Abstract
Potassium channel blockade has long been considered a potential therapeutic strategy for treatment of multiple sclerosis (MS) based on the pathophysiology of demyelinated axons. Dalfampridine, which is also known as fampridine or 4-aminopyridine (4-AP), is the potassium channel blocker that has been studied most extensively in MS and other demyelinating neurologic disorders. An extended-release formulation of dalfampridine was recently approved by the US Food and Drug Administration to improve walking in patients with MS. In randomized, double-blind, placebo-controlled trials, with dalfampridine extended release tablets 10 mg taken twice daily, about 12 h apart, walking speed was improved in approximately one-third of treated patients; in these patients, average walking speed on therapy was about 25% above baseline. This improvement was clinically meaningful as assessed by concurrent measurement of patient-reported severity of walking-related disability. Dalfampridine extended release tablets were generally well tolerated, with a range of adverse effects that appear to be related to increases in central nervous system excitation. There is a dose-dependent increase in the occurrence of seizures at doses higher than the recommended 10 mg twice daily.
Keywords: 4-aminopyridine, dalfampridine, fampridine, multiple sclerosis, potassium channel blockade, walking speed
Introduction
Multiple sclerosis (MS) is estimated to affect more than 2.1 million people worldwide and is the most common disabling neurologic disease in young adults [NMSS, 2010; Frohman, 2003]. Demyelination in the central nervous system is a pathologic hallmark of MS. The axonal conduction delay or block that results from demyelination is believed to play a major role in producing the characteristic symptoms of the disease, including visual deficits, weakness, paralysis, numbness, and other sensory disturbances [Compston and Coles, 2008; Smith and McDonald, 1999; McDonald, 1974]. At the level of individual nerve fibers, demyelination exposes voltage-dependent potassium channels, which leads to larger transmembrane voltage changes and increased potassium current from the axon. This results in action potential conduction delay or block [Rasband, 2005; Waxman, 1982; Bostock et al. 1981; Sherratt et al. 1980]. Based on these electrophysiological mechanisms, blockade of potassium channels to promote conduction in demyelinated axons has been considered as a potential therapeutic strategy for treatment of MS for more than 35 years [Bever, 2005; Kocsis et al. 1986; Waxman, 1982; Bostock et al. 1981, 1978].
Most studies addressing the treatment of demyelination with K+ channel blockade have used the drug 4-aminopyridine (4-AP). While the mechanism of action of this drug remains uncertain, experimental studies in isolated nerve preparations showed that 4-AP selectively blocked voltage-sensitive potassium channels, reduced potassium current, and increased action-potential duration in nonmyelinated systems [Bever, 2009; Meves and Pichon, 1977; Yeh et al. 1976; Pelhate, 1975]. In early clinical trials, intravenous or oral 4-AP was shown to improve muscle strength and neuromuscular transmission in patients with neuromuscular disorders, such as myasthenia gravis, congenital myasthenia, and Lambert–Eaton syndrome [Murray and Newsom-Davis, 1981; Lundh et al. 1979]. The drug was also used in some countries as an analeptic agent in anesthesiology, specifically to reverse the neuromuscular blocking effects of D-tubocurarine [Lundh, 1978]. However, in clinical studies, high doses of 4-AP were associated with seizures and other central nervous system adverse events (AEs), raising questions as to its clinical utility [Murray and Newsom-Davis, 1981; Ball et al. 1979]. A number of investigators subsequently reported certain beneficial effects of 4-AP in MS when the drug was carefully titrated to obtain maximal therapeutic effect while avoiding higher plasma concentrations associated with AEs, such as dizziness, nausea, or seizure, which were indicative of excessive nervous system excitation [Polman et al. 1994; van Diemen et al. 1992; Stefoski et al. 1987].
The original United States Adopted Name (USAN) for 4-AP was fampridine. The US Food and Drug Administration (FDA), at the time of approval of the drug in MS, required the name to be changed to avoid potential confusion with the existing drug famotidine. The name dalfampridine was proposed to the USAN Committee and the name change was accepted. Outside the USA, the drug will continue to be known by the International Nonproprietary Name fampridine. In January 2010, dalfampridine extended release tablets for use at 10 mg twice daily were approved by the FDA for the improvement of walking in patients with MS, as demonstrated by an increase in walking speed in two phase 3 randomized trials [Goodman et al. 2010, 2009]. For the purposes of this review, the name used will reflect the historical context of the works under discussion, but it should be recognized that 4-AP, fampridine, and dalfampridine are names used at different times and in different parts of the world for the same compound.
Experimental studies of 4-AP in models of demyelination
The concept of treating conduction failure in demyelinated axons arose from the observation that conduction could be improved by lowered temperatures, which prolong the action potential [Schauf and Davis, 1974; Rasminsky, 1973]. The first experiment to examine a pharmacological approach used scorpion venom to reduce sodium inactivation [Bostock et al. 1978]. This observation was followed by experimental studies showing that 4-AP, as well as tetraethylammonium chloride, another potassium channel blocker, prolonged compound action potentials in both demyelinated and unmyelinated isolated sensory nerve fibers from rat spinal roots [Bostock et al. 1981; Sherratt et al. 1980]. In subsequent studies, 4-AP also demonstrated the ability to restore conduction in isolated demyelinated rat sciatic nerves [Targ and Kocsis, 1985], and in central demyelinated axons in feline and guinea-pig models of spinal-cord injury [Jensen and Shi, 2003; Shi and Blight, 1997; Shi et al. 1997; Blight, 1989]. Only two of these studies incorporated concentrations of 4-AP low enough to be relevant to the submicromolar tissue concentrations that are practically tolerable with systemic delivery [Shi and Blight, 1997; Shi et al. 1997].
