Abstract
The focus of effective management of inflammatory bowel disease, especially Crohn’s disease, has shifted from short-term symptom control to long-term modification of disease course and complications. Intestinal healing has achieved prominence as a goal of therapy that influences long-term disease course. We review the natural history of inflammatory bowel disease, the markers of disease control that may reflect outcomes and the specific role of mucosal healing. We may aim at better mucosal healing by appropriate choice of therapy, appropriate combinations of therapy and intervening early in the disease course.
Keywords: anti-tumour necrosis factor therapy, Crohn’s disease, healing, immunosuppressive drugs, long-term disease course, mucosal ulcers, remission, ulcerative colitis
Introduction: natural history of Crohn’s disease and ulcerative colitis
Crohn’s disease and ulcerative colitis are chronic idiopathic inflammatory diseases of the gastrointestinal tract characterized by the presence in the gut of extensive ulcerations. Intestinal ulceration can result in bleeding and anemia, perforation with abscess or fistula formation, or subsequent fibrosis with intestinal obstruction. The natural history of Crohn’s disease suggests that 33% of patients with chronic or intermittently active disease develop complications requiring hospitalization and surgery in the first year after diagnosis, 13% in the second year, and 3% in each subsequent year. Surgery is rarely curative and does not stop the progression of disease. In fact endoscopic recurrence was found to occur in 75% of patients at 1 year after ileal resectional surgery, and symptomatic recurrence in 50% of patients at 5 years [Travis et al. 2006; Bassi et al. 2004; Cosnes et al. 2002; Munkholm et al. 1995; Rutgeerts et al. 1990]. In ulcerative colitis, approximately 15% of patients may develop an acute attack of severe colitis, and 30–40% of these patients do not respond to corticosteroid therapy and end up requiring colectomy. Many patients after colectomy do not regain their previous quality of life with a normally functioning colon [Caprilli et al. 2007]. Inflammatory bowel disease (IBD) is associated with significant healthcare costs [Bassi et al. 2004] mainly due to hospitalization and surgery resulting from the consequences of extensive ulcerations. Damaged intestinal mucosa is capable of restitution and repair but is also prone to fibrosis and scarring.
Assessment and quantification of mucosal healing
It is important to predict the long-term outcome of IBD in an individual patient so that treatment can be individualized and tailored to the predicted disease course. Mucosal healing is thought to be an important prognostic feature of the efficacy of treatment in IBD. Mucosal healing is assessed by endoscopy and is a component of intestinal healing which is composed of endoscopic healing, histological healing, transmural healing and fistula healing (Table 1). There is a growing body of evidence to suggest that mucosal healing is an appropriate parameter for assessing treatment efficacy and a secondary endpoint in clinical trials in patients with Crohn’s disease or ulcerative colitis. If the mucosa heals, it is thought that disease complications are unlikely to occur and future hospitalization and surgery requirements should decrease. If this can be established conclusively, mucosal healing may well become the most important parameter used to assess treatment efficacy because it may best reflect modification of the natural history of the disease as mentioned above. However it is unlikely to be the only factor indicative of long-term outcome. Genetics and the anatomical site of involvement are also important.
Table 1.
Markers of intestinal healing.
| • Endoscopic healing: colonoscopy, endoscopy, capsule endoscopy |
| • Histologic healing: biopsies |
| • Transmural healing: MRI, CT |
| • Surrogate markers: fecal calprotectin, lactoferrin |
| • Others: intestinal permeability, whole gut lavage cytokines, mucosal TNF |
CT, computed tomography; MRI, magnetic resonance imaging; TNF, tumour necrosis factor.
Traditionally, the principal goal of treatment has been symptomatic control of disease activity but clinical remission alone may not determine clinical course and does not predict the outcome of disease. A dilemma arises regarding management when a patient in apparent symptomatic remission of disease does not show mucosal healing, which is often the case with steroid therapy. As in the management of asthma, stopping therapy on clinical improvement may leave a considerable reservoir of disease, predisposing the patient to future complications.
Many disease activity indices have been created over the last 30 years to assess clinical disease activity in Crohn’s disease and ulcerative colitis. These disease activity indices are often composite scores, consisting of endoscopic disease activity score (in the case of ulcerative colitis) and clinical and laboratory parameters of disease activity. These are often used to determine the entry criteria and outcome measurements in clinical trials of new drugs. Clinical, laboratory, endoscopic and histologic parameters have been validated to varying extents. Recent advances have included validation of magnetic resonance imaging criteria, and extensive use of ultrasonography with or without contrast and computed tomography (CT) enterography, reflecting aspects of intestinal healing, superficial or histologic or transmural.
The Crohn’s Disease Activity Index (CDAI) is the most commonly used and broadly accepted clinical disease activity score worldwide in clinical trials of Crohn’s disease [Best et al. 1976]. In the CDAI, the physician’s overall evaluation of clinical status is correlated with eight independent variables, including the number of liquid stools, the severity of abdominal pain, general wellbeing, the need for antidiarrheal drugs, the presence of abdominal mass, extraintestinal manifestations, the haematocrit value and body weight. The calculation of the CDAI is based on a 7-day diary. A CDAI score of 150 or less indicates remission, a score of greater than 150 and up to 220 indicates mild disease activity, a score of greater than 220 and less than 450 indicates moderate disease activity, and a score of 450 or greater indicates severe disease activity. However the CDAI is poorly correlated with mucosal healing, can be subjective, may be influenced by factors such as bile salt malabsorption, and has limitations as the primary endpoint in clinical trials. Interestingly, the association between symptoms and mucosal healing is often poor in patients with Crohn’s disease. For example, the Groupe d’Etudes Therapeutiques des Affections Inflammatoires Digestives (GETAID) study showed that endoscopic disease activity is rather weakly correlated with the CDAI [Cellier et al. 1994].
The endoscopic substudy of a Crohn's disease clinical trial evaluating infliximab in a new long-term treatment regimen (ACCENT I) also showed that mucosal healing did not correlate accurately with the CDAI. At 10 weeks, only 36% of patients with mucosal healing were in remission as defined by the CDAI, and 40% of patients in clinical remission according to the CDAI did not have endoscopic remission. At week 54, 67% of patients with mucosal healing were in remission according to the CDAI while 56% of patients in remission according to the CDAI did not have mucosal healing. Therefore, long-term outcome studies may increasingly have to rely on mucosal healing assessment in addition to the CDAI.
The Harvey Bradshaw Index (HBI) is a simplified version of a clinical disease activity index often used in long-term open-label studies [Harvey and Bradshaw, 1980]. This index consists of five of the eight items of the CDAI. It is independent of laboratory criteria of inflammatory activity and the correlation with the CDAI is excellent (r = 0.88; p < 0.01) [Gomes et al. 1986]. Several other clinical indices have been developed, such as the van Hees or Dutch Index, the Cape Town index, the Bristol score, and the St Mark’s Index, which are similar to the CDAI. The CDAI and HBI do not include endoscopic assessment and this reflects the prevailing view at the time of development that clinical improvement is the most important goal and not endoscopic appearance. These indices are often not used formally in routine clinical practice.
