Abstract
Background
Because activation of T cells is associated with human immunodeficiency virus (HIV) pathogenesis, CD4 and CD8 activation levels in patients coinfected with HIV and hepatitis C virus (HCV) may explain conflicting reports regarding effects of HCV on HIV disease progression.
Methods
Kaplan-Meier and multivariate Cox regression models were used to study the risk of incident clinical AIDS and AIDS-related deaths among 813 HCV-negative women with HIV infection, 87 HCV-positive nonviremic women with HIV coinfection, and 407 HCV-positive viremic women with HIV coinfection (median follow-up time, 5.2 years). For 592 women, the percentages of activated CD4 and CD8 T cells expressing HLA-DR (DR) and/or CD38 were evaluated.
Results
HCV-positive viremic women had a statistically significantly higher percentage of activated CD8 T cells (P < .001) and a statistically significantly higher incidence of AIDS compared with HCV-negative women (P < .001 [log-rank test]). The AIDS risk was greater among HCV-positive viremic women in the highest tertile compared with the lowest tertile (>43% vs <26%) of CD8+CD38+DR+ T cells (hazard ratio, 2.94 [95% confidence interval, 1.50–5.77]; P =.001). This difference was not observed in the HCV-negative women (hazard ratio, 1.87 [95% confidence interval, 0.80–4.35]; P =.16). In contrast, CD4 activation predicted AIDS in both groups similarly. Increased percentages of CD8+CD38−DR+, CD4+CD38−DR−, and CD8+CD38−DR− T cells were associated with a >60% decreased risk of AIDS for HCV-positive viremic women and HCV-negative women.
Conclusion
HCV-positive viremic women with HIV coinfection who have high levels of T cell activation may have increased risk of AIDS. Earlier treatment of HIV and HCV infection may be beneficial.
Persistent generalized immune activation is a hallmark of human immunodeficiency virus (HIV) infection and is associated with CD4 cell count and HIV RNA level [1–7]. Activation of T cells accelerates their maturation and is associated with decreased CD4 level, increased HIV replication, more rapid HIV disease progression, and a decreased amount of CD4 gain following highly active antiretroviral therapy. Animal studies have suggested that immune activation, rather than viral load, is linked to HIV disease progression [8].
Worldwide, many HIV-infected individuals are coinfected with hepatitis C virus (HCV). However, studies of the effect of HCV on HIV disease progression have been conflicting [9–19]. In contrast, all studies find liver disease to be accelerated in HIV-coinfected patients compared with patients singly infected with HCV [20–22]. Determining the association between HCV and HIV is critical for clinical management of both infections in coinfected patients. We assessed the effect of HIV and HCV on HIV disease progression in a prospective cohort of women, and we determined the percentages of CD4 and CD8 T cells that expressed the cell surface markers HLA-DR (DR) and/or CD38 as measures of immune activation. We hypothesized that the combined effects of these 2 viruses would be increased immune activation and higher likelihood of HIV disease progression.
METHODS
Study design
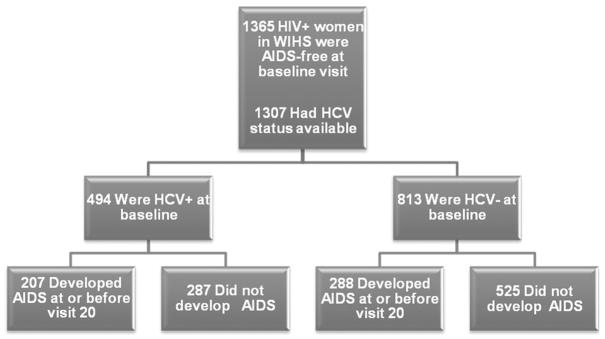
The methods and baseline cohort characteristics of the Women’s Interagency HIV Study have been described elsewhere [23]. Women were enrolled during 1994–1995 or 2001–2002 at 6 sites in the United States. Only women enrolled during 1994–1995 were included in this study. Approval from the local institutional review boards was obtained, as was informed consent from all study participants. Clinical and laboratory evaluations, including CD4 cell counts, HIV RNA levels, and platelet counts, were performed at 6-month intervals, and aspartate and alanine aminotransferase levels were determined every 6–12 months. Among 1365 HIV-positive women who had not experienced an AIDS-defining clinical condition at or before study entry, baseline HCV seropositivity and HCV RNA status were available for 1307 women (Figure 1). Through September 2004, the median follow-up time was 5.2 years (range, 0.35–9.9 years) with 14,420 person-years of observation. Immune activation marker data from 3 published substudies were pooled (1972 evaluations; median, 3 measures/person) [7, 24, 25] (Figure 2).
Figure 1.
AIDS development among AIDS-free women infected with human immunodeficiency virus (HIV) at baseline. HCV, hepatitis C virus; HCV+, HCV-positive; HCV−, HCV-negative; HIV+, HIV-positive; WIHS, Women’s Interagency HIV Study.
Figure 2.
AIDS development among human immunodeficiency virus (HIV)–infected women with immune activation data. Baseline hepatitis C virus (HCV) data were missing for 11 of the 592 woman with immune activation data. HCV+, HCV-positive; HCV+RNA+, HCV-positive viremic; HCV+RNA−, HCV-positive nonviremic; HCV−, HCV-negative; HIV+, HIV-positive.
Laboratory evaluations
HIV RNA levels were determined using isothermal nucleic acid sequence–based amplification (Organon Teknika) in laboratories certified by the National Institute of Allergy and Infectious Diseases. Baseline HCV antibody testing was performed using enzyme immunoassays (version 2.0 or 3.0; Abbott). HCV RNA levels were measured in a single laboratory (principal investigator: A. Kovacs) by means of polymerase chain reaction (Roche Diagnostics) as described elsewhere [26]. For 260 HCV-positive viremic women, HCV genotype was determined using the NC Trugene HCV 5′ NC genotyping kit (Bayer HealthCare). The AIDS Clinical Trials Group (ACTG) Consensus Protocol was used for 3-color flow cytometry in laboratories certified by the National Institute of Allergy and Infectious Diseases [27] (FACSCalibur; Becton Dickinson) by means of the following fluorochrome-conjugated antibodies: anti-CD3, anti-CD4, anti-CD8, anti–HLA-DR, and anti-CD38 (Becton Dickinson or PharMingen) as reported elsewhere [7, 24, 25].
Clinical outcomes
The first self-reported AIDS-defining clinical condition or AIDS-related death (if death was the first event), excluding CD4 cell count criteria, defined AIDS onset [28, 29]. Death certificates were requested for women who died, as reported elsewhere [28]. The date of AIDS onset was imputed as the midpoint between the date of the visit at which an AIDS-defining condition was reported (or the date of AIDS-related death) and the date of the previous visit [28]. AIDS-free survival was defined as the time between the date of enrollment in the Women’s Interagency HIV Study and the date of the earliest occurrence of an AIDS-defining condition, the date of AIDS-related death, the date of death not due to AIDS, or the date of the last visit at which the patient was AIDS free (through September 2004).
Parameterization of T cell data
HIV RNA level was evaluated as a time-dependent variable. CD4 cell count was modeled as a time-dependent categorical variable with respect to a value of <200 cells/μL at any time during the follow-up period: never <200 cells/μL, <200 cells/μL only previously, <200 cells/μL only currently, or <200 cells/μL previously and currently. This parameterization of CD4 cell count showed a stronger association with AIDS risk than did CD4 cell count categorized at each visit as <200 cells/μL vs 200–350, 351–500, or >500 cells/μL (data not shown). Percentages of CD4 and CD8 T lymphocytes that expressed HLA-DR and/or CD38 were categorized using tertiles and modeled as time-dependent variables.
