Abstract
Hepatic encephalopathy (HE) is a common problem in patients with chronic liver disease and is characterized by diminished mentation and neuromuscular abnormalities. Symptoms range from subtle cognitive changes to coma and death. Gut-derived toxins such as ammonia are thought to play a central role in the pathogenesis of HE. Treatment strategies are directed at increased elimination or reduction of gut-derived ammonia in addition to correction of dynamic conditions that provoke bouts of HE. The standard of care for treatment of acute HE is lactulose, a nonabsorbable disaccharide that is thought to increase elimination and reduce absorption of ammonia. Although lactulose seems to work in the acute setting, the rate of recurrent HE on maintenance lactulose is high. Medications have been sought that reduce the rate of recurrent HE in patients at high risk for HE but none have been identified. Rifaximin is a poorly absorbed antibiotic that is thought to reduce ammonia production by eliminating ammonia-producing colonic bacteria. Many small studies have suggested that rifaximin is effective in treating acute HE and is extremely well tolerated. This led to a randomized, placebo-controlled, multicenter, multinational trial investigating the efficacy of rifaximin over a 6-month period in reducing the risk of recurrent HE in patients at baseline, but with a history of at least two bouts of acute HE in the previous 6 months prior to enrollment. Lactulose could be administered at the discretion of the investigator. A total of 299 patients were randomized to receive rifaximin or placebo; 91% of patients in each group received lactulose. Compared with placebo, patients at high risk for recurrent HE in the rifaximin group had highly statistically significant reductions in bouts of acute HE (58%) and reductions in hospitalizations related to HE (50%) over a 6-month period. The medication was well tolerated with a side-effect profile comparable to placebo. This led to the approval of rifaximin for reduction of risk of recurrent HE by the US Food and Drug Administration in March 2010. It is recommended that patients with a history of recurrent acute HE should be maintained on rifaximin with or without lactulose to reduce the risk of recurrent HE and related hospitalization.
Keywords: ammonia, cirrhosis, hepatic encephalopathy, lactulose, rifaximin
Introduction
Overt hepatic encephalopathy (HE) is a common complication of chronic liver disease, occurring in 30–45% of patients with cirrhosis [Poordad, 2007]. It is characterized by a spectrum of clinical manifestations that are potentially reversible, including changes in mentation and neuromuscular abnormalities. Symptoms range from mild cognitive abnormalities to coma and death [Ferenci et al. 1998]. The pathogenesis remains incompletely understood, although gut-derived toxins such as ammonia are thought to play a central role. Not only is HE difficult to manage and treat, it is also associated with increased mortality. Management strategies consist of identifying and treating precipitating factors and reducing the effects of gut-derived toxins. Many therapeutic alternatives are available for treatment of acute HE, although the failure rate of therapy is high.
Rifaximin is a poorly absorbed antibiotic that has been used to treat acute HE. A recent randomized, placebo-controlled, multicenter, multinational trial supports the administration of rifaximin to prevent recurrent bouts of HE in patients at high risk for HE.
Pathogenesis
The pathophysiology of HE remains incompletely understood despite decades of study. Ammonia, a gut-derived nitrogenous toxin, is thought to play a central role [Keiding et al. 2006]. Gut-derived nitrogenous toxins reach the systemic circulation from the portal venous circulation as a result of impaired liver function and portal-systemic shunting that is present in the setting of portal hypertension. Ammonia is taken up by astrocytes in the brain and combined with glutamate. Glutamine is produced causing astrocyte swelling that, in turn, is thought to lead to the symptomatic presentation of HE. Ammonia alone, however, does not account for the development of HE. Inflammatory mediators are also thought to play a role [Shawcross et al. 2004]. Oxidative stress, endogenous opioids and/or benzodiazepine-like ligands, gamma-aminobutyric acid-like molecules, abnormal histamine and serotonin neurotransmission, and manganese toxicity have also been implicated [Cordoba and Blei, 2007].
Classification and grading
HE is characterized by a spectrum of both neurocognitive and neuromuscular abnormalities. Three categories of HE have been proposed (Table 1) [Ferenci et al. 1998]. Type C is the category associated with cirrhosis and portal hypertension and is the patient group discussed in this manuscript.
Table 1.
