Abstract
Diarrhoea, as a common side effect of antibiotics, increases treatment costs and length of stay in acute healthcare facilities. One potential strategy to prevent this side effect is the concurrent use of probiotic bacteria or yeast. This review discusses the evidence for the efficacy of probiotics in the prevention of antibiotic-associated diarrhoea and Clostridium difficile infection; the potential mechanisms by which probiotics may work; their safety; what future research is required; and recommendations for use in clinical practice.
Keywords: antibiotic-associated diarrhoea, Clostridium difficile, hospitalized adults, probiotics
Introduction
Diarrhoea, as a common side effect of antibiotics, causes increased treatment costs and extended length of stay in acute healthcare facilities. Clostridium difficile infection as a cause of diarrhoea has become a major issue in many countries, resulting in a search for the best way to prevent its occurrence. Prevention primarily revolves around control of antibiotic use, followed by comprehensive infection control procedures once outbreaks occur. Nevertheless, other potential factors have been explored, including the use of probiotic bacteria and yeast. This review discusses the evidence for the efficacy of probiotics in the prevention of antibiotic-associated diarrhoea (AAD) and Clostridium difficile-associated diarrhoea (CDAD); the potential mechanisms by which probiotics may work; their safety; what future research is required; and recommendations for their use in clinical practice.
Antibiotic-associated diarrhoea
Diarrhoea is a common complication of antibiotics. It occurs in between 5% and 39% of patients depending on the population and type of antibiotic [McFarland, 1998]: adults over the age of 65 years are known to be at the top end of this range (Bignardi, 1998], and broad spectrum antibiotics also impart a greater risk than narrow spectrum, in particular, clindamycin, cephalosporins and fluoroquinolones [Graul et al. 2009]. There is no universal agreement on which antibiotics impart greatest risk and any antibiotic may disrupt the colonic microbiota resulting in diarrhoea [Shannon-Lowe et al. 2010]. AAD can occur up to 2–3 weeks following cessation of antibiotic therapy rather than during the treatment [Wistrom et al. 2001]. It is a particular problem in hospitals, among the older frail and ill patients, and is an extremely unpleasant and debilitating side effect. The usual treatment is to withdraw antibiotics if they are still being taken, which can result in incomplete courses and corresponding difficulties with treating the underlying infection, potentially leading to increased length of stay and costs of care. It has also been shown that hospital patients are at greater risk of future infections and increased mortality [McFarland, 1998].
Clostridium difficile-associated diarrhoea
CDAD is a severe form of diarrhoea caused by the C. difficile bacteria. These bacteria produce a toxin resulting in symptoms ranging from mild diarrhoea to inflammation of the bowel (pseudomembranous colitis), which can cause death. CDAD is responsible for around 10–20% of all cases of AAD [Bartlett, 2002] and it can occur up to 8 weeks after antibiotic therapy [Gerding et al. 1986]. There are three key risk factors for the development of this infection; antibiotic use [Bartlett, 2006], increasing age [Karlstrom et al. 1998], and hospitalization [Wistrom et al. 2001]. C. difficile is contagious and spreads easily from patient to patient, thus initial outbreaks can rapidly spread to other patients unless strict and comprehensive infection control measures are put in place immediately.
CDAD has become and remains a serious problem for acute healthcare providers, leading to concerns around patient safety and increased medical treatment costs. The latest estimates of the cost of C. difficile infection in the USA are $3.2 billion [O'Brien et al. 2007], $2454 per CDAD case [Dubberke et al. 2008] and for the UK £4107 per case [Wilcox et al. 1996]. CDAD is treated by withdrawal of the precipitating antibiotic, avoidance of antiperistaltic agents and then treatment either with metronidazole for mild to moderate cases or vancomycin for severe cases or in people who do not respond to metronidazole [Cohen et al. 2010]. A total of 75–80% of patients respond well to one of these treatments but 20–25% may develop recurrent CDAD [Bartlett, 2002]. In recent years there has been an emergence of hypervirulent strains that have lead to increased incidence of CDAD, more severe disease, higher relapse rates, increased mortality, and greater resistance to fluoroquinolone antibiotics [Cartman et al. 2010]. The most notorious of these strains [classified as either restriction-endonuclease analysis BI, North American pulse-field type 1 (BI/NAP1) or PCR ribotype 027] is thought to have developed due to excessive use of quinolone antibiotics. Other strains continue to emerge, such as ribotype 078 and 106, which also cause severe disease but are currently less widespread [Cartman et al. 2010].
Probiotics
Probiotics are defined as ‘live microorganisms, which, when administered in adequate amounts, confer a health benefit on the host’ [FAO/WHO, 2001]. A variety of bacteria have been studied to explore their probiotic effect, including Lactobacillus rhamnosus GG, various Lactobacillus and Bifidobacterium strains and the yeast Saccharomyces boulardii. An important consideration when evaluating this research is that the effects of any bacteria are strain specific, meaning the data from research relates only to that specific strain. Research results cannot be extrapolated to other species or strains. For example, L. rhamnosus GG is a specific bacterial strain (the nomenclature includes genus, species and strain) which demonstrates a probiotic effect in the prevention of AAD [McFarland, 2006]. Other strains of L. rhamnosus species may not have this effect, and likewise other species in the genus of Lactobacillus may not act as probiotics. This is because individual strains exhibit different specific characteristics, such as resistance to gastric acid and bile, ability to colonize the mucosa, and antimicrobial activity [Jacobsen et al. 1999]. A useful overview of the history of probiotic use and the formulation and delivery of probiotics can be found in a review by s and Luack [Verna and Luack, 2010].
