Abstract
Background: Epidemiologic data suggest that colonic adenomas have an increased tendency to occur in patients who are obese, African American, or have a positive family history of colon cancer, or diabetes mellitus. Recent data suggest that impaired glucose tolerance, dyslipidemia, and metabolic syndrome are associated with a higher risk for colonic adenomas. Patients with nonalcoholic fatty liver disease (NAFLD) often share several of the aforementioned risk factors for colonic adenomas. However, data are lacking about the relationship between NAFLD and colonic adenomas. The aim of this study was to systematically evaluate whether NAFLD is an independent risk factor for colonic adenomas.
Methods: We performed a retrospective cohort observational study on 233 patients who underwent screening colonoscopies at Brooke Army Medical Center from November 2007 to March 2010 to assess for the association between NAFLD and colonic adenomas. Patients who had previously been found to have biopsy-proven simple steatosis (n = 65) or nonalcoholic steatohepatitis (NASH) (n = 29) were compared with a control group without fatty liver disease on sonographic imaging (n = 139). Patients were stratified based on gender, race, body mass index (BMI), and family history and adjusted for variables previously known to be associated with increased adenoma risk.
Results: The mean age was 54.7 ± 6.0 years (48.5% women). Racial demographics were: 62.7% White, 18.5% Hispanic, 13.7%, African American, and 5.2% other. The mean BMI was 29.7 ± 5.8. The prevalence of colonic adenomas was 25.1% in the control group and 24.4% in the NAFLD group to include simple steatosis and NASH (p = 1.00). Furthermore, when adjusting for known confounders to include race, BMI, and family history no significant differences were found (p = 0.33). However, the ultrasound-negative patients ranked lower in the number of adenomas per person (p = 0.016).
Conclusions: There was no difference in the prevalence of colonic adenomas when comparing the NAFLD group who had undergone colonoscopy with a group of control patients without NAFLD who had undergone colonoscopy. However, patients with negative ultrasounds appeared to have a lower polyp burden.
Keywords: colonic adenomas, metabolic syndrome, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis
Introduction
Colon cancer remains one of the most common causes of cancer worldwide and the second most common cause of cancer death in the United States [Labianca et al. 2010; Nelson and Thorson, 2009]. One million new cases are diagnosed each year [Boyle and Leon, 2002]. Although increased age, black race, smoking and low fiber diets are well established risk factors, data suggest that obesity, insulin resistance and metabolic syndrome are potential risk factors as well [Reilly and Rader, 2003]. Metabolic syndrome – to include at least three of the following factors: increased waist circumference, hypertriglyceridemia (triglycerides ≥150 mg/dl), low high-density lipoprotein (HDL) cholesterol (≤50 mg/dl in women and ≤40 mg/dl in men), hypertension (130/≥85 mmHg), and elevated fasting glucose – is a sequelae of conditions that has plagued the Western world. Its incidence is increasing in populations that are obese and overweight. In the United States, metabolic syndrome affects nearly 25% of people over the age of 20 and close to 45% of persons over the age of 50 [Giovannucci, 2007].
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome and the main cause for elevated liver enzymes [Rubinstein et al. 2008]. The prevalence of NAFLD ranges from 20% to 46% in the Western world [Williams et al. 2011; Patel et al. 2009; Powell et al. 2005]. Many studies have correlated metabolic syndrome with increased risk for colon cancer or precancerous polyps [Larsson and Wolk, 2007; Ahmed et al. 2006]. Kim and colleagues [Kim et al. 2007] found a prevalence of colonic adenomas to be 17% in patients with metabolic syndrome compared with 11% in the control population without metabolic syndrome. However, there is a paucity of literature analyzing NAFLD and its relationship to colorectal neoplasms. Knowing that metabolic syndrome alone is a risk factor for adenomatous colon polyps, this investigation aimed to determine if NAFLD is an independent risk factor for colorectal adenomatous polyps.
Patients and methods
Study participants
We performed a retrospective cohort observational study on patients who underwent screening colonoscopies at Brooke Army Medical Center (BAMC) from November 2007 to March 2010. Study participants were previously enrolled in a recently published NAFLD prevalence study [Williams et al. 2011]. Of the 400 patients enrolled, 233 had colonoscopies within the study dates. Patients were included only if they had undergone a colonoscopy at our facility within the study dates, completed a right upper quadrant ultrasound to evaluate for fatty liver disease, and completed a subsequent liver biopsy to exclude nonalcoholic steatohepatitis (NASH) if fatty liver was found to be present on ultrasound.