Pioneering work in the development of the Crohn’s Disease Endoscopic Index of Severity (CDEIS) has been performed by the French GETAID [Mary and Modigliani, 1989]. This group evaluated and validated the importance of predefined lesions: pseudopolyps, healed ulcerations, erythema, mucosal oedema, aphthoid ulcerations, superficial and deep ulcerations and stenoses. The index was refined by incorporating the percentage of involvement of all the endoscopic segments (ileum, ascending colon, transverse colon, descending and sigmoid colon, rectum). The CDEIS correlated well with a global evaluation of lesion severity, measured on a visual analogue scale. The index was found to have an excellent interobserver agreement and is now most often used as an endoscopic severity index in clinical trials, generally as a substudy, or a standalone study. However, the CDEIS is cumbersome and not often used in clinical practice. It is also prone to interobserver nonconformity.
Recently Daperno and colleagues reported on a Simplified Endoscopic Severity Index for Crohn’s Disease (SES-CD). This index evaluates the size and the penetration of ulcerations, which are taken to reflect severity of Crohn’s disease (aphthoid <0.5 cm, large 0.5–2 cm, very large >2 cm, deep and superficial). The SES-CD has excellent interobserver agreement for all selected variables (κ coefficient 0.791–1.000). It is also well correlated with the CDEIS. However, the CDEIS and SES-CD scores tend to overestimate the disease of patients with extensive colitis and underestimate endoscopic severity in patients with ileitis and limited colonic involvement [Daperno et al. 2004]. An approach taken in clinical trials has been to document the complete absence of ulcerations as mucosal healing, sometimes allowing for more superficial erosions.
For ulcerative colitis, a wide range of different instruments have been developed to assess disease activity and these generally include endoscopic evaluation of mucosal appearance. However there is no absolute gold standard for measuring disease activity. In 1955 Truelove and Witts described the widely used score classifying ulcerative colitis into one of three categories: mild, moderate, or severe. This score has not been formally validated, is not quantitative, does not include endoscopic criteria and never includes remission criteria [Truelove and Witts, 1955]. The score is most often used to identify acute severe colitis for hospitalization and intensive medical therapy. The American College of Gastroenterologists (ACG) made a minor modification by adding a fulminant category. Subsequently it has been recognized that deep mucosal ulcerations despite treatment for acute severe colitis provide an additional perspective of prognosis.
The Powell-Tuck Index, commonly called the St Mark’s Index, added physical examination, temperature, and eight other components but has been never validated [Powell-Tuck et al. 1978]. Sutherland and colleagues developed the Ulcerative Colitis Disease Activity Index (UCDAI) and Schroeder and colleagues developed the Mayo Disease Activity Index, both of which have four components and include endoscopy [Sutherland et al. 1991; Schroeder et al. 1987]. The Mayo Disease Activity Index includes a four-point scale (0–3) describing the appearance of the rectosigmoid mucosa. This endoscopic score allows for friability grade 1 of activity. Similarly, the UCDAI score describes sigmoidoscopic appearance on a four-point scale (0–3), with friability grade 1. With friability being subjective, and potentially scoring either 1 or 2 depending on severity, some trials have modified the UCDAI by scoring 2 for friability. Attempts are now under way to evolve a validated endoscopic score that may be universally adopted.
Seo and colleagues developed the first noninvasive quantitative index in ulcerative colitis based on symptoms and levels of haemoglobin, albumin, and erythrocyte sedimentation rate. Walmsley and colleagues developed the Simple Clinical Colitis Activity Index (SCCAI), a survey consisting of six questions about symptoms, and showed that it correlated well with the St Mark’s Index, the Seo Index, and with a validated quality of life measure, the IBD Quality of Life Index (IBDQ) [Walmsley et al. 1998; Seo et al. 1992] However the Mayo score is the most widely used of the ulcerative colitis clinical indices, and therefore, unlike Crohn’s disease, mucosal appearance at flexible sigmoidoscopy forms an integral part of assessment of response in ulcerative colitis therapeutic trials.
Histological healing also may be important for guiding therapy and for evaluation of true remission in patients with IBD. Many histological scores have subsequently been proposed and most have been designed for ulcerative colitis, such as the Baron score. For Crohn’s disease, a microscopic score reflecting the segmental nature of the disease, which may be transmural, poses a challenge. The scoring system, according to Gomes and colleagues, can be divided into two items which are graded using a stepwise system: the disease activity and/or severity are divided into normal mucosa, quiescent, inactive disease chronic persistent disease and active disease [Gomes et al. 1986]. Active disease is further subdivided into mild, moderate, or severely active disease. In the scoring system advocated by Odze and colleagues activity is defined by the presence of focal crypitis, crypt abscesses or surface erosions [Geboes et al. 2000; Odze et al. 1993; Gomes et al. 1986].
The correlation between endoscopy and histology in IBD is not perfect and may be variable. In ulcerative colitis, persistent microscopic evidence of inflammation is common in quiescent colitis. Overall, however, a good correlation has been found in several studies: in 28 patients with ulcerative colitis the correlation between endoscopy and histology was good (r = 0.61, p < 0.001). Similarly, a good correlation has been reported by D’Haens and colleagues between histology and endoscopy in Crohn’s disease (r = 0.54, p = 0.004) [D’Haens et al. 1999; Odze et al. 1993; Floren et al. 1987].
None of these disease activity indices, invasive or noninvasive, endoscopic or histologic have been formally well validated in terms of reflecting the natural history of the disease in the long term. In the absence of a validated gold standard for disease activity, experts in the field have advocated different indices, leading clinical trials to measure multiple indices in their patients, such as clinical disease activity as well as mucosal healing. This is especially true for Crohn’s disease because clinical disease activity indices do not include endoscopic assessment.
The recent advent of high-definition endoscopy and filter techniques such as i-SCAN and narrow band imaging allow the resolution to be enhanced significantly and therefore may result in a revised definition of mucosal appearance in the future.
Noninvasive investigations to assess intestinal healing
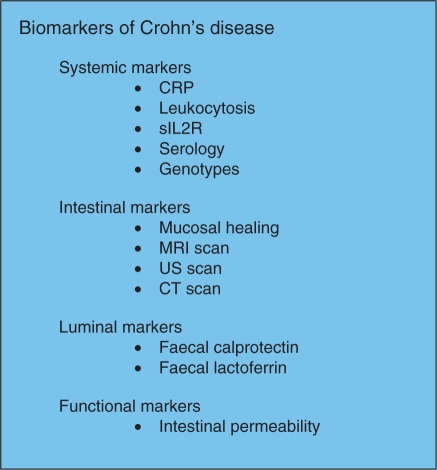
Endoscopic assessment is inconvenient and patients do not readily accept this as a method of repeated assessment. Surrogate markers such as C-reactive protein (CRP), faecal calprotectin and faecal lactoferrin may provide indirect evidence of intestinal healing. Structural parameters assessed by cross-sectional radiological techniques such as magnetic resonance (MR) enterography and computed tomographic (CT) enterography may increasingly influence the management of inflammatory bowel disease in the future. These techniques are now required to identify transmural intestinal inflammation and complications such as fibrotic strictures and fistulae. Contrast enhanced ultrasonography is also valuable, especially for small intestinal disease, but is subject to operator experience and interpretation (Figure 1).