Classification of hepatic encephalopathy (HE). Copyright © [2011] Massachusetts Medical Society. All rights reserved.
| Type A: HE associated with acute liver failure |
| Type B: HE associated with portosystemic bypass and no intrinsic liver disease |
| Type C: HE associated with cirrhosis and portal hypertension |
Neurocognitive abnormalities are graded on a clinical basis by the West Haven criteria (also known as the Conn score) (Table 2) [Conn, 1994]. Clinical manifestations range from impaired memory, poor concentration, and subtle changes in personality to lethargy, confusion, gross disorientation and coma. Neuromuscular manifestations include asterixis, slow monotonous speech, loss of fine motor skills and hyperreflexia in milder cases to decorticate and decerebrate posturing in severe cases [Mullen, 2007].
Table 2.
West Haven criteria for grading hepatic encephalopathy. Copyright © [2011] Massachusetts Medical Society. All rights reserved.
| Grade 0 |
| Lack of detectable changes in personality or behavior |
| No asterixis |
| Grade 1 |
| Trivial lack of awareness, shortened attention span, sleep disturbance, and altered mood |
| Asterixis may be present |
| Grade 2 |
| Lethargy, disorientation to time, amnesia of recent events, impaired simple computations, inappropriate behavior, slurred speech |
| Asterixis is present |
| Grade 3 |
| Somnolence, confusion, disorientation to place, bizarre behavior, clonus, nystagmus, positive Babinski sign |
| Asterixis usually absent |
| Grade 4 |
| Coma and lack of verbal, eye, and oral response |
Clinical presentation
HE is generally an acute process that is reversible. The spectrum of clinical symptoms is wide [Conn and Lieberthal, 1979]. Symptoms may be subtle and include poor short-term memory and concentration or mild confusion. Manifestations may be more severe and include slurred speech, personality changes, or gross disorientation, and, in life-threatening situations, coma. Asterixis, a flapping tremor, may be observed on physical examination.
Although chronic liver disease and portal hypertension that develop over many years underlie the predisposition to HE, patients may progress from baseline mentation to coma in a matter of minutes. Patients can also improve remarkably quickly. This is not because liver function changes on a moment-to-moment basis. Rather, HE occurs usually because one or more dynamic precipitating factors increase both the amount and effect of gut-derived nitrogenous toxins such as ammonia. Common precipitating factors include dehydration and electrolyte abnormalities (perhaps exacerbated by diuretic therapy), infection (including spontaneous bacterial peritonitis), upper gastrointestinal bleeding, and psychoactive medications (including narcotics, benzodiazepines, antihistamines and sleeping medications). Identification and correction of possible precipitating factors are critical to the management of acute HE. In addition, attempts to reduce production and absorption of gut-derived ammonia and facilitate its elimination are central to the treatment of acute HE.
Pharmacological treatment of acute hepatic encephalopathy
There are many pharmacological treatment options for acute HE. Strategies include attempts to reduce nitrogen production or absorption from the colon, increase ammonia metabolism, alter neurotransmission, and liver transplantation. Pharmacological therapy that is most commonly administered includes the nonabsorbable dissacharide lactulose and antibiotics.
Lactulose, a nonabsorbable disaccharide, is considered first-line therapy for pharmacological therapy of acute HE. It has been approved for treatment of HE for more than 30 years despite a paucity of strong data supporting its efficacy [Als-Nielsen et al. 2004]. The mechanism of action of lactulose remains poorly understood. Lactulose is not absorbed in the small bowel because people do not have the disaccharidase that metabolizes the sugar. Lactulose then is available for colonic bacteria that can break it down. Postulated mechanisms of activity of lactulose include metabolism by colonic bacteria to fatty acids, with consequent decreased pH in the colon. This results in formation of poorly absorbed ammonium ion from ammonia, and therefore decreases overall absorption of ammonia [Mortensen, 1992]. In addition, lactulose causes changes in the colonic bacterial milieu via its cathartic effect that favorably affect toxin elimination. Lactulose is poorly tolerated because it has an unpleasant sweet taste, causes flatus, abdominal cramps and is dosed to two to three loose bowel movements daily [Fritz et al. 2005]. Not only are many patients noncompliant with the medication, diarrhea is common and actually may lead to dehydration and cause worsened encephalopathy [Bajaj et al. 2010]. Although many patients seem to benefit from lactulose therapy in the acute setting, it has also been observed on an anecdotal basis that patients maintained on lactulose with a history of HE and who thus are at high risk for recurrent HE frequently break through and suffer from recurrent bouts.