Measurements
The body mass index (BMI) for all patients was calculated in kg/m2. Hepatic ultrasonography scanning (ATL HDI 5000, Philips Medical Systems with a C4-2 MHz curved array transducer; iU22 Ultrasound system, Philips Medical System with a C5-1 MHz curved array transducer (Andover, MA, USA); and the Acuson S2000 Siemens Ultrasound System (Malvern, PA, USA)) was performed on all participants by one of five sonographers with 5–22 years of experience. Fatty liver was diagnosed if there was significantly increased echogenicity relative to the renal parenchyma, the ultrasound beam was attenuated with the diaphragm indistinct, or the echogenic walls of the portal veins were less visible. A single expert hepatopathologist reviewed all liver biopsies and utilized the scoring system established by Brunt and colleagues [Brunt et al. 1999] for grading and staging of steatohepatitis if present.
Colonoscopy
Each patient underwent colonoscopy after completing a bowel preparation with 4 L polyethelyne glycol lavage solution. Colonoscopies were performed by one of seven staff gastroenterologists at BAMC. Each colonoscopy report was examined for the presence of polyps, polyp location, size (<5 mm, 5–10 mm, or >10 mm), number, attachment (flat, sessile, or pedunculated), and histology. The location of polyps was divided into ascending, hepatic flexure, transverse, splenic flexure, descending, sigmoid, and rectal. Family history of adenomas was also recorded.
Statistical analysis
All calculations were performed with the use of Windows SPSS 16.0 statistical software (Chicago, Illinois, USA). The statistical results are presented as the mean ± standard deviation or percentages. The statistical analyses included one-way analysis of variance (ANOVA), Student’s t-test, Pearson Chi-square test, Kruskal–Wallis ANOVA, and Mann–Whitney rank sum. The Spearman rank test was used to assess the correlation between NAFLD and colonic adenomas after adjusting for independent variables previously known to be associated with increased colon cancer risk, such as age, race, BMI, and family history. Each odds ratio (OR) is presented with its 95% confidence interval (CI). A value of p < 0.05 was considered statistically significant. The study was approved by the Institutional Review Board of BAMC, and all participants provided permission for their medical information to be used for research.
Results
Of the 233 patients with colonoscopies included in this study, 139 (59.6%) did not have fatty liver on ultrasound and served as the control group while 94 (40.3%) had positive ultrasounds for fatty liver. Of the patients with positive ultrasounds, 65 (69.1%) were biopsy proven simple steatosis and 29 (30.9%) had NASH [Stage 0–1 (n = 22), Stage 2–4 (n = 7)]. Patients were stratified based on gender, race, BMI and family history, and adjusted for variables previously known to be associated with increased adenoma risk. The mean age was 54.7 ± 6.0 years, 48.5% female, mean BMI 29.7 ± 5.8. The study population included 62.7% Whites, 18.5% Hispanics, 13.7% African Americans, and 5.2% other. Of all the ethnic groups, the highest percentage of NAFLD existed among Hispanics (58.2%). Compared with the control group, those with NAFLD had a significantly higher BMI (p < 0.001) and were more likely to have metabolic syndrome (p < 0.001). Baseline characteristics of the study participants are summarized in Table 1.
Table 1.
Baseline characteristic of patients taking part in study.