Other model covariates
Race/ethnicity, baseline HCV serology, and HCV RNA level were modeled as fixed variables. Women were categorized as HCV-negative, HCV-positive nonviremic, or HCV-positive viremic by quartiles (HCV RNA level: ≤983,000, 983,001–2,395,000, 2,395,001–3,980,000, or >3,980,000 IU/mL). Other time-dependent factors included antiretroviral therapy (ART), age, smoking, alcohol use, injection drug use, and antibiotic prophylaxis. The aspartate to platelet ratio index, a marker of liver fibrosis [30], was assessed as a time-dependent covariate.
Statistical analysis
SAS software (version 9.1; SAS Institute) was used for the statistical analyses. Demographic characteristics, clinical characteristics, and AIDS outcomes were compared between HCV-negative, HCV-positive nonviremic, and HCV-positive viremic women by use of χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables. Kaplan-Meier analyses were used to estimate the unadjusted probability of remaining AIDS-free or of remaining alive by HCV status. Cox proportional hazards models were used to evaluate the relationship between the onset of an AIDS-defining condition (or AIDS-related death if first event) and exposures of interest with hazard ratios (HRs) and 95% confidence intervals (CIs). AIDS diagnosis was analyzed as a single outcome adjusted for age, race, and HCV status. Other model covariates were included if they were statistically significantly associated with AIDS or if they altered the HR for the exposures of interest by >10%. AIDS risk at a given event time was associated with exposures measured at the preceding 6-month visit. If these data were missing, we used data from the preceding 1-year visit; we did not carry forward exposure data beyond 1 year. To evaluate the effect of HCV status on AIDS in ART-naive women, separate analyses were conducted with censoring at ART initiation.
Correlates of CD4 and CD8 activation were determined using generalized estimating equations that accounted for within-subject correlation of the measures taken over time. An exchangeable correlation matrix was assumed between any 2 measures of a given subject. Mean percentages of CD4 and CD8 cells expressing CD38 and/or HLA-DR over time were reported for the categories of each independent variable. Correlates of baseline HCV status were determined using χ2 tests. Factors related to both T cell activation and HCV status were included in multivariate generalized estimating equation models in which T cell activation markers were the outcomes of interest and HCV status was the primary exposure of interest.
Risk of AIDS by tertiles of CD4 and CD8 activation markers was evaluated using Cox proportional hazards models adjusted for age, race, ART, smoking, and HCV RNA level as defined above (hereafter model 1). Additional models tested for the effect of either HIV RNA level or CD4 cell count (or both). Stratified analyses were also conducted for HCV-negative and HCV-positive viremic women. Separate Cox regression analyses evaluated whether HR estimates for immune activation markers varied between fresh samples or frozen samples (data not shown). Because there were no substantial differences, data were pooled in our final models.
RESULTS
Demographic and baseline clinical characteristics of study participants
Among the 1307 women included in the study, there were 813 HCV-negative women (62%), 87 HCV-positive nonviremic women (7%), and 407 HCV-positive viremic women (31%). Among the 260 HCV-positive viremic women for whom HCV genotype was determined, 210 (80%) were infected with genotype 1. HCV-positive women were older and more likely to be injection drug users than HCV-negative women (Table 1). Number of sexual partners and CD4 and CD8 cell counts were similar among the groups. The overall prevalence of injection drug use during the follow-up period was 0.4% (range, 0%–1%) in HCV-negative women, 10.5% (range, 0%–21%) in HCV-positive nonviremic women, and 12.7% (range, 6%–26%) in HCV-positive viremic women (P < .001 [χ2 test]; data not shown). Although anti-HCV therapy was not generally given during the period of this study, 19 of 1307 women received interferon, the majority after the 10th visit. Among these 19 women, there were 14 HCV-positive viremic women, 1 HCV-positive nonviremic woman, and 4 HCV-negative women. None of these women received ribavirin.
Table 1.
Demographic and Clinical Characteristics of HIV-Infected Women at Baseline
| Characteristic | HCV-negative (N =813) | HCV-positive nonviremic (N =87) | HCV-positive viremic (N =407) |
|---|---|---|---|
| Age, years | |||
| 16–21 | 35 (4) | 0 (0) | 0 (0) |
| 22–34 | 453 (56) | 23 (27) | 97 (24) |
| 35–54 | 304 (37) | 62 (71) | 306 (75) |
| >55 | 21 (3) | 2 (2) | 4 (1) |
| Median (range) | 33 (17–73) | 40 (27–67)a | 39 (23–65)b |
| Race or ethnicity | |||
| White | 145 (18) | 14 (16) | 67 (16) |
| African American | 433 (53) | 47 (54) | 247 (61)b |
| Hispanic | 207 (26) | 24 (28) | 88 (22) |
| Other | 28 (3) | 2 (2) | 5 (1) |
| No. of sexual partners | |||
| 0 | 9 (1) | 1 (1) | 6 (1) |
| 1–4 | 394 (49) | 42 (48) | 177 (44) |
| 5–10 | 196 (24) | 11 (13) | 98 (24) |
| 11–100 | 169 (21) | 20 (23) | 92 (23) |
| >100 | 44 (5) | 13 (15)a | 34 (8) |
| Median (range) | 3 (1–5) | 3 (1–5) | 3 (1–5) |
| Data missing | 1 (0.1) | 0 (0) | 0 (0) |
| Transmission risk | |||
| Injection drug use | 36 (5) | 58 (67)a | 312 (77)b |
| Heterosexual contact | 458 (56) | 25 (29) | 73 (18) |
| Transfusion | 39 (5) | 1 (1) | 6 (1) |
| Not identified | 270 (33) | 3 (3) | 16 (4) |
| Data missing | 10 (1) | 0 (0) | 0 (0) |
| Antiretroviral therapy in past 6 months | |||
| None | 331 (41) | 31 (36) | 184 (45) |
| Monotherapy | 257 (32) | 30 (34) | 136 (33) |
| Combination (2 drugs) | 223 (27) | 26 (30) | 83 (21) |
| HAART | 2 (0.3) | 0 (0) | 2 (0.5) |
| Data missing | 0 (0) | 0 (0) | 2 (0.5) |
| Plasma HIV RNA level, copies/mL | |||
| ≤4000 | 255 (31) | 38 (44) | 132 (32) |
| 4001–20,000 | 168 (21) | 21 (24) | 90 (22) |
| 20,001–55,000 | 141 (17) | 15 (17) | 51 (12) |
| 55,001–100,000 | 77 (9) | 5 (6) | 43 (11) |
| >100,000 | 160 (20) | 8 (9) | 88 (22) |
| Median (range) | 17,000 (80–3,200,000) | 7600 (80–3,600,000)a | 14,000 (80–2,800,000) b |
| Data missing | 12 (2) | 0 (0) | 3 (1) |
| CD4 T cell count, cells/μL | |||
| <200 | 237 (29) | 28 (32) | 122 (30) |
| 200–350 | 190 (23) | 21 (24) | 87 (21) |
| 351–500 | 195 (24) | 21 (24) | 100 (25) |
| >500 | 169 (21) | 14 (16) | 88 (22) |
| Median (range) | 371 (0–1823) | 408 (6–1933) | 366 (0–1972) |
| Data missing | 22 (3) | 3 (3) | 10 (3) |
| CD8 T cell count, cells/μL | |||
| ≤580 | 198 (24) | 16 (18) | 101 (25) |
| 581–820 | 205 (25) | 15 (17) | 104 (26) |
| 821–1140 | 193 (24) | 29 (33) | 95 (23) |
| >1140 | 194 (24) | 24 (28) | 97 (24) |
| Median (range) | 806 (21–4133) | 932 (108–3866)a | 806 (58–3003) c |
| Data missing | 23 (3) | 3 (3) | 10 (2) |
NOTE. Data are no. (%) of women unless otherwise indicated. HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
P < .05 for comparison between HCV-negative women and HCV-positive nonviremic women.