Why diarrhoea occurs and how probiotics may prevent it
Antibiotics disrupt the normal colonic microflora and consequently alter carbohydrate metabolism and antimicrobial activity in the colon, potentially leading to osmotic diarrhoea or diarrhoea caused by pathogenic bacteria [Hogenauer et al. 1998]. In the first case, reduced metabolism of fermentable carbohydrate leads to reduced short-chain fatty acids (bacterial source of energy) and increased nonabsorbable carbohydrate in the lumen of the gut. The increased osmotic pressure reduces water absorption from the gut, liquefying the stools [Binder, 2010]. Secondly, the protective barrier provided by the normal intestinal microflora is disrupted and this leads to a reduction in the ability of the gut to resist colonization by pathogens. As a result opportunistic growth of pathogens occurs, for example C. difficile, Salmonella, Staphyloccus aureus, or Clostridium perfringens, and toxins result in mucosal damage and inflammation leading to diarrhoea. In addition to these effects, drugs which increase gut motility can exacerbate the situation and worsen or cause diarrhoea. Examples include the action of erythromycin and clavulanate (found in Augmentin (GSK, Brentford, UK)/Co-Amoxiclav (non-proprietary)) [Caron et al. 1991].
Older adults are at greater risk of developing diarrhoea and this may be because ageing is associated with changes to the microbiota of the gut [Mueller et al. 2006], which makes the old person more vulnerable to the effects of antibiotics. The main changes are decreases in total numbers and species diversity of Bacteroides and Bifidobacteria associated with decreased amylolytic activity; with corresponding increases in facultative anaerobes, Fusobacteria, Clostridia, and Eubacteria. There are further changes observed in elderly patients treated with antibiotics, including decreased short-chain fatty acid production and increased proteolytic activity [Guigoz et al. 2008]. All these changes may result in an increased risk of diarrhoea; production of toxic metabolites which could increase cancer risk; altered colonization resistance thus leading to reduced resistance to disease and increases in pathogenic bacteria in the gut; and finally changes in immunity within the gut, increasing infection risk.
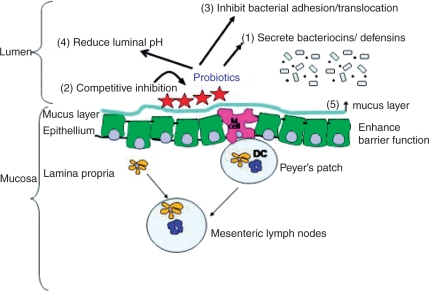
Researchers have proposed that probiotics may prevent diarrhoea by interrupting either of the the potential mechanisms; by maintaining the flora of the gut and ongoing carbohydrate fermentation; and/or by competitively inhibiting the growth of pathogens. The exact mechanism of action is as yet unknown and may vary between strains of bacteria. Ng and colleagues, and Oelschlaeger have written comprehensive reviews about the evidence supporting proposed mechanisms of probiotic action [Oelschlaeger, 2010; Ng et al. 2008]. There are three broad areas: modulation of the host’s immune system; antimicrobial activity; and other mechanisms relating to indirect action on pathogens, the host or food components. These are summarized in Figure 1.
Figure 1.
Inhibition of enteric bacteria and enhancement of barrier function by probiotic bacteria. Schematic representation of the crosstalk between probiotic bacteria and the intestinal mucosa. Antimicrobial activities of probiotics include (1) the production of bacteriocins/defensins, (2) competitive inhibition with pathogenic bacteria, (3) inhibition of bacterial adherence or translocation, and (4) reduction of luminal pH. Probiotic bacteria can also enhance intestinal barrier function by (5) increasing mucus production. Reproduced from Ng [2008], with permission of John Wiley & Sons, Inc.
To date, most studies in this area have been in vitro or animal studies. These have provided a useful framework and scientific basis for modes of action; however this work needs to be extended to human studies. Parkes and colleagues specifically review the evidence for the mechanisms of action in the prevention of CDAD [Parkes et al. 2009]. There are several lines of evidence, primarily for S. boulardii, and L. rhamnosus GG, which suggest that stimulation of immune factors, and suppression of pathogenic colonization are key. For example, S. boulardii has been shown in two studies to upregulate antitoxin A secretory immunoglobulin A expression in animal models of CDAD [Qamar et al. 2001; Buts et al. 1994] and in another study to directly inhibit C. difficile toxin A binding to the epithelium [Pothoulakis et al. 1993]. L. rhamnosus GG has been shown to increase gut mucin production [Mack et al. 1999], which improves the barrier defences of the epithelium, and increases colonic water absorption [Madsen et al. 2001], which directly reduces diarrhoea.
Summary of the evidence for probiotic prevention of antibiotic-associated diarrhoea
Several systematic reviews on adult and paediatric AAD suggest that probiotic bacteria offer a solution. Data indicate that Lactobacillus strains in particular seem to be effective, however the largest body of evidence is for the yeast S. boulardii [McFarland, 2010]. The latest meta-analysis of 10 randomized control trials testing the efficacy of S. boulardii in preventing AAD shows an overall, pooled relative risk of 0.47 [95% confidence interval (CI) = 0.35, 0.63; p < 0.001]. McFarland’s previous meta-analysis [McFarland, 2006] examined other bacteria as well and found the relative risk of getting AAD is 0.31 (95% CI = 0.13, 0.72; p = 0.006) while taking L. rhamnosus GG (data combined from six trials). Data from seven other trials using various mixtures of bacteria were combined and showed a relative risk of 0.51 (95% CI = 0.38, 0.68; p < 0.0001). Finally, six further trials of various single bacterial strains were combined to give a relative risk of AAD of 0.46 (95% CI = 0.21, 1.03; p = 0.06). Caution is required in interpreting meta-analyses of more than one strain because each strain has specific actions. Previously the small number of trials testing each individual strain led to meta-analyses combining results from various stains, but now, as the numbers of trials increase, it is encouraging to see that new single-strain meta-analyses are being published. It can be concluded from these results that several bacterial strains and one yeast have the potential to prevent AAD. The strongest evidence is for S. boulardii and L. rhamnosus GG because these have been used in most studies.