Clinical studies of immediate-release 4-AP in MS
Phase 1 and 2 trials
Quickly following the first studies in animal models of peripheral demyelination, 4-AP was evaluated in a small clinical trial involving two groups of five patients with MS: one with temperature-sensitive optic neuritis, the other with spastic paraparesis as a result of spinal MS [Jones et al. 1983]. The optic-neuritis group was treated in a crossover design with placebo followed by orally administered 4-AP, 10–20 mg daily for up to 7 days; the spastic-paraparesis group was also treated first with placebo then orally administered 4-AP up to 60 mg for 7 days. The results showed improvement with 4-AP treatment in some visual tests for the optic-neuritis group, including a reduction in the size of scotomata. Among spastic-paraparesis patients, there was no change in the Kurtzke Expanded Disability Status Scale (EDSS) [Kurtzke, 1983], following either placebo or 4-AP treatment, although one patient noted improvement in walking, and one reported a return of sacral sensation with 4-AP treatment. Overall, the investigators concluded that the potential risk of seizures, shown in other studies with higher doses [Ball et al. 1979], meant that the drug was unlikely to have practical utility in MS.
Other investigators continued to study the potential of 4-AP in MS using careful dose titration to minimize the potential for AEs [Stefoski et al. 1987]. They evaluated the effects of intravenous injection of 7–35 mg of 4-AP in 1–5 mg doses every 10–60 min over 1.5–3.5 h in 12 men with temperature-sensitive MS and five healthy men; five additional men with MS were administered single-blind placebo. 4-AP treatment was associated with mild to marked improvement in 10 out of the 12 patients; seven with vision improvement, five with oculomotor function improvement, and five with motor function improvement. One patient with severe paraparesis also experienced gait improvement. No serious AEs (SAEs) were observed.
A follow-up, double-blind, dose-ranging study in 20 men with temperature-sensitive MS investigated the effects of single, oral doses of 4-AP ranging from 10-25 mg using 2.5-mg and 5-mg capsules (n = 15) compared with placebo (n = 5) [Davis et al. 1990]. In this study, within 7 h after administration of a single dose of 4-AP, the investigators noted improved motor function including gait, power, and coordination in nine patients, improved vision in 11 patients, and improved oculomotor function in one out of two patients in which this endpoint was evaluated. No changes were observed in the placebo-treated group. No seizures were reported, and 4-AP was well tolerated. An additional double-blind study was performed using multiple doses of 4-AP 7.5–52.5 mg administered in one to three daily doses over 1–5 days in both men and women with temperature-sensitive MS. Clinically important motor and visual improvements were reported in 13 out of 17 4-AP-treated patients compared with 3 out of 9 placebo-treated patients [Stefoski et al. 1991]. Average peak improvement scores on a 0–6 scale of function were 0.40 for 4-AP and 0.12 for placebo (p = 0.03); no SAEs were reported.
Subsequent to these small, short-term studies in selected patients, a randomized, double-blind, placebo-controlled, 24-week crossover trial of immediate-release 4-AP was conducted in 70 patients with clinically definite MS [van Diemen et al. 1992]. Patients were randomized 1:1 to either 12 weeks of placebo then crossover to 12 weeks of 4-AP, or 4-AP followed by placebo on the same schedule. 4-AP was orally administered starting at 10–15 mg daily in two to three divided doses, then titrated up to a maximum dose of 0.5 mg/kg of body weight. The primary efficacy measure was change in the EDSS score.
After 24 weeks, EDSS score, which was 5.0 at baseline, had improved (decreased) by 0.28 points while on 4-AP compared with placebo (p = 0.001). Ten patients experienced a clinically significant decrease of ≥1.0 points in EDSS during 4-AP treatment; this degree of improvement was not seen in any patients receiving placebo (p < 0.05). In addition, 18 patients reported subjective improvement while receiving 4-AP compared with only one patient receiving placebo (p < 0.05). A total of 18 (29.5%) patients responded to 4-AP, defined as either a decrease of 1.0 in EDSS score or a positive subjective response during 4-AP treatment. No seizures or other SAEs were reported, and AEs were generally mild, leading to withdrawal in three patients receiving 4-AP and one patient receiving placebo. They concluded that patients with temperature-sensitive symptoms, patients characterized by having a longer duration of disease, and patients in progressive disease had a higher probability of improvement.
A long-term, open-label, follow-up extension study was conducted in 31 patients, including 19 who had participated in the preceding 24-week crossover study and expressed a positive subjective response to 4-AP, and an additional 12 patients with MS who met the same eligibility criteria as those who participated in the crossover study [Polman et al. 1994]. Patients were administered either the last dose of 4-AP they had been taking in the preceding crossover trial, or were started with a dose of 10–15 mg in two to three divided doses and titrated up to a maximum dose of 0.5 mg/kg of body weight. Efficacy was assessed by patient self-report and EDSS score. At the end of the extension study, 23 patients had been treated with 4-AP for more than 6 months and up to 32 months. Of these patients, 13 reported subjective improvement in ambulation, 13 in fatigue, and five in visual function. The mean change in EDSS during follow-up was an increase of 0.35 per year. Seizures occurred in two patients who had continued 4-AP treatment from the preceding trial. With the exception of one patient who experienced severe liver dysfunction, no other SAEs were reported [Polman et al. 1994].
Addressing the narrow therapeutic index
As seizures had been reported with immediate-release 4-AP treatment in patients with MS [Polman et al. 1994], as well as other neuromuscular disorders [Murray and Newsom-Davis, 1981], clinical studies were conducted to determine the dose that would provide the optimal balance of efficacy and safety. One 12-week study investigated the relationship between dosage, serum level, efficacy, and safety of intravenously and orally administered 4-AP in 70 patients with MS who had participated in the 24-week crossover study described above [van Diemen et al. 1993, 1992]. In this study, immediate-release 4-AP administered in oral doses three times daily was associated with large fluctuations in drug serum levels. Efficacy, as assessed by smooth pursuit gain of eye movements and incidence of AEs was correlated with higher serum levels. They also concluded that higher dosages and serum levels were more likely to produce greater improvement in those patients who were capable of responding.
