Abstract
When an odorant is presented to one side of the nose and air to the other, the ability to localize which side received the odorant depends upon trigeminal nerve stimulation. It has been shown that performance on this lateralization task increases as stimulus concentration increases. In this study, we determined the influences of stimulus volume and sex on the ability to localize each of 8 odorants presented at neat concentrations: anethole, geraniol, limonene, linalool, menthol, methyl salicyclate, phenyl ethanol, and vanillin. At a low stimulus volume (11 mL), only menthol was localized at an above-chance level. At a high stimulus volume (21 mL), above-chance localization occurred for all odorants except vanillin. Women were significantly better than men in localizing menthol. Stimuli rated as most intense were those that were most readily localized. The detection performance measures, as well as rated intensity values, significantly correlated with earlier findings of the trigeminal detectability of odorants presented to anosmic and normosmic subjects. This study suggests that differences in stimulus volume may explain some discrepant findings within the trigeminal chemosensory literature and supports the concept that vanillin may be a “relatively pure” olfactory stimulus.
Keywords: nose, olfaction, scent tracking, smell, trigeminal system
Introduction
As with the case of vision, audition, and the other major senses, the sense of smell is bilaterally organized. Thus, humans have 2 openings into the nasal chambers (nares), 2 olfactory mucosae, and 2 olfactory bulbs. Whereas the triangulation or spatial localization functions of bilaterality are obvious in senses such as vision and audition, this is not immediately obvious for olfaction in humans, given the closeness of the nares and the noncoherence of odorant plumes in most ecological situations.
Most odorants stimulate, at high concentrations, both the olfactory (cranial nerve [CN] I) and trigeminal (CN V) nerves (von Skramlik 1924; Elsberg et al. 1935; Doty et al. 1978; Kobal et al. 1989), although localization of a chemosensory stimulus to a given side of the nose depends largely upon CN V (von Skramlik 1924; Kobal et al. 1989). Thresholds for trigeminal sensations, such as burning, cooling, stinging, and fullness, are generally higher than thresholds for olfactory sensations (Cometto-Muniz and Cain 1990). Studies claiming localization to “pure” olfactory stimulants in both humans (von Békésy 1964; Porter et al. 2005) and rats (Rajan et al. 2006) have, in fact, employed stimuli known to be capable of stimulating the trigeminal nerve (Doty et al. 1978; Kobal and Hummel 1992; Yang et al. 2003).
A number of investigators have shown that performance on an odor localization task is influenced by stimulus concentration (Cometto-Muniz and Cain 1990; Hummel et al. 2003; Frasnelli and Hummel 2005). However, Cometto-Muniz and Cain (1984) suggested that the trigeminal system may detect the overall mass of a stimulus rather than its concentration (Cometto-Muniz and Cain 1984). If this is true, changing the volume of the stimulus at a given concentration should have the same effect as changing its concentration.
In this study, we tested the influence of stimulus volume on nasal localization of 8 odorants commonly used in olfactory research. The stimuli ranged from ones with little or no trigeminal activity (e.g., vanillin and phenyl ethanol) to ones with distinct trigeminal activity (e.g., menthol) (Doty et al. 1978). We also determined whether localization ability is related to the perceived intensity and pleasantness of the stimuli, as well as the sex of the subjects, and whether the test measures correlate with earlier findings of the trigeminal detectability of odorants presented to anosmic and normosmic subjects (Doty et al. 1978).
Materials and methods
Subjects
Forty subjects participated (20 women and 20 men; mean [range] age = 24 18–44 years). Only those with a normal sense of smell were included, as assessed by the University of Pennsylvania Smell Identification Test (Doty et al. 1984). Exclusion criteria were neurological or rhinological conditions associated with olfactory disorders, including major septal deviations, as assessed using acoustic rhinometry. Subjects were asked to refrain from smoking, eating, or drinking anything other than water for at least 1 h prior to testing.
Odorants
Eight odorants, certified by the manufacturer as having 99% or better purity, were chosen for study: anethole, geraniol, limonene, linalool, menthol, methyl salicylate, phenyl ethanol, and vanillin (Sigma-Aldrich, St Louis, MO). They were chosen on the basis of their frequent use in other olfactory research (e.g., Doty 1975) and their wide range of trigeminal stimulation capabilities (Doty et al. 1978).