Since the McFarland (2006) meta-analysis, 11 further randomized controlled trials testing various bacteria for preventative effects in adults have been published. Seven tested various strains of Lactobacillus and/or Bifidobacterium and all showed a preventative effect (see Table 1 for details). Three other trials failed to show an effect (see Table 2 for details). However, there were flaws in these three study designs, including relatively small bacterial doses compared with successful trials and limited follow up. One abstract provides very limited methodological information [Stein et al. 2007]. Finally, one trial tested S. boulardii and is included in the recent meta-analysis [McFarland, 2010] and therefore is not detailed here.
Table 1.
Trials in adults, published since 2007, showing a preventative effect of the tested bacteria.
| Reference | Bacteria being tested (product containing them) | Patient group | Population daily dose | **Duration of probiotic treatment after antibiotic treatment complete (days) | Follow-up after antibiotic treatment complete (days) | AAD in treatment (%) | AAD in controls (%) | CDAD in Treatment (%) | CDAD in control (%) |
|---|---|---|---|---|---|---|---|---|---|
| Gao et al. [2010] | Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R (Bio-K+ CL1285, Bio-K + International, Laval, Quebec, Canada) | Hospitalized, 50–70 years | Dose 1: 5 × 1010 cfu/day Dose 2: 1 × 1011 cfu/day | 5 | 26 | Dose 1: 24/85 (28.2%) Dose 2: 13/86 (15.5%) | 37/84 (44.1%) | Dose 1: 8/85 (9.4%) Dose 2: 1/86 (1.2%) | 20/84 (23.8%) |
| Psaradellis et al. [2010] | Lactobacillus acidophilus CL1285 and Lactobacillus casei (strain not given) (Bio-K+ CL1285, Bio-K + International, Laval, Quebec, Canada) | Hospitalized, >18 years | 5 × 1010 cfu/day | 5 | 21 | 17/216 (7.9%) | 30/221 (13.6%) | 1/16 (6.2%) | 4/30 (13.3%) |
| Safdar et al. [2008] | Lactobacillus acidophilus (strain not given) (Florajen, American Lifeline, Inc. Baraboo, Wisconsin, USA) | Hospitalized, >18 years | 6 × 1010 cfu/day | 14 | 14 | 4/23 (17%) | 6/16 (37%) | 0/3 (0%) 1 case not tested | 1/4 (25%) 2 cases not tested |
|
Wenus et al. [2008] |
Lactobacillus rhamnosus GG, Lactobacillus acidophilus La-5 and Bifidobacterium Bb-12 (Biola TINE BA, Oslo) |
Hospitalized, >18 years |
2.5 × 1010 cfu/day of LGG and Bb-12 2.5 × 109 cfu/day of La-5, |
14 days’ probiotic treatment, data on length of antibiotic treatment not given |
Data not given |
2/46 (5.9%) |
8/41 (27.6%) |
ND |
ND |
| Stockenhuber et al. [2008] (only abstract available – individual patients were not randomized, only wards, and no placebo given) | Lactobacillus casei shirota (Yakult, Yakult Honsha Co., Ltd, Japan) | Hospitalized, mean age 71 years | 6.5 × 109 cfu/day | 3 | Data not given | 17/340 (5%) | 63/338 (18%) | ND | ND |
| Beausoleil et al. [2007] | Lactobacillus acidophilus CL1285 and Lactobacillus casei (strain not given) (Bio-K+ CL1285, Bio-K + International, Laval, Quebec, Canada) | Hospitalized, mean age 70 years | 5 × 1010 cfu/day | 0 (only during abx course) | 21 | 7/44 (15.9%) | 16/45 (35.6%) | 1/44 (2.3%) | 7/45 (15.6%) |
| Hickson et al. [2007] | Lactobacillus casei DN-114 001, Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus (Actimel, Danone, France) | Hospitalized, mean age 74 years | 1.94 × 1010cfu/d of L.casei DN114001, 1.94 × 1010cfu/d of L.bulgaricus, 1.94 × 109cfu/d of S.thermophilus | 7 | 28 | 7/57 (12%) | 19/56 (34%) | 0/56 (0%) 1 case not tested | 9/53 (17%) 3 cases not tested |
All studies gave probiotic during antibiotic treatment
AAD, antibiotic-associated diarrhoea; CDAD, Clostridium difficile-associated diarrhoea; cfu, colony forming units; ND, no data available or CDAD not an outcome.
Table 2.