Another randomized, placebo-controlled, double-blind, crossover trial compared the short-term safety and efficacy of sequential treatment for 1 day each with placebo, with 4-AP at a low-target serum level of 30–59 ng/mL (actual range 35–64 ng/mL), and 4-AP at a high-target serum level of 60–100 ng/mL (actual range 57–114 ng/mL) in eight MS patients with temperature-sensitive visual and motor deficits [Bever et al. 1994]. This study recorded seven AEs during the placebo phase, nine during the low–serum-concentration phase, and 36 during the high–serum-concentration phase. Among these, two seizure-related SAEs occurred during the high–serum-concentration phase: a grand mal tonic-clonic seizure, which occurred at a 4-AP serum peak level of 104 ng/mL, and an episode of encephalopathy at a 4-AP peak serum level of 114 ng/mL. The investigators reported only a small association between improvements in visual and motor functions and 4-AP serum levels, and there were no changes in the Ambulatory Index or EDSS scores at either dosing level. These findings suggested that control of 4-AP serum levels by modulating drug delivery could minimize AEs while maintaining efficacy [Bever et al. 1994; van Diemen et al. 1993]. Thus, new formulations of 4-AP, then known as fampridine, were developed to achieve efficacy without exceeding plasma concentrations of 100 ng/mL [Bever and Judge, 2009].
A number of capsule and tablet formulations with more prolonged-release characteristics were developed and tested in pharmacokinetic studies, and one was tested in a larger study of efficacy in 1994. This multicenter, placebo-controlled, double-blind study was conducted in 161 patients with stable MS who were randomized to an early sustained-release formulation of fampridine or placebo for 6 weeks (data unpublished but mentioned in Schwid et al. [1997]). In this 1994 study, the percentage of subjects showing improvement in EDSS scores, the primary outcome, was 22% in both the fampridine and placebo groups. However, it was considered that the EDSS may have been an inadequate primary measure for this trial, based on reports of substantial intra-rater and inter-rater variability observed for this scale [Francis et al. 1991; Noseworthy et al. 1990; Amato et al. 1989], and its potential insensitivity to change because of its ordinal design [CCMSSG, 1991].
Clinical trials of dalfampridine extended release tablets in MS patients
A more stable formulation of fampridine tablets, then known as fampridine sustained-release or fampridine-SR, was developed. This formulation was essentially the same as the currently marketed drug, dalfampridine extended release tablets, although it was tested in a range of dosage strengths, including the current 10 mg.
The first study with this formulation incorporated a crossover design and was conducted in 10 patients with clinically definite, stable MS, and limb weakness as a major symptom. Patients were randomized to double-blind treatment with fampridine 17.5 mg twice daily or placebo for 7 days, followed by a washout period, and then crossed over for another 7 days [Schwid et al. 1997]. The outcome measures in this trial included the EDSS, but also time-to-walk-8-m test, as well as maximum voluntary isometric contraction test (MVICT), manual muscle test (MMT), and grip strength measurement. Comparing the two periods of crossover treatment, 9 out of the 10 patients had faster walking time on the 8-m timed-walk test with fampridine treatment, compared with placebo (p = 0.02). EDSS scores improved in three patients with fampridine treatment, which indicated a trend towards a treatment effect. MVICT, MMT, and grip strength all improved with fampridine, although not significantly, compared with placebo in these small groups. It was reported that treatment appeared particularly efficacious in subjects with serum 4-AP levels above 60 ng/mL. No SAEs were reported. Overall, these results were interpreted as suggesting that timed-walk testing was a more sensitive measure of functional change than EDSS in studies of fampridine [Schwid et al. 1997].
Later analyses by other researchers have supported this assessment of the two measures. One study in MS patients found that maximum walking distance at the speed most comfortable for them, which is the major ambulation assessment included in EDSS, varied substantially on a day-to-day basis, while average walking speed, as derived by dividing mean maximum distance by mean walking time in these patients, was a more stable and reliable measure of disability [Albrecht et al. 2001]. The timed 25-foot walk (T25FW) of the MS Functional Composite (MSFC) assesses change in ambulatory function [Polman and Rudick, 2010], and is considered a measure of maximal walking speed [Cohen, 2010; Dobkin, 2006]. Independent studies have demonstrated that a greater than 20% change in T25FW time is clinically meaningful [Kragt et al. 2006; Hoogersvorst et al. 2004; Schwid et al. 2002; Kaufman et al. 2000]. Other studies found that the EDSS had poor responsiveness to clinical change and limited validity [Hobart et al. 2000; Sharrack et al. 1999].
Dose-ranging phase 2 studies
Study MS-F201
Following the pilot study of motor effects [Schwid et al. 1997], a phase 2, randomized, double-blind, placebo-controlled, dose-ranging study (MS-F201) was conducted in 36 patients with MS who were randomized to fampridine (n = 25) at doses escalating from 10–40 mg twice daily in weekly increments of 5 mg, or to placebo (n = 11) for 8 weeks [Goodman et al. 2007]. This study included the MSFC and Lower-Extremity Manual Muscle Test (LEMMT) as measures of efficacy, together with a number of measures of fatigue. There were no significant differences between the treatment groups in the planned efficacy analyses, which were based on analysis of variance. However, a post hoc repeated-measures analysis showed significant improvement with fampridine treatment in walking speed, based on the T25FW, a component of the MSFC (p = 0.03), and in the LEMMT (p = 0.01). Average changes in walking speed and strength were apparent at the 10-mg twice-daily dosing, and increased during the 15-mg and slightly during the 20-mg twice-daily dosing periods, but did not further increase at doses higher than 20 mg.
