Abstract
Neurocysticercosis (NCC) is the most common cause of acquired epilepsy in developing countries. It can present variably depending on the location and stage of cysts in the nervous system, and the host immune response. The most common presentation of parenchymal NCC is with seizures that are usually focal and brief; status epilepticus occurs in some cases. About a third of cases have headache and vomiting. Diagnosis is made by either CT or MRI. Single, small, contrast enhancing lesions are the most common; visualization of a scolex is diagnostic. Some cases have multiple cysts with a characteristic starry-sky appearance. Although treatment with cysticidal therapy continues to be debated, there is increasing evidence that it helps through increased and faster resolution of CT lesions; whether there is any improvement in long-term seizure control needs further study. It should not be used in cysticercus encephalitis or in ophthalmic NCC and used with caution in extraparenchymal NCC. It is of no use in calcified lesions. Corticosteroids are used simultaneously to reduce cerebral oedema. Seizures respond well to a single antiepileptic, and the seizure recurrence rate is low in cases with single lesions; those with multiple, persistent or calcified lesions usually have recurrent seizures. Extraparenchymal NCC is often associated with intracranial hypertension, hydrocephalous and chronic meningitis; it has a guarded prognosis; surgical intervention is required in many cases. Management of NCC needs to be individualized. NCC is potentially eradicable; proper sanitation, hygiene and animal husbandry are warranted.
Keywords: cysticidal therapy, epilepsy, neurocysticercosis
Introduction
Neurocysticercosis (NCC) is one of the oldest known and perhaps the most common parasitic infections of the human nervous system. It is a major cause of epilepsy and neurological disease in many developing countries [White, 1997; Commission on Tropical Disease of the International League Against Epilepsy, 1994]. The World Health Organization (WHO) has estimated that NCC accounts for over 50,000 deaths per year and for active epilepsy in many times this number of people [Román et al. 2000].
It is endemic in Latin America, South East Asia, India, Nepal, China, Africa and many other developing countries. With increasing globalization and international travel, NCC is now being reported from many developed countries such as the USA, UK and many European countries.
Aetiology
NCC is caused by infection of the human central nervous system (CNS) with encysted larvae of the tapeworm Taenia solium. Humans are the definite hosts and acquire intestinal T. solium (taeniasis) from pigs by ingestion of undercooked pork infected with cysticerci. These cysticerci release larvae that develop into adult tapeworms that mature in the intestine, and shed thousands of extremely contagious eggs in the faeces. NCC is acquired by eating food accidently contaminated with these eggs, either through already contaminated food or through transmission from tapeworms carriers in the household [Garcia et al. 2003].
The eggs hatch in the human intestine and release larvae that penetrate the intestinal mucosa, and migrate throughout the body to produce human cysticercosis. The cysts have a predilection to lodge in areas where the blood flow is high. Most mature cysts are found in the CNS, skeletal muscle, subcutaneous tissue, and the eyes, although they may be found in any human tissue.
The life cycle of the parasite is completed when pigs ingest parasitic eggs passed in human faeces, by coprophagia or by grazing on contaminated soil. The eggs hatch in the intestine into larvae that invade the mucosa, to reach various tissues, where they mature into cysticerci.
Pathogenesis
Most cysticerci lodge in the brain parenchyma (parenchymal NCC) particularly at the grey–white cortical junction. In general, cysticerci live asymptomatically within host tissues for prolonged periods because of various protective mechanisms. Studies on murine models using the cestode Mesocestoides corti suggest that there is a rapid release of certain glycoconjugates (GCs) from the tegument of the parasite when it penetrates into the CNS. The tegument is partially lost and there is a temporary reduction in the rate of tegument build up, and thus less antigenic exposure in the early stages of parasite invasion. In addition, there is a continuous release of the parasite GCs during the course of NCC which leads to a suppressive and immunoregulatory environment that protects the parasite from damaging inflammatory responses. Death of the parasite ends this balance and an adverse inflammatory reaction ensues [Alvarez et al. 2008]. Immunomodulation through the production of alternately activated macrophages (AAMs) that lead to the production of downregulatory cytokines and activation of the alternative arginaze 1 pathway has also been suggested as an immunoprotective mechanism of the parasite [Rodriguez-Sosa et al. 2002].
Although animal models are not an accurate replica of natural infection in human beings, certain features in these models correlate with some studies performed in humans infected with T. solium, and these models do provide some insight into the pathophysiology of NCC [Mahanty and Garcia, 2010].
The immune response in symptomatic human NCC is thought to be of the Th1 phenotype predominantly, which is responsible for severe neuropathology including the formation of granulomas [White et al. 1997]. Toll-like receptors (TLRs), particularly TLR 4 polymorphisms, have been reported to be significantly associated with symptomatic NCC [Verma et al. 2010]. Newer mechanisms are being explored to understand the complex neuropathology and heterogeneity of NCC.
In the brain parenchyma, the cyst evolves through four stages. In the vesicular stage, the cyst is filled with clear fluid, has a thin semitransparent wall and an eccentric opaque 4–5 mm scolex. Vesicular cysts are viable, produce scarce inflammatory changes and are usually asymptomatic. When the host immune system overcomes the protective mechanisms of the cyst, the cyst starts degenerating and an inflammatory response is elicited, the larva undergoes hyaline degeneration and the clear cyst fluid is replaced with gelatinous material; this is termed the colloidal stage. The cyst then contracts, the walls are replaced by focal lymphoid nodules and necrosis, and the scolex is transformed into coarse mineralized granules forming the granular nodular stage. Finally the granulation tissue is replaced by collagenous structures and calcification giving rise to the nodular calcified stage [Escobar, 1983]. At this time the oedema subsides but there are perilesional astrocytic changes. When cysts lodge outside the brain parenchyma (extraparenchymal NCC), such as in the ventricles, subarachnoid space, or cisterns, they tend to grow irregularly depending on the space available and usually elicit a strong inflammatory response. Occasionally the cysts enlarge considerably, become racemose without scolices and cause mass effects. Hydrocephalous can result either because of direct obstruction of cerebrospinal fluid (CSF) pathways by intraventricular cysts or secondary to inflammatory obstruction.
Clinical manifestations
The clinical presentation of NCC is variable and ranges from asymptomatic cases to cases with severe neurological problems. The clinical manifestations are determined by the location, number and viability of the cysts, and the host immune response. There is also clinical heterogeneity across countries; most cases from the Indian subcontinent present with single degenerating lesions whereas those from Latin America and China present with few viable lesions [Singh, 1997]. Extraparenchymal NCC is frequently seen in Latin American countries whereas it is uncommon in the Indian subcontinent. These variations are perhaps due to complex interactions between the host, parasite and environmental factors [Fleury et al. 2010]. Genetic differences in T. solium cysticerci have been reported from different countries [Maravilla et al. 2008; Vega et al. 2003] and may contribute towards the clinical variations across countries. A genetic susceptibility to NCC has been suggested by a reported positive association of HLA-DRBII 13 with single, small, contrast enhancing CT lesions (SSECTLs) [Jain et al. 1999]. However, familial aggregation of seizures in first degree relatives of cases of NCC with seizures was not found [Kelvin et al. 2009].
NCC is common in adults, as well as in children. Although it usually manifests after 5 years of age, symptomatic NCC is seen even in preschoolers and in infants. The prevalence of epilepsy in Mexico was seen to increase with age [Fleury et al. 2006]. There are also age-related differences in the manifestation of NCC. Most cases of childhood NCC present with mild to moderate symptomatology and single lesions; also extraparenchymal NCC seen in adults is rarely seen in children [Saenz et al. 2006].
The clinical manifestations of parenchymal NCC are quite different from those of extraparenchymal NCC
Parenchymal NCC
The most common presentation of parenchymal NCC is with seizures. Seizures are reported in 70–90% cases; in a series of 500 children from India, seizures were reported in 94.8% cases [Singhi et al. 2000]. In most cases, seizures occur suddenly in an otherwise healthy individual. Seizures are often focal, with or without secondary generalization; sometimes they are generalized. The higher frequency of generalized seizures (60%) reported in adults [Del Brutto et al. 1992] may be related to a higher number of cases with multiple lesions. Children usually present with a single brief seizure lasting less than 5 minutes; however, some children may have repeated seizures; clustering of seizures is common in adults. Status epilepticus has been reported in 1.7–32% of children [Talukdar et al. 2002; Morales et al. 2000; Singhi et al. 2000; Baranwal et al. 1998].