Trials in adults, published since 2007, showing no effect of tested bacteria.
| Reference | Bacteria being tested (product containing them) | Patient group | Population daily dose | Duration of probiotic treatment after antibiotic treatment complete* (days) | Follow up after antibiotic treatment complete (days) | AAD in treatment (%) | AAD in controls (%) | CDAD in treatment (%) | CDAD in controls (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lonnermark et al. [2010] | Lactobacillus plantarum 299v (Skånemejerier, Sweden) | Outpatients, infectious diseases clinic, >16 years | 1 × 1010 cfu/day | 7 | 28 | 6/83 (7.5%) | 5/80 (6.0%) | ND | ND |
| Conway et al. [2007] | Bio yogurt: Streptococcus thermophillus, Lactobacillus acidophilus, and Bifidobacteria anamalis lactus, Commercial yogurt: Streptococcus thermophillus, and Lactobacillus delbrueckii bulgaris (Yeo Valley Organics, Somerset, UK) | GP practice, children (26%) and adults (74%) | 1 × 109 cfu/day | 5 | 5 | Bio: 9/131 (7%) Commercial: 13/118 (11%) | 17/120 (14%) | ND | ND |
| Stein et al. [2007] (abstract only – full article in Hebrew; minimal methodological information provided) | No data given on product or bacterial content | Hospitalized, >18 years | No data given | 21 days’ probiotic treatment, data on length of antibiotic treatment not given | No data on antibiotic treatment given | 3/21 (14.3%) | 1/21 (4.8%) | ND | ND |
All studies gave probiotic during antibiotic treatment
AAD, antibiotic-associated diarrhoea; CDAD, Clostridium difficile-associated diarrhoea; cfu, colony forming units; ND, no data available or CDAD not an outcome.
It is worth noting that yoghurt is made by the fermentation of milk with the starter cultures Lactobacillus delbrueckii subspecies bulgaricus and Streptococcus thermophilus. Although yogurt is a common product in which probiotic bacteria can be delivered to the human lower gut, there is little evidence that these particular strains offer beneficial effects. Some evidence exists for assisting with rehabilitation following malnutrition in children [Hamilton-Miller, 2004], however most research has focused not on these starter cultures but on the other bacteria added to the yogurt product. The one study testing yogurt in the prevention of AAD in the community [Conway et al. 2007] did not show a beneficial effect for either standard yogurt or bioyogurt (which contains the additional strains Lactobacillus acidophilus and Bifidobacteria anamalis lactus), but the study was underpowered (see Table 2). Nevertheless, an earlier trial [Beniwal et al. 2003] conducted in hospital patients, using bioyogurt containing a combined dose of 2.27 × 108 colony forming units (cfu)/day of L. acidophilus, L. delbrueckii subspecies bulgaricus, and S. thermophilus reduced AAD incidence from 24% to 12%. However, this trial did not control the quality of the bioyogurt to verify bacterial content, failed to use a control product and was not blinded. Therefore, the evidence for bioyogurt in the prevention of AAD is equivocal but suggests if patients choose to eat yogurt they should be advised to eat live bioyogurts containing L. acidophilus.
The focus of this review is diarrhoea in adults, but it is worth noting that the conclusion of the Cochrane systematic review for AAD in paediatrics:
Probiotics show promise for the prevention of paediatric AAD. While per protocol analysis yields treatment effect estimates that are both statistically and clinically significant, as does analysis of high quality studies, the estimate from the intention to treat analysis was not statistically significant. Future studies should involve probiotic strains and doses with the most promising evidence (e.g., Lactobacillus GG, Lactobacillus sporogenes, Saccharomyces boulardii at 5 to 40 billion colony forming units/day). Research done to date does not permit determination of the effect of age (e.g., infant versus older children) or antibiotic duration (e.g., 5 days versus 10 days). Future trials would benefit from a validated primary outcome measure for antibiotic-associated diarrhoea that is sensitive to change and reflects what treatment effect clinicians, parents, and children consider important. The current data are promising, but it is premature to routinely recommend probiotics for the prevention of paediatric AAD [Johnston et al. 2007, p. 2].
The study of probiotics in adults would also benefit from improved trial methodology and this is discussed in a later section.
Summary of the evidence of efficacy for Clostridium difficile-associated diarrhoea prevention
The data for C. difficile infection is less robust with fewer trials conducted and most being underpowered. It is important to note there are two categories of studies around CDAD: primary prevention, and treatment and prevention of recurrent CDAD.
The Cochrane Group produced a systematic review [Pillai and Nelson, 2008] to evaluate the evidence for the treatment of CDAD. The authors reviewed four studies [Lawrence et al. 2005; Wullt et al. 2003; Surawicz et al. 2000; McFarland et al. 1994] and concluded that there was insufficient evidence to support the use of probiotic therapy as an adjunct to antibiotic therapy, and no evidence for the use of probiotics alone, in the treatment of C. difficile colitis.
There are two recent papers evaluating the evidence on the prevention of CDAD [Parkes et al. 2009; Tung et al. 2009]. Parkes and colleagues reviewed five studies, three testing mixtures of bacteria and two examining S. boulardii. The results were varied with one mixture [Plummer et al. 2004] and neither S. boulardii trial [Kotowska et al. 2005; Surawicz et al. 1989] showed a significant effect. The other two trials of mixtures showed a significant reduction in CDAD rates [Hickson et al. 2007; Rafiq et al. 2007]. However, one trial is only available as an abstract and has limited methodological and bacterial species information [Rafiq et al. 2007], and the other trial had CDAD as a secondary outcome and the results are based on only nine cases of CDAD [Hickson et al. 2007]. Tung and colleagues specifically reviewed the data on S. boulardii and its role in both prevention of primary and recurrent C. difficile infection [Tung et al. 2009]. They included the two primary prevention studies [McFarland et al. 1995; Surawicz et al. 1989], neither of which showed a significant preventative effect. Both Tung and colleagues and Parkes and colleagues concluded that the evidence to date, although showing some promise for primary prevention, is not yet sufficient to make specific recommendations. Further large, well powered studies with rigorous methodology are required for each specific bacterial strain or mixture with promising preliminary data.