Figure 1.
Four domains of markers to assess inflammation in inflammatory bowel disease. CRP, C-reactive protein; CT, computed tomography; MRI, magnetic resonance imaging; sIL2R, soluble interleukin-2 receptor; US, ultrasound.
Faecal markers, which are noninvasive laboratory markers derived from mucosal inflammation, seem promising and more specific in detecting gut inflammation, in predicting the disease course and in monitoring the effect of treatment in patients with IBD [D’Haens, 1996]. However faecal markers may be affected by lesions elsewhere, such as esophagitis, and reflect active inflammation with mucosal ulcerations rather than chronic intestinal changes.
A large study of 203 patients referred for symptoms of lower bowel disease showed that CRP was a good marker in differentiating IBD from irritable bowel syndrome. Although CRP is the most sensitive marker of active inflammation, it is raised in only 50–60% of patients with active ulcerative colitis. It is raised more frequently, between 70% and 100%, in patients with Crohn’s disease but less often in clinical trials recruitment [Vermiere et al. 2006].
An important study by Langhorst and colleagues confirmed that the faecal markers lactoferrin, calprotectin, polymorphonuclear neutrophil elastase, as well as CRP are able to differentiate active from inactive IBD [Langhorst et al. 2008]. Faecal calprotectin as a marker showed high diagnostic accuracy in Crohn’s disease (81.4%) whereas faecal lactoferrin was superior to the other markers in ulcerative colitis (83.3%). A combination of stool markers, CRP and activity index may increase the accuracy of measuring endoscopic inflammation and may be an important avenue for improving noninvasive monitoring of intestinal inflammation [Solem et al. 2005]. Such assessment may also indicate risk of relapse after attaining clinical remission if CRP and/or faecal calprotectin are raised.
CT, especially CT enterography, and magnetic resonance imaging (MRI), especially MR enteroclysis/enterography, are excellent minimally invasive tools for diagnosing Crohn’s disease, and for subsequent assessment of the disease, before and after therapy. However, there is a downside to repeated radiation exposure produced by the CT scan. A recent study showed that patients with Crohn’s disease may be exposed to high doses of radiation with the potential for radiation-induced malignant complications [Desmond et al. 2008]. MR enterography can provide detailed cross-sectional images similar to those of CT enterography without exposure to ionizing radiation. Early results are very encouraging for demonstrating transmural inflammation and possibly fibrosis. In a recent study, a CT enteroclysis protocol and CT protocol using oral contrast medium were compared with histological and endoscopic assessment for detection of active disease in the terminal ileum. The sensitivity and specificity with CT oral was 78% and 83% respectively, while with enteroclysis the sensitivity and specificity were 75% and 100% respectively (compared with conventional small bowel radiology and terminal ileoscopy) for detection of active disease [Wold et al. 2003]. In another study, MR enteroclysis was prospectively compared with multidetector spiral CT enteroclysis in patients with suspected small bowel disease. CT enteroclysis had a mean sensitivity of 72.2% whereas MR enteroclysis had a mean sensitivity of only 44.3%. Specificities of the two techniques were 93.2% and 94.7% respectively. Comparison was with pathological examination and clinical evolution. The lower sensitivity for MR enteroclysis was possibly influenced by the level of experience in interpreting the scans and by inadequate and incomplete intestinal dilatation. For detection and assessment of the perianal fistulizing disease MRI has became a mainstay in evaluation and future studies of fistula healing outcomes will require the use of MRI scans of the pelvis [Schmidts et al. 2003]. In a recent study the sensitivities of MR enterography and CT enterography for detecting active small-bowel Crohn’s disease were similar (90.5% versus 95.2% respectively; p = 0.32). However the image quality scores for MR enterography examinations were significantly lower than those for CT enterography (p = 0.005) [Siddiki et al. 2009]. The reference standard was the clinical diagnosis by the referring gastroenterologist after reviewing all available information. In the case of small bowel disease, the lack of a uniform gold standard for comparison is a significant problem.
Solem and colleagues, in an important comprehensive study, have correlated CRP with clinical, endoscopic, histologic and radiographic [small bowel follow through (SBFT) or CT enterography] activity in IBD. The study concluded that CRP elevation in patients with IBD is associated with clinical disease activity, endoscopic inflammation, severely active histologic inflammation but not with radiographic activity [Solem et al. 2005]. Serum and faecal biomarkers correlate with endoscopic indices but not clinical disease activity indices. Serum CRP was dependent on genotypic determinants. The CRP 717 mutant homozygote and heterozygote status was associated with significantly lower concentrations of high-sensitivity CRP (p = 0.02). There was a trend toward higher high-sensitivity CRP concentrations in the CRP 286 heterozygous adenine mutant-type mutant genotype, but this did not reach statistical significance [Jones et al. 2008].
A diagnosis of Crohn’s disease in clinical practice is made using a combination of clinical, endoscopic, radiologic, histological, and biochemical tests. However, evaluation of the small intestine with traditional radiologic procedures has been limited and endoscopic evaluation is confined to only the most distal and proximal small bowel. More recently, the development of capsule endoscopy has revolutionized enteroscopy, providing for the first time a noninvasive method for the complete endoscopic evaluation of the small bowel mucosa. This technology has been shown to be more sensitive than standard endoscopy or radiological methods such as push enteroscopy, CT or MR enteroclysis [Albert et al. 2005].
A recent meta-analysis has demonstrated the superiority of capsule endoscopy in diagnosing and assessing mucosal ulcerations in patients with small bowel Crohn’s disease. Nine studies have been considered, and overall, capsule endoscopy was superior to small bowel barium radiography, colonoscopy with ileoscopy, CT enterography and push enteroscopy for diagnosing small bowel Crohn’s disease. However, subanalysis of patients with a suspected initial presentation of Crohn’s disease showed no statistically significant difference between the yield of capsule endoscopy and barium radiography (p = 0.09), colonoscopy with ileoscopy (p = 0.48), CT enterography (p = 0.07), or push enteroscopy (p = 0.51). In contrast, subanalysis of patients with established Crohn’s disease and suspected small bowel recurrence revealed a statistically significant difference in yield in favour of capsule endoscopy compared with all other modalities (barium radiography, p < 0.001); colonoscopy with ileoscopy, p = 0.002; CT enterography, p < 0.001; and push enteroscopy, p < 0.001).
Capsule endoscopy is more sensitive than small bowel X-ray studies but specificity and positive predictive values have not been established. Undoubtedly, capsule endoscopy could diagnose small bowel disease in some instances when Crohn’s disease has been considered and conventional tests are negative [Makins and Blanshard, 2006; Triester et al. 2006]. Double balloon enteroscopy is providing a valuable technique for therapeutic intervention when necessary, such as dilatation of strictures.