Seizures are thought to be evoked by the inflammatory response elicited by degeneration of the cysts; however, they can occur with any stage of the cyst. On the other hand, some individuals with degenerating cysts in their brain can remain asymptomatic. Seizures associated with vesicular cysts on neuroimaging are frequently reported from Latin American countries [Carpio et al. 2008; Garcia et al. 2004] whereas in Asian countries, seizures are usually associated with degenerating cysts [Singhi et al. 2000; Singh, 1997]. Almost a third of NCC cases are associated with headache and vomiting [Talukdar et al. 2002; Singhi et al. 2000; Baranwal et al. 1998]. Raised intracranial pressure with papilloedema is reported in 2.3–6.6% of children; in adults, earlier studies reported much higher figures of 28%; however, these included cases with extraparenchymal NCC [Sotelo et al. 1985a].
Depending on the location of the cysts, various neurological deficits can occur but are uncommon (4–6%) in children. Transient hemiparesis and monoparesis have been described. Cysts in the midbrain can cause ptosis [Singhi et al. 2008]. Young children and adolescents, especially females, sometimes have an acute encephalitic presentation, with numerous cysts and diffuse cerebral oedema, and severe acute raised intracranial pressure.
Extraparenchymal NCC
This is usually seen in adults and is uncommon in children. The manifestations include raised intracranial pressure and hydrocephalous particularly with intraventricular cysts; arachnoiditis and chronic meningitis can occur with subarachnoid cysts. Stroke can occur secondary to vasculitis caused by inflammatory occlusion of the arteries at the base of the brain secondary to arachnoiditis.
Other locations
Cysts within or compressing on the spinal cord occur in 1–5% cases of adult NCC. They can cause various symptoms and signs of spinal dysfunction. Those within the eyes and in the extraocular muscles can cause visual deficits, other eye symptoms, and limitation of eye movements. Unusual presentations of NCC such as behavioural changes, neurocognitive deficits, dystonia, reported in adults, are rare in children.
Diagnosis
Diagnosis is mainly made by neuroimaging. In most endemic countries a CT scan is performed and is usually sufficient for diagnosis although it can miss posterior fossa lesions. MRI is more sensitive than CT for detection of the scolex and for the diagnosis of extraparenchymal NCC. It may at times miss small calcified lesions.
Parenchymal NCC
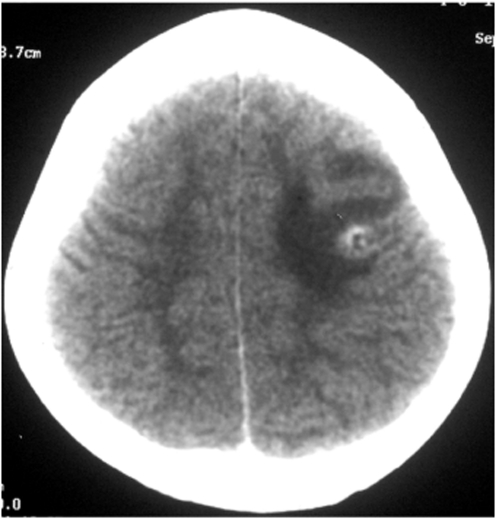
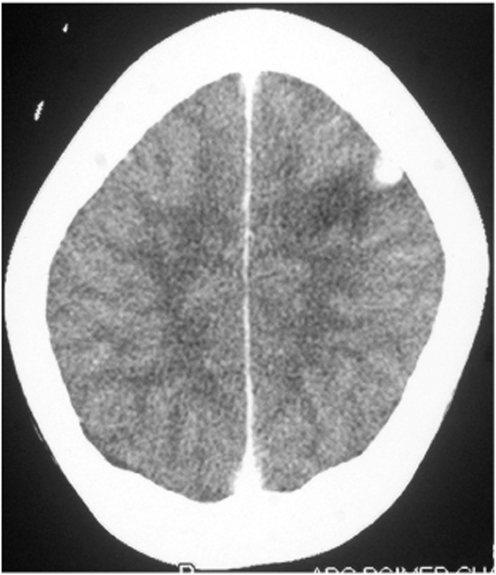
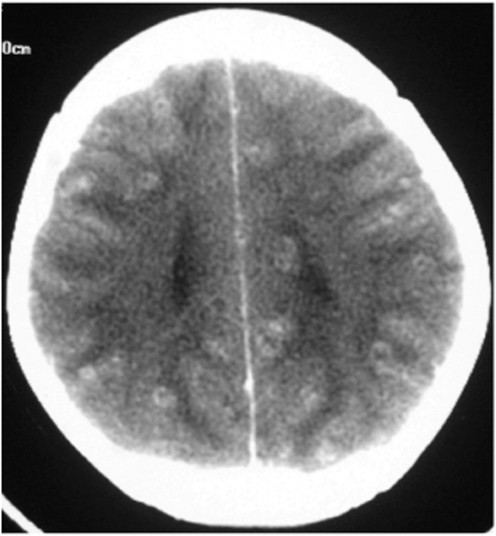
In cases with symptomatic parenchymal NCC, the most common CT finding is a single, small (<20 mm), low-density lesion with ring or disc enhancement which has been termed as a SSECTL. The scolex appears as a bright high-density eccentric nodule and is pathognomonic of NCC (Figures 1 and 2). A SSECTL represents a degenerating cyst and is often associated with perilesional oedema which is usually mild to moderate, occasionally severe. Multiple lesions are seen in some cases; numerous cysts of varying stages may give the so-called ‘starry-sky’ appearance which is typical of NCC (Figure 3). Vesicular cysts generally appear as small round lesions with CSF density cystic fluid; the wall is isodense to the brain parenchyma; they either do not enhance or are minimally enhanced on contrast and are not surrounded by oedema. Calcified cysts are usually punctate or a few millimetres in size, hyperdense dots, single or multiple, generally without any surrounding oedema. Oedema may be seen around calcified lesions in almost a third of cases with active seizures [Nash et al. 2001].
Figure 1.
A single, small, contrast enhancing CT lesion: ring with scolex and perilesional oedema.
Figure 2.
A single, small, contrast enhancing CT lesion: disc.
Figure 3.
CT scan showing multiple neurocysticercosis.
On MRI the scolex is seen as a nodule that is isointense or hyperintense relative to white matter. It is better seen on fluid attenuated inversion recovery (FLAIR) or diffusion weighted images, as the bright perilesional oedema and the high-intensity cystic fluid obscure the scolex on T2 weighted images. Gadolinium-enhanced MRI shows ring enhancement of lesions. A study on the evolution of MRI characteristics in single-lesion NCC showed that cyst contents were initially T1 hypointense and T2 hyperintense with inversion on FLAIR in 30.8% cases but on later scans the contents were T2 hypointense. The cyst wall appeared initially as a T2 hypointense rim but subsequently became hyperintense. The enhancement pattern of the lesion changed from an initial ring to subsequent disc enhancement [de Souza et al. 2010]. On MRI, vesicular cysts appear as round lesions either isointense or slightly hyperintense to the CSF. Calcified lesions appear hypointense on all MRI sequences and small lesions may at times be missed.
Extraparenchymal NCC
The cysts may appear as multilobed CSF isointense lesions that occupy the cisterns, Sylvian fissure or cerebellopontine angle. The findings of arachnoiditis and chronic meningitis such as enhancement of tentorium and basal cisterns, hydrocephalous and occasionally infarcts may be seen. Intraventricular cysts causing obstruction and hydrocephalous may be visualized.