The safety implications of probiotic use in patients at risk of antibiotic-associated diarrhoea
Probiotic bacterial strains used in food products are generally regarded as safe in healthy populations, demonstrated by their extensive use over centuries, with few reported adverse consequences. However, by definition, healthy populations are not at risk of developing AAD, and so it is important to consider the risks of probiotic administration in the at risk group. Risk factors for AAD and CDAD include duration and type of antibiotic course, increasing age, severity of underlying illnesses, duration of hospital stay, presence of a nasogastric tube, and use of proton pump inhibitors [Bignardi, 1998]. In short, the population at greatest risk is old, hospitalized patients treated with antibiotics.
Probiotic bacteria can cause infective episodes if they translocate from the gastrointestinal tract to extraintestinal sites, such as regional lymph nodes, spleen, liver, bloodstream, heart valves, or other tissues. Bacterial translocation is caused by a defective intestinal barrier, immunosuppression, or gut prematurity, and may result in bacteraemia, sepsis, and multiple organ failure [Liong, 2008]. However, cases of probiotic administration leading to bacteraemia or fungaemia are rare. In 2003 an expert panel concluded that ‘Current evidence suggests that the risk of infection with probiotic Lactobacilli or Bifidobacteria is similar to that of infection with commensal strains, and that consumption of such products presents a negligible risk to consumers, including immunocompromised hosts’ [Borriello et al. 2003, p. 779].
Since this time, several further reviews have been published summarizing the reported cases of probiotic-related infections and reported adverse events from clinical trials. Only one of these is a systematic review [Whelan and Myers, 2010], which specifically reviewed the safety of probiotics in artificially fed patients. This review identified reports of 32 patients with probiotic infections concurrent with probiotic consumption and artificial nutrition support. All of these cases were infections of L. rhamnosus GG or S. boulardii, but this is most likely due to their extensive use rather than particular virulence. Identified risks included central venous catheter in situ and increased risk of bacterial translocation caused by critical illness or impaired immune function. Delivery of large doses of bacteria through post pyloric feeding tubes was identified as a possible risk factor because of an increase in noninfectious complications [Rayes et al. 2005] and mortality [Besselink et al. 2008] in severely ill patients. Nevertheless, other trials have delivered probiotic bacteria through jejunal feeding tubes with no reported adverse events [Whelan and Myers, 2010].
The other three recent reviews [Liong, 2008; Boyle et al. 2006; Hammerman et al. 2006] explored the safety of probiotics in all patient groups. L. rhamnosus GG, L. casei, and Bacillus subtilis are identified as species and strains that have caused bacteraemia, and S. boulardii in causing fungaemia. Immunocompromised adults and neonates are identified as at risk, but there is no clear description of how to define immunocompromise. The presence of a central venous catheter, impaired intestinal barrier, post pyloric delivery of the probiotic and cardiac valve disease are also highlighted as increasing the risk of infection. However, the reviews also note that infections are very rare and are not reported in most trials of probiotics, even those studying immunocompromised groups, such as HIV and neonates.
It should be noted that there are difficulties in linking infections to the specific probiotic strain, particularly if only phenotypic identification techniques are used. Ideally, genotypic methods should be used in order to identify the precise strain causing the infection and matching it to the probiotic strain. Lactobacillus bacteria are ubiquitous in the human diet and intestine and many strains are indistinguishable using only phenotypic techniques. The data in the literature do not always refer to certain probiotic infection since the infective bacteria may not have been conclusively identified. Strain specificity is critical when evaluating the benefits of bacteria and it is equally important in considering safety profiles, and so the safety of each proposed probiotic bacteria should be individually assessed.
As outlined previously in this paper, there is substantial evidence for the benefit of certain strains of bacteria in preventing AAD and CDAD, therefore the risk of using a probiotic should be carefully weighed against the benefits it may provide in avoiding these serious and unpleasant side effects.
Current clinical recommendations
In 2008 an expert group was convened at Yale University to examine and grade the evidence for use of probiotics in healthcare [Floch et al. 2008]. Evidence was graded as: A – strong, positive, well conducted, controlled studies in primary literature when the full paper is available; B – positive, controlled studies but the presence of some negative studies; C – some positive studies but clearly an inadequate amount of work to establish either A or B grade. The group identified grade A evidence for the prevention of AAD in ambulatory and hospitalized adult patients. They stated L. rhamnosus GG and S. boulardii have been shown to be effective, noting that evidence for the mixture of L. casei DN-114 001, L. delbrueckii subspecies bulgaricus and S. thermophilus was also good [Hickson et al. 2007]. It should be noted that L. casei DN-114 001 is deemed the ‘probiotic’ in this mixture; it is unlikely that the standard yogurt cultures also included have an effect. Nevertheless, it is impossible to state conclusively that only L. casei DN-114 001 has the beneficial effects, since the bacteria are delivered as part of a yoghurt drink, which contains all three strains.
For the prevention of CDAD and its use in recurrent C. difficile disease the panel identified only grade B evidence and highlighted that the best data were for L. rhamnosus GG and S. boulardii.
The UK Health Protection Agency good practice guidance for the management of C. difficile infection [Department of Health and Health Protection Agency, 2008] does not support the use of any probiotic in the prevention or treatment of C. difficile infection. It also notes that S. boulardii is not licensed in the UK and is associated with fungaemia in immunosuppressed groups, so does not recommend its use. Similarly the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America do not recommend probiotics for primary prevention of C. difficile infection and also note a concern over possible bloodstream infections [Cohen et al. 2010].