| Variables | Ultrasound (−) (N = 139) | Ultrasound (+) |
p value | |
|---|---|---|---|---|
| Non-NASH (N = 65) | NASH (N = 29) | |||
| Women | 78 (56.1%) | 25 (38.5%) | 10 (20.9%) | <0.017 |
| Age (mean years) | 54.4 ± 5.83 | 55.5 ± 6.15 | 54.7 ± 6.04 | <0.445 |
| Body mass index (mean kg/m2) | 27.5 ± 4.90 | 32.0 ± 5.25 | 34.9 ± 5.86 | <0.000 |
| Ethnicity | ||||
| African American (n = 32) | 18 (12.9%) | 9 (13.8%) | 5 (17.2%) | <0.040 |
| White (n = 146) | 94 (67.6%) | 40 (61.5%) | 12 (41.4%) | |
| Hispanic (n = 43) | 18 (12.9%) | 14 (21.5%) | 11 (37.9%) | |
| Other (n = 12) | 9 (6.5%) | 2 (3.1%) | 1 (3.4%) | |
| Diabetes (n = 35) | 9 (6.5%) | 19 (29.2%) | 7 (24.1%) | <0.001 |
| Hypertension (n = 109) | 45 (32.3%) | 42 (64.6%) | 22 (75.8%) | <0.001 |
| Low high-density lipoprotein (n = 49) | 15 (10.8%) | 20 (30.8%) | 14 (48.3%) | <0.001 |
| Hypertriglyceridemia (n = 80) | 20 (14.4%) | 41 (63.1%) | 19 (65.5%) | <0.001 |
| Metabolic syndrome (n = 40) | 4(2.9%) | 25 (38.5%) | 11 (37.9%) | <0.001 |
NASH, nonalcoholic steatohepatitis.
Among the 233 patients included in the study, 58 had adenomatous polyps (24.8%) on colonoscopy. Of these, 35 were in the control group, and 23 were in the ultrasound-positive group to include simple steatosis and NASH. The prevalence of colonic adenomas was 25.1% in the control group (35 of the 139 patients) and 24.4% (23 of the 94 patients) in the NAFLD group. The p value between the two groups was not statistically significant (p = 1.00) (Table 2). No significant differences were found when adjusting for known confounders to include ethnicity, age, BMI, and family history (p = 0.33).
Table 2.
Prevalence of colonic adenomas between patients with and without nonalcoholic steatohepatitis.
| Variables | Ultrasound (–) (N = 139) | Ultrasound (+) |
p value | |
|---|---|---|---|---|
| Non-NASH (N = 65) | NASH (N = 29) | |||
| Number of patients with adenomas (n = 58) | 35 (60.3%) | 13 (22.4%) | 10 (17.2%) | |
| Prevalence | 25.1% | 24.4% | 1.00 | |
NASH, nonalcoholic steatohepatitis.
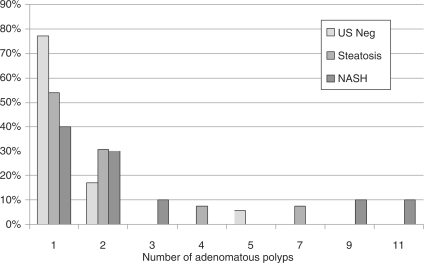
However, there were significantly more adenomas within the NAFLD group when adenoma mean ranks from the control group were compared with those of the NAFLD group (p = 0.016) (Figure 1).
Figure 1.
Number of adenomatous polyps with respect to ultrasound (US) findings in three groups. NASH, nonalcoholic steatohepatitis.
When analyzing adenoma size, most adenomas were less than 5 mm across all three groups. Adenomas were distributed equally between right (ascending, hepatic flexure, transverse, and splenic flexure) and left (descending, sigmoid, and rectal) sides of the colon. Most adenomas were either flat or sessile between groups. The majority of adenomas were tubular adenomas. Two patients in the ultrasound-negative group demonstrated tubulovillous histology. This is summarized in Table 3 and reported as the number of patients with each given adenoma characteristic.
Table 3.
Adenoma characteristics in sample patient population.
| Variables | Ultrasound (–) (N = 139) | Ultrasound (+) |
|
|---|---|---|---|
| Non-NASH (N = 65) | NASH (N = 29) | ||
| *Adenoma size | |||
| (≤5 mm) | 52 (37.4) | 21 (32.3) | 12 (41.3) |
| (5–10 mm) | 14 (10.0) | 5 (7.6) | 2 (6.8) |
| (>10 mm) | 1 (0.70) | 0 | 0 |
| *Adenoma location | |||
| Right | 36 (25.8) | 13 (0.2) | 12 (41.3) |
| Left | 39 (28) | 11 (16.9) | 10 (34.4) |
| *Adenoma attachment | |||
| Flat | 35 (25.1) | 11 (16.9) | 9 (31.0) |
| Sessile | 31 (22.3) | 14 (21.5) | 5 (17.2) |
| Pedunculated | 6 (4.3) | 5 (7.6) | 1 (3.4) |
Reported as number of patients (%) not individual polyps.
NASH, nonalcoholic steatohepatitis.
