulated by FO treatment. Atrial MPO is implicated in atrial fibroblast proliferation, the development of interstitial fibrosis, and AF.23 FO treatment attenuated atrial MPO staining, particularly in the areas remote from the surgical wound (Figure 3), probably as a result of decreased LTB4 production.
4.3. Impact of FO on atrial Cx43
Changes in the trafficking and degradation of Cx43 following surgery may contribute to conduction heterogeneity and AF. In both the sterile pericarditis8 and atrial incision7 models, MPO-positive cellular infiltration was associated with increased conduction heterogeneity. In this study, changes in Cx43 were more subtle than those observed in the sterile pericarditis model.8 FO treatment did not affect basal LAA Cx43 expression. Cx43 in the LA and RA at terminal surgery was lower than the expression of LAA in all animals. There was a non-significant trend for FO treatment to increase the npCx43/t-Cx43 ratio on POD-2. As S368 is a protein kinase C (PKC) target site, lower phosphorylation may reflect less PKC activity. PKC isoforms are activated by Ca2+ and by Gq-coupled receptors (e.g. angiotensin-II, ET-1); less PKC activity would be consistent with less receptor activation. In NC animals, the abundance of lateralized Cx43 was increased after surgery, with especially heterogeneous and elevated Cx43 expression near the wound. FO treatment attenuated Cx43 lateralization, resulting in a more normal distribution pattern following surgery (Supplementary material online, Figure S2).
4.4. Impact of FO on atrial ET-1 system
ET-1 binding to ETAR and ETBR receptors activates PKC/IP3/NADPH oxidase pathways. We recently reported that atrial ET-1 abundance was increased in AF patients, and that atrial ET-1 content was associated with atrial rhythm, size, and fibrosis.17 Here, atrial but not plasma ET-1 levels were higher following surgery (in both study groups). FO reduced both plasma and atrial ET-1. Plasma ET-1 was reported to predict AF recurrence following pulmonary vein isolation;24 this suggests that ET-1 may also promote POAF. Here, FO reduced basal atrial ET-1 expression by 42%. Following surgery, the changes were less significant. Intriguingly, RA ET-1 levels were higher than in the LA, suggesting that there are regional differences in this system.
In patients who experienced POAF, RA ETAR and ETBR mRNA expression was lower than in those who did not.24 Here, ETAR and ETBR expression were lower in the LA following surgery in NC animals, while IP3R-I and IP3R-II were higher. After binding to its receptors, IP3 modulates calcium release from intracellular stores. By increasing IP3 production, elevated ET-1 can enhance atrial contractility, Ca2+ transients, and perhaps arrhythmia inducibility. In cardiac diseases including AF and heart failure, expression of IP3Rs and membrane receptors that couple to phospholipase C signalling is increased, suggesting a role for IP3 signalling in the aetiology of these diseases.25 ET-1 both modulates Ca2+ homeostasis and promotes fibrosis, resulting in impaired atrial conduction and AF inducibility. While FO had no impact on ETBR or IP3R-I expression, it markedly reduced IP3R-II expression in the post-surgical LA. In most species, IP3R-II is the predominant atrial isoform.25 Cardiac myocytes overexpressing IP3RII are hypertrophic and vulnerable to arrhythmogenic triggers upon ET-1 stimulation. FO reduced atrial IP3R-II expression and may attenuate arrhythmogenic disturbances associated with enhanced IP3 signalling or intracellular Ca2+ release.
In vitro studies have shown that treatment of cultured myocytes with EPA (ω3-PUFA) prevents ET-1-mediated induction of iNOS expression and limits cardiac myocyte hypertrophy.26 Increased iNOS expression may result in increased atrial oxidative stress. Here, we observed that FO attenuated both ET-1 production and iNOS expression. ET-1 receptor activation also stimulates NADPH oxidase activity.27 As both iNOS and ET-1 levels were reduced by FO treatment, it is likely that FO treatment attenuates atrial and systemic oxidant stress.