The World Gastroenterology Organization (WGO) produced a practice guideline [World Gastroenterology Organization, 2008] which stated that there was strong evidence of efficacy for the prevention of AAD for S. boulardii or L. rhamnosus GG in adults or children who are receiving antibiotic therapy, and that the mixture containing L. casei DN-114 001 is effective in hospitalized adult patients for preventing AAD and CDAD.
These recommendations show considerable consistency. There is minor disagreement about the level of evidence required to make a recommendation regarding C. difficile infection. The WGO support the use of the mixture containing L. casei DN-114 001, whereas none of the other recommendations consider the one paper providing evidence sufficient. Because this paper [Hickson et al. 2007] only presents data on nine cases of CDAD, it would seem prudent to seek further confirmatory data before making firm recommendations.
Some of the trials listed in Table 1 were not published when these recommendations were prepared. For example, there is increasing evidence for the mixture containing Lactobacillus acidophilus CL1285, with two recent trials showing positive results.
The difficulty in using such recommendations is that many probiotic strains are not readily available for use in healthcare institutions. There are few controls on the labelling and quality of probiotic bacteria, thus care is needed in ensuring that the products used contain only the claimed probiotic bacteria, in the claimed numbers, and will deliver viable bacteria to the lower gut.
Future research
Future research needs to establish which species and strains are best at preventing and treating AAD and CDAD. Furthermore, research is needed to verify the best dose to use, to establish an ongoing robust safety record in groups at most risk of AAD and CDAD (by documenting risks and adverse events), and to explore the cost–benefit relationship of providing such preventative treatment.
Many of the summaries of evidence are confounded by poor trial methodology, lack of strain or dose definition, short follow up, absence of quality control for the probiotic product, and low statistical power. Future work should aim to eradicate these flaws and further investment in research is needed from the companies that market such products.
Clinical trials are, by their nature, highly controlled and always exclude some patients, thus work is required to test the use of probiotic products in routine clinical practice. It is important to show that the products can be easily acquired and successfully delivered to patients in the healthcare setting. Data are also needed to demonstrate that the probiotics are consumed in sufficient amounts, that any costs are outweighed by the savings, as well as demonstrating efficacy in reducing diarrhoea incidence.
Conclusions
L. rhamnosus GG, S. boulardii and two mixtures, one containing L. casei DN-114 001 and the other Lactobacillus acidophilus CL1285, all have good evidence of efficacy in preventing AAD in clinical trials, but evidence of feasibility and efficacy in routine practice is required. The evidence for prevention and treatment of CDAD is currently equivocal. There may be other strains that have equal or better efficacy and research is required to establish which strains are the best to use. Healthcare providers want a well defined, proven intervention, and to deliver this goal more high-quality research is needed.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
M Hickson has a consultancy contract with Danone Ltd to provide advice on scientific matters related to the effects of Actimel on human health.
References
- Bartlett J.G. (2002) Clinical practice: Antibiotic associated diarrhoea. N Engl J Med 346: 334–339 [DOI] [PubMed] [Google Scholar]
- Bartlett J.G. (2006) Narrative review: The new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med 145: 758–764 [DOI] [PubMed] [Google Scholar]
- Beausoleil M., Fortier N., Guenette S., L'Ecuyer A., Savoie M., Franco M., et al. (2007) Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: A randomized, double-blind, placebo-controlled trial. Can J Gastroenterol 21: 732–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniwal R.S., Arena V.C., Thomas L., Narla S., Imperiale T.F., Chaudhry R.A., et al. (2003) A randomized trial of yogurt for prevention of antibiotic-associated diarrhea. Dig Dis Sci 48: 2077–2082 [DOI] [PubMed] [Google Scholar]
- Besselink M.G., van Santvoort H.C., Buskens E., Boermeester M.A., van G.H., Timmerman H.M., et al. (2008) Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 371: 651–659 [DOI] [PubMed] [Google Scholar]
- Bignardi G.E. (1998) Risk factors for Clostridium difficile infection. J Hosp Infect 40: 1–15 [DOI] [PubMed] [Google Scholar]
- Binder H.J. (2010) Role of colonic short-chain fatty acid transport in diarrhea. Ann Rev Physiol 72: 297–313 [DOI] [PubMed] [Google Scholar]
- Borriello S.P., Hammes W.P., Holzapfel W., Marteau P., Schrezenmeir J., Vaara M., et al. (2003) Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis 36: 775–780 [DOI] [PubMed] [Google Scholar]
- Boyle R.J., Robins-Browne R.M., Tang M.L. (2006) Probiotic use in clinical practice: What are the risks? Am J Clin Nutr 83: 1256–1264 [DOI] [PubMed] [Google Scholar]
- Buts J.P., De K.N., De R.L. (1994) Saccharomyces boulardii enhances rat intestinal enzyme expression by endoluminal release of polyamines. Pediatr Res 36: 522–527 [DOI] [PubMed] [Google Scholar]