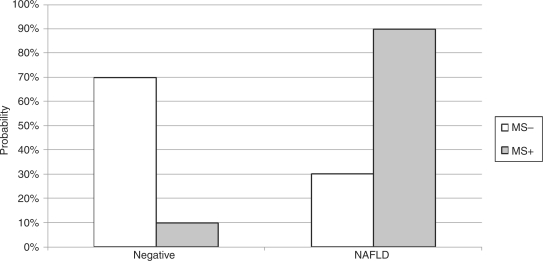
Diabetes was identified in 35 of 233 patients (15.0%) and hypertension in 109 of 233 patients (46.8%). Low HDL and hypertriglyceridemia were seen in 49 of 233 (21.0%) and 80 of 233 patients (34.3%) respectively. Metabolic syndrome was present in 40 of 233 patients (17.1%). Individual components of metabolic syndrome to include impaired fasting glucose, low HDL, hypertension, and hypertriglyceridemia were associated with increased risk of fatty liver disease, as illustrated in Table 1. Patients with metabolic syndrome had a 90% risk of also having NAFLD (Figure 2). However, there was no significant increase in the risk for colonic adenomas when comparing individual components for metabolic syndrome and metabolic syndrome alone (Table 4). Waist circumference was not obtained in our previously referenced study and therefore was not utilized in our analysis [Williams et al. 2011].
Figure 2.
Percentage of patients with nonalcoholic fatty liver disease (NAFLD) with metabolic syndrome (MS).
Table 4.
Risk of adenomatous polyp by individual factors of metabolic syndrome.
| Individual metabolic syndrome components* | Adenoma presence |
p value | |
|---|---|---|---|
| No | Yes | ||
| Diabetes | |||
| No | 149 | 49 (24.7%) | <1.000 |
| Yes | 26 | 9 (25.7%) | |
| Hypertension | |||
| No | 94 | 30 (24.2%) | <0.911 |
| Yes | 81 | 28 (25.7%) | |
| Low high-density lipoprotein | |||
| No | 137 | 47 (25.5%) | <0.795 |
| Yes | 38 | 11 (22.4%) | |
| Hypertriglyceridemia | |||
| No | 112 | 41 (26.8%) | <0.441 |
| Yes | 63 | 17 (21.2%) | |
| Metabolic syndrome | |||
| No | 145 | 48 (24.9%) | <1.000 |
| Yes | 30 | 10 (25.0) | |
Waist circumference not obtained.
Overall, this study confirmed an overwhelming relationship between fatty liver disease and individual components of metabolic syndrome and metabolic syndrome alone. Although an increased risk for colonic adenomas was not found in patients with NAFLD or metabolic syndrome, a higher burden of adenomas was reflected in patients with NAFLD.
Discussion
This is the first study to evaluate the relationship between biopsy-confirmed fatty liver disease and colonic adenomas. Hwang and colleagues conducted a large population-based study in Korea comparing the relationship between ultrasound-diagnosed NAFLD and colorectal adenomas. They found a direct association with NAFLD and colonic adenomas and in particular, an increase in adenoma polyp numbers in patients with fatty liver disease [Hwang et al. 2010]. Our study found similar associations with the addition of biopsy proven fatty liver disease. The results of our study show that patients with NAFLD to include simple steatosis and NASH do not have a higher prevalence of precancerous polyps but rather have an increase in overall number of adenomatous polyps. Currently, patients with more than three adenomas of any size are subject to colonoscopic screening every 3 years [Rex et al. 2009]. Thus, patients with NAFLD may need more stringent endoscopic follow up and this may have implications for current screening guidelines.
NAFLD is commonly referred to as the hepatic manifestation of metabolic syndrome and patients with metabolic syndrome have been shown to have higher risks of colorectal cancer, as illustrated by several studies [Colangelo et al. 2002; Trevisan et al. 2001]. The mechanism that joins the two entities is most likely linked to insulin resistance [Pais et al. 2009]. Perhaps the factors that lead to metabolic syndrome also play a distinct role in carcinogenesis.