The results of noninvasive mucosal healing predictive factors in IBD such as faecal calprotectin are most encouraging, but for now, endoscopic assessment still remains the gold standard in clinical diagnosis and management by providing direct mucosal healing evidence. The advantages are direct visualization of the mucosa and the possibility of tissue sampling and therapeutic intervention if necessary. Molecular biological techniques such as proinflammatory cytokine mRNA expression arrays in mucosa may provide evidence of subtle inflammatory activity and may relate to outcomes, but these techniques are unlikely to become routine in clinical practice.
Novel endoscopic techniques: ‘confocal endomicroscopy in vivo’ in assessment of mucosal healing
Confocal endomicroscopy (CLE) has recently been developed and provides high-magnification images of the gastrointestinal epithelium in vivo. This novel technique has the capability to obtain ‘real-time’ histology like images of cellular and subcellular structures of the gastrointestinal mucosa.
The great potential of CLE is to observe physiologic and pathophysiologic changes of the epithelial cells in vivo during the endoscopy examination. The intestinal epithelium forms a barrier between the gut lumen and the body. The barrier is potentially challenged by the high turnover of epithelial cells being shed because cell shedding is a normal physiologic process in the gut. After cell shedding, an epithelial gap occurs [Watson et al. 2005]. Examination of the CLE images of the intestine has identified the endomicroscopic differences in shape between a goblet cell and an epithelial gap. It is known that proinflammatory cytokines such as tumour necrosis factor-α (TNF-α) play a significant role in the pathogenesis of IBD, which includes changes in the intestinal epithelial barrier function. Previous studies have shown alterations in tight junctions causing an increase in paracellular permeability [Turner, 2006; Schreiber et al. 2005). Cell shedding and gap formation are known to increase after exposure to TNF-α. Kiesslich and colleagues reported, using CLE dynamic observation, that a single injection of TNF-α into mice substantially increased cell shedding and compromised local barrier function at gaps. The loss of barrier function at sites of cell shedding could increase epithelial permeability and is postulated by some to play an important role in the pathogenesis of IBD [Kiesslich et al. 2007]. Patients with IBD also showed malfunction of gap closure, which could lead to bacterial invasion into the lamina propria. Moussata and colleagues have demonstrated that the translocation of bacteria into the lamina propria was present in 58.8% and 61.3% of patients with ulcerative colitis and Crohn’s disease respectively compared with 14% in normal controls (p < 0.001). The bacterial translocation could be demonstrated in the rectum and right colon for ulcerative colitis and Crohn’s disease respectively [Moussata et al. 2009]. Endomicroscopy can visualize not only tissue and cellular architecture but also bacterial interaction with the mucosal layer. Therefore such novel imaging through CLE has the potential to demonstrate another dimension of mucosal healing.
Disease course after mucosal healing prior to biological therapy era
Whether endoscopic healing predicts a long-term favourable outcome has not been conclusively established, and such data are lacking for cross-sectional imaging or histologic healing. In a pivotal Norwegian population-based prospective cohort study, Froslie and colleagues documented a role of mucosal healing in monitoring treatment efficacy and long-term disease outcome, before the biological therapy era, in patients during 5 years of follow up. In patients with ulcerative colitis 50% had confirmed mucosal healing after 1 year; educational level and extensive disease at diagnosis were recognized as predictive factors of mucosal healing (p = 0.004 and p = 0.2 respectively). The evidence that socioeconomic factors influence the course of ulcerative colitis might suggest that psychoimmunological factors may play a role in disease modulation. Most importantly, patients with mucosal healing had a significantly lower risk of future colectomy than patients without mucosal healing (p = 0.02). In patients with Crohn’s disease mucosal healing was detected in 38% and lack of mucosal healing in 62% at 1 year; fever at diagnosis and medical treatment without the use of steroids were significant predictors (p = 0.03 and p = 0.01 respectively) of mucosal healing. Patients with mucosal healing had less inflammatory activity at endoscopy after 5 years and decreased requirement for future steroid treatment; patients with mucosal healing had a trend towards fewer surgical resections on follow up [Froslie et al. 2007]. This new evidence from a prospective study suggests that early potent suppression of inflammation leading to mucosal healing may change the long-term outcome of disease and similar evidence is now required in the context of long-term biological therapy. Deep colonic ulcerations in Crohn’s disease also have adverse prognostic outcomes. It is possible that mucosal healing after biological therapy or fistula healing demonstrated on MRI scans may be one of the factors influencing the decision to discontinue biological therapy with a relatively low risk of relapse.
Mucosal healing with different therapies
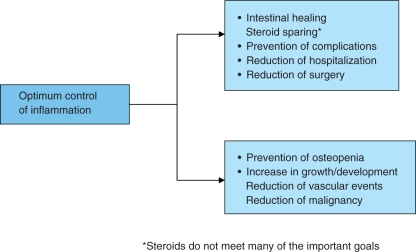
Optimal control of inflammation has considerable target organ as well as potential systemic benefits (Figure 2). One of the principal reasons why mucosal healing was not considered a goal of IBD treatment in the past was that most available medications were not able to heal the bowel mucosa in a significant proportion of patients. Conventional drugs used as first-line treatment include 5-aminosalicylic acid (5-ASA) or antibiotics but these generally have modest clinical efficacy, particularly in patients with small bowel or ileocolonic Crohn’s disease or patients with moderate to severe active IBD [Malchow et al. 1984]. Even in ulcerative colitis, the ASCEND II trial demonstrated mucosal healing after treatment with 5-ASA in only 20–40% of patients with ulcerative colitis and moderately active disease. In this study 71.8% (89/124) of patients with moderately active ulcerative colitis treated with 4.8 g/day of mesalamine (800 mg tablet) achieved overall improvement at 6 weeks, but only 20.2% (25/89) showed complete remission [Hanauer et al. 2005]. More recently, a new mesalamine formulation, mesalamine with MMX Multi Matrix System, offering a novel high-strength (1.2 g/tablet) delivery system, has been used in patients with active, mild-to-moderate ulcerative colitis. The results of two large studies reported by Lichtenstein and colleagues (SPD476-301) [Lichtenstein et al. 2007] and Kamm and colleagues (SPD476-302) [Kamm et al. 2007] have shown that MMX mesalamine 2.4 g/day (given once daily, or as 1.2 g given twice daily) or 4.8 g/day (once daily) is well tolerated and efficacious for the induction of clinical and endoscopic remission in patients with active, mild-to-moderate ulcerative colitis. The UCDAI score was modified by including the friability parameter as score 2. Approximately 64% of patients in these studies did not achieve strictly defined remission according to endoscopic and clinical parameters [Lichtenstein et al. 2007; Kamm et al. 2007]. The 8-week complete mucosal healing rate was 32% in both MMX mesalazine groups, which was twice the rate of 16% in the placebo group [Sandborn et al. 2007]. The PODIUM study using pentasa showed again that once daily 5-ASA can maintain mucosal healing in ulcerative colitis [Dignass et al. 2009].
Figure 2.
Intestinal healing and prevention of complications is one of the many benefits of optimum control of inflammation.