P < .05 for comparison between HCV-negative women and HCV-positive viremic women.
P < .05 for comparison between HCV-positive nonviremic women and HCV-positive viremic women.
The 592 women with immune activation data were slightly older (mean age, 37.4 vs 35.5 years); were more likely African American or Hispanic (82.5% vs 77.5%), injection drug users (53% vs 27%), and smokers (58% vs 49%); and had higher CD4 cell counts (mean, 439 vs 403 cells/μL) and CD8 cell counts (mean, 964 vs 867 cells/μL) than women without these data (data not shown).
Disease progression and HCV
Among the 1307 women who were AIDS free at baseline, 495 developed AIDS, including 162 women who died an AIDS-related death during 10 years of follow-up. Although at the time of first AIDS diagnosis there were no statistically significant differences in reported individual AIDS-defining conditions between HCV-negative women and HCV-positive women, by the end of follow-up HCV-positive viremic women were more likely to have bacterial pneumonia (20% vs 13%; P =.002), dementia and/or encephalopathy (11% vs 7%; P =.02), and wasting syndrome (12% vs 8%; P =.03) than were HCV-negative women. By the end of follow-up, HCV-positive viremic women were also more likely to have tuberculosis than were HCV-negative women; however, the difference was borderline statistically significant (6% vs 4%; P =.06). HCV-positive viremic women were less likely to have any cancer (5% vs 8%; P =.03) and toxoplasmosis (0.6% vs 2%; P =.03) than were HCV-negative women.
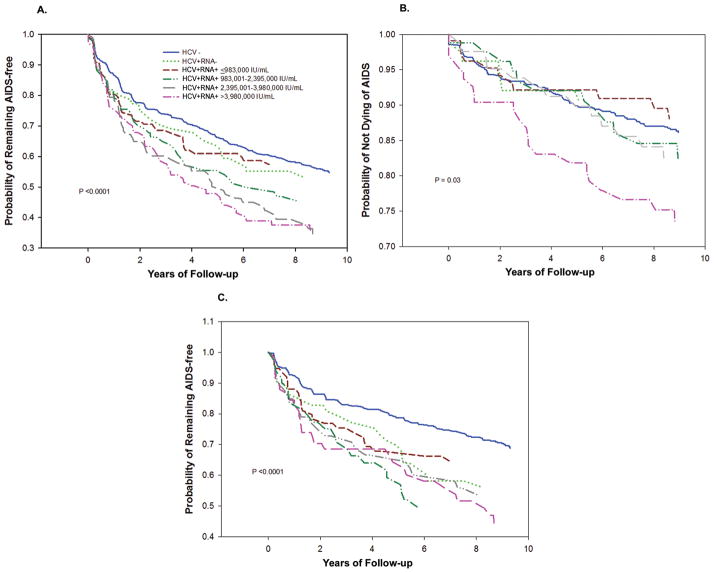
Kaplan-Meier analyses showed that HCV-positive viremic women were more likely to develop AIDS with an HCV RNA level of >2.3 million IU/mL (Figure 3A) and were more likely to die an AIDS-related death with an HCV RNA level of >3.98 million IU/mL (Figure 3B) than were women in the other groups (P < .001 and P =.03, respectively [log-rank test]). Among women who never had a CD4 cell count of <200 cells/μL, HCV-positive women (regardless of HCV RNA status) were more likely to develop AIDS than were HCV-negative women (Figure 3C), with rates at 1, 3, and 5 years after study entry of 13% vs 7%, 26% vs 16%, and 33% vs 21%, respectively (P < .001).
Figure 3.
Kaplan-Meier estimates of the probability of remaining AIDS-free and of not dying of AIDS by hepatitis C virus (HCV) status at baseline. A, Probability of remaining AIDS-free by HCV status for 1307 women infected with human immunodeficiency virus (HIV). The number of women who developed AIDS-defining conditions was 495: 288 HCV-negative women; 31 HCV-positive nonviremic women; and 39, 40, 51, and 46 HCV-positive viremic women with RNA levels of ≤983,000, 983,001–2,395,000, 2,395,001–3,980,000, and >3,980,000 IU/mL, respectively (P < .001 [log-rank test]). B, Probability of not dying of AIDS by HCV status for these 1307 women. The number of women who died an AIDS-related death was 162: 97 HCV-negative women; 7 HCV-positive nonviremic women; and 11, 12, 13, and 22 HCV-positive viremic women with RNA levels of ≤983,000, 983,001–2,395,000, 2,395,001–3,980,000, and >3,980,000 IU/mL, respectively (P =.03 [log-rank test]). C, Probability of remaining AIDS-free by HCV status among 881 women who never had a CD4 T cell count of <200 cells/μL. Of these 881 women, the number of women who developed AIDS-defining conditions was 264: 145 HCV-negative women; 24 HCV-positive nonviremic women; and 25, 23, 27, and 20 HCV-positive viremic women with RNA levels of ≤983,000, 983,001–2,395,000, 2,395,001–3,980,000, and >3,980,000 IU/mL, respectively (P < .001 [log-rank test]). HCV−, HCV-negative; HCV+RNA−, HCV-positive nonviremic; HCV+RNA+, HCV-positive viremic.
After adjusting for age, race, smoking, and ART, we found HCV status, injection drug use, CD4 cell count, and HIV plasma RNA level to be independent risk factors for AIDS-defining conditions in the Cox model (Table 2). Compared with women who never had a CD4 cell count of <200 cells/μL, women with a current and previous CD4 cell count of <200 cells/μL had a 3-fold increased risk of AIDS and AIDS-related death (HR, 3.48 [95% CI, 2.77–4.36]). This was confirmed in a separate model that assessed visit-specific CD4 cell counts of <200 vs >500 cells/μL (HR, 3.00 [95% CI, 2.26–4.00]; data not shown). Alcohol use, prophylactic antibiotic use, and aspartate to platelet ratio index were not statistically significant predictors of AIDS, and their inclusion in multivariate models did not alter HR estimates for injection drug use, HCV status, CD4 cell count, and HIV RNA levels. Among ART-naive women, HCV-positive viremic women had a 2-fold higher risk of AIDS compared with HCV-negative women (data not shown).
Table 2.
Risk Factors for AIDS Development in HIV-Infected Women
| Risk factor | Adjusted HR (95% CI) | Pa |
|---|---|---|
| Injection drug use in past 6 months | NA | |
| No | 1.00 | |
| Yes | 1.57 (1.11–2.22) | |
| Baseline HCV RNA status | .01 | |
| HCV-negative | 1.00 | |
| HCV-positive nonviremic | 1.14 (0.76–1.70) | |
| HCV-positive viremic: RNA level, IU/mL | ||
| ≤983,000 | 1.20 (0.83–1.73) | |
| 983,001–2,395,000 | 1.35 (0.93–1.95) | |
| 2,395,001–3,980,000 | 1.53 (1.10–2.14) | |
| >3,980,000 | 1.34 (0.94–1.90) | |
| CD4 T cell count of <200 cells/μL | NA | |
| Never | 1.00 | |
| Only previously | 2.50 (1.70–3.68) | |
| Only currently | 1.52 (1.00–2.31) | |
| Previously and currently | 3.48 (2.77–4.36) | |
| Plasma HIV RNA level, copies/mL | <.001 | |
| ≤4000 | 1.00 | |
| 4001–20,000 | 1.65 (1.24–2.21) | |
| 20,001–55,000 | 1.87 (1.37–2.57) | |
| 55,001–100,000 | 2.38 (1.68–3.39) | |
| >100,000 | 2.93 (2.18–3.94) |
NOTE. CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio from multivariate Cox regression analysis adjusted for age (time dependent), race, smoking (time dependent), and antiretroviral therapy (time dependent); NA, not applicable.