Stimulus presentation device
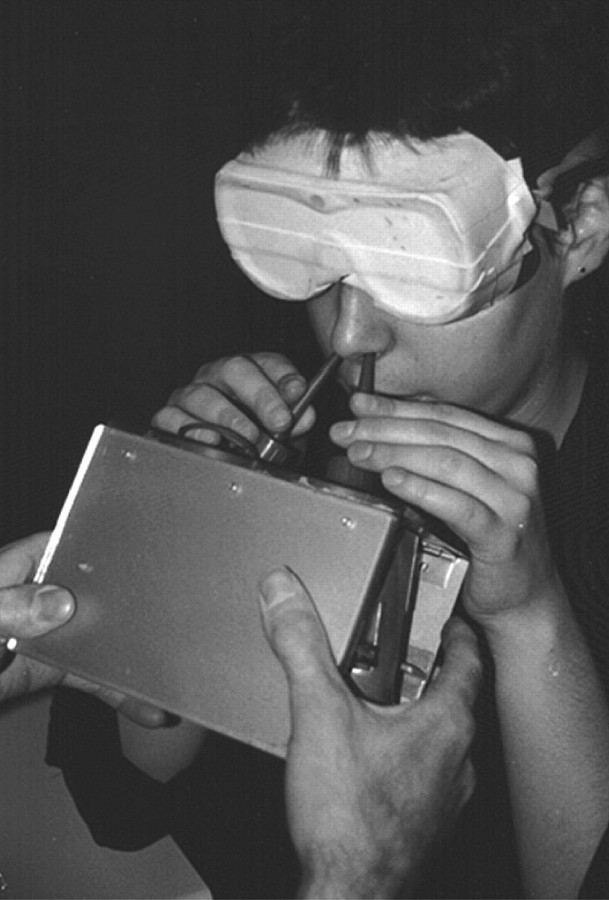
The stimuli were delivered by the handheld device pictured in Figure 1 (Roscher et al. 1996; Krone et al. 2001; Hummel et al. 2003; Livermore and Hummel 2004; Frasnelli et al. 2009). This device contained 2 removable, parallel, and high-density polypropylene squeeze bottles (total volume 250 mL), the spouts of which were angled so that the vapors from one could be directed into the left naris and the vapors of the other into the right naris. One bottle contained no odorant and the other an odorant. In the case of vanillin and menthol, the odorant consisted of 5 mg of the crystalline agent. In all other cases, 20 mL of liquid odorant was employed. Air from the headspace of both bottles was released in a uniform manner by pressing the hinged top and bottom of the device in a practiced manner. The vertical movement of the device could be calibrated so that 11 or 21 mL of the bottles was delivered. The subject held onto the spouts to prevent movements that might accompany squeezing of the bottles, thereby mitigating confounding mechanical stimulation (Hummel et al. 2003). Subjects were stimulated passively and instructed not to breathe during stimulation.
Figure 1.
Picture of the stimulus presentation device used in this study (Roscher et al. 1996). The subject is holding onto the spouts of the bottles so that the squeezing of the device would not produce mechanical irritation at the nostrils.
Test procedure
The subjects wore opaque goggles during testing. Each subject was tested on 4 consecutive days, 2 odorants (vs. air) per day. The presentation order of the 2 stimuli and the side to which they were presented were counterbalanced using a pseudorandom sequence. Half of the subjects were tested with the 11-mL stimulus volume and half with the 21-mL stimulus volume. A total of 40 stimulus presentations were made for each odorant, with an interstimulus interval of ∼30 s. After each stimulus presentation, subjects were asked to identify the side of the nose where the odorant had been presented. The 2 odorant test sessions of a given day were separated by an interval of 30 min (Kobal et al. 1989; Hummel et al. 2003). Following each session, the subjects rated the average intensity and pleasantness of the stimuli using 10-cm-long horizontal visual analogue scales with the extremes anchored with the terms “no smell”/“extremely strong” and “extremely unpleasant”/“extremely pleasant,” respectively.