Serological tests
The development of numerous serodiagnostic tests using different parasitic antigens is indicative of the fact that none of them are 100% sensitive and specific, particularly in cases with single-lesion parenchymal NCC. For multiple lesions, the enzyme-linked immunoelectrotransfer blot (EITB) assay using purified glycoprotein antigens from T. solium cysticerci was reported to be highly specific (100%) and nearly 98% sensitive as assessed for active lesions in hospitalized patients. The sensitivity was less for single lesions and for calcified lesions [Tsang et al. 1989]. A comparative study of enzyme-linked immunosorbent assay (ELISA) and dot-blot assay in children found that both were more sensitive in cases with multiple brain lesions (100%) than in those with a single lesion (87%) [Mandal et al. 2006]. The sensitivity of antibody detecting EITB assays is not better with the use of CSF samples as compared with serum samples. Detection of circulating cysticercosis antigens using ELISA has a modest sensitivity especially for parenchymal lesions. The antigen detecting ELISA has a better sensitivity with the use of CSF samples as compared with serum samples. However, ELISA has less sensitivity as compared with EITB for serum as well as CSF samples, for both intraparenchymal and extraparenchymal NCC [Rodriguez et al. 2009]. In our practice, we do not routinely use CSF samples for serological tests.
It has been suggested that the use of excretory secretory (ES) antigens, rather than somatic antigens might improve the serodiagnosis of cases with vesicular stages of the parasite [Sahu et al. 2009]. The use of ES antigens for the detection of antibodies in serum was found be more useful than that in urine in patients with enhancing lesions [Atluri et al. 2009]. Three ES peptides, namely 67 kDa, 43 kDa and 32 kDa, were reported to have high sensitivity and specificity in both serum and CSF reactivity; 43 kDa was particularly useful in the diagnosis of NCC [Sahu et al. 2010]. It has been suggested that synthetic peptide selected by phage display may be useful in the immunodiagnosis of NCC [Hell et al. 2009].
There is as yet no ideal immunodiagnostic test for single-lesion NCC. In our unit we perform NCC serology tests for all cases, keeping in mind that a positive serodiagnostic test supports the diagnosis of NCC; however, a negative test does not exclude the diagnosis of NCC. It should also be remembered that in endemic areas individuals may be seropositive without having NCC.
Other tests
Other tests that may support the diagnosis of NCC include biopsy of subcutaneous nodules (if present) to look for the parasite, and, in adults, X-rays of skeletal muscles to detect calcified cysts. Blood counts may show peripheral eosinophilia in about a third of cases. Finding tapeworms in stools is indicative of taeniasis but does not necessarily indicate the presence of NCC. In our previous studies we did not find these tests to be very useful, hence we do not perform them routinely.
In cases with NCC meningitis, CSF may show mild elevation of protein, hypoglycorrhachia and pleocytosis which may be lymphocytic, monocytic or polymorphonuclear. In cases of parenchymal NCC, the CSF is usually normal and is therefore not routinely indicated.
Diagnostic criteria for NCC incorporating clinical, radiological, immunologic and epidemiologic data of patients have been proposed, to provide a definite or probable diagnosis [Del Brutto et al. 2001].
Differential diagnosis
Owing to its pleomorphic presentation, NCC needs to be considered in the aetiological differential diagnosis of several neurologic symptoms and signs particularly seizures, headache and vomiting, especially in endemic countries.
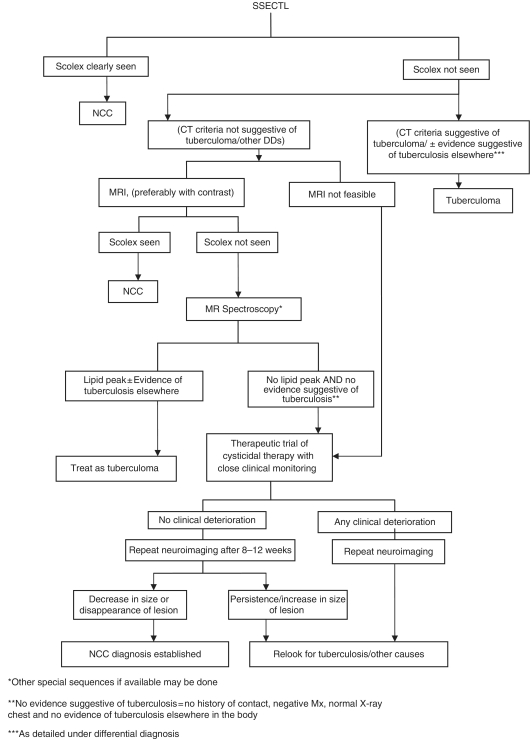
In cases with a SSECTL, visualization of the scolex is diagnostic of NCC. In cases where the scolex is not seen, the most common differential diagnosis is that of a tuberculoma, particularly in countries where tuberculosis is endemic. Certain criteria such as the presence of raised intracranial pressure, progressive focal neurodeficit, size of CT lesion >20 mm, lobulated irregular shape and marked oedema causing midline shift may indicate a tuberculoma [Rajshekhar et al. 1993]. Also tuberculomas are usually seen in the base of the brain whereas NCC lesions are commonly seen at the grey–white junction of the cortex. However, none of these criteria are diagnostic of a tuberculoma. It is our practice to obtain a detailed history of contact, a Mantoux test and a chest X-ray in all cases to exclude tuberculosis. An MRI is indicated in cases with SSECTL where a scolex is not seen. However, if it is not feasible to obtain an MRI scan, and if the diagnosis of a tuberculoma is reasonably excluded (by CT criteria, a negative Mantoux test, normal chest X-ray and no history of contact with a case of tuberculosis), a therapeutic trial of cysticidal therapy with close clinical monitoring can be given, and a CT scan repeated after 3 months. A reduction in size or disappearance of the lesion confirms the diagnosis of NCC.
Other conditions such as microabscess, toxoplasmosis, fungal lesion, low-grade astrocytoma and cystic cerebral metastasis may also be considered in the differential diagnosis of enhancing lesions [Singhi and Baranwal, 2001]. However, most of conditions can usually be diagnosed based on the associated clinical setting and often definitive tests.
Other MR sequences including diffusion weighted MRI [Gupta et al. 2005; Raffin et al. 2001], magnetization transfer ratio (MTR) [Kathuria et al. 1998], proton magnetic resonance spectroscopy (MRS) [Pretell et al. 2005] and 3D constructive interference in steady state (3DCISS) [Robbani et al. 2004; Govindappa et al. 2000] are being investigated for use in cases where the scolex is not well seen and it is difficult to make the diagnosis of NCC with certainty. Some of these may be helpful. The presence of a lipid peak on MRS indicates a tuberculoma [Pretell et al. 2005].
NCC meningitis and hydrocephalous need to be differentiated from other causes of chronic meningitis, and acquired hydrocephalus. A practical schema of approach to a case with SSECTL that we often use is depicted in Figure 4. If after this it is still not possible to reach a diagnosis, other differential diagnoses need to be reconsidered and relevant investigations need to be done. Stereotactic biopsy of the lesion, where feasible, is indicated when the diagnosis cannot be reached in spite of all other investigations.
Figure 4.
Practical schema for a diagnostic approach to a single, small, contrast enhancing CT lesion in countries where neurocysticercosis and tuberculosis are endemic.
Management
Management of NCC involves the use of cysticidal therapy and symptomatic therapy.
Cysticidal therapy
Medical treatment of NCC has been available for the past 25 years and has been a matter of debate since then. The two main aspects to be considered are (i) whether it is beneficial in cyst destruction and (ii) whether it improves the clinical outcome.
Destruction of cysts
Some of the earliest reports showed that praziquantel was highly effective in the destruction of viable parenchymal cysts and somewhat less effective in meningeal cysticercosis [Sotelo et al. 1985b, 1984]. Subsequently albendazole was reported to be more effective than praziquantel [Cruz et al. 1991; Sotelo et al. 1990; Escobedo et al. 1987]. Further studies confirmed the efficacy of cysticidal therapy in the destruction of viable cysts [Medina et al. 1993; Takayanagui and Jardim, 1992]. However, the efficacy of cysticidal therapy was debated by proponents for [Del Brutto 1995] and against [Kramer, 1995] it.