Adiponectin is an adipokine that is found in decreased concentrations in those who are obese and have diabetes or insulin resistance. Decreased adiponectin leads to increased insulin levels due to marked insulin resistance and in turn increases insulin growth factor-1 (IGF-1) [Kaaks et al. 2000]. Adiponectin also directly inhibits tumor necrosis factor alpha (TNF-α), which plays a role in tumor cell proliferation and angiogenesis [Ferroni et al. 2007; Rose et al. 2004]. In a study by Ferroni and colleagues [Ferroni et al. 2007], low adiponectin levels were inversely related to colonic tumor stage and independently predicted cancer recurrence [Stattin et al. 2004]. Insulin binds to IGF-1 receptors and plays an important role in cell proliferation, apoptosis and increased production of vascular endothelial growth factor, an angiogenic factor that supports cancer growth [Grimberg and Cohen, 2000; Warren et al. 1996].
Inflammatory cytokines may also play a role in colorectal carcinogenesis and are also related to metabolic syndrome in that adipocytes secrete various cytokines such as TNF-α, interleukin-6 (IL-6), IL-8 and IL-10 [Cowey and Hardy, 2006; Trayhurn and Wood, 2004]. It is postulated that groups of adipocytes become hypoxic and secrete cytokines to stimulate angiogenesis into the adipose cells and these cytokines in turn promote insulin resistance and increase circulating triglycerides. This proinflammatory state influences growth, apoptosis, and proliferation of tumor cells in many cancers, including colorectal carcinoma [Shoelson et al. 2006; Pittas et al. 2004; Sonnenberg et al. 2004]. Although more studies are needed to discern the exact mechanisms for increased risk of colorectal adenomas in patients with metabolic syndrome, an association is clinically recognizable.
Our study has several limitations. The population size was too small to accurately reflect some known risk factors to be associated with adenomas, such as family history and ethnicity. The retrospective design made it difficult to conclude causality between NAFLD and the risk of colonic adenomas in the setting of independent confounders. Previously published data suggest that independent components of metabolic syndrome are associated with increased adenoma risk [Liu et al. 2010; Pittas et al. 2004], however our study did not show this association. Our study population was also skewed in age in that most of the study participants were over the age of 50. Perhaps if we offered colonoscopy to a younger NAFLD patient group we may have uncovered different relationships.
In conclusion, our study is the first to show an association with biopsy-proven NAFLD and the burden of colonic adenomas. Although the prevalence of adenomas was not increased in either group, we showed that there was a potentially increased polyp burden in patients with fatty liver disease compared with the ultrasound-negative group. Additional prospective studies are needed to better define a true association between NAFLD and adenomatous colon polyps. However, data still suggest that metabolic syndrome and its components are associated with increased risk for colonic neoplasm. This relationship has an important impact on society and further emphasizes the importance of a healthy lifestyle to prevent and treat metabolic syndrome and its hepatic manifestation, NAFLD.
What is already known
Metabolic syndrome has been associated with increased risk of cancer overall.
NAFLD is the hepatic manifestation of metabolic syndrome.
Colon cancer is the second most common cause of cancer death in USA.
What is new here
Patients with NAFLD do not appear to be at increased risk for colonic adenomas.
No single component of metabolic syndrome increases the risk for colonic adenomas.
NAFLD appears to be associated with a higher polyp burden than in patients without NAFLD.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the US Department of the Army, Air Force or the US Department of Defense.