Statistical analysis
SPSS 16.0 for Windows (SPSS Inc., Chicago, IL) was used for statistical analyses. Initially, we examined whether the mean number of correct responses given to each odorant differed from chance performance of 20 correct responses in 40 trials (1-sample t-tests). We then counted the number of subjects performing above chance based on the binomial distribution. Subsequently, a repeated measures analysis of variance (ANOVA) with sex and stimulation volume (small: 11 mL; large: 21 mL) as between-subject factors and odorant type as within-subject factor was applied to the number of correct responses. Normal distribution of the scores was ascertained by a Kolmogorov–Smirnoff test. For post hoc testing, we used t-tests with Bonferroni correction for inflated alpha. Finally, Pearson correlation coefficients were computed between the mean lateralization detection scores and the mean intensity and pleasantness ratings. Analogous correlations were computed between the dependent measures and the percentage of anosmic subjects who were able to detect a given odorant, as well as the percentage of normosmic subjects who reported trigeminal sensations after stimulation with the same odorant, as reported previously by Doty et al. (1978).
Results
Independent of stimulus volume, the mean laterality test scores were significantly above-chance level (20 correct responses) for the following odorants: menthol (P < 0.001), limonene (P < 0.001), phenyl ethanol (P = 0.002), anethole (P = 0.007), and linalool (P = 0.008). For the other odorants, the average scores did not differ from chance. The numbers of subjects performing above (>25, P = 0.04), below (<15, P = 0.04), or at chance levels (binomial distribution) are presented in Table 1 for each odorant.
Table 1.
Number of subjects performing above chance (score > 25), below chance (score < 15), or at chance level based on binomial distribution (P < 0.04)
| All subjects (n = 40) |
Small testing volume (n = 20) |
Large testing volume (n = 20) |
||||||||||
| Above chance | At chance | Below chance | Average score (SEM) | Above chance | At chance | Below chance | Average score (SEM) | Above chance | At chance | Below chance | Average score (SEM) | |
| Vanillin | 5 | 22 | 13 | 18.4 (1.0) | 0 | 14 | 6 | 18.0 (0.9) | 5 | 8 | 7 | 18.9 (1.7) |
| Geraniol | 6 | 32 | 2 | 21.4 (0.8) | 1 | 17 | 2 | 19.7 (1.2) | 5 | 15 | 0 | 23.1 (0.9) |
| Methyl salicylate | 11 | 23 | 6 | 21.8 (1.2) | 3 | 13 | 4 | 19.2 (1.1) | 8 | 10 | 2 | 24.3 (1.9) |
| Phenyl ethanol | 14 | 25 | 1 | 22.6 (0.8) | 3 | 17 | 0 | 21.1 (1.0) | 11 | 8 | 1 | 24.1 (1.1) |
| Anethole | 11 | 27 | 2 | 22.4 (1.4) | 4 | 15 | 1 | 21.7 (1.0) | 7 | 12 | 1 | 23.2 (1.4) |
| Linalool | 11 | 27 | 2 | 23.0 (1.1) | 2 | 17 | 1 | 21.1 (1.2) | 9 | 10 | 1 | 24.9 (1.7) |
| Limonene | 16 | 23 | 1 | 24.3 (1.0) | 3 | 17 | 0 | 22.5 (1.2) | 13 | 6 | 1 | 26.1 (1.4) |
| Menthol | 28 | 10 | 2 | 30.1 (1.4) | 10 | 8 | 2 | 27.1 (2.3) | 18 | 2 | 0 | 33.2 (1.4) |
SEM, standard error of the mean.