A randomized, controlled trial from Ecuador in which neither praziquantel nor albendazole was found to be effective for the destruction of viable cysts questioned the efficacy of cysticidal therapy [Carpio et al. 1995]. This evoked a strong comment in favour of cysticidal therapy [Del Brutto, 1997]. In a recent randomized trial from Ecuador by the same authors on 176 patients, 31% of those who received albendazole were free of ‘active’ (viable) cysts versus 7% in the placebo group (p = 0.001) [Carpio et al. 2008].
While it is generally accepted that both praziquantel and albendazole are effective in destroying viable cysts, their use in cases with enhancing lesions has been debated as these lesions are considered to represent degenerating cysts, many of which resolve spontaneously.
In a double-blind, placebo-controlled trial in 63 children with single-lesion NCC, the use of albendazole (15 mg/kg/day for 4 weeks) within 3 months of onset of seizures was associated with a significantly increased and faster disappearance of lesions after 1 month (41%) as compared with placebo (16.2%; p < 0.05), and after 3 months (albendazole 64.5% versus placebo 37.5%; p < 0.05) [Baranwal et al. 1998]. Subsequently several other trials have confirmed this [Chaurasia et al. 2010; Thussu et al. 2008; Kalra et al. 2003]. Other studies have not found any beneficial effect of albendazole therapy [Das et al. 2007; Padma et al. 1994]; however one of these has methodological issues which include wide variations in the age and in duration of epilepsy in the cases included [Padma et al. 1994].
Clinical outcome
The role of cysticidal therapy in control of seizures secondary to NCC has been debated [Carpio et al. 1998]. An improved seizure control with the use of cysticidal drugs was found in adults with epilepsy associated with vesicular lesions [Medina et al. 1993; Del Brutto et al. 1992; Vazquez and Sotelo, 1992] as well as after enhancing lesions [Del Brutto et al. 1992]. In a double-blind, placebo-controlled trial of 120 patients with vesicular lesions, cysticidal therapy was effective, at least in reducing the number of seizures with generalization [Garcia et al. 2004]. Other studies have not, however, found any significant improvement in seizure control both in patients with live cysts and multiple or single enhancing lesions [Carpio et al. 2008, 1995; Das et al. 2007]. In a meta-analysis of all therapeutic trials of NCC from 1966–1999 in NCC, the authors found only four studies that used placebo-controlled cysticidal therapy. They concluded that there was a lower risk of cyst persistence in CT at 6 months among those who received cysticidal therapy, but there was no decrease in seizure recurrence; however, the numbers studied were small [Salinas et al. 1999]. In a repeat meta-analysis in 2007, the same authors concluded that there is insufficient evidence to assess whether cysticidal therapy in neurocysticerosis is associated with beneficial effects [Salinas and Prasad, 2007]. A separate meta-analysis of randomized, placebo-controlled trials between 1979 and 2005 that included six trials of patients with vesicular cystic lesions and five trials of patients with enhancing (colloidal) lesions, found that cysticidal therapy was associated with a significantly higher rate of complete resolution of vesicular cystic lesions (44% versus 19%; p = 0.025) and a trend toward increased resolution of enhancing lesions (72% versus 63%; p = 0.38) that became statistically significant when an outlier trial was excluded from the analysis (69% versus 55%; p = 0.006). The risk for seizure recurrence was lower after cysticidal therapy in patients with enhancing lesions (14% versus 37%; p < 0.001). In the single trial that evaluated the frequency of seizures in patients with vesicular cystic lesions, there was a 67% reduction in the rate of generalized seizures with treatment (p = 0.006) [Del Brutto et al. 2006]. A recent Cochrane review of 21 randomized, controlled trials of cysticidal therapy [Abba et al. 2010] concluded that in adults with viable lesions the use of albendazole was associated with a decrease in the number of lesions but not in the recurrence of seizures. There were no trials in adults with nonviable lesions. In children, there were no trials for viable lesions; for nonviable lesions, the use of albendazole was associated with a decrease in seizure recurrence, but not a reduction in lesions. This is difficult to explain, particularly in view of the fact that seizure recurrences are related to persistent lesions. Also some of the studies included in this review have included heterogeneous populations and also extraparenchymal NCC, and as acknowledged by the authors themselves, ‘unexplained heterogeneity was introduced by one relatively large but poorly reported trial [Das et al. 2007] with results mostly running in the opposite direction to those reported in other trials’. It is therefore difficult to generalize these conclusions.
On the balance of evidence, cysticidal therapy seems to be effective in reducing the number of lesions, but its role in improving long-term seizure control needs further larger studies. Cysticidal drugs have also been found to be effective in the treatment of extraparenchymal NCC including orbital, spinal, intraventricular and subarachnoid NCC, and even for giant cysts [Proaño et al. 2001] although resistance has been reported by some [Cárdenas et al. 2010].
Cysticidal drugs
Albendazole and praziquantel are the two main cysticidal drugs used for the treatment of NCC. Albendazole was reported to have increased efficacy as compared with praziquantel; however, a recent evaluation of published studies concluded that the superiority of either of these drugs cannot be established with certainty [Garcia, 2008]. Albendazole is, however, preferred as it less expensive, better tolerated and has better penetration into the subarachnoid space. Also, the bioavailability of albendazole is increased by co-administration of steroids [Jung et al. 1990] and is not affected by phenytoin or carbamezepine, whereas that of praziquantel decreases with co-administration of steroids [Vazquez et al. 1987], as well as by phenytoin and carbamezepine [Bittencourt et al. 1992], which are often used as first-line anticonvulsants in children with seizures due to NCC.
Albendazole is used at a dose of 15 mg/kg/day (maximum 800 mg) in two or three divided doses in children. In adults a dose of 400 mg twice a day is used; higher doses are also being evaluated. Albendazole is absorbed variably after oral administration; its absorption is increased with a fatty meal. In studies on population pharmacokinetics of albendazole, it was found that almost a quarter of the population has only 30% of drug absorption relative to others [Castro et al. 2009]; this may be responsible for some treatment failures reported in some studies. Albendazole is rapidly metabolized in the liver to albendazole sulfoxide which has potent anthelmintic activity. Albendazole sulfoxide has a plasma half life of 8–9 hours (range 4–15 hours) and is excreted mainly in the urine. Albendazole has an excellent safety profile; side effects occur rarely, in less than 1% cases and include transient gastrointestinal symptoms such as nausea, diarrhoea and vomiting. However, side effects also depend on the dose and duration of albendazole therapy; prolonged use may cause an increase in serum aminotransferase, jaundice, headaches, loss of hair, leukopenia and thrombocytopenia.
Allergic phenomena such as urticaria, rashes and oedema can occur, but are extremely rare. Albendazole is teratogenic and embryotoxic in animals and should not be used in pregnant women. The duration of albendazole therapy has varied. It is used usually for 28 days [García et al. 2002], although shorter durations of 8–14 days have also been used. In a placebo-controlled trial of 1 week versus 4 weeks of albendazole therapy in children with one to three enhancing lesions, both regimens were found to be equally effective both in terms of resolution of lesions as well as seizure control at 1 year [Singhi et al. 2003a]. A 3-day albendazole therapy has also been found to be effective in increasing the cyst resolution as compared with placebo [Chaurasia et al. 2010].
Praziquantel is used at a dose of 50 mg/kg/day. It is readily absorbed after oral administration and reaches peak plasma levels within 2 hours. It is rapidly metabolized by first-pass metabolism to several inactive metabolites which are excreted mainly in the urine and to some extent in the bile. Praziquantel is generally well tolerated. Dose-related side effects include abdominal discomfort, headache, dizziness and drowsiness. Idiosyncratic side effects include pruritus, fever, rashes and muscle and joint pains. Side effects are uncommon. The usual duration of therapy is for a period of 15 days. A single day therapy (25 mg/kg/dose in three doses) was found to be as effective as a 7-day treatment with albendazole [Prettel et al. 2000]; however, it was not found to be effective for multiple lesions [Prettel et al. 2001].
In the first placebo-controlled, randomized trial of a combination therapy of albendazole and praziquantel in children with SSECTL, there was a trend towards higher resolution of lesions in the combination therapy group as compared with albendazole alone. However, this was not statistically significant. There was no difference in seizure outcome in the two groups [Kaur et al. 2009].
