Abstract
Introduction
Sunitinib is an approved treatment for metastatic renal cell carcinoma (RCC). A prospective clinical trial was conducted to evaluate the safety and clinical response to sunitinib administered prior to nephrectomy in patients with localized or metastatic clear cell RCC.
Methods
Patients with biopsy-proven clear cell RCC were enrolled and treated with sunitinib malate, 37.5 mg daily, for 3 months prior to nephrectomy. The primary endpoints was safety.
Results
Twenty patients were enrolled during an 18 month period. The most common toxicities were GI symptoms and hematologic. Grade 3 toxicities occurred in 6 patients (30%). No surgical complications were attributable to treatment with sunitinib. Seventeen of the 20 patients (85%) experienced a decrease in tumor diameter (mean change −11.8%, range −27% to 11%) and cross-sectional area (mean change −27.9%, range −43% to 23%). Enhancement on contrast-enhanced CT decreased in 15 patients, with a mean change in Hounsfield units of −22% (range −74% to 29%). Following a decrease in tumor size, 8 patients with cT1b tumors underwent laparoscopic partial nephrectomy. Surgical parameters such as blood loss, transfusion rate, operative time, and complications were similar to those who underwent surgery during the study period and were not enrolled on the trial.
Conclusions
Preoperative treatment with sunitinib is safe. Sunitinib decreased the size of primary RCC in 17 of 20 patients, and future trials can be considered to evaluate the use of neoadjuvant sunitinib to maximize nephron-sparing and decrease recurrence risk for high-risk, localized RCC.
Keywords: sunitinib, neoadjuvant, nephrectomy, renal cell carcinoma
INTRODUCTION
There are approximately 58,000 new cases of kidney cancer diagnosed in the United States annually, and approximately 13,000 patients die of their disease each year.1 Widespread use of abdominal imaging has resulted in an increase in incidence of renal tumors and a parallel increase in surgical interventions.2 However, the mortality associated with larger renal tumors remains largely unaffected. Therefore, surgical resection alone may be inadequate for curing high-risk renal cell carcinoma (RCC). The risk of metastatic recurrence is directly related to tumor size for patients with clinically localized RCC, and 25% to 50% of patients treated for localized disease eventually recur.3, 4 There are no effective therapies to reduce the risk of metastatic recurrence.
In 2006, the FDA approved sunitinib for the treatment of advanced kidney cancer based on the results of 2 clinical trials in patients who were previously treated with cytokine therapy.5, 6 Unlike immune cytokines such as interleukin-2 and interferon, which produce minimal tumor responses7, sunitinib decreased the size of metastatic deposits in the majority of cases. These observations provided a rationale for investigating sunitinib as an adjuvant therapy patients at high risk of recurrence after nephrectomy for clinically localized RCC. A large phase III study is currently underway to evaluate adjuvant sunitinib, sorafenib, or placebo in patients with unfavorable, clinically localized RCC.8
There are several theoretical advantages of neoadjuvant therapy over adjuvant therapy. Preoperative treatment with sunitinib may allow for earlier treatment and even cure of microscopic metastases. Downsizing of a bulky primary tumor may facilitate surgical resection and allow for nephron-sparing surgery. This is particularly relevant since maximizing renal function is now well recognized as an important goal of RCC surgery.9 For metastatic RCC, upfront treatment with systemic therapy may allow for identification of patients with rapidly progressing disease who are unlikely to benefit from cytoreductive nephrectomy.10
Given the rationale for preoperative therapy with sunitinib, we evaluated the safety of sunitinib prior to renal surgery in patients with biopsy proven clear cell RCC. We also determined the response of the primary tumor to sunitinib. This is the first prospective evaluation of preoperative sunitinib in patients with surgically resectable RCC.
MATERIALS AND METHODS
Population
Between September 2007 and April 2009, patients with biopsy proven clear cell RCC were enrolled in an Institutional Review Board approved (I95206) study of preoperative sunitinib. Patients were eligible for enrollment if they were at least 18 years of age and were candidates for a nephrectomy. All patients had to have clinical T1b, T2, or T3 RCC and Eastern Cooperative Oncology Group – Performance Status (ECOG-PS) of 0 or 1. Patients were eligible regardless of clinical N or M categories. All patients had adequate liver function, hematologic parameters, and renal function.