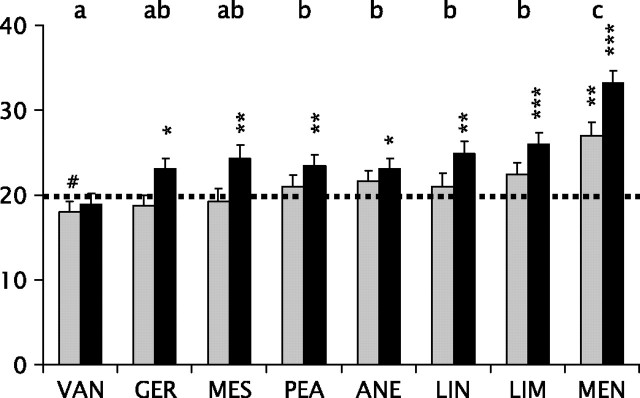
The ANOVA revealed significant main effects of odorant (F[7,252] = 14.8; P < 0.001) and volume (F[1,36] = 13.8; P = 0.001) and a significant odorant by sex 2-way interaction (F[7,252] = 5.5; P < 0.001). The main effect of odorant reflected performance differences among odorants. Specifically, higher scores occurred for menthol than for each of the other odorants (all P values < 0.05) and lower scores for vanillin than for each of the other odorants, with the exception of geraniol and methyl salicylate (all P values < 0.05). No other significant differences were present (Figure 2). The main effect of volume reflected better performance, on average, during the large than during the small volume presentations (respective mean [standard deviation {SD}] number of correct responses = 24.7 [0.7] and 21.2 [0.7]). The significant odorant by sex interaction was explained by the fact that women outperformed men only for the menthol stimulus (mean [SD] number of correct trials = 35.1 [1.4] and 25.2 [1.9], respectively; P < 0.001), although the average performance of men was still significantly above chance (P = 0.001).
Figure 2.
All subjects: mean scores (error bars indicate standard error) for 40 subjects localizing monorhinally presented odorants vanillin (VAN), phenyl ethyl alcohol (PEA), geraniol (GER), menthol (MEN), anethol (ANE), linalool (LIN), limonene (LIM), and methyl salicylate (MES). Gray and black bars indicate mean scores for subjects who received small and large stimulation volumes, respectively. Significant differences in post hoc comparisons between compounds (averaged for 40 subjects) are indicated by different letters (P < 0.05; Bonferroni corrected). The dotted line represents chance performance. Asterisks indicate performance above chance (*, P < 0.05; **, P < 0.01; ***, P < 0.001); the pound sign indicates performance below chance (#, P < 0.05).
In the group tested with the small volume, subjects performed, on average, above chance only for menthol (27.0 [2.3]; P = 0.007; 1-sample t-test). Interestingly, for the odorant vanillin, they performed significantly below chance (17.9 [0.9]; P = 0.04; 1-sample t-test). In fact, in the small volume group, different scores were only obtained between menthol on one hand and vanillin and methyl salicylate on the other hand (both P values < 0.05, paired t-tests, corrected). In the large volume group, subjects performed significantly above chance for all odorants except vanillin (all P values < 0.05). Subjects performed significantly better for menthol than for any other odorant (all P values < 0.05). For limonene, they performed significantly better than for vanillin (P = 0.001); there was no significant difference between the other odorants.
When average localization scores for each odorant were plotted against their average intensity ratings, a significant correlation was observed (r[8] = 0.72, P = 0.043; Figure 3). No such correlation was present between the average pleasantness ratings and the lateralization scores (r[8] = −0.25, P > 0.5). Additionally, the average localization scores were correlated with both the number of anosmic subjects detecting these specific odorants and the number of normosmic subjects reporting trigeminal sensation reported by Doty et al. (1978) (respective r[8] = 0.76 and 0.88, P values < 0.05).
Figure 3.
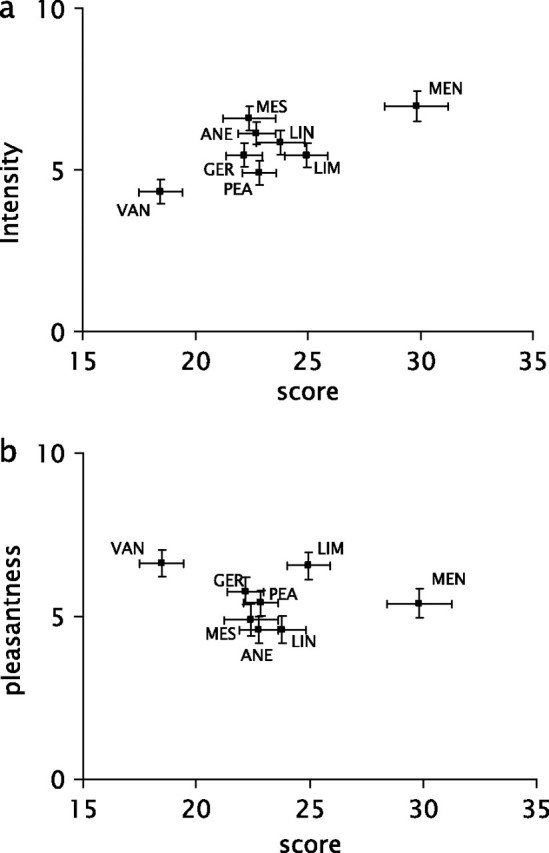
(a) Correlation between mean scores (maximum: 40, chance: 20) and average intensity (0–10) for 40 subjects. Error bars indicate standard error of the mean. (b) Correlation between mean scores (maximum: 40, chance: 20) and average pleasantness (0–10) for 40 subjects. Error bars indicate standard error of the mean.