Test for trend.
Association between level of immune activation and HCV status
HCV status was statistically significantly correlated with CD8+CD38+DR+ and CD4+CD38+DR+ T cell levels in univariate analyses (Table 3). In adjusted generalized estimating equation models, percentage of CD8+CD38+DR+ T cells was statistically significantly positively associated with HCV status (P =.04). Other independent correlates of CD8 activation included HIV RNA level and ART. For CD4+CD38+DR+ T cells, there was no statistically significant positive trend overall by HCV status. However, after the HCV-negative group was removed from the analysis, the trend was statistically significant among HCV-positive women (P =.02).
Table 3.
Correlates of CD8+CD38+DR+ and CD4+CD38+DR+ T Cells among HIV-Infected Women (N =592)
| Correlate | CD8+CD38+DR+ |
CD4+CD38+DR+ |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis
|
Multivariate analysisa |
Univariate analysis
|
Multivariate analysisa |
|||||
| Mean (SEM), % | P | Mean (SEM), % | P | Mean (SEM), % | P | Mean (SEM), % | P | |
| Baseline HCV RNA status | <.001 | .04 | .01 | .42 | ||||
|
| ||||||||
| HCV-negative | 33.80 (1.07) | 35.09 (1.14) | 14.40 (0.69) | 15.91 (1.07) | ||||
|
| ||||||||
| HCV-positive nonviremic | 34.30 (2.05) | 33.82 (2.21) | 12.40 (0.93) | 13.66 (1.30) | ||||
|
| ||||||||
| HCV-positive viremic: RNA level, IU/mL | .03b | .02b | ||||||
|
| ||||||||
| ≤983,000 | 36.50 (1.70) | 35.13 (1.92) | 13.90 (1.21) | 14.57 (1.54) | ||||
|
| ||||||||
| 983,001–2,395,000 | 37.23 (1.95) | 35.02 (2.05) | 15.10 (1.44) | 14.69 (1.57) | ||||
|
| ||||||||
| 2,395,001–3,980,000 | 40.06 (1.68) | 37.88 (1.88) | 16.90 (1.22) | 16.48 (1.78) | ||||
|
| ||||||||
| >3,980,000 | 42.15 (1.62) | 38.98 (1.90) | 17.40 (1.31) | 16.75 (1.54) | ||||
|
| ||||||||
| CD4 T cell count, cells/μL | <.001 | <.001 | ||||||
|
| ||||||||
| >500 | 29.70 (0.80) | NA | 9.22 (0.31) | NA | ||||
|
| ||||||||
| 351–500 | 34.80 (0.85) | NA | 12.60 (0.41) | NA | ||||
|
| ||||||||
| 200–350 | 39.29 (0.95) | NA | 15.90 (0.53) | NA | ||||
|
| ||||||||
| <200 | 44.90 (1.14) | NA | 24.30 (0.93) | NA | ||||
|
| ||||||||
| HIV RNA, copies/mL | <.001 | <.001 | <.001 | <.001 | ||||
|
| ||||||||
| ≤4000 | 30.90 (0.70) | 28.07 (1.23) | 11.60 (0.38) | 10.16 (1.02) | ||||
|
| ||||||||
| 4001–20,000 | 38.30 (0.93) | 35.27 (1.32) | 15.00 (0.59) | 13.65 (1.15) | ||||
|
| ||||||||
| 20,001–55,000 | 41.10 (1.10) | 36.62 (1.50) | 17.00 (0.70) | 15.19 (1.19) | ||||
|
| ||||||||
| 55,001–100,000 | 42.50 (1.30) | 38.34 (1.60) | 18.10 (0.90) | 16.54 (1.33) | ||||
|
| ||||||||
| >100,000 | 45.70 (1.20) | 41.62 (1.69) | 22.50 (1.11) | 21.18 (1.57) | ||||
|
| ||||||||
| Age, years | .14 | .13 | .11 | .03 | ||||
|
| ||||||||
| 15–22 | 17.80 (3.20) | 22.67 (2.59) | 7.42 (2.12) | 8.69 (2.78) | ||||
|
| ||||||||
| 23–35 | 35.92 (1.16) | 38.74 (1.21) | 14.37 (0.74) | 15.83 (0.78) | ||||
|
| ||||||||
| 36–55 | 36.90 (0.82) | 40.26 (0.84) | 15.13 (0.52) | 17.32 (0.56) | ||||
|
| ||||||||
| >55 | 37.80 (3.10) | 42.26 (3.00) | 17.21 (2.92) | 19.53 (2.69) | ||||
|
| ||||||||
| Race or ethnicityc | ||||||||
|
| ||||||||
| White | 34.63 (1.59) | … | NA | 13.76 (1.50) | … | NA | ||
|
| ||||||||
| African American | 37.36 (0.85) | .13 | NA | 15.25 (0.58) | .008 | NA | ||
|
| ||||||||
| Hispanic | 35.44 (1.29) | .69 | NA | 14.84 (0.86) | .03 | NA | ||
|
| ||||||||
| Other | 24.51 (4.40) | .03 | NA | 10.81 (4.59) | .48 | NA | ||
|
| ||||||||
| Injection drug use | ||||||||
|
| ||||||||
| No | 36.10 (0.69) | … | NA | 14.80 (0.45) | … | NA | ||
|
| ||||||||
| Yes | 37.60 (1.60) | .50 | NA | 14.90 (0.87) | .90 | NA | ||
|
| ||||||||
| Antiretroviral therapy | <.001 | <.001 | <.001 | |||||
|
| ||||||||
| None | 39.58 (0.84) | 34.49 (1.25) | 15.38 (0.50) | 14.61 (1.05) | .87 | |||
|
| ||||||||
| Monotherapy | 37.66 (1.14) | 36.55 (1.42) | 16.60 (0.82) | 16.16 (1.22) | ||||
|
| ||||||||
| Combination (2 drugs) | 35.06 (0.93) | 35.38 (1.36) | 15.42 (0.60) | 16.10 (1.12) | ||||
|
| ||||||||
| HAART | 32.55 (0.85) | 34.52 (1.42) | 13.01 (0.53) | 14.51 (1.17) | ||||
NOTE. Generalized estimating equations model: dependent variable, immune activation marker; independent variables, baseline hepatitis C virus (HCV) status, CD4 T cell count, human immunodeficiency virus (HIV) RNA level, age, injection drug use, and antiretroviral therapy (all but baseline HCV status and race were time varying). DR, HLA-DR; HAART, highly active antiretroviral therapy; NA, not applicable; SEM, standard error of the mean.
The multivariate model included factors that were statistically significantly associated with both baseline HCV status and T cell activation.
P value for trend among HCV-positive women.
The P values for the African American, Hispanic, and other race categories are compared with those for the white race category.
Immune activation and risk of AIDS
Among the 592 women with immune activation data, AIDS risk was statistically significantly increased among women in the highest tertile of CD8+CD38+DR+ T cells compared with women in the lowest tertile of CD8+CD38+DR+ T cells, and among women in the highest tertile of CD4+CD38+DR+ T cells compared with women in the lowest tertile of CD4+CD38+DR+ T cells (Table 4). In contrast, higher percentages of CD8+CD38−DR−, CD8+CD38−DR+, and CD4+CD38−DR− T cells were statistically significantly protective against AIDS. When we adjusted for HIV RNA level, these associations remained unchanged. However, after adjusting for CD4 cell count or CD4 cell count and HIV RNA level, HRs remained statistically significant only for CD8+CD38−DR+ and CD4+CD38+DR+ T cells. Of note, HRs for each HCV RNA level were not statistically significant in any immune marker models: in model 1 for CD8+CD38+DR+ T cells, the HR was 0.94 (95% CI, 0.48–1.82) for HCV-positive nonviremic women and 0.88 (95% CI, 0.45–1.73), 1.67 (95% CI, 0.62–2.20), 1.14 (95% CI, 0.61–2.17), and 1.14 (95% CI, 0.60–2.17) for HCV-positive viremic women with RNA levels of ≤983,000, 983,001–2,395,000, 2,395,001–3,980,000, and >3,980,001 IU/mL, respectively.