All surgeries were performed by a single surgeon (HLK). To confirm that surgical parameters were typical for patients undergoing standard management, results were compared to a control group (historic control), which included patients who underwent nephrectomy by the same surgeon (HLK) during the study period. Information for historic controls was derived retrospectively from a prospectively maintained institutional database (I53605).
Procedure
Twenty patients enrolled in a single-arm, open-label study. Patients were given 37.5 mg of oral sunitinib malate (Sutent, Pfizer Inc, New York, NY) daily for 90 days prior to surgery. As indicated in the protocol, in the first 5 patients, sunitinib was discontinued 5 days prior to surgery and in the last 15 patients, sunitinib was continued up to the day before surgery. The primary objectives of the study were to determine the safety of sunitinib in patients with a primary tumor in place and the safety of surgery after 90 days of sunitinib treatment. The response of the primary tumor to treatment was a secondary endpoint.
A baseline CT of the abdomen and pelvis (GE LightSpeed VCT 64 row scanner), with intravenous contrast, was obtained prior to starting treatment. A CT was repeated on the same scanner prior to surgery and after at least 2 months of treatment with sunitinib. All CT scans were performed using a standard protocol that involved injecting at least 150cc of contrast at a standard rate (5cc/s) using an 18-gauge needle. Change in size of the primary tumor was determined using 2 different methods: [1] by measuring the longest cross-sectional diameter of the tumor (RECIST criteria: Response Evaluation Criteria In Solid Tumors), and [2] calculating the bidimensional product of the cross-section of the tumor (WHO criteria: World Health Organization).11, 12 Based on the size and location of the primary tumor on the final preoperative CT, the treating physician recommended radical or partial nephrectomy.
Tumor enhancement during contrast-enhanced CT was quantified by drawing a region of interest around the cross-sectional margin of the tumor and measuring the mean Hounsfield units (HU). As proposed by Choi et al, treatment-induced decrease in tumor perfusion was calculated as percent change in enhancement.13 Radiographic findings were correlated with histologic necrosis, calculated as the average percent necrosis in 4 quadrants of a cross-section obtained from the tumor midpoint. All pathologic specimens were reviewed by one of two genitourinary pathologists and characterized using the 2004 American Joint Committee on Cancer TNM staging system.
Statistical analysis
To compare study patients with historic controls, student’s t-test and fisher’s exact test were used. Correlations between one-dimensional (RECIST) and two-dimensional (WHO) responses, and changes in HUs and size were measured using Spearman’s rho correlation. Creatinine clearance (CrCl) was calculated using the Cockroft-Gault formula based on the patient’s actual body weight (ABW):
Age is in years and Scr is serum creatinine. Statistical analyses were performed with Stata, version 10.0 (College Station, TX). P-value < 0.05 was considered significant.
RESULTS
Twenty patients enrolled in the study. A historic control group was used for comparison of surgical parameters. Patient characteristics are summarized in table 1. In the study group, 4 patients had radiographic evidence of metastatic disease at presentation. Study patients who underwent laparoscopic partial nephrectomy had significantly larger tumors when compared to historic controls undergoing the same procedure. The tumor size in table 1 is measured from the pretreatment CT and does not reflect tumor shrinkage from sunitinib treatment.
Table 1.
Patient demographics by surgical type.