References
- Ahmed R.L., Shmitz K.H., Anderson K.E., Rosamond W.D., Folsom A.R. (2006) The metabolic syndrome and risk of incident colorectal cancer. Cancer 107: 28–36 [DOI] [PubMed] [Google Scholar]
- Boyle P., Leon M.E. (2002) Epidemiology of colorectal cancer. Br Med Bull 64: 1–25 [DOI] [PubMed] [Google Scholar]
- Brunt E.M., Janney C.G., DiBisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. (1999) Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am J Gastroenterol 94: 2467–2474 [DOI] [PubMed] [Google Scholar]
- Colangelo L.A., Gapstur S.M., Gann P.H., Dyer A.R., Liu K. (2002) Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev 11: 385–391 [PubMed] [Google Scholar]
- Cowey S., Hardy R. (2006) Metabolic syndrome, a high risk state for cancer? Am J Pathol 169: 1505–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferroni P., Palmirotta R., Spila A., Martini F., Raparelli V., Fossile E., et al. (2007) Prognostic significance of adiponectin levels in non-metastatic colorectal cancer. Anticancer Res 27: 483–489 [PubMed] [Google Scholar]
- Giovannucci E. (2007) Metabolic syndrome, hyperinsulinemia, and colon cancer: A review. Am J Clin Nutr 86: s836–s842 [DOI] [PubMed] [Google Scholar]
- Grimberg A., Cohen P. (2000) Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol 183: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.T., Cho Y.K., Park J.H. (2010) Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J Gastroenterol Hepatol 25: 562–567 [DOI] [PubMed] [Google Scholar]
- Kaaks R., Toniolo P., Akhmedkhanov A. (2000) Serum C-peptide, insulin growth factor (IGF-1), IGF-binding proteins and colorectal cancer risk in women. J Natl Cancer Inst 92: 1592–1600 [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lim Y.J., Kim Y.H. (2007) Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev 16: 1543–1546 [DOI] [PubMed] [Google Scholar]
- Labianca R., Beretta G.D., Kildani B. (2010) Colon cancer. Crit Rev Oncol Hematol 74: 106–133 [DOI] [PubMed] [Google Scholar]
- Larsson S.C., Wolk A. (2007) Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am J Clin Nutr 86: 556–565 [DOI] [PubMed] [Google Scholar]
- Liu C.S., Hsu H.S., Li C.I. (2010) Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol 10: 51–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R.S., Thorson A.G. (2009) Colorectal cancer screening. Curr Oncol Rep 1: 482–489 [DOI] [PubMed] [Google Scholar]
- Pais R., Silaghi H., Silaghi A.C., Rusu M.L., Dumitrascu D.L. (2009) Metabolic syndrome and the risk of subsequent colorectal cancer. World J Gastroenterol 15: 5141–5148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.A., Torres D.M., Harrison S.A. (2009) Effect of weight loss on nonalcoholic fatty liver disease. J Clin Gastroenterol 43: 970–974 [DOI] [PubMed] [Google Scholar]
- Pittas A.G., Joseph N.A., Greenberg A.S. (2004) Adipocytokines and insulin resistance. J Clin Endocrinol Metab 89: 447–452 [DOI] [PubMed] [Google Scholar]
- Powell E.E., Jonsson C.R., Clouston A.D., Powell E.E., Jonsson J.R., Clouston A.D. (2005) Dangerous liaisons: The metabolic syndrome and nonalcoholic fatty liver disease. Ann Intern Med 143: 753–754 [DOI] [PubMed] [Google Scholar]
- Reilly M.P., Rader D.J. (2003) The metabolic syndrome: more than the sum of its parts? Circulation 108: 1546–1551 [DOI] [PubMed] [Google Scholar]
- Rex D.K., Johnson D.A., Anderson J.C. (2009) American College of Gastroenterology guidelines on colorectal screening. Am J Gastroenterol 104: 739–750 [DOI] [PubMed] [Google Scholar]
- Rose D.P., Komninou D., Stephenson G.D. (2004) Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev 5: 153–165 [DOI] [PubMed] [Google Scholar]
- Rubinstein E., Lavine J.E., Schwimmer J.B. (2008) Hepatic, cardiovascular, and endocrine outcomes of the histologic subphenotype of nonalcoholic fatty liver disease. Sem Liv Dis 28: 380–385 [DOI] [PubMed] [Google Scholar]
- Shoelson S.E., Lee J., Goldfine A.B. (2006) Inflammation and insulin resistance. J Clin Invest 116: 1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg G.E., Krakower G.R., Kissebah A.H. (2004) A novel pathway to the manifestations of metabolic syndrome. Obes Res 12: 180–186 [DOI] [PubMed] [Google Scholar]
- Stattin P., Lukanova A., Biessy C. (2004) Obesity and colon cancer: Does leptin provide a link? Int J Cancer 109: 149–152 [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Wood I.S. (2004) Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92: 347–355 [DOI] [PubMed] [Google Scholar]
- Trevisan M., Liu J., Muti P., Misciagna G., Menottim A., Fucci F., et al. (2001) Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev 10: 937–941 [PubMed] [Google Scholar]
- Warren R.S., Yuan H., Matli M.R., Ferrara N., Donner D.B. (1996) Induction of vascular endothelial growth factor by insulin growth factor 1 in colorectal carcinoma. J Biol Chem 271: 29483–29488 [DOI] [PubMed] [Google Scholar]
- Williams C.D., Stengel J., Asike M.I., Torres D.M., Shaw J., Contreras M., et al. (2011) Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 140: 124–131 [DOI] [PubMed] [Google Scholar]