Enteral nutrition as a primary therapy for patients with active Crohn’s disease has its proponents, especially in paediatric practice, but few studies have assessed mucosal healing with this therapy. Elemental diet is a liquid diet, with essential amino acids, sugars and fatty acids, trace elements and vitamins. These can be absorbed without further digestion, and may cause a remission of the disease, acting as anti-inflammatory agents. In the study by Yamamoto and colleagues 28 consecutive patients with active Crohn’s disease were treated with an elemental diet for 4 weeks. Clinical remission was achieved in 71% of patients. Endoscopic healing and improvement rates were 44% and 76% in the terminal ileum and 39% and 78% in the colon [Yamamoto et al. 2005]. However, in a systematic review Zachos and colleagues found that corticosteroid therapy is more effective than enteral nutrition for inducing remission of active Crohn’s disease. This meta-analysis of the available trials did not demonstrate any significant benefit based the composition of nutritional therapies, but a nonsignificant trend favouring very low fat and/or very low long-chain triglyceride content [Zachos et al. 2005].
Corticosteroids are generally poor in their ability to improve the endoscopic severity of ilecolonic lesions. Although corticosteroids are highly effective in quickly suppressing symptoms of acute inflammation, they have shown no benefit in maintaining remission, in preventing new flares or in inducing mucosal healing. The lack of correlation between endoscopic and clinical outcomes may be explained by the inability of corticosteroids to modify mucosal and submucosal inflammatory processes [Agrawal et al. 2005; Rutgeerts, 2001]. This poor correlation between clinical remission and mucosal healing was demonstrated in two studies conducted by GETAID. A total of 71% of patients who achieved clinical remission of active Crohn’s disease with corticosteroid therapy continued to have active endoscopic lesions after 7 weeks of therapy. Only 13% of the patients in clinical remission after corticosteroid therapy showed evidence of complete healing of mucosal lesions at endoscopy [Modigliani et al. 1990]. Budesonide, a corticosteroid with high first pass metabolism and fewer systemic side effects than prednisolone can be tolerated for a longer period than conventional steroids. It is effective in inducing remission in right-sided colonic and ileal disease, but failed to maintain remission and attain mucosal healing after 1 year of treatment [Sandborn et al. 2005]. It is likely that corticosteroids are unable to maintain remission because of their poor mucosal healing potential. It is also possible that corticosteroids adversely affect mucosal healing when used as monotherapy.
Immunosuppressive agents, such as azathioprine, 6-mercaptopurine and methotrexate are prescribed in patients with steroid resistance and steroid dependence. They have slow onset of action, they have been shown to maintain remission in IBD, and are able to induce mucosal healing [Lichtenstein et al. 2007]. Studies investigating mucosal healing with immunosuppressive agents are generally small and uncontrolled. D'Haens and colleagues reported on 20 patients with Crohn’s colitis or ileocolitis treated with azathioprine. They demonstrated that in the colon, 70% of patients had complete mucosal healing, while in the ileum, 54% had complete healing [D’Haens et al. 1999; Vermiere et al. 2007]. As described below, however, in the recently concluded SONIC study, mucosal healing with azathioprine monotherapy was disappointing.
Mucosal healing has been studied more extensively in the biological era. Remarkable MH was reported with initial use of infliximab in CD and this provided a compelling evidence for use of infliximab in clinical practice. In the pivotal ACCENT I maintenance study, scheduled 8-weekly infusions of infliximab led to superior remission and response rates, superior mucosal healing, and decreased need for hospitalization and surgery compared with placebo or episodic infusions of infliximab, probably as a result of superior mucosal healing. In the scheduled treatment group, 31% of patients showed evidence of complete mucosal healing at 10 weeks and 50% of patients had complete mucosal healing at 54 weeks. Patients with mucosal healing had fewer hospitalizations than patients without healing (18.8% versus 28%, p = not significant) [Rutgeerts et al. 2005] and a trend towards fewer surgical procedures.
The recently reported SONIC study was a randomized, double-blind, controlled trial comparing infliximab plus azathioprine with infliximab alone or azathioprine alone in Crohn’s disease. In this study, 508 patients naïve to immunomodulators and biological therapies were randomized to receive azathioprine 2.5 mg/kg, azathioprine 2.5 mg/kg plus infliximab 5 mg/kg infusions, or infliximab 5 mg/kg alone. The primary endpoint was the proportion of patients in steroid-free remission (CDAI < 150) at week 26. The azathioprine plus infliximab group (56.8%) was superior to the infliximab monotherapy group (44.4%, p = 0.022), which in turn was superior to the azathioprine alone group (30.6%, p = 0.009), in achieving steroid-free remission. Mucosal healing was also statistically superior with combination therapy, with 43.9%, 30.1%, and 16.5% of patients treated with azathioprine plus infliximab, infliximab alone, and azathioprine alone respectively showing mucosal healing. This may serve as a compelling argument for choosing combination therapy in immunomodulator and biological therapy naïve patients, after consideration of risk versus benefit, because combination therapy may be associated with a small increase in risk for infection and possibly lymphoma [Colombel et al. 2010b].
The potential for biological therapies to induce mucosal healing and potentially change the natural history of Crohn’s disease has been further confirmed by a top-down/step-up trial [D’Haens et al. 2008]. This is an open-label, multicentre trial conducted in 26 centres in the Netherlands and Belgium. Patients with predominantly newly diagnosed active Crohn’s disease were randomized to a top-down arm (infliximab induction 0, 2, 6 weeks with azathioprine maintenance, with on-demand infliximab for flares; systemic steroids were added only if disease did not respond to infliximab and azathioprine) or a step-up arm (prednisone 40 mg daily induction; permitted two steroid tapers before starting azathioprine; and then infliximab if disease fails to respond to immunosuppressive drugs). At 6 and 12 months, significantly more top-down patients were in steroid-free remission (75% and 77%, respectively) than in the step-up group (48% and 64%, respectively). At 24 months, no statistically significant difference was found between the two groups. The most remarkable finding in this cohort was the result of the endoscopic substudy, in which 71% (17/24) of patients in the top-down arm achieved mucosal healing versus 30% (6/20) in the step-up arm at year 2, and mucosal healing predicted sustained clinical remission for a further 2 years [Baert et al. 2010]. Some of the patients in the top-down arm had only received an induction dose of infliximab. The results suggest that if mucosal healing is established as a desirable outcome of therapy, steroid induction even for newly presenting patients will not achieve this desirable outcome. This study is also interesting because although anti-TNF trough levels have been associated with mucosal healing, in the step-up top-down study the patients had only received induction dosing.
Studies in the paediatric population also suggest that early treatment may alter the course of Crohn’s disease, and response to therapy may be related to disease duration. In the REACH (Response and Remission Related to Infliximab in Pediatric Patients with Moderate to Severe Crohn’s Disease) study, 112 paediatric patients were evaluated and received infliximab 5 mg/kg every 8 weeks or every 12 weeks. Short-term efficacy was 88% clinical response and 59% clinical remission rate at 10 weeks. At the end of 1 year, patients receiving infliximab every 8 weeks had a 64% response rate and 56% remission rate [Hyams et al. 2007]. These response and remission rates are superior to those seen in the ACCENT I study of infliximab in adults with a median disease duration of more than 7 years, where the 10-week remission rate was 40% and the 54-week remission rate was 30% [Rutgeerts et al. 2004]. It is possible that mucosal healing is better in the paediatric population. In addition, surrogate markers will be accepted more readily than endoscopic assessment in this population.