It is feared that the use of cysticidal therapy may aggravate the host inflammatory response and worsen the clinical condition; however, most cases of parenchymal NCC do not show any such worsening particularly when steroids are used concomitantly. Cysticidal therapy should not be used in cases (i) with markedly elevated intracranial pressure, particularly in disseminated NCC with extensive cerebral oedema or (ii) in ophthalmic (intraocular) NCC, to avoid any worsening of intracranial pressure and damage to the eye, respectively. Steroids alone are used in these situations. It should be used with caution in cases with extraparenchymal NCC as any increase in the inflammatory response may lead to the development of infarct; pretreatment with steroids and management of intracranial hypertension is warranted. Cysticidal therapy is of no use for calcified lesion(s). There is no universally agreed single protocol for the treatment of NCC. Consensus guidelines for the treatment of NCC recommend an individualized approach [Nash et al. 2006]. It is our practice to treat all symptomatic NCC cases with viable or enhancing lesions with albendazole, unless it is contraindicated (intraocular NCC, NCC encephalitis).
Symptomatic therapy
Antiepileptic therapy
Seizures are often well controlled with a single antiepileptic drug (AED), and the recurrence rate after AED withdrawal is low in cases with single-lesion NCC both in children [Talukdar et al. 2002; Baranwal et al. 2001] as well as in adults [Goel et al. 2010]. Although a high recurrence rate after AED withdrawal (76%) was reported in a study on 203 adults from Mexico [Del Brutto, 1994], this study had included cases with pre-existing single or multiple calcified lesions (53% cases); in addition, of the 110 cases with vesicular cysts, 73% had multiple lesions. Recurrence of seizures after AED withdrawal is correlated with the presence of multiple lesions prior to starting cysticidal therapy, and persistence or calcification of lesions after therapy [Goel et al. 2010; Singhi et al. 2003b; Del Brutto, 1994].
The optimal duration of AED therapy has been a matter of debate. Although the usual practice has been to use AED for 2 years seizure-free interval, shorter durations of AED have been proposed. In a randomized study of 106 children with SSECTL, seizure recurrence after AED therapy for 1 year versus 2 years seizure-free interval was not significantly different and correlated significantly with persistence or calcification of lesions and an abnormal EEG at the time of withdrawal [Singhi et al. 2003b]. It seems prudent to withdraw anticonvulsants after a 1-year seizure-free interval in cases where the lesion has disappeared and the EEG has normalized; longer durations are needed for those with persistent or calcified lesions.
Corticosteroids
Oral corticosteroids are administrated generally a couple of days before and a few days along with anticysticercal therapy so as to prevent any adverse reactions that may occur due to the host inflammatory response. Pretreatment with steroids also increases plasma levels of albendazole sulfoxide. Usually oral prednisolone 1–2 mg/kg is used; intravenous dexamethasone may be used if there are features of raised intracranial pressure. In cases with disseminated lesions and extensive cerebral oedema, steroids may be required for a prolonged period.
The role of steroids in the resolution of cysts remains controversial. In a randomized trial in adults with live cysts wherein albendazole (15 mg/kg/day for 8 days) and praziquantel (50 mg/kg/day for 15 days), with oral prednisolone, were compared with oral prednisolone only (1 mg/kg/day for 15 days) no significant difference was found in the proportion of patients free of cysts at 6 months and 1 year and in the proportion of patients free of seizures over a 18-month follow up [Carpio et al. 1995]. In a randomized trial among 133 children with SSECTL, disappearance of lesions at 3-month follow up was somewhat higher (62.9%) in the group that received corticosteroid plus albendazole as compared with albendazole alone (59.5%) or steroids alone (52.6%); however, this was not statistically significant. Children in the corticosteroid group had significantly more seizure recurrences while on AEDs [Singhi et al. 2004]. In another open-label randomized trial on 100 adults with SSECTL a significantly higher resolution was seen in the group that received prednisolone (68.1%) as compared with the group that received only antiepileptic monotherapy (60.9%) (p < 0.05) [Kishore and Misra, 2007]. In an open-label study a significantly better resolution and decreased risk of seizure recurrence were seen in prednisolone-treated patients as compared with those who were not given prednisolone [Mall et al. 2003]. This aspect therefore needs to be further studied. Our usual practice is to start oral prednisolone 2 mg/kg/day, 2–3 days before starting albendazole, and continue it for the next 3–4 days. Children with moderate to severe oedema are kept under observation for 24–48 hours after starting albendazole. Prolonged steroid therapy is used in some children with extensive oedema.
Role of surgery
Surgical intervention is required in some cases particularly in intraventricular and subarachnoid NCC. A ventriculoperitoneal shunt is needed for hydrocephalous; simultaneous use of steroids and albendazole and recurrent courses of steroids reduce the risk of frequent obstructions and shunt revisions. Endoscopic removal of cysts is the least invasive and is therefore the procedure of choice [Goel et al. 2008; Suri et al. 2008]. Excision of giant cysts that fail to respond to medical therapy may be required.
Follow up
All cases on treatment require periodic follow up. A repeat CT scan after 3–6 months is generally indicated to determine whether the lesions have resolved. A repeat course of cysticidal therapy is often given in cases with persistent (but not calcified) lesions.
Prognosis
Cases with single lesions generally have a good prognosis: seizures are usually well controlled and lesions disappear within 6 months in over 60% cases. Cases with multiple lesions, and those with calcifications often have frequent seizure recurrences. Cysticercus encephalitis and extraparenchymal NCC have a guarded prognosis.
Prevention
The only way to reduce the burden of disease due to NCC is to prevent it. Public education regarding proper hygiene and sanitation, and enforcing strict animal husbandry and meat inspection procedures are warranted. Mass treatment programs of the population to eliminate tapeworms with the use of niclosamide [Allan et al. 1997] or with praziquantel [Sarti et al. 2000; Cruz et al. 1989] have been successful in temporarily reducing the transmission of the disease, but the effect was not sustainable. Treatment of pigs with cysticidal therapy such as oxfendazole has been investigated. A combined human (praziquantel 5 mg single dose) and porcine (oxfendazole 30 mg/kg in two rounds) chemotherapy program in Peru led to a decrease in the prevalence and incidence of porcine infection for almost 2 years but its magnitude was small [Garcia et al. 2006]. There is a potential danger of causing symptoms in asymptomatic cases of NCC when praziquantel is used and therefore some prefer the use of niclosamide for such programs. However, no such significant incidents have been reported in the studies conducted so far. In the study by Cruz and colleagues, of the 10,173 persons treated, there was one case of seizures; however, the authors could not relate this directly to treatment.
Vaccination of pigs with different vaccines against T. solium has been tried with varying rates of success. The most effective of these has been the TSOL 18 vaccine. In a field trial, the use of TSOL 18 vaccine together with a single treatment of pigs with oxfendazole was effective in stopping the transmission of T. solium by pigs [Lightowlers, 2010]. Simultaneous mass treatment of the population with anthelminthics, and vaccination of pigs has been suggested and is in progress in Peru [Mahanty and Garcia, 2010]. All of these methods have obvious limitations in resource poor countries where the disease is endemic.
Conclusion
NCC is the most common cause of acquired epilepsy in developing countries. The use of albendazole is associated with increased and faster resolution of lesions. However, whether it leads to a better control of seizures needs further study. Successful medical therapy has limited the need for surgery in NCC. Corticosteroids are used for the reduction of cerebral oedema associated with NCC. Seizures are well controlled in most cases of single-lesion NCC but frequently recur in cases with multiple lesions. Prevention of NCC is essential to reduce the global burden of the disease.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
None declared.