Discussion
This study had 4 main findings: first, larger stimulation volumes facilitated the nasal localizability of the target odorants; second, when such volumes were used, odorants considered by some as purely olfactory stimulants could be localized (e.g., phenyl ethanol); third, odor intensity was positively correlated with localization performance; and fourth, positive correlations were present between the lateralization scores of this study and both the number of anosmic subjects who could detect the odor and the number of normosmic subjects who reported a trigeminal sensation in an earlier study (Doty et al. 1978).
Our finding that the 21-mL stimulus volume resulted in better localization performance than the 11-mL stimulus volume could reflect several factors. First, the larger stimulus volume may have simply resulted in the stimulus reaching a larger area of the nasal epithelium, in effect activating a larger number of receptors. Second, assuming that the same general areas of the epithelium received the stimulus, the trigeminal system may integrate the number of the incoming molecules within a given, as yet unknown, period of time (see Cometto-Muniz and Cain 1984). Other things being equal, longer or more concentrated stimuli, or stimuli with a higher flow rate, produce larger trigeminal responses than shorter, less concentrated, or less rapidly flowing stimuli, regardless of whether trigeminal activity is measured behaviorally (Cometto-Muniz and Cain 1984; Frasnelli et al. 2003; Wise et al. 2004, 2006) or electrophysiologically (Kobal 1981; Prah and Benignus 1984; Lorig et al. 1993; Pause et al. 1997; Frasnelli et al. 2003). Thus, the trigeminal system may integrate stimulus concentration, duration, and volume to signal the overall number (mass) of stimulus molecules. For a low-concentration stimulus to be perceived as strong as a high concentration one, it has to either be presented for a longer period of time or to be applied in a larger volume or both (Frasnelli et al. 2003).
When we applied a high stimulus volume, subjects could detect, with the exception of vanillin, all odors more frequently than expected by chance. It is particularly noteworthy that phenyl ethanol was also localized laterally at above-chance levels at this high volume. This odorant is widely used in olfactory research and is considered by some to be a pure olfactory stimulus that cannot be so localized (Porter et al. 2005; Frasnelli et al. 2008, 2009; but see also Kobal and Hummel 1992). In previous instances where such localization has been found in humans and rats, the authors have assumed that phenyl ethanol is a pure odorant and therefore such localization was based on olfactory stimulation (Porter et al. 2005; Rajan et al. 2006). Our study suggests that internasal localization is mediated by the trigeminal, not the olfactory, system. Support for this concept and against the idea that phenyl ethanol is a pure olfactory stimulus at all concentrations comes from earlier work in which both anosmic and normosmic subjects were investigated. One of 15 anosmic subjects could perceive phenyl ethanol. Similarly, 4 of 15 normosmic subjects who had been instructed to focus on trigeminal sensations reported such sensations while smelling this chemical (Doty et al. 1978).
The observation that localization performance was significantly below chance for vanillin is of considerable interest. Although it is possible that this effect was a chance phenomenon and that no differences in sensation between the 2 nasal chambers were present, other explanations are worthy of consideration. Here it should be pointed out that 5 of 20 subjects who received the large test volume also localized vanillin above chance. Three of these 5 also localized phenyl ethanol above chance. Hence, it is conceivable that these subjects were particularly sensitive to both stimuli and were able to localize them via the trigeminal nerve. One has also to keep in mind that vanillin was used in its crystalline form; the amount of vanillin in the gas phase may be lower than that available had a solution been used.