Although analysis of certolizumab pegol (PRECISE 1 and PRECISE 2 studies) and adalimumab (CLASSIC II and CHARM studies) has demonstrated similar clinical efficacy results, formal mucosal healing data with both these agents are awaited [Schreiber et al. 2007; Colombel et al. 2007]. Such studies have been completed recently and preliminary data suggest that adalimumab also induces mucosal healing at a superior rate compared with placebo both at 12-week and 1-year endpoints. In the EXTEND study of moderate to severe ileocolonic Crohn’s disease, 135 patients received open-label adalimumab 160 mg/80 mg at 0 and 2 weeks. At week 4, 129 patients were randomized to either maintenance adalimumab 40 mg every other week or placebo. At week 12, 16% of patients receiving adalimumab every other week had both clinical remission and mucosal healing, while the figure was 10% for placebo (p = 0.34). At week 52, 19% of patients on maintenance adalimumab had clinical remission and mucosal healing while the figure was 0% for placebo (p < 0.001) [Colombel et al. 2010a]. Full publications are awaited and study differences will make any comparison between the three anti-TNF agents difficult regarding mucosal healing efficacy. While anti-TNF therapies are a step forward in achieving mucosal healing as an endpoint, it is clear that these are not magic bullets. With these data, it is unlikely we will achieve histologic remission as a further endpoint in the near future. In addition, standardization of histologic readouts continues to pose challenges.
In ulcerative colitis, two large studies – Active Ulcerative Colitis (ACT) I and II – demonstrated that remission rates were significantly better with infliximab than with placebo and such remission was maintained with infliximab. In ACT I, clinical remission at week 8 for placebo, 5 mg/kg infliximab, and 10 mg/kg infliximab was 15%, 39% and 32% respectively, whereas mucosal healing was 34%, 62%, and 59% respectively. Similar results were found in ACT II [Rutgeerts et al. 2005]. In the ACT studies an endoscopy subscore of 0 or 1 was taken to indicate mucosal healing, but if only an endoscopic subscore of 0 was considered, the results were comparable to clinical remission rates, both in the short and long term.
Conclusions
Optimizing outcomes in IBD requires rapid and sustained control of inflammation. Currently the best indicator of this appears to be mucosal healing.
Conventional therapy is not fully effective in IBD and is often continued for long periods without optimum efficacy or assessment of mucosal healing. Consequently many patients are undertreated and remain clinically active without a healed mucosa, increasing the risk of hospitalization, surgery and complications. Increasingly, evidence supports mucosal healing as a predictive factor for disease course in IBD. Lack of mucosal healing may represent an indication for intensified therapeutic strategies to prevent serious complications of disease. The past decade has seen an explosion of therapies aimed at altered immune response observed in patients with IBD. The goal of this highly effective and targeted approach is to induce rapid remission in a steroid-free environment and to promote mucosal healing. This new ‘top-down’ strategy has been associated with encouraging medium-term rates of mucosal healing and could potentially modify the natural history of the disease by leading to remission with fewer complications and reduced surgical intervention. It is likely that assessment of mucosal healing will increase in the future (Table 2) and will be adopted into routine clinical practice. In Crohn’s disease, cross-sectional imaging of transmural disease will become increasingly important to assess intestinal damage comprehensively, with scores enabling this to be developed.
Table 2.
When is assessment of mucosal healing indicated?
| • When contemplating change in therapy |
| • When contemplating change in dose |
| • When assessment of a discrepancy between symptoms and inflammatory parameters is required |
| • When contemplating start of new therapy |
| • When contemplating discontinuation of effective therapy |
| • To determine risk of future dysplasia |
In the near future, the universal therapeutic goals for ulcerative colitis and Crohn’s disease will include inducing rapid response, maintaining remission without the use of steroids, achieving and maintaining complete mucosal healing, avoiding complications, hospitalizations and surgery, preventing disease-related mortality and improving patient-related quality of life (Figure 3). Early treatment, judicious use of combination therapy, avoiding inappropriate use of steroids and appropriate surgical intervention will provide the best outcomes and will require precise decision making.
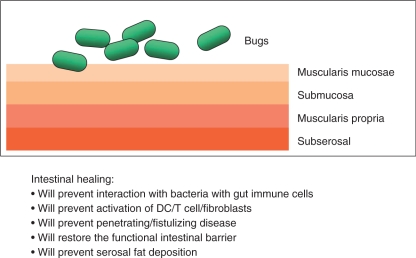
Figure 3.
Intestinal healing as an essential component of long-term treatment goals. DC, dendritic cell.
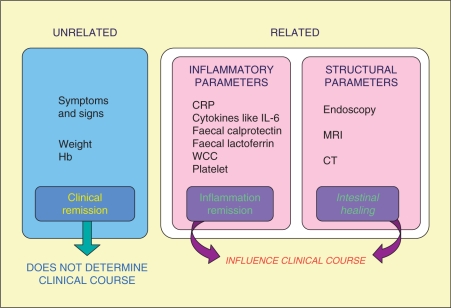
Although mucosal healing appears to be attractive as a concept and should logically lead to improved outcomes, this is not currently common practice. The argument that mucosal healing is a valid therapeutic endpoint is supported by many independent lines of evidence as discussed above. These include: higher relapse rates and surgery if mucosal healing is not achieved; significant mucosal ulcerations postoperatively being predictive of higher clinical relapse and surgery rates; a step-up/top-down study showing the relevance of mucosal healing in predicting outcome over 3–4 years; continuing mucosal activity in ulcerative colitis being predictive of neoplastic changes. However, it may not be feasible to perform routine repeated endoscopic or radiographic assessments for mucosal healing. Endoscopy is invasive, time consuming and requires extensive staff training, with the potential for serious complications. Unfortunately, at present, an ideal noninvasive surrogate marker of mucosal healing is lacking. Understanding the interrelationship between assessment parameters and alteration of disease course is evolving and will lead to a long-term approach to disease control (Figure 4). Fine definition of mucosal healing using novel techniques such as confocal endomicroscopy or other new imaging methods, and transmural healing by cross-sectional imaging have the potential to introduce new concepts in the pathogenesis of ulcerations and different evolution of healing processes.
Figure 4.
Assessment parameters and long-term disease course. CRP, C-reactive protein; CT, computed tomography; Hb, haemoglobin; IL-6, interleukin-6; MRI, magnetic resonance imaging; WCC, white cell count.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
Dr S. Ghosh has received honorarium for lectures from Abbott, Shire, Merck Pharmaceuticals and received research funds from Abbott and Merck. He has participated in Ad-Hoc Advisory boards of Merck, Abbott and Shire. Dr M. Iacucci has no conflicts of interest.