4.5. Limitations
Owing to technical limitations, baseline heart rate/HRV data were not obtained prior to surgery. Electrophysiological testing was performed only in a subset of the animals, and AF was inducible only in four of six NC animals. The small sample size limited our ability to evaluate changes in atrial ERP beyond POD-2, and other factors associated with AF inducibility. To shift the atrial lipid content within 3 weeks required an FO dose larger than what has been used in any of the clinical trials using dietary PUFA (0.6 g/kg/day). In a canine ventricular tachypacing model, 2 weeks pre-treatment with ∼0.2 g/kg/day ω3 PUFA effectively minimized haemodynamic changes, atrial fibrosis, and AF inducibility. Additional studies are needed to clarify the impact of dietary ω3-PUFA on the substrate for AF in the absence of an initial surgery, as well as to determine the threshold ω6/ω3 ratio needed to impact post-surgical atrial arrhythmogenesis, and the duration of pre-treatment needed.
4.6. Conclusions
Cardiac surgery elicits profound changes in plasma and atrial inflammatory markers. Use of dietary FO at levels that shifted atrial ω3-content to levels similar to those of individuals consuming a Mediterranean diet decreased the post-operative NLR and evidence of atrial neutrophil infiltration. FO slowed post-operative heart rate and enhanced HRV, in a manner consistent with improved vagal tone. FO also reduced plasma and atrial ET-1 levels, and the atrial expression of iNOS and IP3R-II. Following surgery, FO treatment was associated with less lateralization of atrial Cx43. Each of these elements has a critical role in atrial calcium cycling or conduction. Increasing atrial and plasma ω3 levels has a favourable impact on the substrate for post-operative atrial arrhythmogenesis. Based on the negative results of recent clinical trials, it seems likely that long-term dietary changes, rather than short-term pharmacological interventions, may provide greater clinical benefit.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: None declared.
Funding
This work was supported by the Atrial Fibrillation Innovation Center, a Wright Center Initiative of the State of Ohio, the Fondation Leducq European-North American Atrial Fibrillation Research Alliance, and by a fellowship from the American Heart Association (F.M.).
Supplementary Material
References
- 1.Bruins P, Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–3548. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 2.Abdelhadi RH, Gurm HS, Van Wagoner DR, Chung MK. Relation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperatively. Am J Cardiol. 2004;93:1176–1178. doi: 10.1016/j.amjcard.2004.01.053. doi:10.1016/j.amjcard.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 3.Goette A, Juenemann G, Peters B, Klein HU, Roessner A, Huth C, et al. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res. 2002;54:390–396. doi: 10.1016/s0008-6363(02)00251-1. doi:10.1016/S0008-6363(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 4.Pichlmaier AM, Lang V, Harringer W, Heublein B, Schaldach M, Haverich A. Prediction of the onset of atrial fibrillation after cardiac surgery using the monophasic action potential. Heart. 1998;80:467–472. doi: 10.1136/hrt.80.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:68–74. doi: 10.1016/j.jacc.2007.07.085. doi:10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- 6.Page PL, Plumb VJ, Okumura K, Waldo AL. A new animal model of atrial flutter. J Am Coll Cardiol. 1986;8:872–879. doi: 10.1016/s0735-1097(86)80429-6. doi:10.1016/S0735-1097(86)80429-6. [DOI] [PubMed] [Google Scholar]