References
- Abba K., Ramaratnam S., Ranganathan L.N. (2010) Anthelmintics for people with neurocysticercosis. Cochrane Database Syst Rev 20: CD000215–CD000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan J.C., Velasquez-Tohom M., Fletes C., Torres-Alvarez R., Lopez-Virula G., Yurrita P., et al. (1997) Mass chemotherapy for intestinal Taenia solium infection: effect on prevalence in humans and pigs. Trans R Soc Trop Med Hyg 91: 595–598 [DOI] [PubMed] [Google Scholar]
- Alvarez J.I., Rivera J., Teale J.M. (2008) Differential release and phagocytosis of tegument glycoconjugates in neurocysticercosis: implications for immune evasion strategies. PLoS Negl Trop Dis 2: e218–e218, doi:10.1371/Journal Pntd.0000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri S.R., Singhi P., Khandelwal N., Malla N. (2009) Neurocysticercosis immunodiagnosis using Taenia solium cysticerci crude soluble extract, excretory secretory and lower molecular mass antigens in serum and urine samples of Indian children. Acta Trop 110: 22–27 [DOI] [PubMed] [Google Scholar]
- Baranwal A.K., Singhi P., Singhi S., Khandelwal N. (2001) Seizure recurrence in children with focal seizures and single small enhancing computed tomographic lesions. Prognostic factors on long-term follow-up. J Child Neurol 16: 443–445 [DOI] [PubMed] [Google Scholar]
- Baranwal A.K., Singhi P.D., Khandelwal N., Singhi S.C. (1998) Albendazole therapy in children with focal seizures and single small enhancing computerized tomographic lesions: a randomized placebo-controlled, double blind trial. Pediatric Infect Dis J 17: 696–700 [DOI] [PubMed] [Google Scholar]
- Bittencourt P.R., Gracia C.M., Martins R., Fernandes A.G., Diekmann H.W., Jung W. (1992) Phenytoin and carbamazepine decrease oral bioavailability of praziquantel. Neurology 42: 492–496 [DOI] [PubMed] [Google Scholar]
- Cárdenas, G., Carrillo-Mezo, R., Jung, H., Sciutto, E., Hernandez, J.L. and Fleury, A. (2010) Subarachnoidal neurocysticercosis non-responsive to cysticidal drugs: a case series. BMC Neurol 10: 16, http://www.biomedcentral.com/1471-2377/10/16. [DOI] [PMC free article] [PubMed]
- Carpio A., Escobar A., Hauser A.W. (1998) Cysticercosis and epilepsy: a critical review. Epilepsia 39: 1025–1040 [DOI] [PubMed] [Google Scholar]
- Carpio A., Kelvin E.A., Bagiella E., Leslie D., Leon P., Andrews H., et al. (2008) Ecuadorian Neurocysticercosis Group. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry 79: 1050–1055 [DOI] [PubMed] [Google Scholar]
- Carpio A., Santillan F., Leon P., Flores C., Hauser W.A. (1995) Is the course of neurocysticercosis modified by treatment with antihelminthic agents. Arch Intern Med 155: 1982–1988 [PubMed] [Google Scholar]
- Castro N., Márquez-Caraveo C., Brundage R.C., González-Esquivel D., Suárez A.M., Góngora F., et al. (2009) Population pharmacokinetics of albendazole in patients with neurocysticercosis. Int J Clin Pharmacol Ther 47: 679–685 [PubMed] [Google Scholar]
- Chaurasia R.N., Garg R.K., Agarwall A., Kohli N., Verma R., Singh M.K., et al. (2010) Three day albendazole therapy in patients with a solitary cysticercus granuloma: a randomized double blind placebo controlled study. Southeast Asian J Trop Med Public Health 41: 517–525 [PubMed] [Google Scholar]
- Commission on Tropical Diseases of the International League Against Epilepsy (1994) Relationship between epilepsy and tropical diseases. Epilepsia 35: 89–93 [PubMed] [Google Scholar]
- Cruz M., Cruz L., Horton J. (1991) Albendazole vs praziquantel in the treatment of cerebral cysticercosis: clinical evaluation. Trans R Soc Trop Med Hyg 85: 244–247 [DOI] [PubMed] [Google Scholar]
- Cruz M., Davis A., Dixon H., Pawlowski Z.S., Proano J. (1989) Operational studies on the control of Taenia solium taeniasis/cysticercosis in Ecuador. Bull World Health Organ 67: 401–407 [PMC free article] [PubMed] [Google Scholar]
- Das K., Mondal G.P., Banerjee M., Mukherjee B.B., Singh O.P. (2007) Role of antiparasitic therapy for seizures and resolution of lesions in neurocysticercosis patients: an 8 year randomised study. J Clin Neurosci 14: 1172–1177 [DOI] [PubMed] [Google Scholar]
- de Souza A., Nalini A., Kovoor J.M., Yeshraj G., Siddalingaiah H.S., Thennarasu K. (2010) Natural history of solitary cerebral cysticercosis on serial magnetic resonance imaging and the effect of albendazole therapy on its evolution. J Neurol Sci 288: 135–141 [DOI] [PubMed] [Google Scholar]
- Del Brutto O.H. (1994) Prognostic factors for seizure recurrence after withdrawal of antiepileptic drugs in patients with neurocysticercosis. Neurology 44: 1706–1709 [DOI] [PubMed] [Google Scholar]
- Del Brutto O.H. (1995) Medical treatment of cysticercosis – effective. Arch Neurol 52: 102–104 [DOI] [PubMed] [Google Scholar]
- Del Brutto O.H. (1997) Is the course of neurocysticercosis modified by treatment with anthelmintic agents? Arch Intern Med 157: 128–130 [DOI] [PubMed] [Google Scholar]
- Del Brutto O.H., Rajshekhar V., White A.C., J, Tsang V.C., Nash T.E., Takayanagui O.M., et al. (2001) Proposed diagnostic criteria for neurocysticercosis. Neurology 57: 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Brutto O.H., Roos K.L., Coffey C.S., García H.H. (2006) Meta-analysis: Cysticidal drugs for neurocysticercosis: albendazole and praziquantel. Ann Intern Med 145: 43–51 [DOI] [PubMed] [Google Scholar]
- Del Brutto O.H., Santibanez R., Noboa C.A., Aguirre R., Diaz E., Alarcon T.A. (1992) Epilepsy due to neurocysticercosis analysis of 203 patients. Neurology 42: 389–392 [DOI] [PubMed] [Google Scholar]
- Escobar A. (1983) The pathology of neurocysticercosis. In: Palacios E., Rodriguez-Carbajal K.J., Taveras J. (eds), Cysticercosis of the central nervous system, Charles C. Thomas: Springfield, IL, 27–54 [Google Scholar]
- Escobedo F., Penagos P., Rodriquez J., Sotelo J. (1987) Albendazole therapy for neurocysticercosis. Arch Intern Med 147: 738–741 [PubMed] [Google Scholar]
- Fleury A., Escobar A., Fragoso G., Sciutto E., Larralde C. (2010) Clinical heterogeneity of human neurocysticercosis results from complex interactions among parasite, host and environmental factors. Trans R Soc Trop Med Hyg 104: 243–250 [DOI] [PubMed] [Google Scholar]
- Fleury A., Morales J., Bobes R.J., Dumas M., Yanez O., Pina J., et al. (2006) An epidemiological study of familial neurocysticercosis in an endemic Mexican community. Trans R Soc Trop Med Hyg 100: 551–558 [DOI] [PubMed] [Google Scholar]
- Garcia H.H. (2008) Antiparasitic drugs in neurocysticercosis: albendazole or praziquantel? Expert Rev Anti Infect Ther 6: 295–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García H.H., Evans C.A., Nash T.E., Takayanagui O.M., White A.C., Jr, Botero D., et al. (2002) Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev 15: 747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia H.H., Gonzalez A.E., Gilman R.H. (2003) The Cysticercosis Working Group in Peru. Diagnosis, treatment and control of Taenia solium cysticercosis. Curr Opin Infect Dis 16: 411–419 [DOI] [PubMed] [Google Scholar]