| Study Patients | Historic Control* | |||
|---|---|---|---|---|
|
Lap radical (n=12) |
Lap partial (n=8) |
Lap radical (n=55) |
Lap partial (n=97) |
|
| Age (range) | 61 (45-85) | 60 (48-73) | 65 (38-88) | 62 (26-89) |
| Sex: | ||||
| Male | 7 (58%) | 4 (50%) | 24 (44%) | 62 (36%) |
| Female | 5 (42%) | 4 (50%) | 31 (56%) | 35 (64%) |
| Tumor size (cm) | 8.1 (4.7-11) | 5.3 (4.4-7.2) | 7.7 (1.8-21) | 2.6 (0.8-6.8)** |
| Dept of invasion (cm) | Not applicable | 2.7 (1.7-4) | Not applicable | 1.8 (.2-16)** |
| Preop CrCl | 98 (64-153) | 103 (50-179) | 83 (8.7–186) | 92 (25-191) |
| Clinical T category | ||||
| cT1a | 0 | 0 | 9 (16%) | 82 (85%) |
| cT1b | 1 (8%) | 7 (87%) | 19 (35%) | 15 (15%) |
| cT2 | 9 (75%) | 1 (13%) | 25 (45%) | 0 |
| cT3a | 0 | 0 | 0 | 0 |
| cT3b | 2 (16%) | 0 | 2 (4%) | 0 |
| Clinical N category | ||||
| cN0 | 11 (92%) | 8 (100%) | 49 (88%) | 95 (98%) |
| cN1 | 0 | 0 | 2 (4%) | 1 (1%) |
| cN2 | 1 (8%) | 0 | 4 (8%) | 1 (1%) |
| Clinical M category | ||||
| cM0 | 8 (67%) | 8 (100%) | 48 (87%) | 96 (99%) |
| cM1 | 4 (33%) | 0 | 7 (13%) | 1 (1%) |
Surgeries performed by the same surgeon at the same institution during the study period
p-value comparison of study patients and historic control patients who underwent lap partial (only p-values<0.05 are noted):
Tumor size <0.001
Dept of invasion 0.002
Abbreviations: Lap radical, laparoscopic radical nephrectomy, Lap partial, laparoscopic partial nephrectomy, CrCl, creatinine clearance
The most common sunitinib-toxicities were GI symptoms (nausea, abdominal pain, dyspepsia, indigestion, vomiting, flatulence, diarrhea, abdominal cramps, and constipation) and hematologic (leukopenia and thrombocytopenia), occurring in 13 (65%) and 11 (55%)patients, respectively (Supplemental Tables 1 and 2, online). Other side effects were fatigue in 9 patients (45%), hand-and-foot syndrome in 3 patients (15%), and hypertension in 5 patients (25%). Grade 3 toxicities were rare and included 2 patients (10%) with neutropenia, 2 patients (10%) with hand-and-foot syndrome and 3 patient (15%) with pancreatitis. The only grade 4 toxicity was an episode of hyponatremia that resolved with fluid restriction. Twelve patients (60%) completed the full 90 day-treatment of oral sunitinib at the full dose. Dose interruptions were required for 5 patients (25%) and dose reductions were required in 2 patients (10%). No patient required a delay in surgery due to toxicities.
Surgical outcomes are summarized in table 2. No intra-operative or postoperative complications were attributable to administration of sunitinib. There was one nonemergent conversion from laparoscopic to open partial nephrectomy in a patient with a 5.5 cm central tumor in a solitary kidney. This patient’s estimated creatinine clearances before and after surgery were 179 and 161, respectively. One patient undergoing laparoscopic partial nephrectomy was reported to have negative margins on intraoperative frozen-section; however the final pathology review of the same margin tissue was determined to be focally positive. Tumor size for the study group was larger than for controls as protocol eligibility required a primary tumor size of 4 cm or greater. There was no significant difference for any surgical parameter between study patients and historic controls with the exception of warm ischemia time (p<0.001).
Table 2.
Surgical and pathologic outcomes of study patients.