The most common treatment-emergent AEs, each occurring more frequently with fampridine than with placebo, were dizziness (36% in the fampridine group, 18% in the placebo group), insomnia (36% fampridine, 9% placebo), paresthesia (32% fampridine, 9% placebo), asthenia and nausea (each 28% fampridine, 9% placebo), and headache and tremor (each 24% fampridine; 9% and 0%, respectively, placebo). Most AEs were mild to moderate in severity, transient, and occurred across the treatment period with no relationship to dose or duration of treatment. Severe AEs occurred only at doses >25 mg twice daily, as did AEs leading to discontinuation, which occurred in five patients: one for dizziness with 25 mg twice daily; one for pain plus asthenia and one for convulsion, both with 30 mg twice daily; one for ataxia plus tremor and one for convulsion, both with 35 mg twice daily.
Study MS-F202
A larger, phase 2, dose-ranging study of fampridine, MS-F202, was conducted in 206 patients with MS at 24 study centers in the USA and Canada [Goodman et al. 2008]. Patients were randomized to receive double-blind treatment with fampridine 10, 15, or 20 mg twice daily or placebo for 15 weeks. The primary efficacy endpoint was the percentage change in walking speed as measured by the T25FW, which has been validated in MS as one of the three components of the MSFC [Fischer et al. 1999]. In this trial, all three fampridine-dose groups showed greater average increases in walking speed as measured by the T25FW compared with placebo, although statistical significance was not met. Average LEMMT score, a prespecified secondary outcome, was significantly improved in the fampridine 10-mg and 15-mg groups compared with placebo (p = 0.02 and p = 0.003, respectively), but not in the 20-mg group.
A novel responder analysis of the walking speed results was conducted retrospectively to assess consistency of walking-speed improvement, rather than percentage change in walking speed. A ‘timed-walk responder’ to active treatment was defined as a patient whose walking speed for at least three visits during the double-blind treatment period was faster than the maximum speed measured in the five off-treatment visits (four prior to treatment and one at 2 weeks after the end of treatment). This post hoc analysis found that the response rates in the fampridine 10-, 15-, and 20-mg twice-daily dose groups were 35.3%, 36.0%, and 38.6%, respectively, and 36.7% for the pooled fampridine-dose groups. Interestingly, the response rates did not appear to increase with dose. These rates were all individually and collectively greater than the 8.5% response rate in the placebo group (p < 0.001 for pooled vs. placebo). Among fampridine-treated timed-walk responders, the mean improvement in walking speed was consistently 25%–29% across the double-blind treatment period, and was significantly greater than in the placebo group (2%–4%) at every visit, both for each fampridine-dose group and for the pooled-dose groups. Fampridine was generally well tolerated, with most AEs mild to moderate in severity. SAEs occurred at higher rates in the 15- and 20-mg dose groups, and at a similar rate in the 10-mg dose group compared with the placebo group. Discontinuations due to AEs included one patient in the placebo group, no patients in the fampridine 10-mg group, and one patient in the 15-mg group who discontinued because of nausea and dizziness. Five patients discontinued in the fampridine 20-mg group, including two who experienced seizure. One of these patients had two separate incidents of complex partial seizures after taking an overdose of the study medication on both occasions.
Phase 3 trials
Study MS-F203
Following the promising results of these phase 2 trials, a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial was conducted in patients with any type of MS to evaluate the safety and efficacy of dalfampridine extended release tablets 10 mg twice daily (n = 229) compared with placebo (n = 72) for 14 weeks [Goodman et al. 2009]. Eligibility criteria included the ability to complete two trials of the T25FW in 8–60 seconds. The primary endpoint was the percentage of timed-walk responders in each treatment group. Secondary endpoints included: LEMMT scores; the 12-item Multiple Sclerosis Walking Scale (MSWS-12) [Motl and Snook, 2008; McGuigan and Hutchinson, 2004; Hobart et al. 2003], a patient-reported outcome used to evaluate the clinical significance of the timed-walk response criterion; the Ashworth score for spasticity; a subject global impression (SGI); and a clinician global impression (CGI). In this trial, as in the post hoc analysis of MS-F202, a timed-walk responder was defined as a patient who had a faster walking speed for at least three out of four visits during the double-blind treatment period than the maximum speed for any of the first five off-treatment visits.
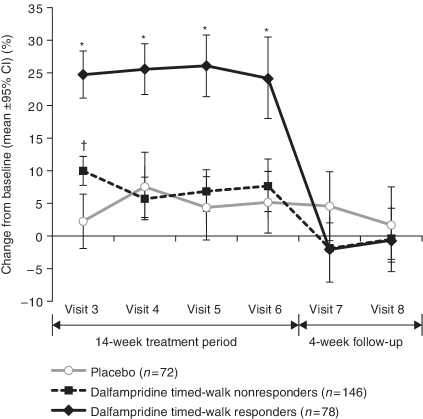
The percentage of timed-walk responders was significantly higher in the fampridine group (35%) compared with the placebo group (8%; p < 0.0001). The average change from baseline in walking speed among the fampridine-treated timed-walk responders during double-blind treatment was 25.2%, or 0.5 ft/s, compared with 4.7%, or 0.1 ft/s, in the placebo group (Figure 1). The increase in walking speed among the fampridine-treated timed-walk responders was maintained throughout the double-blind treatment period (Figure 1). MSWS-12 scores during treatment were reduced (improved) by an average of −6.84 for timed-walk responders versus an increase of 0.05 for timed-walk nonresponders (p = 0.0002), regardless of treatment group; scores were consistently lower for all 12 items of the scale among timed-walk responders compared with nonresponders. Timed-walk responders also had more positive mean SGI scores than nonresponders (4.88 vs. 4.43, respectively; p = 0.001), and more improved mean CGI scores (3.28 vs. 3.74, respectively; p < 0.0001). The average improvement in the LEMMT scores among fampridine-treated timed-walk responders was 0.18 compared with 0.04 for the placebo group (p = 0.0002).
Figure 1.
Percentage change in walking speed as measured by the timed 25-foot walk at each visit after randomization (MS-F203). *p < 0.001 versus placebo and fampridine-treated timed-walk nonresponders; †p < 0.001 versus placebo only. (Reproduced with permission from Goodman et al. [2009].) CI, confidence interval.