- Caron F., Ducrotte P., Lerebours E., Colin R., Humbert G., Denis P. (1991) Effects of amoxicillin-clavulanate combination on the motility of the small intestine in human beings. Antimicrob Agents Chemother 35: 1085–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartman S.T., Heap J.T., Kuehne S.A., Cockayne A., Minton N.P. (2010) The emergence of “hypervirulence” in Clostridium difficile. Int J Med Microbiol 300: 387–395 [DOI] [PubMed] [Google Scholar]
- Cohen S.H., Gerding D.N., Johnson S., Kelly C.P., Loo V.G., McDonald L.C., et al. (2010) Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 31: 431–455 [DOI] [PubMed] [Google Scholar]
- Conway S., Hart A., Clark A., Harvey I. (2007) Does eating yogurt prevent antibiotic-associated diarrhoea? A placebo-controlled randomised controlled trial in general practice. Br J Gen Pract 57: 953–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Health Protection Agency (2008) Clostridium difficile infection: How to deal with the problem. Best Practice Guidelines, Department of Health: London [Google Scholar]
- Dubberke E.R., Reske K.A., Olsen M.A., McDonald L.C., Fraser V.J. (2008) Short- and long-term attributable costs of Clostridium difficile-associated disease in nonsurgical inpatients. Clin Infect Dis 46: 497–504 [DOI] [PubMed] [Google Scholar]
- FAO/WHO (2001) Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with live Lactic Acid Bacteria, Food and Agriculture Organization of the United Nations and World Health Organization, Joint FAO/WHO Expert Consultation Group: Cordoba, Argentina [Google Scholar]
- Floch M.H., Walker W.A., Guandalini S., Hibberd P., Gorbach S., Surawicz C., et al. (2008) Recommendations for probiotic use – 2008. J Clin Gastroenterol 42(Suppl. 2): S104–S108 [DOI] [PubMed] [Google Scholar]
- Gao X.W., Mubasher M., Fang C.Y., Reifer C., Miller L.E. (2010) Dose–response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 105: 1636–1641 [DOI] [PubMed] [Google Scholar]
- Gerding D.N., Olson M.M., Peterson L.R., Teasley D.G., Gebhard R.L., Schwartz M.L., et al. (1986) Clostridium difficile-associated diarrhea and colitis in adults. A prospective case-controlled epidemiologic study. Arch Intern Med 146: 95–100 [PubMed] [Google Scholar]
- Graul T., Cain A.M., Karpa K.D. (2009) Lactobacillus and bifidobacteria combinations: A strategy to reduce hospital-acquired Clostridium difficile diarrhea incidence and mortality. Med Hypotheses 73: 194–198 [DOI] [PubMed] [Google Scholar]
- Guigoz Y., Dore J., Schiffrin E.J. (2008) The inflammatory status of old age can be nurtured from the intestinal environment. Curr Opin Clin Nutr Metab Care 11: 13–20 [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J.M. (2004) Probiotics and prebiotics in the elderly. Postgrad Med J 80: 447–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerman C., Bin-Nun A., Kaplan M. (2006) Safety of probiotics: Comparison of two popular strains. Br Med J 333: 1006–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson M., D'Souza A.L., Muthu N., Rogers T.R., Want S., Rajkumar C., et al. (2007) Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. Br Med J 335: 80–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenauer C., Hammer H.F., Krejs G.J., Reisinger E.C. (1998) Mechanisms and management of antibiotic-associated diarrhea. Clin Infect Dis 27: 702–710 [DOI] [PubMed] [Google Scholar]
- Jacobsen C.N., Rosenfeldt N.V., Hayford A.E., Moller P.L., Michaelsen K.F., Paerregaard A., et al. (1999) Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol 65: 4949–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B.C., Supina A.L., Ospina M., Vohra S. (2007) Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev 2: CD004827–CD004827 [DOI] [PubMed] [Google Scholar]
- Karlstrom O., Fryklund B., Tullus K., Burman L.G. (1998) A prospective nationwide study of Clostridium difficile-associated diarrhea in Sweden. The Swedish C. difficile Study Group. Clin Infect Dis 26: 141–145 [DOI] [PubMed] [Google Scholar]
- Kotowska M., Albrecht P., Szajewska H. (2005) Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea in children: A randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther 21: 583–590 [DOI] [PubMed] [Google Scholar]
- Lawrence S.J., Korzenik J.R., Mundy L.M. (2005) Probiotics for recurrent Clostridium difficile disease. J Med Microbiol 54: 905–906 [DOI] [PubMed] [Google Scholar]
- Liong M.T. (2008) Safety of probiotics: Translocation and infection. Nutr Rev 66: 192–202 [DOI] [PubMed] [Google Scholar]
- Lonnermark E., Friman V., Lappas G., Sandberg T., Berggren A., Adlerberth I. (2010) Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics. J Clin Gastroenterol 44: 106–112 [DOI] [PubMed] [Google Scholar]
- Mack D.R., Michail S., Wei S., McDougall L., Hollingsworth M.A. (1999) Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol 276: G941–G950 [DOI] [PubMed] [Google Scholar]
- Madsen K., Cornish A., Soper P., McKaigney C., Jijon H., Yachimec C., et al. (2001) Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121: 580–591 [DOI] [PubMed] [Google Scholar]
- McFarland L.V. (1998) Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis 16: 292–307 [DOI] [PubMed] [Google Scholar]
- McFarland L.V. (2006) Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol 101: 812–822 [DOI] [PubMed] [Google Scholar]
- McFarland L.V. (2010) Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol 16: 2202–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland L.V., Surawicz C.M., Greenberg R.N., Elmer G.W., Moyer K.A., Melcher S.A., et al. (1995) Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol 90: 439–448 [PubMed] [Google Scholar]
- McFarland L.V., Surawicz C.M., Greenberg R.N., Fekety R., Elmer G.W., Moyer K.A., et al. (1994) A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 271: 1913–1918 [PubMed] [Google Scholar]