| Study patients | Historic control* | ||||
|---|---|---|---|---|---|
| Lap radical (n=12) |
Lap partial (n=8) |
Lap radical (n=55) |
Lap partial (n=97) |
||
| Surgical | Operative time (min) | 252 (159-376) | 211 (170-275) | 222 (100-605) | 197 (95-316) |
| Parameters | EBL (ml) | 430 (20-1400) | 384 (100-700) | 464 (5-7000) | 258 |
| No. patients transfused | 0 | 0 | 6 (11%) | 3 (3%) | |
| Warm ischemia (min) | Not applicable | 35 (13-51) | Not applicable | 20 (8-50)** | |
| Hospital stay (days) | 2.0 (1-4) | 1.9 (1-6) | 2.5 (1-20) | 1.7 (1-11) | |
| Postop CrCl | 71 (46-94) | 89 (43-171) | 69 (27-138) | 85 (18-147) | |
| Pathology | Pathologic T category | ||||
| pT1a | 0 | 5 (63%) | 7 (13%) | 67 (69%) | |
| pT1b | 1 (8%) | 2 (25%) | 14 (26%) | 6 (6%) | |
| pT2 | 5 (42%) | 0 | 12 (22%) | 0 | |
| pT3a | 3 (25%) | 1 (13%) | 10 (18%) | 5 (5%) | |
| pT3b | 1 (8%) | 0 | 3 (5%) | 0 | |
| pT4 | 2 (16%) | 0 | 4 (7%) | 0 | |
| Clinical N category | |||||
| pNx | 0 | 6 (75%) | 9 (16%) | 77 (79%) | |
| pN0 | 10 (83%) | 2 (25%) | 36 (65%) | 0 | |
| pN1 | 0 | 0 | 0 | 0 | |
| pN2 | 2 (17%) | 0 | 5 (9%) | 1 (1%) | |
| Positive margins | 0 | 1 (13%) | 4 (7%) | 2 (2%) | |
| No. lymph nodes examined |
13.5 (3-24) | Not applicable | 14 (3-24) | Not applicable | |
Surgeries performed by the same surgeon at the same institution during the study period
p-value comparing study patients and historic control patients who underwent lap partial (only p-value<0.05 are noted):
Warm ischemia <0.001
Abbreviations: Lap radical, laparoscopic radical nephrectomy, Lap partial, laparoscopic partial nephrectomy, EBL, estimated blood loss, CrCl, creatinine clearance
Surgical complications were typical for patients undergoing nephrectomy. In patients undergoing radical nephrectomy, complications included pneumothorax(1), urinary retention(1), bile leak from the liver that spontaneously resolved(1), and deep venous thrombosis(1). In patients undergoing partial nephrectomy, complications included conversion to open surgery(1), AV fistula requiring selective embolization(1), and ventral hernia(1).
All study patients undergoing radical nephrectomy had formal retroperitoneal lymph node dissection; one patient with clinically enlarged nodes (cN2) had pathologically proven lymph node metastases (pN2). A second patient with clinically normal nodes also had pathologically proven nodal disease (pN2). Long-term follow-up for oncologic efficacy was not a primary endpoint of this study. Both the mean and median postoperative follow-up was 6.5 months. At last follow-up, all patients were alive. One patient treated for clinically localized disease recurred in the lung 9 months following surgery, and another recurred in the psoas muscle 12 months after surgery.
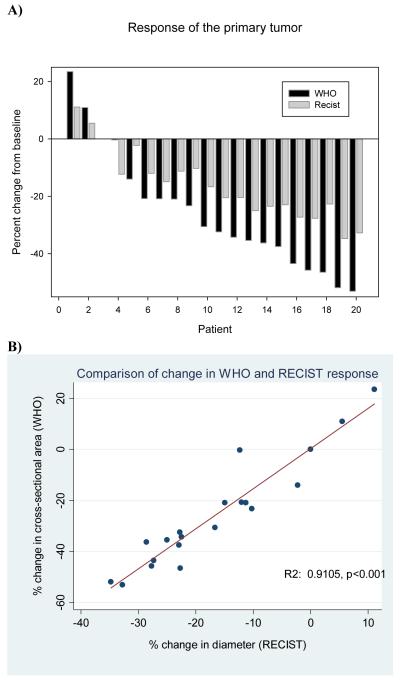
Seventeen of 20 patients (85%) had a decrease in tumor size on 2 month follow-up CT (Figure 1A and Supplemental Table 3, online,). The mean change in tumor diameter was −11.8% (range −27 to 11%), with negative number indicating a decrease in size; one patient had a formal partial response and all others had stable disease as defined by the RECIST criteria. The mean change in tumor cross-sectional area was −27.9% (range −43% to 23%); two patients had a formal partial response as defined by the WHO criteria. In 2 patients (5%), the tumor grew while on sunitinib. Fifteen patients exhibited a decrease in CT density, with a mean percent change in HU of −22% (range −74% to 29%). The average cross-sectional area that was necrotic was 58.8% (range 0%-94%).