Fampridine was generally well tolerated. A total of 11 (5%) fampridine-treated patients were withdrawn due to AEs, which included sepsis, ankle fracture, balance disorder, confusional state, dizziness, headache, and anxiety. SAEs that were possibly or probably related to treatment occurred in two patients, including one who experienced severe anxiety. This individual had been taking diazepam as needed for anxiety and insomnia before the study. The other patient contracted sepsis secondary to community-acquired pneumonia and during this event experienced a focal seizure that was judged to be possibly related to treatment.
Study MS-F204
A second phase 3 trial of dalfampridine extended release tablets, 10 mg twice daily, MS-F204, was conducted in 239 patients aged 18–70 years with ambulatory deficits due to MS. Eligibility criteria included completion of the T25FW in 8–45 seconds at screening [Goodman et al. 2010, 2009, 2008]. Patients were randomized to double-blind treatment with placebo (n = 119) or dalfampridine 10 mg (n = 120) twice daily for 9 weeks. The primary outcome was the percentage of timed-walk responders in each treatment group, defined as in the MS-F202 and MS-F203 trials [Goodman et al. 2010]. The secondary measure of efficacy was LEMMT score in dalfampridine-treated timed-walk responders versus nonresponders. The MSWS-12 was also used to confirm the clinical meaningfulness of the primary efficacy endpoint as in the MS-F203 trial.
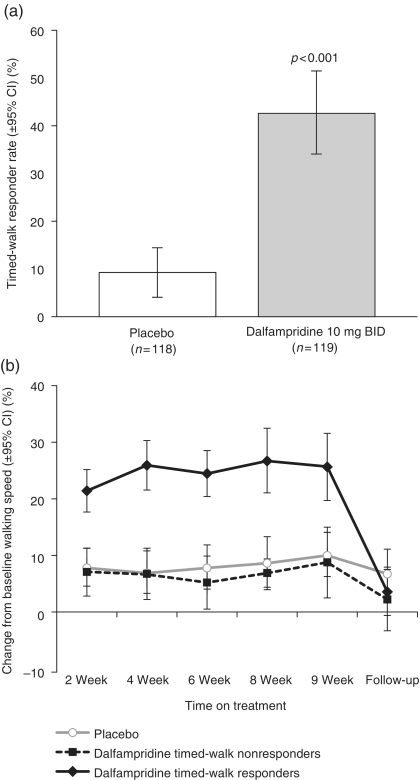
Outcomes from this trial were consistent with the MS-F203 trial. The response rate in the dalfampridine group was higher compared with the placebo group (42.9% vs. 9.3%, respectively; p < 0.001) (Figure 2(a)). The average improvement from baseline in walking speed among dalfampridine-treated timed-walk responders was 25% compared with 6% for nonresponders and 8% for the placebo group [Goodman et al. 2010] (Figure 2(b)). Leg strength, as measured by LEMMT, was also improved among the dalfampridine-treated timed-walk responders, compared with placebo-treated patients (p = 0.028). In addition, timed-walk responders, irrespective of treatment group, had greater improvements than nonresponders in average change from baseline in MSWS-12, SGI, and CGI [Goodman et al. 2010]. The AE profile of dalfampridine in this trial was similar to that in other trials. The most common AEs in the dalfampridine-treated group that were also more frequent than in the placebo group were urinary tract infections, insomnia, and headache [Goodman et al. 2010]. Three (2.5%) patients in the dalfampridine group did not complete the study due to a treatment-emergent AE or SAE, including patellar fracture, symptomatic hypotension, and headache; no seizures occurred in the dalfampridine group, but there was one instance of partial complex seizure reported in the placebo group [Goodman et al. 2010].
Figure 2.
(a) Percentage of timed-walk responders in placebo- and dalfampridine-treated patients. (b) Percentage change from baseline walking speed at each visit following randomization, by responder analysis group. The dalfampridine-treated timed-walk responder showed sustained improvement during treatment that was completely reversed at the 2-week follow-up visit. The dalfampridine-treated timed-walk nonresponder showed a smaller increase, similar to that seen in the placebo-treated group. (Adapted with permission from Goodman et al. [2010].) BID, twice daily; CI, confidence interval.
Summary and appraisal of data
Data from the two phase 3 clinical trials demonstrated that dalfampridine 10 mg twice daily improved walking speed (i.e. reduced the time to walk 25 feet) in 35% and 42.3% of people with MS. The average improvement in T25FW speed in those responding to dalfampridine was approximately 25% across a wide range of baseline deficits. As noted above, changes of greater than 20% in T25FW time have been shown to be clinically meaningful [Kragt et al. 2006; Hoogersvorst et al. 2004; Schwid et al. 2002; Kaufman et al. 2000]. Time to complete the T25FW was also found to correlate significantly with ambulation in the community setting in patients with Friedreich’s ataxia, as measured by accelerometry, which is the current gold standard for measurement of real-world ambulation [Fahey et al. 2007; Pearson et al. 2004]. In stroke patients, moreover, gait velocity as measured in meters per second has been shown to be a leading indicator of real-world walking function and is used as the basis of classifications, including household ambulation (<0.4 m/s), limited community ambulation (0.4–0.8 m/s), and full community ambulation (>0.8 m/s) [van de Port et al. 2008; Schmid et al. 2007]. While these classifications have not been widely applied to MS patients, a study of 166 people with MS found that only 13% of participants had a walking speed of >1.5 m/s, which, the investigators noted, is the minimum speed necessary to traverse a crosswalk in Europe before the traffic light has changed [Einarsson et al. 2006]. Hence, walking speed has become increasingly viewed as a key indicator of community ambulatory function in various disease populations, although the magnitude of this association in MS patients is yet to be fully determined. It should be noted that a 20% increase in walking speed on the T25FW is equivalent but opposite to (would be negated by) a 20% increase in walking time.