- Garcia H.H., Gonzalez A.E., Gilman R.H., Moulton L.H., Verastegui M., Rodriguez S., et al. (2006) Cysticercosis Working Group in Peru. Combined human and porcine mass chemotherapy for the control of T. solium. Am J Trop Med Hyg 74: 850–855 [PubMed] [Google Scholar]
- Garcia H.H., Pretell E.J., Gilman R.H., Martinez S.M., Moulton L.H., Del Brutto O.H., et al. (2004) Cysticercosis Working Group in Peru. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med 350: 249–258 [DOI] [PubMed] [Google Scholar]
- Goel D., Mittal M., Bansal K.K., Singhal A. (2010) Natural history of solitary cerebral cysticercosis cases after albendazole therapy: a longitudinal follow-up study from India. Acta Neurol Scand 121: 204–208 [DOI] [PubMed] [Google Scholar]
- Goel R.K., Ahmad F.U., Vellimana A.K., Suri A., Chandra P.S., Kumar R., et al. (2008) Endoscopic management of intraventricular neurocysticercosis. J Clin Neurosci 15: 1096–1101 [DOI] [PubMed] [Google Scholar]
- Govindappa S.S., Narayanan J.P., Krishnamoorthy V.M., Shastry C.H., Balasurbramaniam A., Krishna S.S., et al. (2000) Improved detection of Intraventricular cysticeral cysts with the use of three-dimensional constructive interference in steady state MR sequences. AJNR Am J Neuroradiol 21: 679–684 [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K., Prakash M., Mishra A.M., Husain M., Prasad K.N., Husain N., et al. (2005) Role of diffusion weighted imaging in differentiation of intracranial tuberculoma and tuberculous abscess from cysticercus granulomas-a report of more than 100 lesions. Eur J Radiol 55: 384–392 [DOI] [PubMed] [Google Scholar]
- Hell R.C., Amim P., de Andrade H.M., de Avila R.A., Felicori L., Oliveira A.G., et al. (2009) Immunodiagnosis of human neurocysticercosis using a synthetic peptide selected by phage-display. Clin Immunol 131: 129–138 [DOI] [PubMed] [Google Scholar]
- Jain S., Padma M.V., Kanga U., Mehra N.K., Maheshwari N.C. (1999) Family studies and human leukocyte antigen class II typing in Indian probands with seizures in association with single small enhancing computed tomography lesions. Epilepsia 40: 232–238 [DOI] [PubMed] [Google Scholar]
- Jung H., Hurtado M., Medina M.T., Sanchez M., Sotelo J. (1990) Dexamethasone increases plasma levels of albendazole. J Neurol 237: 279–280 [DOI] [PubMed] [Google Scholar]
- Kalra V., Dua T., Kumar V. (2003) Efficacy of albendazole and short-course dexamethasone treatment in children with 1 or 2 ring-enhancing lesions of neurocysticercosis: a randomized controlled trial. J Pediatr 143: 111–114 [DOI] [PubMed] [Google Scholar]
- Kathuria M.K., Gupta R.K., Roy R., Gaur V., Husain N., Pradhan S. (1998) Measurement of magnetization transfer in different stages of neurocysticercosis. J Magn Reson Imaging 8: 473–479 [DOI] [PubMed] [Google Scholar]
- Kaur S., Singhi P., Singhi S., Khandelwal N. (2009) Combination therapy of praziquantel and albendazole versus albendazole alone in children with single lesion NCC—a randomized placebo controlled double blind trial. Ped Infect Dis J 28: 403–406 [DOI] [PubMed] [Google Scholar]
- Kelvin E.A., Carpio A., Hesdorffer D.C., Bagiella E., Leslie D., Leon P., et al. (2009) Investigation of familial aggregation of seizures in neurocysticercosis patients. Epilepsy Res 84: 67–71 [DOI] [PubMed] [Google Scholar]
- Kishore D., Misra S. (2007) Short course of oral prednisolone on disappearance of lesion and seizure recurrence in patients of solitary cysticercal granuloma with single small enhancing CT lesion: an open label randomized prospective study. J Assoc Physicians India 55: 419–424 [PubMed] [Google Scholar]
- Kramer L.D. (1995) Medical treatment of cysticercosis – ineffective. Arch Neurol 52: 101–102 [DOI] [PubMed] [Google Scholar]
- Lightowlers M.W. (2010) Eradication of Taenia solium cysticercosis: a role for vaccination of pigs. Int J Parasitol 40: 1183–1192 [DOI] [PubMed] [Google Scholar]
- Mahanty S., Garcia H.H. (2010) Cysticercosis Working Group in Perú. Cysticercosis and neurocysticercosis as pathogens affecting the nervous system. Prog Neurobiol 91: 172–184 [DOI] [PubMed] [Google Scholar]
- Mall R.K., Agarwal A., Garg R.K., Kar A.M., Shukla R. (2003) Short course of prednisolone in Indian patients with solitary cysticercus granuloma and new-onset seizures. Epilepsia 44: 1397–1401 [DOI] [PubMed] [Google Scholar]
- Mandal J., Singhi P.D., Khandelwal N., Malla N. (2006) Evaluation of ELISA and dot blots for the serodiagnosis of neurocysticercosis, in children found to have single or multiple enhancing lesions in computerized tomographic scans of the brain. Ann Trop Med Parasitol 100: 39–48 [DOI] [PubMed] [Google Scholar]
- Maravilla P., Gonzalez-Guzman R., Zuniga G., Peniche A., Dominguez-Alpizar J.L., Reyes-Montes R., et al. (2008) Genetic polymorphism in Taenia solium cysticerci recovered from experimental infections in pigs. Infect Genet Evol 8: 213–216 [DOI] [PubMed] [Google Scholar]
- Medina M.T., Genton P., Montoya M.C., Cordova S., Dravet C., Sotelo J., et al. (1993) Effect of anticysticercal treatment on the prognosis of epilepsy in neurocysticercosis: A pilot trial. Epilepsia 34: 1024–1027 [DOI] [PubMed] [Google Scholar]
- Morales N.M., Agapejev S., Morales R.R., Padula N.A., Lima M.M. (2000) Clinical aspects of neurocysticercosis in children. Pediatr Neurol 22: 287–291 [DOI] [PubMed] [Google Scholar]
- Nash T.E., Pretell J., Garcia H.H. (2001) Calcified cysticerci provoke perilesional edema and seizures. Clin Infect Dis 33: 1649–1653 [DOI] [PubMed] [Google Scholar]
- Nash T.E., Singh G., White A.G., Rajshekhar V., Loeb J.A., Proaño J.V., et al. (2006) Treatment of neurocysticercosis: current status and future research needs. Neurology 67: 1120–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padma M.V., Behari M., Misra N.K., Ahuja G.K. (1994) Albendazole in single CT ring lesions in epilepsy. Neurology 44: 1344–1346 [DOI] [PubMed] [Google Scholar]
- Pretell E.J., Garcia H.H., Custodio N., Padilla C., Alvarado M., Gilman R.H., et al. (2000) Short regimen of praziquantel in the treatment of single brain enhancing lesions. Clin Neurol Neurosurg 102: 215–218 [DOI] [PubMed] [Google Scholar]
- Pretell E.J., Garcia H.H., Gilman R.H., Saavedra H., Martinez M. (2001) The Cysticercosis Working Group in Peru. Failure of one-day praziquantel treatment in patients with multiple neurocysticercosis lesions. Clin Neurol Neurosurg 103: 175–177 [DOI] [PubMed] [Google Scholar]
- Pretell E.J., Martinot C., Jr, Garcia H.H., Alvarado M., Bustos J.A., Martinot C. (2005) Cysticercosis Working Group in Peru. Differential diagnosis between cerebral tuberculosis and neurocysticercosis by magnetic resonance spectroscopy. J Comput Assist Tomogr 1: 112–114 [DOI] [PubMed] [Google Scholar]
- Proaño J.V., Madrazo I., Avelar F., López-Félix B., Díaz G., Grijalva I., et al. (2001) Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med 345: 879–885 [DOI] [PubMed] [Google Scholar]
- Raffin L.S., Bacheschi L.A., Machado L.R., Nobrega J.P.S., Coelho C., Leite C.C. (2001) Diffusion-weighted MR imaging of cystic lesions of neurocysticercosis. Arq Neuropsiquiatr 59: 839–842 [DOI] [PubMed] [Google Scholar]