Figure 1.
A) Waterfall plot of the response of the primary tumor to sunitinib determined from baseline CT and CT after at least 2 months of therapy. Response was determined using the RECIST and WHO criteria. B) Comparison of primary tumor response determined using the WHO criteria and the RECIST criteria.
There was a range of correlations between the differing CT scan response criteria (RECIST, WHO, and Hounsfield unit changes) applied to the primary tumors. The percent change in tumor diameter (RECIST) correlated highly with the percent change in cross-sectional area (WHO) (R2 0.9105, p<0.001, Figure 1B). However, neither the percent change in tumor diameter nor cross-sectional area correlated significantly with percent decrease in CT scan density (R2 0.4074, p=0.0746 and R2 0.3005, p=0.1980; respectively). There was a statistically significant correlation between the final, contrast-enhanced CT density and histologic necrosis (R2 0.2408, p=0.0280).
DISCUSSION
Sunitinib is an effective therapy for RCC. Our study indicates that sunitinib can be used safely prior to radical or partial nephrectomy. No patient experienced any adverse intraoperative or postoperative events attributed to the use of sunitinib. Surgical parameters such as blood loss, operative time, transfusion rate, and length of postoperative hospital stay were not significantly different when compared to historic controls. Patients on the study who underwent laparoscopic partial nephrectomy had slightly longer warm ischemia times when compared to control patients undergoing the same operation. This reflects the fact that study patients had larger tumors that were more centrally located, which increased the technical demands of the operation. However, it is important to note that study patients undergoing partial nephrectomy had good postoperative renal function, which was better than that of study patients who underwent radical nephrectomy.
The safety of targeted therapies in the preoperative setting was addressed in a recent retrospective study. Margulis et al. reviewed the outcomes of patients who received a number of different targeted therapies, such as sunitinib, sorafenib, or bevacizumab, prior to surgery.14 There was no statistically significant difference in perioperative morbidity when patients treated with preoperative targeted therapy were compared to patients who underwent up-front resection. Specifically, there was no statistically significant difference in perioperative mortality, readmissions, re-explorations, thromboembolic events, medical complications, infections, or incision-related complications between the two groups. In our study, sunitinib was generally well tolerated, with 12 of 20 patients (60%) completing the course without dose modification. GI and hematologic toxicities were common (65% and 55%, respectively) and fatigue were noted in 45% of patients. These toxicities are similar to those reported in large, multicenter studies of sunitinib for advanced RCC.5,6,15
In our study, 17 of the 20 patients (85%) demonstrated a decrease in primary tumor size on follow-up CT scan performed after 2 months of therapy. The average tumor shrank by approximately 12% in diameter and 28% in cross-sectional area. Similarly, van der Veldt et al. reported that in patients with metastatic RCC, the primary tumor volume decreased in 16 of 17 patients who received sunitinib with their primary tumor in place.16 These observations suggest new roles for targeted therapies that are based on their effects on the primary tumor. Targeted therapies may be useful for down-staging the tumor, allowing resection of tumors considered unresectable at initial presentation. They may also downstage localized tumors, making partial nephrectomies feasible for larger tumors. For patients with metastatic disease who are candidates for cytoreductive nephrectomy, Jonash et al. demonstrated in a phase II study that a brief course of targeted therapies can be used to identify patients with rapidly progressive disease who are unlikely to benefit from surgical debulking, sparing them the morbidity of surgery.10
The most widely accepted criteria for response to antineoplastic agents are based on changes in tumor size. The RECIST criteria are based on measurements of tumor diameter, while the WHO criteria are based on measurements of cross-sectional area. Both the RECIST and WHO criteria accurately reflected changes in tumor size and there was excellent correlation between the criteria when applied to the primary tumor. However, both criteria underestimate tumor shrinkage when large primary tumors are assessed. Therefore, formal cutoffs used to classify partial response for metastatic disease are not relevant when assessing primary tumor response. Furthermore, in this study, tumor size was reassessed after patients received 2 out of the 3 months of treatment. Therefore, the tumor likely continued to shrink after the followup CT; the majority of patients with metastatic disease experience their first partial response following more than 2 months of sunitinib treatment.5
In addition to tumor shrinkage, another indication of response is decrease in tumor perfusion. Following treatment with sunitinib, we found that 15 of 20 tumors (75%) had decrease in contrast-enhanced CT density. There was only modest correlation between change in tumor size and change in CT density, suggesting that change in tumor perfusion should be an additional criterion for treatment response. In a study of gastrointestinal stromal tumors, decrease in CT density was correlated with improved survival and was validated as a criteria for treatment response.13 This proposal is further supported by our observation that contrast-enhanced CT density after 2 months of sunitinib therapy correlated with percent histologic necrosis determined pathologically. In patients undergoing up-front nephrectomy, histologic necrosis has been associated with poor prognosis.17 However, histologic necrosis in untreated tumors likely reflects a bulky lesion that has outgrown its vascular supply, while the necrosis in patients who have been treated with targeted therapies may also reflect treatment effect.