Importantly, the improvements in T25FW time associated with dalfampridine were also shown to be clinically meaningful as measured by patient self-report using the MSWS-12, a well-validated measure of the impact of MS on walking quality and response to treatment [Motl and Snook, 2008; McGuigan and Hutchinson, 2004; Hobart et al. 2003]. The MSWS-12 has also correlated strongly with accelerometry data in multiple studies [Sosnoff et al. 2010; Snook et al. 2009; Motl and Snook, 2008].
Dalfampridine is generally well tolerated, but there is a dose-dependent increase in the risk of seizures at doses higher than the recommended dose of 10-mg, twice-daily, extended release formula. The mechanism of action of dalfampridine remains uncertain but probably includes improvement in action-potential conduction in demyelinated nerve fibers.
Concluding remarks
Walking impairment is among the most disabling problems associated with MS, and is associated with substantial adverse impact on patients’ lives and healthcare costs [Zwibel, 2009]. Treatment with dalfampridine extended release tablets 10 mg twice daily improves walking in approximately one-third of treated patients across MS disease types as measured by walking speed. This improvement is clinically meaningful, as indicated by patient self-report and by the average 25% improvement in walking speed among timed-walk responders. Use of dalfampridine extended release tablets at the recommended dose of 10 mg twice daily is important to avoid the increased risk of seizure observed at higher doses.
Acknowledgment
Editorial assistance in preparation of this manuscript was provided by the Curry Rockefeller Group, LLC.
Funding
Editorial assistance was funded by Acorda Therapeutics, Inc.
Conflict of interest statement
AB is an employee of Acorda Therapeutics, Inc., and holds stock in the company.
References
- Albrecht H., Wötzel C., Erasmus L.P., Kleinpeter M., König N., Pöllmann W. (2001) Day-to-day variability of maximum walking distance in MS patients can mislead to relevant changes in the Expanded Disability Status Scale (EDSS): Average walking speed is a more constant parameter. Mult Scler 7: 105–109 [DOI] [PubMed] [Google Scholar]
- Amato M.P., Fratiglione L., Groppi C., Siracuse G., Amaducci L. (1989) Interrater reliability in assessing functional systems and disability on the Kurtzke Scale in multiple sclerosis. Arch Neurol 45: 746–748 [DOI] [PubMed] [Google Scholar]
- Ball A.P., Hopkinson R.B., Farrell I.D., Hutchison J.G., Paul R., Watson R.D., et al. (1979) Human botulism caused by Clostridium botulinum type E: The Birmingham outbreak. QJM 48: 473–491 [PubMed] [Google Scholar]
- Bever C.T., Jr (2005) Clinical pharmacology of abnormal potassium channel organization in demyelinated axons. In: Waxman S.G. (ed.). Multiple Sclerosis as a Neuronal Disease, Elsevier Academic Press: Amsterdam [Google Scholar]
- Bever C.T., Jr (2009) 10 questions about 4-aminopyridine and the treatment of multiple sclerosis. Neurologist 15: 161–162 [DOI] [PubMed] [Google Scholar]
- Bever C.T., Jr, Judge S.I. (2009) Sustained-release fampridine for multiple sclerosis. Expert Opin Investig Drugs 18: 1013–1024 [DOI] [PubMed] [Google Scholar]
- Bever C.T., Jr, Young D., Anderson P.A., Krumholz A., Conway K., Leslie J., et al. (1994) The effects of 4-aminopyridine in multiple sclerosis patients: Results of a randomized, placebo-controlled, double-blind, concentration-controlled, crossover trial. Neurology 44: 1054–1059 [DOI] [PubMed] [Google Scholar]
- Blight A.R. (1989) Effect of 4-aminopyridine on axonal conduction-block in chronic spinal cord injury. Brain Res Bull 22: 47–52 [DOI] [PubMed] [Google Scholar]
- Bostock H., Sears T.A., Sherratt R.M. (1981) The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J Physiol 313: 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Sherratt R.M., Sears T.A. (1978) Overcoming conduction failure in demyelinated nerve fibres by prolonging action potential. Nature 274: 385–387 [DOI] [PubMed] [Google Scholar]
- Canadian Cooperative Multiple Sclerosis Study Group (CCMSSG) (1991) The Canadian cooperative trial of cyclophosphamide and plasma exchange in progressive multiple sclerosis. Lancet 337: 441–446 [PubMed] [Google Scholar]
- Cohen J.T. (2010) Walking speed and economic outcomes for walking-impaired patients with multiple sclerosis. Expert Rev Pharmacoecon Outcomes Res 10: 595–603 [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A. (2008) Multiple sclerosis. Lancet 372: 1502–1517 [DOI] [PubMed] [Google Scholar]
- Davis F.A., Stefoski D., Rush J. (1990) Orally administered 4-aminopyridine improves clinical signs in multiple sclerosis. Ann Neurol 27: 186–192 [DOI] [PubMed] [Google Scholar]
- Dobkin B.H. (2006) Short-distance walking speed and timed walking distance: Redundant measures for clinical trials? Neurology 66: 584–586 [DOI] [PubMed] [Google Scholar]
- Einarsson U., Gottberg K., von Koch L., Fredrikson S., Ytterberg C., Jin Y.P., et al. (2006) Cognitive and motor function in people with multiple sclerosis in Stockholm County. Mult Scler 12: 340–353 [DOI] [PubMed] [Google Scholar]