Antibiotics have been administered in the treatment of HE, usually employed as second-line therapy. The mechanism is thought to relate to decreased colonic deaminating bacteria that produce nitrogenous compounds (i.e. ammonia) by metabolism of urea. The most common antibiotics used on a historic basis for HE include neomycin and metronidazole. Neomycin is approved as adjuvant therapy in hepatic coma. Metronidazole is not approved for HE. Neomycin is better studied than metronidazole [Strauss et al. 1992; Conn et al. 1977]. There are several controlled trials involving neomycin. Most studies, including those with metronidazole, are small and uncontrolled. Few support the use of either antibiotic for treatment of HE. Furthermore, long-term use of neomycin is limited by ototoxicity and nephrotoxicity. Long-term use of metronidazole is limited by gastrointestinal upset and neurotoxicity.
Rifaximin
Rifaximin is a poorly absorbed, oral antibiotic [Williams and Bass, 2005]. It is derived from rifamycin and has a broad spectrum of activity against Gram-positive and Gram-negative, aerobic and anaerobic, enteric bacteria. It is thought to diminish deaminating enteric bacteria to decrease production of nitrogenous compounds that are subsequently absorbed and cause HE.
Data are available to support the usage of rifaximin in the treatment of acute HE [Sama et al. 2004; Loguercio et al. 2003; Mas et al. 2003; Williams et al. 2000; Bucci and Palmieri, 1993; Massa et al. 1993; Pedretti et al. 1991]. Further, rifaximin is well tolerated. In fact, rifaximin has been approved for treatment of HE in many countries and was granted orphan drug status for the treatment of HE in the USA in 1998.
Reduction in risk of recurrent hepatic encephalopathy
Despite the availability of many therapeutic alternatives for the treatment of acute HE, the aforementioned therapies are limited by poor efficacy and poor patient compliance due to intolerable side effects. Recurrent bouts of HE that require evaluation and hospitalization cause a significant burden to patients, their families and caregivers as well as to the healthcare system. Effective therapy is sought to decrease the incidence of recurrent HE in a high-risk population, that is, patients that have had previous bouts of HE.
The number and length of hospitalizations for acute HE were significantly reduced in patients treated with rifaximin in comparison to lactulose [Leevy and Phillips, 2007; Neff et al. 2006]. Since rifaximin has efficacy in the treatment of acute HE and has a favorable side-effect profile that lends itself to long-term therapy, a trial was undertaken to examine the effect of rifaximin on the reduction of risk of recurrence of HE in a group of patients at high risk.
The efficacy and safety of rifaximin in maintaining remission of HE over 6 months in patients at high risk were assessed within the context of a phase 3, randomized, double-blind, placebo-controlled, multicenter, multinational trial [Bass et al. 2010].
Eligible patients
Patients were eligible for enrollment if they were at least 18 years old and had had at least two episodes of overt HE (Conn score of 2 or more) in the setting of chronic liver disease (cirrhosis or portal hypertension) within 6 months of screening. Patients must have had a Model for End-Stage Liver Disease (MELD) score of 25 or lower. Exclusion criteria included conditions known to provoke HE such as gastrointestinal bleeding, anemia (hemoglobin <8 g/dl), electrolyte abnormalities (sodium, Na < 125 mmol/l), renal insufficiency (creatinine, Cr > 2.0 mg/dl), infection or respiratory insufficiency. A Transjugular Intrahepatic Portosystemic Shunt (TIPS) could be present, but it could not have been placed or revised within the past 3 months. Patients could not be on daily prophylactic antibiotic therapy at the outset. At the time of screening, the patient must have been in remission as defined by a Conn score of 0 or 1.
Objectives
The primary objective was to assess the time to the first breakthrough to overt HE as defined by an increase in the Conn score from 0 or 1 to 2 or more, or an increase in the Conn score from 0 to 1 and an increase in the asterixis score of one grade.
The key secondary objective was to assess the time to the first HE-related hospitalization. Other secondary objectives included time to any increase from baseline in Conn score, time to any increase from baseline in asterixis grade, mean change in baseline in fatigue domain scores on the Chronic Liver Disease Questionnaire (CLDQ) at the end of treatment and mean change from baseline in venous ammonia concentration at the end of treatment.