- Mueller S., Saunier K., Hanisch C., Norin E., Alm L., Midtvedt T., et al. (2006) Differences in fecal microbiota in different European study populations in relation to age, gender, and country: A cross-sectional study. Appl Environ Microbiol 72: 1027–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.C., Hart A.L., Kamm M.A., Stagg A.J., Knight S.C. (2008) Mechanisms of action of probiotics: Recent advances. Inflamm Bowel Dis 15: 300–310 [DOI] [PubMed] [Google Scholar]
- O'Brien J.A., Lahue B.J., Caro J.J., Davidson D.M. (2007) The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: Clinical and economic consequences. Infect Control Hosp Epidemiol 28: 1219–1227 [DOI] [PubMed] [Google Scholar]
- Oelschlaeger T.A. (2010) Mechanisms of probiotic actions – a review. Int J Med Microbiol 300: 57–62 [DOI] [PubMed] [Google Scholar]
- Parkes G.C., Sanderson J.D., Whelan K. (2009) The mechanisms and efficacy of probiotics in the prevention of Clostridium difficile-associated diarrhoea. Lancet Infect Dis 9: 237–244 [DOI] [PubMed] [Google Scholar]
- Pillai A., Nelson R. (2008) Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev 1: CD004611–CD004611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer S., Weaver M.A., Harris J.C., Dee P., Hunter J. (2004) Clostridium difficile pilot study: Effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol 7: 59–62 [PubMed] [Google Scholar]
- Pothoulakis C., Kelly C.P., Joshi M.A., Gao N., O'Keane C.J., Castagliuolo I., et al. (1993) Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology 104: 1108–1115 [DOI] [PubMed] [Google Scholar]
- Psaradellis E., Sampalis J. (2010) Efficacy of BIO K+ CL1285® in the reduction of antibiotic associated diarrhea – a placebo controlled double-blind randomized, multi-center study. Arch Med Sci 6: 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar A., Aboudola S., Warny M., Michetti P., Pothoulakis C., LaMont J.T., et al. (2001) Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect Immun 69: 2762–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq, R., Pandey, D., Mahgoubosman, S., Masood, R., Donepundi, I., Norkus, E. et al. (2007) Prevention of Clostridium difficile diarrhoea with probiotics in hospitalized patients treated with antibiotics. Gastroenterology 132 (suppl 2): A187.
- Rayes N., Seehofer D., Theruvath T., Schiller R.A., Langrehr J.M., Jonas S., et al. (2005) Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation – a randomized, double-blind trial. Am J Transplant 5: 125–130 [DOI] [PubMed] [Google Scholar]
- Safdar N., Barigala R., Said A., McKinley L. (2008) Feasibility and tolerability of probiotics for prevention of antibiotic-associated diarrhoea in hospitalized US military veterans. J Clin Pharm Ther 33: 663–668 [DOI] [PubMed] [Google Scholar]
- Shannon-Lowe J., Matheson N.J., Cooke F.J., Aliyu S.H. (2010) Prevention and medical management of Clostridium difficile infection. Br Med J 340: c1296–c1296 [DOI] [PubMed] [Google Scholar]
- Stein G.Y., Nanim R., Karniel E., Moskowitz I., Zeidman A. (2007) [Probiotics as prophylactic agents against antibiotic-associated diarrhea in hospitalized patients]. Harefuah 146: 520–522, 575 [PubMed] [Google Scholar]
- Stockenhuber, A., Kamhuber, C., Leeb, G., Adelmann, K., Prager, E., Mach, K. et al. (2008) Preventing diarrhoea associated with antibiotics using a probiotic Lactobacillus casei preparation. Gut 57 (suppl 2): A20.
- Surawicz C.M., Elmer G.W., Speelman P., McFarland L.V., Chinn J., van Belle G. (1989) Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: A prospective study. Gastroenterology 96: 981–988 [DOI] [PubMed] [Google Scholar]
- Surawicz C.M., McFarland L.V., Greenberg R.N., Rubin M., Fekety R., Mulligan M.E., et al. (2000) The search for a better treatment for recurrent Clostridium difficile disease: Use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis 31: 1012–1017 [DOI] [PubMed] [Google Scholar]
- Tung J.M., Dolovich L.R., Lee C.H. (2009) Prevention of Clostridium difficile infection with Saccharomyces boulardii: A systematic review. Can J Gastroenterol 23: 817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna E.C., Lucak S. (2010) Use of probiotics in gastrointestinal disorders: What to recommend? Ther Adv Gastroenterol 3: 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenus C., Goll R., Loken E.B., Biong A.S., Halvorsen D.S., Florholmen J. (2008) Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur J Clin Nutr 62: 299–301 [DOI] [PubMed] [Google Scholar]
- Whelan K., Myers C.E. (2010) Safety of probiotics in patients receiving nutritional support: A systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am J Clin Nutr 91: 687–703 [DOI] [PubMed] [Google Scholar]
- Wilcox M.H., Cunniffe J.G., Trundle C., Redpath C. (1996) Financial burden of hospital-acquired Clostridium difficile infection. J Hosp Infect 34: 23–30 [DOI] [PubMed] [Google Scholar]
- Wistrom J., Norrby S.R., Myhre E.B., Eriksson S., Granstrom G., Lagergren L., et al. (2001) Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: A prospective study. J Antimicrob Chemother 47:( 43–50 [DOI] [PubMed] [Google Scholar]
- World Gastroenterology Organization (2008) Probiotics and prebiotics: Practice Guideline. Available at: http://www.worldgastroenterology.org/probiotics-prebiotics.html (accessed 23 November 2010)
- Wullt M., Hagslatt M.L., Odenholt I. (2003) Lactobacillus plantarum 299v for the treatment of recurrent Clostridium difficile-associated diarrhoea: A double-blind, placebo-controlled trial. Scand J Infect Dis 35: 365–367 [DOI] [PubMed] [Google Scholar]