This pilot study showed that preoperative sunitinib therapy and surgery following sunitinib are safe. It also defined the extent of the clinical response that can be expected with preoperative therapy. These observations pave the way for future studies that can explore novel treatment paradigms that were never previously explored as historic treatments for RCC rarely produced clinical responses.
CONCLUSIONS
There were no intraoperative or postoperative surgical complications attributed to the use of preoperative sunitinib. Sunitinib decreased the size of the renal tumor in 17 of 20 patients (85%), and future trials may be considered to evaluate neoadjuvant sunitinib for facilitating nephron-sparing surgery and improving progression-free survival in patients with high-risk, localized RCC.
Supplementary Material
Acknowledgments
Funding: Pfizer, NIH (R01CA133072-01)
Footnotes
Conflict of interest: Research funding: H.L.K., Pfizer
Supplemental tables: http://www.csmc.edu/rcc
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–4. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 3.Hock LM, Lynch J, Balaji KC. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167:57–60. [PubMed] [Google Scholar]
- 4.Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. J Clin Oncol. 1994;12:206–12. doi: 10.1200/JCO.1994.12.1.206. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. Jama. 2006;295:2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 7.Dutcher J, Atkins MB, Margolin K, Weiss G, Clark J, Sosman J, et al. Kidney cancer: the Cytokine Working Group experience (1986-2001): part II. Management of IL-2 toxicity and studies with other cytokines. Med Oncol. 2001;18:209–19. doi: 10.1385/MO:18:3:209. [DOI] [PubMed] [Google Scholar]
- 8.Haas NB, Uzzo R. Adjuvant therapy for renal cell carcinoma. Curr Oncol Rep. 2008;10:245–52. doi: 10.1007/s11912-008-0037-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim HL, Shah SK, Tan W, Shikanov SA, Zorn KC, Shalhav AL, et al. Estimation and prediction of renal function in patients with renal tumor. J Urol. 2009;181:2451–60. doi: 10.1016/j.juro.2009.01.112. discussion 60-1. [DOI] [PubMed] [Google Scholar]
- 10.Jonasch E, Wood CG, Matin SF, Tu SM, Pagliaro LC, Corn PG, et al. Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4076–81. doi: 10.1200/JCO.2008.21.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 14.Margulis V, Matin SF, Tannir N, Tamboli P, Swanson DA, Jonasch E, et al. Surgical morbidity associated with administration of targeted molecular therapies before cytoreductive nephrectomy or resection of locally recurrent renal cell carcinoma. J Urol. 2008;180:94–8. doi: 10.1016/j.juro.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 16.van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Bex A, de Gast G, Haanen JB, et al. Sunitinib for treatment of advanced renal cell cancer: primary tumor response. Clin Cancer Res. 2008;14:2431–6. doi: 10.1158/1078-0432.CCR-07-4089. [DOI] [PubMed] [Google Scholar]
- 17.Sengupta S, Lohse CM, Leibovich BC, Frank I, Thompson RH, Webster WS, et al. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005;104:511–20. doi: 10.1002/cncr.21206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.