Table 4.
Multivariate Associations between Immune Activation Markers and Incident AIDS among HIV-Infected Women
| Immune activation marker, model | Total a (N =592)
|
HCV-positive viremicb (N =278)
|
HCV-negativec (N =242)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Tertile 2 adjusted HR (95% CI)d | Tertile 3 adjusted HR (95% CI)d | P | Tertile 2 adjusted HR (95% CI)d | Tertile 3 adjusted HR (95% CI)d | P | Tertile 2 adjusted HR (95% CI)d | Tertile 3 adjusted HR (95% CI)d | P | |
| CD8+CD38+DR+
| |||||||||
| Model 1 | 1.24 (0.78–1.99) | 1.83 (1.17–2.88) | .007 | 2.06 (1.02–4.15) | 2.94 (1.50–5.77) | .001 | 0.78 (0.31–1.95) | 1.87 (0.80–4.35) | .16 |
|
| |||||||||
| Model 1 with HIV viral load | 1.02 (0.63–1.64) | 1.24 (0.77–1.99) | .04 | 1.64 (0.81–3.33) | 1.94 (0.96–3.90) | .07 | 0.57 (0.22–1.45) | 1.36 (0.57–3.27) | .48 |
|
| |||||||||
| Model 1 with CD4 cell count | 1.02 (0.62–1.62) | 1.13 (0.70–1.84) | .52 | 1.77 (0.87–3.59) | 1.98 (0.97–4.04) | .08 | 0.53 (0.20–1.29) | 1.04 (0.43–2.53) | .88 |
|
| |||||||||
| Model 1 with HIV viral load and CD4 cell count | 0.92 (0.57–1.48) | 0.95 (0.58–1.56) | .67 | 1.56 (0.77–3.17) | 1.59 (0.77–3.26) | .27 | 0.44 (0.17–1.14) | 0.91 (0.37–2.25) | .92 |
|
| |||||||||
| CD8+CD38−DR−
| |||||||||
| Model 1 | 0.52 (0.34–0.80) | 0.51 (0.33–0.77) | .002 | 0.58 (0.35–0.96) | 0.48 (0.27–0.84) | .009 | 0.26 (0.10–0.72) | 0.32 (0.14–0.73) | .005 |
|
| |||||||||
| Model 1 with HIV viral load | 0.64 (0.42–0.98) | 0.77 (0.49–1.20) | .01 | 0.73 (0.43–1.22) | 0.78 (0.43–1.41) | .36 | 0.28 (0.10–0.79) | 0.43 (0.18–1.02) | .04 |
|
| |||||||||
| Model 1 with CD4 cell count | 0.69 (0.44–1.07) | 0.83 (0.53–1.32) | .28 | 0.74 (0.43–1.27) | 0.76 (0.41–1.41) | .36 | 0.34 (0.12–0.94) | 0.53 (0.22–1.27) | .11 |
|
| |||||||||
| Model 1 with HIV viral load and CD4 cell count | 0.74 (0.48–1.15) | 1.00 (0.63–1.61) | .42 | 0.82 (0.47–1.41) | 0.99 (0.52–1.86) | .91 | 0.35 (0.12–0.98) | 0.59 (0.25–1.41) | .17 |
|
| |||||||||
| CD8+CD38+DR−
| |||||||||
| Model 1 | 1.09 (0.71–1.69) | 1.22 (0.79–1.88) | .34 | 1.07 (0.61–1.86) | 1.19 (0.68–2.09) | .54 | 1.26 (0.50–3.13) | 1.21 (0.53–2.79) | .66 |
|
| |||||||||
| Model 1 with HIV viral load | 1.09 (0.71–1.68) | 1.13 (0.74–1.75) | .56 | 1.14 (0.65–1.99) | 1.15 (0.65–2.00) | .53 | 1.04 (0.41–2.62) | 0.94 (0.40–2.20) | .97 |
|
| |||||||||
| Model 1 with CD4 cell count | 1.17 (0.76–1.80) | 1.22 (0.79–1.88) | .36 | 1.17 (0.66–2.04) | 1.16 (0.65–2.06) | .63 | 0.98 (0.39–2.44) | 0.97 (0.42–2.56) | .94 |
|
| |||||||||
| Model 1 with HIV viral load and CD4 cell count | 1.14 (0.74–1.75) | 1.14 (0.74–1.76) | .56 | 1.19 (0.68–2.08) | 1.12 (0.63–2.00) | .71 | 0.89 (0.35–2.26) | 0.83 (0.35–1.98) | .68 |
|
| |||||||||
| CD8+CD38−DR+
| |||||||||
| Model 1 | 0.44 (0.29–0.66) | 0.36 (0.23–0.55) | <.001 | 0.46 (0.28–0.77) | 0.34 (0.19–0.61) | <.001 | 0.32 (0.14–0.78) | 0.29 (0.12–0.68) | .002 |
|
| |||||||||
| Model 1 with HIV viral load | 0.55 (0.36–0.84) | 0.50 (0.32–0.78) | .001 | 0.58 (0.35–0.98) | 0.46 (0.25–0.86) | .009 | 0.40 (0.16–0.98) | 0.41 (0.16–1.02) | .04 |
|
| |||||||||
| Model 1 with CD4 cell count | 0.54 (0.35–0.82) | 0.47 (0.30–0.74) | .001 | 0.54 (0.32–0.92) | 0.43 (0.23–0.80) | .005 | 0.44 (0.18–1.11) | 0.47 (0.19–1.16) | .07 |
|
| |||||||||
| Model 1 with HIV viral load and CD4 cell count | 0.60 (0.39–0.92) | 0.56 (0.36–0.89) | .01 | 0.61 (0.36–1.03) | 0.51 (0.27–0.96) | .03 | 0.48 (0.19–1.22) | 0.55 (0.21–1.42) | .17 |
|
| |||||||||
| CD4+CD38+DR+
| |||||||||
| Model 1 | 1.26 (0.73–2.18) | 2.89 (1.76–4.72) | <.001 | 1.94 (0.86–4.39) | 4.12 (1.92–8.84) | <.001 | 0.80 (0.27–2.40) | 4.04 (1.65–9.85) | .008 |
|
| |||||||||
| Model 1 with HIV viral load | 1.06 (0.61–1.85) | 1.94 (1.15–3.26) | <.001 | 1.56 (0.67–3.59) | 2.71 (1.22–6.00) | .005 | 0.67 (0.22–2.02) | 2.71 (1.06–7.00) | .02 |
|
| |||||||||
| Model 1 with CD4 cell count | 1.02 (0.58–1.78) | 1.67 (0.95–2.94) | .02 | 1.63 (0.71–3.76) | 2.71 (1.17–6.28) | .01 | 0.57 (0.18–1.74) | 1.59 (0.57–4.41) | .24 |
|
| |||||||||
| Model 1 with HIV viral load and CD4 cell count | 0.95 (0.54–1.67) | 1.40 (0.79–2.48) | .04 | 1.46 (0.63–3.37) | 2.18 (0.93–5.15) | .05 | 0.52 (0.17–1.61) | 1.41 (0.50–3.92) | .34 |
|
| |||||||||
| CD4+CD38−DR−
| |||||||||
| Model 1 | 0.66 (0.43–0.99) | 0.49 (0.32–0.75) | .002 | 0.67 (0.40–1.13) | 0.41 (0.23–0.71) | .002 | 0.61 (0.28–1.33) | 0.43 (0.17–1.07) | .06 |
|
| |||||||||
| Model 1 with HIV viral load | 0.78 (0.51–1.20) | 0.74 (0.47–1.16) | .02 | 0.81 (0.48–1.38) | 0.63 (0.34–1.14) | .12 | 0.68 (0.31–1.51) | 0.63 (0.25–1.59) | .28 |
|
| |||||||||
| Model 1 with CD4 cell count | 0.79 (0.51–1.21) | 0.75 (0.47–1.18) | .20 | 0.79 (0.46–1.35) | 0.60 (0.33–1.09) | .09 | 0.77 (0.35–1.71) | 1.07 (0.40–2.84) | .95 |
|
| |||||||||
| Model 1 with HIV viral load and CD4 cell count | 0.86 (0.56–1.32) | 0.94 (0.58–1.50) | .45 | 0.87 (0.50–1.49) | 0.77 (0.41–1.43) | .40 | 0.82 (0.37–1.83) | 1.21 (0.45–3.29) | .86 |
|
| |||||||||
| CD4+CD38+DR−
| |||||||||
| Model 1 | 1.02 (0.68–1.52) | 0.72 (0.45–1.14) | .21 | 1.14 (0.68–1.91) | 0.92 (0.52–1.63) | .81 | 0.76 (0.32–1.79) | 0.39 (0.15–0.99) | .05 |