References
- Agrawal A., Durrani S., Leiper K., Ellis A., Morris A.I., Rhodes J.M. (2005) Effect of systemic corticosteroid therapy on risk for intra-abdominal or pelvic abscess in non-operated Crohn’s disease. Clin Gastroenterol Hepatol 3: 1215–1220 [DOI] [PubMed] [Google Scholar]
- Albert J.G., Martiny F., Krummenerl A., Stock K., Leßke J., Göbel C.M., et al. (2005) Diagnosis of small bowel Crohn’s disease: A prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut 54: 1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert F., Moortgat L., Van Assche G., Caenepeel P., Vergauwe P., De Vos M., et al. (2010) Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 138: 463–468 [DOI] [PubMed] [Google Scholar]
- Bassi A., Dodd S., Williamson P., Bodger K. (2004) Cost of illness of inflammatory bowel disease in the UK: A single centre retrospective study. Gut 53: 1471–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best W.R., Becktel J.M., Singleton J.W., Kerm F. (1976) Development of Crohn’s disease activity index. Gastroenterology 70: 439–444 [PubMed] [Google Scholar]
- Caprilli R., Viscido A., Latella G. (2007) Current management of severe ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 4: 92–101 [DOI] [PubMed] [Google Scholar]
- Cellier C., Sahmoud T., Froguel E., Adenis A., Belaiche J., Bretagne J.F., et al. (1994) Correlation between clinical activity, endoscopy, severity, and biological parameters in colonic and ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. Groupe d’Etudes Therapeutiques des Affections Inflammatoires Digestives. Gut 35: 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel J.F., Rutgeerts P., Sandborn W.J., Camez A., Pollack P., Chen N., et al. (2010a) Deep remission for adalimumab-treated patients with moderate to severe ileocolonic Crohn’s disease: Results from EXTEND. J Crohn’s Colitis 4: S10–S10 [Google Scholar]
- Colombel J.F., Sandborn W.J., Reinisch W., Mantzaris G.J., Kornbluth A., Rachmilewitz D., et al. (2010b) Infliximab, azathioprine or combination therapy for Crohn’s disease. N Engl J Med 362: 1383–1395 [DOI] [PubMed] [Google Scholar]
- Colombel J.F., Sandborn W.J., Rutgeerts P., Enns R., Hanauer S.B., Panaccione R., et al. (2007) Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: The Charm Trial. Gastroenterology 132: 52–65 [DOI] [PubMed] [Google Scholar]
- Cosnes J., Cattan S., Blain A., Beaugerie L., Carbonnel F., Parc R., et al. (2002) Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 8: 244–250 [DOI] [PubMed] [Google Scholar]
- Daperno M., D'Haens G., Van Assche G., Baert F., Bulois P., Maunoury V., et al. (2004) Development and validation of a new, simplified endoscopic activity score for Crohn's disease: The Ses-Cd. Gastrointest Endosc 60: 505–512 [DOI] [PubMed] [Google Scholar]
- Desmond A.N., O'Regan K., Curran C., McWilliams S., Fitzgerald T., Maher M.M., et al. (2008) Crohn's disease: Factors associated with exposure to high levels of diagnostic radiation. Gut 57: 1524–1529 [DOI] [PubMed] [Google Scholar]
- D’Haens G. (1996) Clinical and immunopathological characteristics of early recurrent Crohn’s disease: In search of pathogenesis. Thesis, K.U. Leuven. [Google Scholar]
- D’Haens G., Baert F., van Assche G., Caenepeel P., Vergauwe P., Tuynman H., et al. (2008) Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: An open randomized trial. Lancet 371: 660–667 [DOI] [PubMed] [Google Scholar]
- D’Haens G., Geboes K., Rutgeerts P. (1999) Endoscopic and histologic healing of Crohn's (ileo-) colitis with azathioprine. Gastrointest Endosc 50: 667–671 [DOI] [PubMed] [Google Scholar]
- Dignass, A.U., Bokemeyer, B., Adamek, H., Mross, M., Vinter-Jensen, L., Börner, N. et al (2009) Mesalamine once daily is more effective than twice daily in patients with quiescent ulcerative colitis. Clin Gastroenterol Hepatol 7(7): 762–769. [DOI] [PubMed] [Google Scholar]
- Floren C.H., Benoni C., William R. (1987) Histological and colonoscopy assessment of disease extension in ulcerative colitis. Scand J Gastroenterol 22: 459–462 [DOI] [PubMed] [Google Scholar]
- Froslie K.F., Jahnsen J., Moum B.A., Vatn M.H. (2007) Mucosal healing in inflammatory bowel disease: Results from a Norwegian population-based cohort. Gastroenterology 133: 412–422 [DOI] [PubMed] [Google Scholar]
- Geboes K., Riddell R., Ost A., Jensfelt B., Persson T., Lofberg R. (2000) A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 47: 404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes P., Du Boulay C., Smith C.L., Holdstock G. (1986) Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut 27: 92–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer S.B., Sandborn W.J., Kornbluth A., Katz S., Safdi M., Woogen S., et al. (2005) Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: The Ascend II Trial. Am J Gastroenterol 100: 2478–2485 [DOI] [PubMed] [Google Scholar]
- Harvey R.F., Bradshaw J.M. (1980) A simple index of Crohn's-disease activity. Lancet I: 514–514 [DOI] [PubMed] [Google Scholar]
- Hyams J., Crandall W., Kugathasan S., Griffiths A., Olson A., Johanns J., et al. (2007) Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology 132: 863–873, quiz 1165–1166. [DOI] [PubMed] [Google Scholar]
- Jones J., Loftus E.V., Jr, Panaccione R., Chen L.S., Peterson S., McConnell J., et al. (2008) Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clin Gastroenterol Hepatol 6: 1218–1224 [DOI] [PubMed] [Google Scholar]
- Kamm M.A., Sandborn W.J., Gassull M., Schreiber S., Jackowski L., Butler T., et al. (2007) Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology 132: 66–75 [DOI] [PubMed] [Google Scholar]
- Kiesslich R., Goetz M., Angus E.M., Hu Q., Guan Y., Potten C., et al. (2007) Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology 133: 1769–1778 [DOI] [PubMed] [Google Scholar]
- Langhorst J., Elsenbruch S., Koelzer J., Rueffer A., Michalsen A., Dobos G.J. (2008) Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: Performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 103: 162–169 [DOI] [PubMed] [Google Scholar]
- Lichtenstein G.R., Kamm M.A., Boddu P., Gubergrits N., Lyne A., Butler T., et al. (2007) Effect of once- or twice-daily MMX mesalamine (Spd476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol 5: 95–102 [DOI] [PubMed] [Google Scholar]
- Makins, R. and Blanshard, C. (2006) Guidelines for capsule endoscopy: diagnoses will be missed. Aliment Pharmacol Ther 24(2): 293–297. [DOI] [PubMed] [Google Scholar]
- Malchow H., Ewe K., Brandes J.W., Goebell H., Ehms H., Sommer H., et al. (1984) European Cooperative Crohn's Disease Study (ECCDS): Results of drug treatment. Gastroenterology 86: 249–266 [PubMed] [Google Scholar]