Methods
Patients were administered rifaximin at a dosage of 550 mg or placebo twice daily for a period of 24 weeks. Patients were withdrawn if a bout of HE occurred. Lactulose usage was not prohibited in the study; rather, it was administered at the discretion of the investigator. Lactulose use was strictly monitored throughout. A total of 299 patients were enrolled; 140 received rifaximin and 159 received placebo. The results were analyzed on an intention to treat basis.
Results
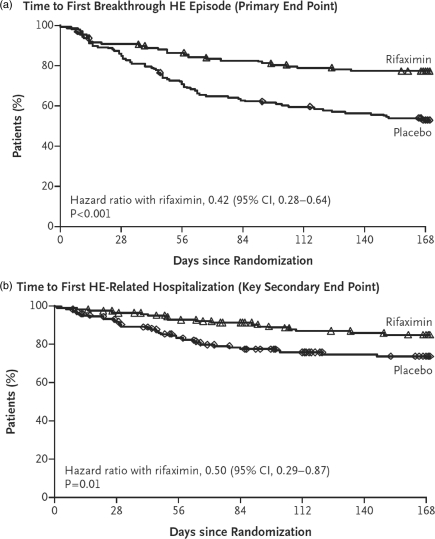
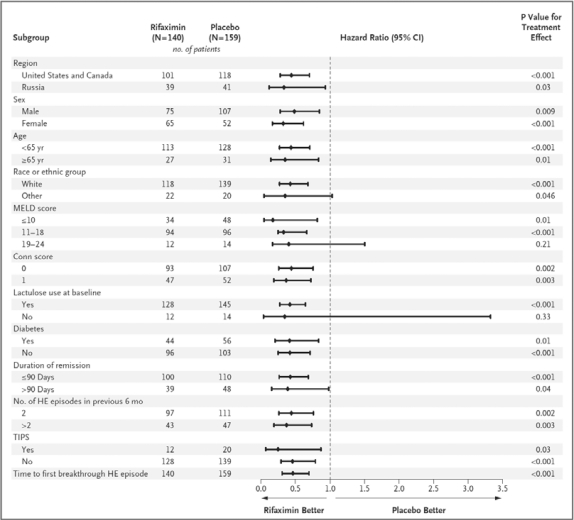
Baseline characteristics were similar in the two groups. Rifaximin treatment yielded a 58% reduction compared with placebo in the risk of breakthrough HE [hazard ratio (HR) 0.421] (see Figure 1) [Bass et al. 2010]. A total of 22% of patients in the treatment group compared with 46% in the placebo group suffered from recurrent HE over the 24-week treatment course. In fact, benefit was derived in all subgroups assessed, including gender, age, race, region, MELD score, initial Conn score, presence or absence of diabetes, duration of current remission at the outset and number of HE episodes prior to enrollment (see Figure 2) [Bass et al. 2010].
Figure 1.
(a) Time to first breakthrough hepatic encephalopathy episode (primary endpoint). (b) Time to first hepatic encephalopathy related hospitalization (key secondary endpoint).
Figure 2.
Subgroup assessment of response (recurrent encephalopathy) comparing rifaximin and placebo groups.
HE-related hospitalizations were reduced by 50% with rifaximin therapy in comparison to placebo (HR 0.500) (Figure 1) [Bass et al. 2010]. A total of 14% of patients in the rifaximin group in comparison to 24% of patients in the placebo group had HE-related hospitalizations. Statistically significant improvements were also observed in Conn score increase from baseline, asterixis grade increase from baseline, fatigue domains as measured by the CLDQ and serum venous ammonia levels in favor of rifaximin.
It is important to note that lactulose was given to 91% of patients in both groups. Furthermore, the amount of lactulose used was statistically equivalent in both groups. Therefore, any differences between the groups could not be attributed to differences in the administration of lactulose.
Tolerability
Adverse events were frequently reported in the trial. This is not surprising because it involved patients with advanced liver disease, many of whom were maintained on lactulose. However, there were no differences in adverse-event profiles in the rifaximin group compared with the placebo group. In addition, there was no indication that Clostridium difficile infection was related to the usage of rifaximin in the study (two cases were reported in the treatment group and zero in the placebo group). In fact, the two patients with C. difficile in the rifaximin group had other risk factors and remained on rifaximin with successful treatment of C. difficile.