|
| |||||||||
| Model 1 with HIV viral load | 0.95 (0.64–1.42) | 0.65 (0.41–1.04) | .08 | 1.16 (0.69–1.94) | 0.82 (0.46–1.46) | .55 | 0.56 (0.23–1.33) | 0.32 (0.12–0.85) | .02 |
|
| |||||||||
| Model 1 with CD4 cell count | 1.05 (0.70–1.57) | 0.81 (0.51–1.29) | .44 | 1.20 (0.71–2.02) | 0.98 (0.55–1.75) | .99 | 0.71 (0.31–1.65) | 0.46 (0.19–1.17) | .10 |
|
| |||||||||
| Model 1 with HIV viral load and CD4 cell count | 0.99 (0.66–1.49) | 0.74 (0.46–1.18) | .23 | 1.21 (0.72–2.05) | 0.91 (0.51–1.63) | .80 | 0.60 (0.25–1.42) | 0.41 (0.16–1.05) | .06 |
|
| |||||||||
| CD4+CD38−DR+
| |||||||||
| Model 1 | 1.51 (0.93–2.44) | 1.33 (0.82–2.18) | .37 | 1.52 (0.51–2.86) | 1.43 (0.75–2.72) | .35 | 0.96 (0.40–2.53) | 1.20 (0.50–2.58) | .67 |
|
| |||||||||
| Model 1 with HIV viral load | 1.37 (0.84–2.22) | 1.19 (0.73–1.95) | .65 | 1.39 (0.74–2.63) | 1.24 (0.65–2.38) | .65 | 0.75 (0.30–1.86) | 1.14 (0.48–2.69) | .72 |
|
| |||||||||
| Model 1 with CD4 cell count | 1.33 (0.82–2.16) | 0.91 (0.55–1.51) | .44 | 1.37 (0.73–2.58) | 0.99 (0.51–1.94) | .75 | 0.79 (0.32–1.95) | 0.74 (0.30–1.79) | .51 |
|
| |||||||||
| Model 1 with HIV viral load and CD4 cell count | 1.29 (0.79–2.09) | 0.94 (0.56–1.55) | .56 | 1.35 (0.71–2.54) | 1.00 (0.51–1.96) | .78 | 0.72 (0.29–1.79) | 0.77 (0.32–1.85) | .59 |
Model 1 for all women: dependent variable, AIDS; independent variables, immune marker, age, race, antiretroviral therapy, smoking, and baseline HCV status (categorized as HCV-negative; HCV-positive nonviremic; or HCV-positive viremic with an RNA level of ≤983,000, 983,001–2,395,000, 2,395,001–3,980,000, or >3,980,000 IU/mL). CI, confidence interval; DR, HLA-DR; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio.
Model 1 for HCV-positive viremic women: dependent variable, AIDS; independent variables, immune marker, age, race, antiretroviral therapy, and smoking.
Model 1 for HCV-negative women: dependent variable, AIDS; independent variables, immune marker, age, race, antiretroviral therapy, and smoking.
Hazard ratio and 95% confidence interval for tertile relative to tertile 1.
Immune activation and risk of AIDS in HCV-negative and HCV-positive viremic women
Stratified analyses showed that among HCV-positive viremic women (n =278), AIDS risk was statistically significantly increased with higher percentages of CD8+CD38+DR+ and CD4+CD38+DR+ T cells (Table 4). However, for HCV-negative women, percentage of CD8+CD38+DR+ T cells was not statistically significantly associated with AIDS. Conversely, higher percentages of CD4+CD38−DR−, CD8+CD38−DR−, and CD8+CD38−DR+ T cells were associated with decreased AIDS risk for both groups. After additional adjustments for HIV RNA level, CD4 cell count, or both, risk was altered but remained statistically significant for CD8+CD38−DR+ and CD4+CD38+DR+ T cells and was borderline statistically significant for CD8+CD38+DR+ T cells only among HCV-positive viremic women. For HCV-negative women (n =242), HRs remained statistically significant only for CD8+CD38−DR+ and CD4+CD38+DR+ T cells after adjusting for HIV RNA level (Table 4). HRs were not statistically significantly different between HCV-positive viremic women and HCV-negative women (P > .50 for all interactions).
DISCUSSION
HIV-positive patients with chronic active or reactivating viral coinfections (eg, cytomegalovirus or herpes simplex virus coinfections) have higher plasma HIV RNA levels, lower T cell counts, and greater progression to AIDS compared with HIV-positive patients without evidence of infection/reactivation [31, 32]. For HCV coinfection, however, there have been conflicting reports [10–19]. Consequently, our findings from this large cohort study, which included 1307 women, are notable. First, women with high baseline HCV RNA levels have an increased risk of AIDS, independent of CD4 cell count and HIV RNA level. Second, there was an almost 2-fold increased probability of developing AIDS for HCV-positive women compared with HCV-negative women who never had a CD4 cell count of <200 cells/μL and for women who remained ART naive. Third, HCV coinfection was associated with increased CD8 activation. Finally, our most important finding was the statistically significant association between the level of activated CD8 T cells and incident AIDS among HCV-positive viremic women. HCV-positive viremic women with >43% activated CD8 T cells had an almost 3-fold increased risk of AIDS-defining conditions and/or AIDS-related deaths compared with HCV-positive viremic women with <26% activated CD8 T cells. This was not found for HCV-negative women. In contrast, high levels of CD4 activation similarly predicted AIDS in both groups of women. These data suggest that the increased risk of HIV disease progression among HCV-coinfected women with high levels of CD8 activation may be due to immune dysfunction. Importantly, we also showed that women with high percentages of CD8+CD38−DR+ and CD4+CD38−DR− or CD8+CD38−DR− T cells had a 50%–70% decreased AIDS risk, which suggests better immunity among this group of women.