An interesting result of this study is the fact that women outperformed men for menthol. Although both sexes were on average above chance, men who were stimulated with the small volume of menthol did not perform any better than chance (19.7 points). Those stimulated with the large volume performed better (30.6), but still weaker, than women who scored high in both groups (34.4 and 35.8 and the low- and high-volume group, respectively). Earlier studies testing menthol or minty odors such as eucalyptol did not report a significant sex difference on localization scores, although women usually outperform men (Krone et al. 2001; Hummel et al. 2003). This is in line with the notion of women being more sensitive to pungent stimuli (Cometto-Muniz and Noriega 1985). Similarly, women were found to have a tendency to exhibit the negative mucosal potential, a peripheral measure of trigeminal responsiveness, at lower concentrations than men following stimulation with menthol (Frasnelli and Hummel 2003). One could therefore speculate that in the low-volume group, the amount of menthol molecules delivered to the nasal mucosa was not high enough to evoke a distinct trigeminal sensation in men but did so in women. More research is needed to clarify this issue.
In this study, odorants that were best localized were also rated as being most intense. This likely reflected a large influence of the trigeminal component of the odor/trigeminal complex on the intensity ratings. In line with this concept is the observation that odorants rated as more intense by normosmic subjects resemble those that are best detected by anosmic subjects (Doty et al. 1978). It is noteworthy that average lateralization scores for the different odors were significantly correlated to the detection rates in anosmic subjects, as well as to reports of trigeminal sensations from normosmic subjects (Doty et al. 1978). This provides supporting evidence that the localization test accurately assesses the degree to which odorants activate the intranasal trigeminal system.
In conclusion, we have demonstrated that stimulation volume affects odor localization. In addition, we have shown that humans are capable of localizing even odorants considered by many to be pure olfactory stimulants when they are presented at high volumes. This suggests that under certain circumstances, such odorants are capable of activating the trigeminal system. Our study suggests that differences in stimulus volume may explain a number of discrepant findings within the trigeminal chemosensory literature and supports the concept that vanillin may be a relatively selective olfactory stimulus.
Funding
Grants (NIDCD PO1 00161, USAMRAA W81XWH-09-1-0467 to R.L.D.); Postdoctoral training grant from Fonds de la Recherche en Santé du Québec (21069) to J.F.; Deutsche Forschungsgemeinschaft (HU441/10-1) to T.H.
Acknowledgments
We thank Gerd Kobal for his support during the initial phase of the study and his generous support of J.B. Disclosure: R.L.D. is President and major shareholder of Sensonics, Inc., the manufacturer and distributor of the odor identification test used in this study. The authors report no other potential conflicts of interest.
References
- Cometto-Muniz E, Cain WS. Temporal integration of pungency. Chem Senses. 1984;8:315–327. [Google Scholar]
- Cometto-Muniz E, Noriega G. Gender differences in the perception of pungency. Physiol Behav. 1985;34:385–389. doi: 10.1016/0031-9384(85)90200-8. [DOI] [PubMed] [Google Scholar]
- Cometto-Muniz JE, Cain WS. Thresholds for odor and nasal pungency. Physiol Behav. 1990;48:719–725. doi: 10.1016/0031-9384(90)90217-r. [DOI] [PubMed] [Google Scholar]
- Doty RL. Intranasal trigeminal detection of chemical vapors by humans. Physiol Behav. 1975;14:855–859. doi: 10.1016/0031-9384(75)90081-5. [DOI] [PubMed] [Google Scholar]
- Doty RL, Brugger WPE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav. 1978;20:175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Elsberg CA, Levy I, Brewer ED. The sense of smell VI. The trigeminal effects of odorous substances. Bull Neurol Inst NY. 1935;4:270–285. [Google Scholar]