- Fahey M.C., Corben L.A., Collins V., Churchyard A.J., Delatycki M.B. (2007) The 25-foot walk velocity accurately measures real world ambulation in Friedreich ataxia. Neurology 68: 705–706 [DOI] [PubMed] [Google Scholar]
- Fischer J.S., Rudick R.A., Cutter G.R., Reingold S.C. (1999) The Multiple Sclerosis Functional Composite Measure (MSFC): An integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 5: 244–250 [DOI] [PubMed] [Google Scholar]
- Francis D.A., Bain P., Swan A.V., Hughes A.C. (1991) An assessment of disability rating scales used in multiple sclerosis. Arch Neurol 48: 291–301 [DOI] [PubMed] [Google Scholar]
- Frohman E.M. (2003) Multiple sclerosis. Med Clin North Am 87: 867–897 [DOI] [PubMed] [Google Scholar]
- Goodman A.D., Brown T.R., Cohen J.A., Krupp L.B., Schapiro R., Schwid S.R., et al. Fampridine MS-F202 Study Group (2008) Dose comparison trial of sustained-release fampridine in multiple sclerosis. Neurology 71: 1134–1141 [DOI] [PubMed] [Google Scholar]
- Goodman A.D., Brown T.R., Edwards K.R., Krupp L.B., Schapiro R.T., Cohen R., et al. (2010) A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol 68: 494–502 [DOI] [PubMed] [Google Scholar]
- Goodman A.D., Brown T.R., Krupp L.B., Schapiro R.T., Schwid S.R., Cohen R., et al. Fampridine MS-F203 Investigators (2009) Sustained-release oral fampridine in multiple sclerosis: A randomised, double-blind, controlled trial. Lancet 373: 732–738 [DOI] [PubMed] [Google Scholar]
- Goodman A.D., Cohen J.A., Cross A., Vollmer T., Rizzo M., Cohen R., et al. (2007) Fampridine-SR in multiple sclerosis: A randomized, double-blind, placebo-controlled, dose-ranging study. Mult Scler 13: 357–368 [DOI] [PubMed] [Google Scholar]
- Hobart J.C., Freeman J., Thompson A. (2000) Kurtzke scales revisited: The application of psychometric methods to clinical intuition. Brain 123: 1027–1040 [DOI] [PubMed] [Google Scholar]
- Hobart J.C., Riazi A., Lamping D.L., Fitzpatrick R., Thompson A.J. (2003) Measuring the impact of MS on walking ability: The 12-item MS Walking Scale (MSWS-12). Neurology 60: 31–36 [DOI] [PubMed] [Google Scholar]
- Hoogervorst E.L.J., Kalkers N.F., Cutter G.R., Uitdehaag B.M.J., Polman C.H. (2004) The patient’s perception of a (reliable) change in the Multiple Sclerosis Functional Composite. Mult Scler 10: 55–60 [DOI] [PubMed] [Google Scholar]
- Jensen J.M., Shi R. (2003) Effects of 4-aminopyridine on stretched mammalian spinal cord: The role of potassium channels in axonal conduction. J Neurophysiol 90: 2334–2340 [DOI] [PubMed] [Google Scholar]
- Jones R.F., Heron J.R., Foster D.H., Snelgar R.S., Mason R.J. (1983) Effects of 4-aminopyridine in patients with multiple sclerosis. J Neurol Sci 60: 353–362 [DOI] [PubMed] [Google Scholar]
- Kaufman M., Moyer D., Norton J. (2000) The significant change for the Timed 25-Foot Walk in the Multiple Sclerosis Functional Composite. Mult Scler 6: 286–290 [DOI] [PubMed] [Google Scholar]
- Kocsis J.D., Bowe C.M., Waxman S.G. (1986) Different effects of 4-aminopyridine on sensory and motor fibers: Pathogenesis of paresthesias. Neurology 36: 117–120 [DOI] [PubMed] [Google Scholar]
- Kragt J.J., van der Linden F.A.H., Nielsen J.M., Uitdehaag B.M.J., Polman C.H. (2006) Clinical impact of 20% worsening on Timed 25-Foot Walk and 9-Hole Peg Test in multiple sclerosis. Mult Scler 12: 594–598 [DOI] [PubMed] [Google Scholar]
- Kurtzke J.F. (1983) Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 33: 1444–1452 [DOI] [PubMed] [Google Scholar]
- Lundh H. (1978) Effects of 4-aminopyridine on neuromuscular transmission. Brain Res 153: 307–318 [DOI] [PubMed] [Google Scholar]
- Lundh H., Nilsson O., Rosén I. (1979) Effects of 4-aminopyridine in myasthenia gravis. J Neurol Neurosurg Psychiatry 42: 171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W.I. (1974) Pathophysiology in multiple sclerosis. Brain 97: 179–196 [DOI] [PubMed] [Google Scholar]
- McGuigan C., Hutchinson M. (2004) Confirming the validity and responsiveness of the Multiple Sclerosis Walking Scale-12 (MSWS-12). Neurology 62: 2103–2105 [DOI] [PubMed] [Google Scholar]
- Meves H., Pichon Y. (1977) The effect of internal and external 4-aminopyridine on the potassium currents in intracellularly perfused squid giant axons. J Physiol 268: 511–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl R.W., Snook E.M. (2008) Confirmation and extension of the validity of the Multiple Sclerosis Walking Scale-12. J Neurol Sci 268: 69–73 [DOI] [PubMed] [Google Scholar]
- Murray N.M., Newsom-Davis J. (1981) Treatment with oral 4-aminopyridine in disorders of neuromuscular transmission. Neurology 31: 265–271 [DOI] [PubMed] [Google Scholar]
- National Multiple Sclerosis Society (NMSS). About MS: Who Gets MS? [ http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/who-gets-ms/index.aspx; accessed 27 July 2010]
- Noseworthy J.H., Vandervoort M.K., Wong C.J., Ebers G.C. Canadian Cooperative MS Study Group (1990) Interrater variability with Expanded Disability Status Scale (EDSS) and Functional Systems (FS) in a multiple sclerosis trial. Neurology 40: 971–975 [DOI] [PubMed] [Google Scholar]
- Pearson O.R., Busse M.E., van Deursen R.W., Wiles C.M. (2004) Quantification of walking mobility in neurological disorders. QJM 97: 463–475 [DOI] [PubMed] [Google Scholar]