To our knowledge, the Swiss HIV Cohort Study [17] was the first large study to demonstrate that HCV accelerated HIV disease independent of injection drug use. Other studies found no effect of HCV on HIV disease progression, although recent studies have reported increased risk [9–19]. Our study, in which only 10%–20% of the participants were active injection drug users during follow-up, demonstrates an increased risk of an AIDS-defining condition with increasing HCV RNA level. Furthermore, our study assessed outcome from the time of study entry to the time of an AIDS-defining condition adjusting for ART in time-dependent Cox models, whereas some studies limited outcome from initiation of highly active ART or excluded women with CD4 cell counts of <200 cells/μL at baseline, neither of which was done in our study. Although the effect of HCV genotype on immune activation is unknown, most women in our study were infected with genotype 1, which is associated with higher HCV RNA levels [33]. In our study, higher HCV RNA levels correlate with higher levels of immune activation. Differences in genotype or in rates of active injection drug use may explain differences between the results of our study and those in previous reports [19].
Immune activation has been closely linked to HIV disease progression [1, 4, 5, 34–36], but to our knowledge this association has not previously been reported in the setting of HCV coinfection. Our finding of increased incidence of AIDS-defining conditions in relation to high levels of immune activation suggests that there is impaired T cell function in HCV-positive viremic women that may potentially put them at higher risk of HIV disease progression compared with HCV-negative women.
There are a number of factors that could potentially influence immune activation and in turn pathogenesis of HIV disease, including age, race, gender, injection drugs, use of highly active ART and antibiotic prophylaxis, smoking, alcohol, HCV genotype, and extent of liver damage. We evaluated for each of these in univariate models and then in multivariate models when appropriate.
In vitro studies have shown that CD8 T cells have a lower threshold for activation and proliferation compared with CD4 T cells [37]. Ongoing antigen-driven activation of CD8 T cells ultimately leads to CD8 T cell exhaustion and replicative senescence, which lead to inability to fight opportunistic pathogens [4]. The factors that influence T cell activation among the women included in our study are most likely multifactorial and may be a direct consequence of activation in the liver or may be related to extrahepatic replication of HCV [38]. Alternatively, host-specific factors (eg, cytokines) may drive persistent T cell activation. Furthermore, factors that drive immune activation may increase the available targets for further viral replication. Finally, HCV infection may impair T cell maturation more globally to a more immature primed activated phenotype and also may impair responses to Toll-like receptors, suggesting that both the innate and adaptive arms are affected [24, 39].
Our study systematically evaluated the association between HCV viremia, immune activation, and AIDS outcome. We determined that (1) HCV viremia is associated with AIDS outcome, independent of injection drug use, HIV RNA level, CD4 cell count, and ART (Table 2); (2) HCV viremia is associated with CD4 and CD8 activation, independent of HIV RNA level (Table 3); and (3) high levels of CD8 activation are associated with AIDS in HCV-positive viremic women but not in HCV-negative women. Our multivariate models showed that the causal pathway between T cell activation and HIV disease progression cannot be completely explained by HIV or HCV viral load, and other mediators of CD8 activation may be involved in increasing AIDS risk. This may be because substantial immune activation occurs in tissues such as the liver, and viral load may not reflect the extent of the activation. For instance, recently there has been evidence that the gastrointestinal tract is a site for activation of T cells as a result of microbial translocation. Gut-associated T cell depletion may also have a significant effect on HIV pathogenesis [8, 40–42]. From the intestines, T cells and microbial antigens circulate directly through the liver, where activation may continue. Whether the liver plays a role in amplifying or modulating this activation, especially with prior HCV infection, is unknown, but a recent study found evidence of increased microbial translocation among HCV-positive individuals with cirrhosis [43]. In our study, liver biopsy data were unavailable. However, we did evaluate for liver disease by use of the aspartate to platelet ratio index [30] and found no statistically significant correlation with AIDS risk. Furthermore, we found no association between alcohol use and AIDS outcome. On the other hand, although at the time of AIDS diagnosis there were no statistically significant differences in individual AIDS-defining conditions noted between HCV-negative women and HCV-positive women, HCV viremic women were more likely to report wasting syndrome, bacterial pneumonia, and encephalopathy at the end of follow-up. This finding suggests that HCV-positive viremic women may be at continued risk for certain AIDS-defining conditions and further supports the importance of treatment in this group. The pathogenesis of these findings may be HCV-related infection of the central nervous system and/or progressive liver disease including cirrhosis. Bacterial pneumonia is increased among HIV-infected individuals, especially injection drug users [44, 45], and cirrhosis may play a role in some who are coinfected with HCV. However, continued immune activation is also associated with encephalopathy, and some studies have suggested that microbial translocation related to gut-associated immunodeficiency may be a contributing factor [43, 46–48]. Furthermore, wasting syndrome may be a manifestation of chronic and severe gut-associated immunodeficiency that may be exacerbated by progressive HCV disease and/or alcohol use.
Our study results confirm those of previous studies that the presence of HLA-DR on CD8 T cells without CD38 expression appears to be protective [36, 49]. This is consistent with reports showing that elite controllers have higher percentages of HIV-specific and global CD8+CD38−DR+ T cells [49]. CD8+CD38−DR+ T cells appear to have high proliferative capacity and cytotoxic activity upon antigenic stimulation. Our study results suggest further research is needed to better understand the path of CD8 activation, especially the protective effect of HLA-DR expression.
Although our study is unique, it has limitations. We did not have information on the timing of HIV or HCV infection. Furthermore, HCV-positive women and HCV-negative women may be different because of differing routes of HIV infection (injection drug use vs sexual). Immune activation markers were measured for only a subset of the study population [7, 24, 25], and not all phenotypic markers were measured at the same time. Nevertheless, our results confirmed previous observations by other investigators [1, 6, 36]. Finally, we evaluated HCV RNA levels only at baseline because not all women underwent multiple measures. We assumed that HCV RNA levels would not change substantially in women who were not treated for HCV infection, on the basis of published studies that found that even with initiation and discontinuation of highly active ART there is only a 0.43–0.59 log change in HCV viral load [50]; such small changes would not affect our findings.
In conclusion, our study demonstrates that HIV-coinfected HCV-positive viremic women are at increased risk for AIDS-defining conditions compared with HCV-negative women, possibly because of high levels of activation of T cells, especially CD8 T cells, which indicates increased immune dysregulation in this population of women. Lower levels of activation of both CD8 and CD4 T cells and activation of CD8 T cells expressing only HLA-DR is protective against AIDS. Further study is needed to understand better the pathogenesis of T cell activation, especially of CD8 T cells in relation to HIV disease. HCV-positive viremic women may benefit from treatment of HIV and HCV infection to prevent significant immunologic changes and improve long-term outcome. Assessing CD4 and CD8 T cell activation could help clinicians evaluate their patients’ risk of developing AIDS.