- 7.Ishii Y, Schuessler RB, Gaynor SL, Yamada K, Fu AS, Boineau JP, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. doi:10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 8.Ryu K, Li L, Khrestian CM, Matsumoto N, Sahadevan J, Ruehr ML, et al. Effects of sterile pericarditis on connexins 40 and 43 in the atria—correlation with abnormal conduction and atrial arrhythmias. Am J Physiol Heart Circ Physiol. 2007;293:H1231–H1241. doi: 10.1152/ajpheart.00607.2006. doi:10.1152/ajpheart.00607.2006. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein RN, Ryu K, Khrestian C, Van Wagoner DR, Waldo AL. Prednisone prevents inducible atrial flutter in the canine sterile pericarditis model. J Cardiovasc Electrophysiol. 2008;19:74–81. doi: 10.1111/j.1540-8167.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 10.Rennison JH, Van Wagoner DR. Impact of dietary fatty acids on cardiac arrhythmogenesis. Circulation: Arrhythm Electrophysiol. 2009;2:460–469. doi: 10.1161/CIRCEP.109.880773. doi:10.1161/CIRCEP.109.880773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- 12.Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. doi:10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 13.Heidarsdottir R, Arnar DO, Skuladottir GV, Torfason B, Edvardsson V, Gottskalksson G, et al. Does treatment with n-3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery? Europace. 2010;12:356–363. doi: 10.1093/europace/eup429. doi:10.1093/europace/eup429. [DOI] [PubMed] [Google Scholar]
- 14.Saravanan P, Bridgewater B, West AL, O'Neill SC, Calder PC, Davidson NC. Omega-3 fatty acid supplementation does not reduce risk of atrial fibrillation following coronary artery bypass surgery: a randomized, double blind, placebo controlled clinical trial. Circ Arrhythm Electrophysiol. 2009;3:46–53. doi: 10.1161/CIRCEP.109.899633. doi:10.1161/CIRCEP.109.899633. [DOI] [PubMed] [Google Scholar]
- 15.Williams FM. Neutrophils and myocardial reperfusion injury. Pharmacol Ther. 1996;72:1–12. doi: 10.1016/s0163-7258(96)00090-3. doi:10.1016/S0163-7258(96)00090-3. [DOI] [PubMed] [Google Scholar]
- 16.Culp BR, Lands WE, Lucches BR, Pitt B, Romson J. The effect of dietary supplementation of fish oil on experimental myocardial infarction. Prostaglandins. 1980;20:1021–1031. doi: 10.1016/0090-6980(80)90056-8. doi:10.1016/0090-6980(80)90056-8. [DOI] [PubMed] [Google Scholar]
- 17.Mayyas F, Niebauer M, Zurick A, Barnard J, Gillinov AM, Chung MK, et al. Association of left atrial endothelin-1 with atrial rhythm, size and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol. 2010;3:369–379. doi: 10.1161/CIRCEP.109.924985. doi:10.1161/CIRCEP.109.924985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zulfiqar U, Jurivich DA, Gao W, Singer DH. Relation of high heart rate variability to healthy longevity. Am J Cardiol. 2010;105:1181–1185. doi: 10.1016/j.amjcard.2009.12.022. doi:10.1016/j.amjcard.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, Stuklis R, et al. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr. 2007;85:1222–1228. doi: 10.1093/ajcn/85.5.1222. doi: [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. doi:10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 21.Takayama K, Yuhki K, Ono K, Fujino T, Hara A, Yamada T, et al. Thromboxane A2 and prostaglandin F2alpha mediate inflammatory tachycardia. Nat Med. 2005;11:562–566. doi: 10.1038/nm1231. doi:10.1038/nm1231. [DOI] [PubMed] [Google Scholar]
- 22.Verkerk AO, Den Ruijter HM, Bourier J, Boukens BJ, Brouwer IA, Wilders R, et al. Dietary fish oil reduces pacemaker current and heart rate in rabbit. Heart Rhythm. 2009;6:1485–1492. doi: 10.1016/j.hrthm.2009.07.024. doi:10.1016/j.hrthm.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph V, Andrie RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch-Hoffmann B, et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med. 2010;4:470–474. doi: 10.1038/nm.2124. doi:10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolettis TM, Kyriakides ZS, Zygalaki E, Kyrzopoulos S, Kaklamanis L, Nikolaou N, et al. Endothelin system and atrial fibrillation post-cardiac surgery. J Interv Card Electrophysiol. 2008;21:203–208. doi: 10.1007/s10840-008-9206-5. doi:10.1007/s10840-008-9206-5. [DOI] [PubMed] [Google Scholar]
- 25.Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–147. doi: 10.1016/j.yjmcc.2008.05.014. doi:10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimojo N, Jesmin S, Zaedi S, Soma M, Kobayashi T, Maeda S, et al. EPA effect on NOS gene expression and on NO level in endothelin-1-induced hypertrophied cardiomyocytes. Exp Biol Med (Maywood) 2006;231:913–918. [PubMed] [Google Scholar]
- 27.Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–313. doi: 10.1097/FJC.0b013e31817f9398. doi:10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.