- Pelhate M., Mony L., Hue B., Chanelet J. (1975) Modification of potassium current by 4-aminopyridine. Case of the isolated giant axon of the cockroach (Periplanata americana). CR Seances Soc Biol Fil 169: 1436–1441 [PubMed] [Google Scholar]
- Polman C.H., Bertelsmann F.W., van Loenen A.C., Koetsier J.C. (1994) 4-aminopyridine in the treatment of patients with multiple sclerosis. Arch Neurol 51: 292–296 [DOI] [PubMed] [Google Scholar]
- Polman C.H., Rudick R.A. (2010) The multiple sclerosis functional composite: A clinically meaningful measure of disability. Neurology 74: S8–S15 [DOI] [PubMed] [Google Scholar]
- Rasband M.N.(2005) Potassium channel organization of myelinated and demyelinated axons. In: Waxman S.G. (ed.). Multiple Sclerosis as a Neuronal Disease, Elsevier Academic Press: Amsterdam [Google Scholar]
- Rasminsky M. (1973) The effects of temperature on conduction in demyelinated single nerve fibers. Arch Neurol 28: 287–292 [DOI] [PubMed] [Google Scholar]
- Schauf C.L., Davis S.A. (1974) Impulse conduction in multiple sclerosis: A theoretical basis for modification by temperature and pharmacological agents. J Neurol Neurosurg Psychiatry 37: 152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Duncan P.W., Studenski S., Lai S.M., Richards L., Perera S., et al. (2007) Improvements in speed-based gait classifications are meaningful. Stroke 38: 2096–2100 [DOI] [PubMed] [Google Scholar]
- Schwid S.R., Goodman A.D., McDermott M.P., Bever C.F., Cook S.D. (2002) Quantitative functional measures in MS: What is a reliable change? Neurology 58: 1294–1296 [DOI] [PubMed] [Google Scholar]
- Schwid S.R., Petrie M.D., McDermott M.P., Tierney D.S., Mason D.H., Goodman A.D. (1997) Quantitative assessment of sustained-release 4-aminopyridine for symptomatic treatment of multiple sclerosis. Neurology 48: 817–821 [DOI] [PubMed] [Google Scholar]
- Sherratt R.M., Bostock H., Sears T.A. (1980) Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibers. Nature 283: 570–572 [DOI] [PubMed] [Google Scholar]
- Sharrack B., Hughes R.A.C., Soudain S., Dunn G. (1999) The psychometric properties of clinical rating scales used in multiple sclerosis. Brain 122: 141–159 [DOI] [PubMed] [Google Scholar]
- Shi R., Blight A.R. (1997) Differential effects of low and high concentrations of 4-aminopyridine on axonal conduction in normal and injured spinal cord. Neuroscience 77: 553–562 [DOI] [PubMed] [Google Scholar]
- Shi R., Kelly T.M., Blight A.R. (1997) Conduction block in acute and chronic spinal cord injury: Different dose-response characteristics for reversal by 4-aminopyridine. Exp Neurol 148: 495–501 [DOI] [PubMed] [Google Scholar]
- Smith K.J., McDonald W.I. (1999) The pathophysiology of multiple sclerosis: The mechanisms underlying the production of symptoms and the natural history of the disease. Philos Trans R Soc Lond B Biol Sci 354: 1649–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook E.M., Motl R.W., Gliottoni R.C. (2009) The effect of walking mobility on the measurement of physical activity using accelerometry in multiple sclerosis. Clin Rehabil 23: 248–258 [DOI] [PubMed] [Google Scholar]
- Sosnoff J.J., Goldman M.D., Motl R.W. (2010) Real-life walking impairment in multiple sclerosis: Preliminary comparison of four methods for processing accelerometry data. Mult Scler 16: 868–877 [DOI] [PubMed] [Google Scholar]
- Stefoski D., Davis F.A., Faut M., Schauf C.L. (1987) 4-aminopyridine improves clinical signs in multiple sclerosis. Ann Neurol 21: 71–77 [DOI] [PubMed] [Google Scholar]
- Stefoski D., Davis F.A., Fitzsimmons W.E., Luskin S.S., Rush J., Parkhurst G.W. (1991) 4-aminopyridine in multiple sclerosis: Prolonged administration. Neurology 41: 1344–1348 [DOI] [PubMed] [Google Scholar]
- Targ E.F., Kocsis J.D. (1985) 4-aminopyridine leads to restoration of conduction in demyelinated rat sciatic nerve. Brain Res 328: 358–361 [DOI] [PubMed] [Google Scholar]
- van de Port I.G., Kwakkei G., Lindeman E. (2008) Community ambulation in patients with chronic stroke: How is it related to gait speed? J Rehabil Med 40: 23–27 [DOI] [PubMed] [Google Scholar]
- van Diemen H.A., Polman C.H., Koetsler J.C., van Loenen A.C., Nauta J.J., Bertelsmann F.W. (1993) 4-aminopyridine in patients with multiple sclerosis: Dosage and serum level related to efficacy and safety. Clin Neuropharmacol 16: 195–204 [DOI] [PubMed] [Google Scholar]
- van Diemen H.A., Polman C.H., van Dongen T.M., van Loenen A.C., Nauta J.J., Taphoorn M.J., et al. (1992) The effect of 4-aminopyridine on clinical signs in multiple sclerosis: A randomized, placebo-controlled, double-blind, cross-over study. Ann Neurol 32: 123–130 [DOI] [PubMed] [Google Scholar]
- Waxman S.G. (1982) Membranes, myelin, and the pathophysiology of multiple sclerosis. N Engl J Med 306: 1529–1533 [DOI] [PubMed] [Google Scholar]
- Yeh J.Z., Oxford G.S., Wu C.H., Narahashi T. (1976) Interactions of aminopyridines with potassium channels of squid axon membranes. Biophys J 16: 77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwibel H. (2009) Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv Ther 261: 1043–1057 [DOI] [PubMed] [Google Scholar]