- Rajshekhar V., Haran R.P., Prakash S., Chandy M.J. (1993) Differentiating solitary small cysticercus granulomas and tuberculomas in patients with epilepsy—clinical and computerized tomographic criteria. J Neurosurgery 78: 402–407 [DOI] [PubMed] [Google Scholar]
- Robbani I., Razdan S., Pandita K.K. (2004) Diagnosis of intraventricular cysticercosis by magnetic resonance imaging: improved detection with three-dimensional spoiled gradient recalled echo sequences. Australas Radiol 48: 237–239 [DOI] [PubMed] [Google Scholar]
- Rodriguez S., Dorny P., Tsang V.C., Pretell E.J., Brandt J., Lescano A.G., et al. (2009) Cysticercosis Working Group in Peru. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis 199: 1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sosa M., Satoskar A.R., Calderon R., Gomez-Garcia L., Saavedra R., Bojalil R., et al. (2002) Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and TH2-biasing ability. Infect Immun 70: 3656–3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román G., Sotelo J., Del Brutto O., Flisser A., Dumas M., Wadia N., et al. (2000) A proposal to declare neurocysticercosis an international reportable disease. Bull World Health Organ 78: 399–406 [PMC free article] [PubMed] [Google Scholar]
- Saenz B., Ruiz-Garcia M., Jimenez E., Hernandez-Aguilar J., Suastegui R., Larralde C., et al. (2006) Neurocysticercosis: clinical, radiologic, and inflammatory differences between children and adults. Pediatr Infect Dis J 25: 801–803 [DOI] [PubMed] [Google Scholar]
- Sahu P.S., Parija S.C., Jayachandran S. (2010) Antibody specific to 43kDa excretory-secretory antigenic peptide of Taenia solium metacestode as a potential diagnostic marker in human neurocysticercosis. Acta Trop 115: 257–261 [DOI] [PubMed] [Google Scholar]
- Sahu P.S., Parija S.C., Narayan S.K., Kumar D. (2009) Evaluation of an IgG-ELISA strategy using Taenia solium metacestode somatic and excretory-secretory antigens for diagnosis of neurocysticercosis revealing biological stage of the larvae. Acta Trop 110: 38–45 [DOI] [PubMed] [Google Scholar]
- Salinas R., Counsell C., Prasad K., Gelband H., Garner P. (1999) Treating neurocysticercosis medically: a systematic review of randomized, controlled trials. Trop Med Int Health 4: 713–718 [DOI] [PubMed] [Google Scholar]
- Salinas R., Prasad K. (2007) Drugs for treating neurocysticercosis (tapeworm infection of the brain). Cochrane Database Syst Rev 18: CD000215–CD000215 [DOI] [PubMed] [Google Scholar]
- Sarti E., Schantz P.M., Avila G., Ambrosio J., Medina-Santillan R., Flisser A. (2000) Mass treatment against human taeniasis for the control of cysticercosis: a population based intervention study. Trans R Soc Trop Med Hyg 94: 85–89 [DOI] [PubMed] [Google Scholar]
- Singh G. (1997) Neurocysticercosis in South–Central America and the Indian subcontinent. A comparative evaluation. Arq Neuropsiquiatr 55: 349–356 [DOI] [PubMed] [Google Scholar]
- Singhi P., Dayal D., Khandelwal N. (2003a) One week versus four weeks of albendazole therapy for neurocysticercosis in children: a randomized placebo controlled double blind trial. Pediatr Infect Dis J 22: 268–272 [DOI] [PubMed] [Google Scholar]
- Singhi P., Dinakaran, Khandelwal N.K. (2003b) One year versus two years of antiepileptic therapy for SSECTL. J Trop Pediatr 5: 274–278 [DOI] [PubMed] [Google Scholar]
- Singhi P., Jain V., Khandelwal N. (2004) Corticosteroids versus albendazole for treatment of single small enhancing CT lesions in children with NCC. J Child Neurol 19: 323–327 [DOI] [PubMed] [Google Scholar]
- Singhi P., Mahajan V., Khandelwal N. (2008) Sudden-onset ptosis caused by midbrain neurocysticercosis in two children. J Child Neurol 3: 334–337 [DOI] [PubMed] [Google Scholar]
- Singhi P., Ray M., Singhi S., Khandelwal N. (2000) Clinical spectrum of 500 children with neurocysticercosis and response to albendazole therapy. J Child Neurol 15: 207–213 [DOI] [PubMed] [Google Scholar]
- Singhi P.D., Baranwal A.K. (2001) Single small enhancing computed tomographic lesion in Indian children—I: Evolution of current concepts. J Trop Pediatr 47: 204–207 [DOI] [PubMed] [Google Scholar]
- Sotelo J., del Brutto O.H., Penagos P., Escobedo F., Torres B., Rodriguez-Carbajal J., et al. (1990) Comparison of therapeutic regimen of anticysticercal drugs for parenchymal brain cysticercosis. J Neurol 237: 69–72 [DOI] [PubMed] [Google Scholar]
- Sotelo J., Escobedo F., Rodriguez-Carbajal J., Torres B., Rubio-Donnadieu F. (1984) Therapy of parenchymal brain cysticercosis with praziquantel. N Engl J Med 310: 1001–1007 [DOI] [PubMed] [Google Scholar]
- Sotelo J., Guerrero V., Rubio F. (1985a) Neurocysticercosis: A new classification based on active and inactive forms. A study of 753 cases. Arch Intern Med 145: 442–445 [PubMed] [Google Scholar]
- Sotelo J., Torres B., Rubio-Donnadieu F., Escobedo F., Rodriquez-Carbajal J. (1985b) Praziquantel in the treatment of neurocysticercosis: long term follow-up. Neurology 35: 752–755 [DOI] [PubMed] [Google Scholar]
- Suri A., Goel R.K., Ahmad F.U., Vellimana A.K., Sharma B.S., Mahapatra A.K., et al. (2008) Transventricular, transaqueductal scope-in-scope endoscopic excision of fourth ventricular neurocysticercosis: a series of 13 cases and a review. J Neurosurg Pediatr 1: 35–39 [DOI] [PubMed] [Google Scholar]
- Takayanagui O.M., Jardim E. (1992) Therapy for neurocysticercosis. Comparison between albendazole and praziquantel. Arch Neurol 49: 290–294 [DOI] [PubMed] [Google Scholar]
- Talukdar B., Saxena A., Popli V.K., Choudhury V. (2002) Neurocysticercosis in children: clinical characteristics and outcome. Ann Trop Pediatr 22: 333–339 [DOI] [PubMed] [Google Scholar]
- Thussu A., Chattopadhyay A., Sawhney I.M., Khandelwal N. (2008) Albendazole therapy for single small enhancing CT lesions (SSECTL) in the brain in epilepsy. J Neurol Neurosurg Psychiatry 79: 238–239 [DOI] [PubMed] [Google Scholar]
- Tsang V.C., Brand J.A., Boyer A.E. (1989) An enzyme-linked immunoelectro-transfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 159: 50–59 [DOI] [PubMed] [Google Scholar]
- Vazquez M.L., Jung H., Sotelo J. (1987) Plasma levels of praziquantel decrease when dexamethasone is given simultaneously. Neurology 37: 1561–1562 [DOI] [PubMed] [Google Scholar]
- Vazquez V., Sotelo J. (1992) The course of seizures after treatment for cerebral cysticercosis. N Engl J Med 327: 696–701 [DOI] [PubMed] [Google Scholar]
- Vega R., Pinero D., Ramanankandrasana B., Dumas M., Bouteille B., Fleury A., et al. (2003) Population genetic structure of Taenia solium from Madagascar and Mexico: implications for clinical profile diversity and immunological technology. Int J Parasitol 33: 1479–1485 [DOI] [PubMed] [Google Scholar]
- Verma, A., Prasad, K.N., Gupta, R.K., Singh, A.K., Nyati, K.K., Rizwan, A. et al. (2010) Toll-like receptor 4 polymorphism and its association with symptomatic neurocysticercosis. J Infect Dis 202: 1219–1225. [DOI] [PubMed]
- White A.C., Jr (1997) Neurocysticercosis: a major cause of neurological disease worldwide. Clin Infect Dis 24: 101–113 [DOI] [PubMed] [Google Scholar]
- White A.C., Jr, Robinson P., Kuhn R. (1997) Taenia solium cysitcercosis host–parasite interactions and the immune response. Chem Immunol 66: 209–230 [PubMed] [Google Scholar]