- Mary J.Y., Modigliani R. (1989) Development and validation of an endoscopic index of the severity for Crohn's disease: A prospective multicentre study. Groupe D'Etudes Therapeutiques Des Affections Inflammatoires Du Tube Digestif (GETAID). Gut 30: 983–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modigliani R., Mary J.Y., Simon J.F., Cortot A., Soule J.C., Gendre J.P., et al. (1990) Clinical, biological, and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Groupe D'Etude Therapeutique Des Affections Inflammatoires Digestives. Gastroenterology 98: 811–818 [DOI] [PubMed] [Google Scholar]
- Moussata D., Goetz M., Watson A.J., Kerner M., Gloeckner A., Hoffman A., et al. (2009) Confocal laser endomicroscopy for the in vivo identification, localization and quantification of tissue based bacteria in patients with different types of colitis. Gastrointest Endosc 69: 131–131, abstract no. [Google Scholar]
- Munkholm P., Langholz E., Davidsen M., Binder V. (1995) Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol 30: 699–706 [DOI] [PubMed] [Google Scholar]
- Odze R., Antonioli D., Peppercorn M., Goldman H. (1993) Effect of topical 5-aminosalicylic acid (5-ASA) therapy on rectal mucosal biopsy morphology in chronic ulcerative colitis. Am J Surg Pathol 17: 869–875 [DOI] [PubMed] [Google Scholar]
- Powell-Tuck J., Bown R.L., Lennard-Jones J. (1978) A comparison of oral prednisolone given a single or multiple daily doses for active proctocolitis. Scand J Gastroenterol 13: 833–837 [DOI] [PubMed] [Google Scholar]
- Rutgeerts P.J. (2001) Review article: The limitations of corticosteroid therapy in Crohn's disease. Aliment Pharmacol Ther 15: 1515–1525 [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Feagan B.G., Lichtenstein G.R., Mayer L.F., Schreiber S., Colombel J.F., et al. (2004) Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology 126: 402–413 [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Geboes K., Vantrappen G., Beyls J., Kerremans R., Hiele M. (1990) Predictability of the postoperative course of Crohn’s disease. Gastroenterology 99: 956–963 [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Sandborn W.J., Feagan B.G., Reinisch W., Olson A., Johanns J., et al. (2005) Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353: 2462–2476 [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Kamm M.A., Lichtenstein G.R., Lyne A., Butler T., Joseph R.E. (2007) MMX Multi matrix system mesalazine for the induction of remission in patients with mild-to-moderate ulcerative colitis: A combined analysis of two randomized, double-blind, placebo-controlled trials. Aliment Pharmacol Ther 26: 205–215 [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Lofberg R., Feagan B.G., Hanauer S.B., Campieri M., Greenberg G.R. (2005) Budesonide for maintenance of remission in patients with Crohn's disease in medically induced remission: A predetermined pooled analysis of four randomized, double-blind, placebo-controlled trials. Am J Gastroenterol 100: 1780–1788 [DOI] [PubMed] [Google Scholar]
- Schmidt S., Lepori D., Meuwly J.Y., Duvoisin B., Meuli R., Michetti P., et al. (2003) Prospective comparison of MR enteroclysis with multidetector spiral-CT enteroclysis: Interobserver agreement and sensitivity by means of ‘sign-by-sign’ correlation. Eur Radiol 13: 1303–1311 [DOI] [PubMed] [Google Scholar]
- Schreiber S., Renisch W., Colombel J.F., Sandborn W.J., Hommes D.W., Li J., et al. (2007) Early Crohn’s disease shows high levels of remission to therapy with adalimumab: Sub-analysis of CHARM. Gastroenterology 132(Suppl 1): A147 (abstract 985). [Google Scholar]
- Schreiber S., Rosenstiel P., Albrecht M., Hampe J., Krawczak M. (2005) Genetics of Crohn disease, an archetypal inflammatory barrier disease. Nat Rev Genet 6: 376–388 [DOI] [PubMed] [Google Scholar]
- Schroeder K.W., Tremaine W.J., Ilstrup D.M. (1987) Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 317: 1625–1629 [DOI] [PubMed] [Google Scholar]
- Seo M., Okada M., Yao T., Ueki M., Arima S., Okumura M. (1992) An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol 87: 971–976 [PubMed] [Google Scholar]
- Siddiki H.A., Fidler J.L., Fletcher J.G., Burton S.S., Huprich J.E., Hough D.M., et al. (2009) Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn's disease. AJR Am J Roentgenol 193: 113–121 [DOI] [PubMed] [Google Scholar]
- Solem C.A., Loftus E.V., Jr, Tremaine W.J., Harmsen W.S., Zinsmeister A.R., Sandborn W.J. (2005) Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis 11: 707–712 [DOI] [PubMed] [Google Scholar]
- Sutherland L., Singleton J., Sessions J., Hanauer S., Krawitt E., Rankin G., et al. (1991) Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut 32: 1071–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis S.P., Stange E.F., Lemann M., Oresland T., Chowers Y., Forbes A., et al. (2006) European evidence based consensus on the diagnosis and management of Crohn's disease: Current management. Gut 55(Suppl 1): i16–i35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triester S.L., Leighton J.A., Leontiadis G.I., Gurudu S.R., Fleischer D.E., Hara A.K., et al. (2006) A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease. Am J Gastroenterol 101(5): 954–964 [DOI] [PubMed] [Google Scholar]
- Truelove S.C., Witts L.J. (1955) Cortisone in ulcerative colitis: Final report on a therapeutic trial. Br Med J 2: 1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.R. (2006) Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am J Pathol 169: 1901–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire S., Van Assche G., Rutgeers P. (2006) Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut 55: 426–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire S., van Assche G., Rutgeerts P. (2007) Review article: Altering the natural history of Crohn’s disease – evidence for and against current therapies. Aliment Pharmacol Ther 25: 3–12 [DOI] [PubMed] [Google Scholar]
- Walmsley R.S., Ayres R.C., Pounder R.E., Allan R.N. (1998) A simple clinical colitis activity index. Gut 43: 29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A.J., Chu S., Sieck L., Gerasimenko O., Bullen T., Campbell F., et al. (2005) Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology 129: 902–912 [DOI] [PubMed] [Google Scholar]
- Wold P.B., Fletcher J.C., Johnson C.D., Sandborn W.J. (2003) Assessment of small bowel Crohn disease non invasive peroral CT enterography compared with other imaging methods and endoscopy – feasibility study. Radiology 229: 275–281 [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nakahigashi M., Umegae S., Kitagawa T., Matsumoto K. (2005) Impact of elemental diet on mucosal inflammation in patients with active Crohn's disease: Cytokine production and endoscopic and histological findings. Inflamm Bowel Dis 11: 580–588 [DOI] [PubMed] [Google Scholar]
- Zachos M., Tondeur M., Griffiths A.M. (2005) Enteral nutritional therapy for induction of remission in Crohn's disease. Inflamm Bowel Dis 11: 580–588 [DOI] [PubMed] [Google Scholar]