- Frasnelli J, Charbonneau G, Collignon O, Lepore F. Odor localization and sniffing. Chem Senses. 2009;34:139–144. doi: 10.1093/chemse/bjn068. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Hummel T. Age-related decline of intranasal trigeminal sensitivity: is it a peripheral event? Brain Res. 2003;987:201–206. doi: 10.1016/s0006-8993(03)03336-5. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Hummel T. Intranasal trigeminal threshold in healthy subjects. Environ Toxicol Pharmacol. 2005;9:575–580. doi: 10.1016/j.etap.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Lotsch J, Hummel T. Event-related potentials to intranasal trigeminal stimuli change in relation to stimulus concentration and stimulus duration. J Clin Neurophysiol. 2003;20:80–86. doi: 10.1097/00004691-200302000-00011. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Ungermann M, Hummel T. Ortho- and retronasal presentation of olfactory stimuli modulates odor percepts. Chemosens Percept. 2008;1:9–15. [Google Scholar]
- Hummel T, Futschik T, Frasnelli J, Huttenbrink KB. Effects of olfactory function, age, and gender on trigeminally mediated sensations: a study based on the lateralization of chemosensory stimuli. Toxicol Lett. 2003;140–141:273–280. doi: 10.1016/s0378-4274(03)00078-x. [DOI] [PubMed] [Google Scholar]
- Kobal G. Elektrophysiologische Untersuchungen des menschlichen Geruchssinns. Stuttgart (Germany): Thieme Verlag; 1981. [Google Scholar]
- Kobal G, Hummel T. Olfactory evoked potential activity and hedonics. In: van Toller S, Dodd GH, editors. Fragrance—the psychology and biology of perfume. London: Elsevier Applied Science; 1992. [Google Scholar]
- Kobal G, Van Toller S, Hummel T. Is there directional smelling? Experientia. 1989;45:130–132. doi: 10.1007/BF01954845. [DOI] [PubMed] [Google Scholar]
- Krone D, Mannel M, Pauli E, Hummel T. Qualitative and quantitative olfactometric evaluation of different concentrations of ethanol peppermint oil solutions. Phytother Res. 2001;15:135–138. doi: 10.1002/ptr.716. [DOI] [PubMed] [Google Scholar]
- Livermore A, Hummel T. The influence of training on chemosensory event-related potentials and interactions between the olfactory and trigeminal systems. Chem Senses. 2004;29:41–51. doi: 10.1093/chemse/bjh013. [DOI] [PubMed] [Google Scholar]
- Lorig T, Sapp A, Campbell J. Event-related potentials to odor stimuli. Bull Psychon Soc. 1993;31:131–134. [Google Scholar]
- Pause BM, Sojka B, Ferstl R. Central processing of odor concentration is a temporal phenomenon as revealed by chemosensory event-related potentials (CSERP) Chem Senses. 1997;22:9–26. doi: 10.1093/chemse/22.1.9. [DOI] [PubMed] [Google Scholar]
- Porter J, Anand T, Johnson B, Khan RM, Sobel N. Brain mechanisms for extracting spatial information from smell. Neuron. 2005;47:581–592. doi: 10.1016/j.neuron.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Prah JD, Benignus VA. Trigeminal sensitivity to contact chemical stimulation: a new method and some results. Percept Psychophys. 1984;35:65–68. doi: 10.3758/bf03205925. [DOI] [PubMed] [Google Scholar]
- Rajan R, Clement JP, Bhalla US. Rats smell in stereo. Science. 2006;311:666–670. doi: 10.1126/science.1122096. [DOI] [PubMed] [Google Scholar]
- Roscher S, Glaser C, Hummel T, Kobal G. An easy method for separating olfactory from trigeminal stimulation. Chem Senses. 1996;21:492. doi: 10.1093/chemse/21.1.75. [DOI] [PubMed] [Google Scholar]
- von Békésy G. Olfactory analogue to directional hearing. J Appl Physiol. 1964;19:369–373. doi: 10.1152/jappl.1964.19.3.369. [DOI] [PubMed] [Google Scholar]
- von Skramlik E. Über die Lokalisation der Empfindungen bei den niederen Sinnen. Z Sinnesphysiol. 1924;56:69. [Google Scholar]
- Wise P, Canty T, Wysocki C. Temporal integration in nasal lateralization of ethanol. Chem Senses. 2006;31:A57. doi: 10.1093/chemse/bjj023. [DOI] [PubMed] [Google Scholar]
- Wise PM, Radil T, Wysocki CJ. Temporal integration in nasal lateralization and nasal detection of carbon dioxide. Chem Senses. 2004;29:137–142. doi: 10.1093/chemse/bjh018. [DOI] [PubMed] [Google Scholar]
- Yang BH, Piao ZG, Kim YB, Lee CH, Lee JK, Park K, Kim JS, Oh SB. Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res. 2003;82:781–785. doi: 10.1177/154405910308201004. [DOI] [PubMed] [Google Scholar]