There were 20 deaths in the study (nine in the rifaximin group and 11 in the placebo group). None of the deaths were attributed to rifaximin.
Conclusions
Rifaximin at a dosage of 550 mg twice daily was highly effective in diminishing the recurrence of HE and decreasing the rate of HE-related hospitalizations over a 24-week period in a group of patients at high risk for HE.
Discussion
In a randomized, placebo-controlled, multicenter, multinational trial, the vast majority of patients in both the rifaximin and placebo groups were maintained on lactulose and usage was monitored closely. In patients at high risk of HE recurrence, nearly 50% who were maintained on lactulose and placebo had recurrent HE and nearly 25% had HE-related hospitalization, showing the substantial failure rate of lactulose in preventing recurrent HE in this population. However, rifaximin not only significantly reduced recurrent HE and hospitalizations secondary to HE, it was also effective when administered with lactulose and was well tolerated. This led to rifaximin being approved for prevention of recurrent HE by the US Food and Drug Administration in March 2010.
This trial confirms that rifaximin is indicated in all patients who have suffered from recurrent acute HE to decrease the risk of recurrence. Rifaximin was not studied in patients who had only had a single bout of acute HE. However, given the high failure rate of lactulose and the significant success rate of rifaximin in preventing recurrent HE in patients who had had multiple bouts of HE, it is reasonable to consider rifaximin for patients after a single bout of acute HE.
The efficacy of rifaximin in patients with highly advanced liver disease (MELD score >25) was not established in this trial. Future studies in this population are awaited. In addition, the results of long-term studies to verify the long-term efficacy and safety of rifaximin beyond 24 weeks are also awaited.
Summary
Overt HE is a common, reversible process observed in patients with cirrhosis and characterized by neurocognitive and neuromuscular symptoms. It is a distressing problem with poor treatment options. The standard of care has been lactulose, which is poorly tolerated and frequently ineffective in the prophylactic setting. Rifaximin, a minimally absorbed oral antibiotic, was shown within the context of a randomized, placebo-controlled trial to significantly reduce the risk of breakthrough HE and hospitalization related to HE over a 6-month period in patients with a history of recurrent HE. It works in conjunction with lactulose and is well tolerated. Therefore, rifaximin should be administered in patients with a history of recurrent HE to decrease the risk of recurrence.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest statement
Speakers bureau and consultant for Salix Pharmaceuticals, Inc.
References
- Als-Nielsen B., Gluud L.L., Gluud C. (2004) Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomized trials. BMJ 328: 1046–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj J.S., Sanyal A.J., Bell D., Gilles H., Heuman D.M. (2010) Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Ailment Pharmacol Ther 31: 1012–1017 [DOI] [PubMed] [Google Scholar]
- Bass N.M., Mullen K.D., Sanyal A., Poordad F., Neff G., Leevy C.B., et al. (2010) Rifaximin treatment in hepatic encephalopathy. N Engl J Med 362: 1071–1081 [DOI] [PubMed] [Google Scholar]
- Bucci L., Palmieri G.C. (1993) Double-blind, double-dummy comparison between treatment with rifaximin and lactulose in patients with medium to severe degree hepatic encephalopathy. Curr Med Res Opin 13: 109–118 [DOI] [PubMed] [Google Scholar]
- Conn H.O. (1994) Hepatic encephalopathy: Syndromes and therapies. In: Conn H.O., Bircher J. (eds). Quantifying the severity of hepatic encephalopathy, Medi-ed Press: Bloomington, IL, 373–383 [Google Scholar]
- Conn H.O., Leevy C.M., Vlahcevic Z.R., Rodgers J.B., Maddrey W.C., Seef L., et al. (1977) Comparison of lactulose and neomycin in the treatment of chronic portal systemic encephalopathy. A double blind controlled trial. Gastroenterology 72: 573–583 [PubMed] [Google Scholar]