Acknowledgments
We thank the participants of this study. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group, with centers (Principal Investigators) located at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Analysis Center (Stephen Gange). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support: National Institute of Allergy and Infectious Diseases (grant R01-A1052065-04 to A.K.; grant R01-AI057006 to H.S.; grants U01-AI-35004, U01-AI-31834, U01-AI-34994, U01-AI-34989, U01-AI-34993, and U01-AI-42590 to the Women’s Interagency HIV Study [WIHS]; and grant K23 AI-66943 to P.C.T.); Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant U01-HD-32632 to the WIHS); National Cancer Institute; National Institute on Drug Abuse; National Institute on Deafness and Other Communication Disorders; National Center for Research Resources (UCSF-CTSI grant UL1 RR024131).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. Elevated levels of CD38+CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study J Acquir Immune Defic Syndr. 1993;6(8):904–912. [PubMed] [Google Scholar]
- 2.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 3.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12(3):289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 4.Papagno L, Spina CA, Marchant A, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2(2):e20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus–infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187(10):1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 6.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17(13):1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 7.Landay A, Benning L, Bremer J, et al. Correlates of immune activation marker changes in human immunodeficiency virus (HIV)–seropositive and high-risk HIV-seronegative women who use illicit drugs. J Infect Dis. 2003;188(2):209–218. doi: 10.1086/376509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117(11):3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershow RC, O’Driscoll PT, Handelsman E, et al. Hepatitis C virus coinfection and HIV load, CD4+ cell percentage, and clinical progression to AIDS or death among HIV-infected women: Women and Infants Transmission Study. Clin Infect Dis. 2005;40(6):859–867. doi: 10.1086/428121. [DOI] [PubMed] [Google Scholar]
- 10.Anderson KB, Guest JL, Rimland D. Hepatitis C virus coinfection increases mortality in HIV-infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta VA Cohort Study. Clin Infect Dis. 2004;39(10):1507–1513. doi: 10.1086/425360. [DOI] [PubMed] [Google Scholar]
- 11.Weis N, Lindhardt BO, Kronborg G, et al. Impact of hepatitis C virus coinfection on response to highly active antiretroviral therapy and outcome in HIV-infected individuals: a nationwide cohort study. Clin Infect Dis. 2006;42(10):1481–1487. doi: 10.1086/503569. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan PS, Hanson DL, Teshale EH, Wotring LL, Brooks JT. Effect of hepatitis C infection on progression of HIV disease and early response to initial antiretroviral therapy. AIDS. 2006;20(8):1171–1179. doi: 10.1097/01.aids.0000226958.87471.48. [DOI] [PubMed] [Google Scholar]
- 13.Law WP, Duncombe CJ, Mahanontharit A, et al. Impact of viral hepatitis co-infection on response to antiretroviral therapy and HIV disease progression in the HIV-NAT. AIDS. 2004;18(8):1169–1177. doi: 10.1097/00002030-200405210-00010. [DOI] [PubMed] [Google Scholar]
- 14.De Luca A, Bugarini R, Lepri AC, et al. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med. 2002;162(18):2125–2132. doi: 10.1001/archinte.162.18.2125. [DOI] [PubMed] [Google Scholar]
- 15.Dorrucci M, Valdarchi C, Suligoi B, et al. The effect of hepatitis C on progression to AIDS before and after highly active antiretroviral therapy. AIDS. 2004;18(17):2313–2318. doi: 10.1097/00002030-200411190-00012. [DOI] [PubMed] [Google Scholar]
- 16.Daar ES, Lynn H, Donfield S, et al. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J Infect Dis. 2001;183(4):589–595. doi: 10.1086/318539. [DOI] [PubMed] [Google Scholar]
- 17.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356(9244):1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 18.Rockstroh JK, Mocroft A, Soriano V, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192(6):992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 19.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288(2):199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 20.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32(3):492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 21.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33(4):562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal E, Pialoux G, Bernard N, et al. Liver-related mortality in human immunodeficiency virus–infected patients between 1995 and 2003 in the French GERMIVIC Joint Study Group Network (MOR-TAVIC 2003 Study) J Viral Hepat. 2007;14(3):183–188. doi: 10.1111/j.1365-2893.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 23.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Inter-agency HIV Study. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 24.Al-Harthi L, Voris J, Du W, et al. Evaluating the impact of hepatitis C virus (HCV) on highly active antiretroviral therapy–mediated immune responses in HCV/HIV-coinfected women: role of HCV on expression of primed/memory T cells. J Infect Dis. 2006;193(9):1202–1210. doi: 10.1086/500843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovacs A, al-Harthi L, Christensen S, Mack W, Cohen M, Landay A. CD8+ T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis. 2008;197(10):1402–1407. doi: 10.1086/587696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Operskalski EA, Mack WJ, Strickler HD, et al. Factors associated with hepatitis C viremia in a large cohort of HIV-infected and -uninfected women. J Clin Virol. 2008;41(4):255–263. doi: 10.1016/j.jcv.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson J, Kidd P, Mandy F, Livnat D, Kagan J. Three-color supplement to the NIAID DAIDS guideline for flow cytometric immunophenotyping. Cytometry. 1996;26(3):227–230. doi: 10.1002/(SICI)1097-0320(19960915)26:3<227::AID-CYTO8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 28.Anastos K, Barron Y, Cohen MH, et al. The prognostic importance of changes in CD4+ cell count and HIV-1 RNA level in women after initiating highly active antiretroviral therapy. Ann Intern Med. 2004;140(4):256–264. doi: 10.7326/0003-4819-140-4-200402170-00007. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 30.Al-Mohri H, Murphy T, Lu Y, Lalonde RG, Klein MB. Evaluating liver fibrosis progression and the impact of antiretroviral therapy in HIV and hepatitis C coinfection using a noninvasive marker. J Acquir Immune Defic Syndr. 2007;44(4):463–469. doi: 10.1097/QAI.0b013e318030ff8e. [DOI] [PubMed] [Google Scholar]
- 31.Corey L. Synergistic copathogens—HIV-1 and HSV-2. N Engl J Med. 2007;356(8):854–856. doi: 10.1056/NEJMe068302. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs A, Schluchter M, Easley K, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1–infected women: Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 1999;341(2):77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo TW, Donfield S, Lail A, Lynn HS, Daar ES. Effect of hepatitis C virus (HCV) genotype on HCV and HIV-1 disease. J Infect Dis. 2005;191(1):4–10. doi: 10.1086/426513. [DOI] [PubMed] [Google Scholar]
- 34.Eggena MP, Barugahare B, Okello M, et al. T cell activation in HIV-seropositive Ugandans: differential associations with viral load, CD4+ T cell depletion, and coinfection. J Infect Dis. 2005;191(5):694–701. doi: 10.1086/427516. [DOI] [PubMed] [Google Scholar]
- 35.Goicoechea M, Smith DM, Liu L, et al. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis. 2006;194(1):29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- 36.Giorgi JV, Ho HN, Hirji K, et al. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ CD38− CD8+ cells is associated with subsequent stable CD4+ cell levels. The Multicenter AIDS Cohort Study Group. J Infect Dis. 1994;170(4):775–781. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 37.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 38.Laskus T, Operskalski EA, Radkowski M, et al. Negative-strand hepatitis C virus (HCV) RNA in peripheral blood mononuclear cells from anti-HCV–positive/HIV-infected women. J Infect Dis. 2007;195(1):124–133. doi: 10.1086/509897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villacres MC, Literat O, Degiacomo M, Du W, Frederick T, Kovacs A. Defective response to Toll-like receptor 3 and 4 ligands by activated monocytes in chronic hepatitis C virus infection. J Viral Hepat. 2008;15(2):137–144. doi: 10.1111/j.1365-2893.2007.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 41.Dandekar S. Pathogenesis of HIV in the gastrointestinal tract. Curr HIV/AIDS Rep. 2007;4(1):10–15. doi: 10.1007/s11904-007-0002-0. [DOI] [PubMed] [Google Scholar]
- 42.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1(1):23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus–related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135(1):226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis. 2004;4(7):445–455. doi: 10.1016/S1473-3099(04)01060-6. [DOI] [PubMed] [Google Scholar]
- 45.Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus: Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333(13):845–851. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 46.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196(12):1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 48.Kovacs A. Early immune activation predicts central nervous system disease in HIV-infected infants: implications for early treatment. Clin Infect Dis. 2009;48(3):347–349. doi: 10.1086/595886. [DOI] [PubMed] [Google Scholar]
- 49.Saez-Cirion A, Lacabaratz C, Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung RT, Evans SR, Yang Y, et al. Immune recovery is associated with persistent rise in hepatitis C virus RNA, infrequent liver test flares, and is not impaired by hepatitis C virus in co-infected subjects. AIDS. 2002;16(14):1915–1923. doi: 10.1097/00002030-200209270-00008. [DOI] [PubMed] [Google Scholar]