- Conn H.O., Lieberthal M.M. (1979) The Hepatic Coma Syndromes and Lactulose, Williams & Wilkins: Baltimore, MD [Google Scholar]
- Cordoba J., Blei A. (2007) Diseases of liver. In: Schiff E.R., Sorell M.F., Maddrey W.C. (eds). Hepatic Encephalopathy, Lippincott Williams and Wilkins: Philadelphia, PA, 569–599 [Google Scholar]
- Ferenci P., Lockwood A., Mullen K., Tarter R., Weissenborn K., Blei A.T. (1998) Hepatic encephalopathy—definition, nomenclature, diagnosis, and quanitification: final report of the working party at the 11th World Congress of Gastroenterology, Vienna, 1998. Hepatology 35: 716–721 [DOI] [PubMed] [Google Scholar]
- Fritz E., Hammer H.F., Lipp R.W., Hogenauer C., Stauber R., Hammer J. (2005) Effects of lactulose and polyethylene glycol on colonic transit. Ailment Pharmacol Ther 21: 259–268 [DOI] [PubMed] [Google Scholar]
- Keiding S., Sorenson M., Bender D., Munk O.L., Ott P., Vilstrup H. (2006) Brain metabolism of 13N-ammonia during acute hepatic encephalopathy in cirrhosis measured by position emission tomography. Hepatology 43: 42–50 [DOI] [PubMed] [Google Scholar]
- Leevy C.B., Phillips J.A. (2007) Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci 52: 737–741 [DOI] [PubMed] [Google Scholar]
- Loguercio C., Federico A., De Girolamo V., Ferrieri A., Del Vecchio Blanco C. (2003) Cyclic treatment of chronic hepatic encephalopathy with rifaximin. Results of a double-blind clinical study. Minerva Gastroenterol Dietol 49: 53–62 [PubMed] [Google Scholar]
- Mas A., Rodes J., Sunyer L., Rodrigo L., Planas R., Vargas V., et al. (2003) Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: Results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol 38: 51–58 [DOI] [PubMed] [Google Scholar]
- Massa P., Vallerino E., Dodero M. (1993) Treatment of hepatic encephalopathy with rifaximin: double-blind, double dummy study versus lactulose. Eur J Clin Res 4: 7–18 [Google Scholar]
- Mortensen P.B. (1992) The effect of oral-administered lactulose on colonic nitrogen metabolism and excretion. Hepatology 16: 1350–1356 [DOI] [PubMed] [Google Scholar]
- Mullen K.D. (2007) Pathogenesis, clinical manifestation, and diagnosis of hepatic encephalopathy. Semin Liver Dis 27(Suppl. 2): 10–17 [Google Scholar]
- Neff G.W., Kemmer N., Zacharias V.C., Kaiser T., Duncan C., McHenry R., et al. (2006) Analysis of hospitalizations comparing rifaximin versus lactulose in the management of hepatic encephalopathy. Transplant Proc 38: 3552–3555 [DOI] [PubMed] [Google Scholar]
- Pedretti G., Calzetti C., Missale G., Fiaccadori F. (1991) Rifaximin versus neomycin on hyperammoniemia in chronic portal systemic encephalopathy of cirrhotics. A double-blind, randomized trial. Ital J Gastroenterol 23: 175–178 [PubMed] [Google Scholar]
- Poordad F.F. (2007) Review article: The burden of hepatic encephalopathy. Ailment Pharmacol Ther 25: 3–9 [DOI] [PubMed] [Google Scholar]
- Sama C., Morselli-Labate A.M., Pianta P., Lambertini L., Berardi S., Martini G. (2004) Clinical effects of rifaximin in patients with hepatic encephalopathy intolerant or nonresponsive to previous lactulose treatment: An open labeled study. Curr Ther Res 65: 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawcross D.L., Davies N.A., Williams R., Jalan R. (2004) Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol 40: 247–254 [DOI] [PubMed] [Google Scholar]
- Strauss E., Tramote R., Silva E.P., Caly W.R., Honain N.Z., Maffei R.A., et al. (1992) Double-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathy. Hepatogastroenterology 39: 542–545 [PubMed] [Google Scholar]
- Williams R., Bass N. (2005) Rifaximin, a nonabsorbed oral antibiotic, in the treatment of hepatic encephalopathy: Antimicrobial activity, efficacy, and safety. Rev Gastroenterol Disord 5(Suppl): 10–18 [PubMed] [Google Scholar]
- Williams R., James O.F., Warnes T.W., Morgan M.Y. (2000) Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: A double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol 12: 203–208 [DOI] [PubMed] [Google Scholar]