Abstract
Children and adolescents who develop schizophrenia tend to have greater symptom severity than adults who develop the illness. Since the brain continues to mature into early adulthood, developmental differences in brain structure and function may provide clues to the underlying neurobiology of schizophrenia. With an emerging body of evidence supporting disrupted connectivity contributing to the underlying pathophysiology of schizophrenia, it was our goal to assess differences in functional connectivity in children and adolescents who develop schizophrenia. Participants included a total of 28 children and adolescents (14 patients with schizophrenia and 14 age- and gender-matched controls). All subjects underwent a functional magnetic resonance imaging scan involving a modified Sternberg Item Recognition Paradigm with 3 working memory (WkM) loads. Patients had poorer performance at all 3 WkM loads without a load by diagnosis interaction. Functional imaging results demonstrated 3 specific brain networks disrupted in children and adolescents with schizophrenia. These networks include 1) the anterior cingulate and the temporal lobes, bilaterally; 2) the cerebellum with subcortical regions; and 3) the occipital lobe and the cerebellum. Patients with early-onset schizophrenia demonstrate abnormal functional connectivity in networks involving limbic, temporal lobe, cerebellum, and early visual processing streams.
Keywords: cerebellum, early-onset schizophrenia, fMRI, limbic system, prefrontal cortex, temporal lobe
Introduction
Much work over the past several decades supports the theory that schizophrenia involves an impairment in orchestrating the multiple neural networks that participate in higher order cognitive functions (Weinberger et al. 1992; Friston and Frith 1995; Andreasen 1997; Fuster 1999). The development of interconnected brain regions arising during normal development may be altered, leading to the recruitment of either inappropriate regions for task execution or, alternatively, adding additional processing requirements in expected regions. Early studies in adults with schizophrenia utilizing 133Xenon found lower blood flow in frontal regions compared with posterior regions, describing an overall “hypofrontality” (Franzen and Ingvar 1975). Later studies using both glucose metabolism (2-Deoxy-2-[18F]fluoro-D-Glucose-PET) and H2O15 PET also demonstrated hypofrontality in the prefrontal cortex (PFC) during tasks of executive function (Buchsbaum and Wu 1987; Weinberger and Berman 1988; Andreasen, Cohen, et al. 1992; Weinberger et al. 1992; Andreasen et al. 1997; Carter et al. 1998).
While these early imaging studies also demonstrated hypofrontality in patients with schizophrenia performing tasks that tap working memory (WkM) (Buchsbaum and Wu 1987; Weinberger and Berman 1988; Andreasen, Rezai, et al. 1992), these studies were designed neither to specifically tap WkM circuits nor were patients and controls matched on performance. There is evidence that once subjects are matched on WkM task performance, patients with schizophrenia demonstrate hyperfrontality (Manoach et al. 1999; Callicott et al. 2000). One thought is that patients recruit greater cognitive resources in order to match their performance with controls; however, once the task difficulty exceeds their capacity, they demonstrate hypofrontality, fitting an inverted “U”-shaped pattern of activity (Manoach 2003).
An alternate thought is that different regions within the PFC show either hypo- or hyperactivity, depending on their location (Ragland et al. 2007). Regions in the anterior, ventral, or medial PFC may demonstrate hyperactivity, while the dorsolateral PFC (DLPFC) shows hypoactivity (Glahn et al. 2005; Ragland et al. 2007). This would imply that patients are tapping different neural networks to compensate for aberrant DLPFC activity. It would also highlight that differences in image processing strategies, such as spatial filtering, region of interest versus voxel-based approaches, and block versus event-related paradigms, could influence the results of the current studies (Carter et al. 2008).
A recent meta-analysis of functional magnetic resonance imaging (fMRI) imaging studies that utilize the n-back paradigm found that patients also show increased activation in the caudal anterior cingulate cortex (ACC) (Glahn et al. 2005). Since a number of studies using a variety of higher order cognitive tasks find hypoactivity in the ACC in patients with schizophrenia, Ragland et al. (2007) postulate that the increased ACC activity may be related to less conflict in controls who are making less errors during the task. Patients, however, who are making more errors, are in turn activating the ACC to a greater extent.
It is an open question whether patients with schizophrenia are tapping different neural networks compared with controls. Recent image processing algorithms have been designed to measure functional connectivity between brain regions and under different conditions (Meyer-Lindenberg et al. 2001; Calhoun et al. 2006). Functional connectivity is defined as the temporal correlation of the hemodynamic waveforms (i.e., blood oxygen level–dependent [BOLD] signal for a specific voxel or regional clusters of voxels over time) between different brain regions (Friston et al. 1993). One technique to study functional connectivity involves an independent component analysis (ICA) and is able to identify spatially distinct and temporally coherent components of brain activity (Calhoun, Adali, Pearlson, and Pekar 2001a, 2001b). When ICA is combined with a specific task, it provides measures of both functional connectivity and task-related functional connectivity. This allows for the identification of neural networks involving a specific cognitive task as well as the ability to test which of these networks are affected by schizophrenia (Calhoun, Adali, McGinty, et al. 2001). Using a verbal WkM paradigm, Kim et al. (2009) found aberrant connectivity in regions of the DLPFC and ventrolateral PFC in adults with schizophrenia. There have been no studies to date evaluating functional connectivity in children and adolescents with schizophrenia also known as early-onset schizophrenia (EOS).
Children acquire specific cognitive abilities during specific points in development (Thompson and Nelson 2001). Gross and fine motor function, language, and the various aspects of social cognition all develop and mature within specific windows of neurodevelopment. Higher order cognitive processes, complex problem solving skills, and the ability to engage in abstract reasoning show the most robust development after the first decade of life (Luciana and Nelson 2000; De Luca et al. 2003). It is known that the PFC has continued development through adolescence shown by studies of synaptic pruning (Huttenlocher 1979), gray matter (GM) changes (Sowell, Thompson, Holmes, Batth, et al. 1999; Sowell, Thompson, Holmes, Jernigan, and Toga 1999), and myelination (Yakovlev and Lecours 1967; Paus et al. 1999; Bartzokis et al. 2001; De Bellis et al. 2001).
There have been 2 fMRI studies to date that utilized fMRI to evaluate WkM in EOS (Haenschel et al. 2007; Pauly et al. 2008). Haenschel et al. (2007) reported abnormal activations during a visual WkM paradigm in temporal and occipital regions during both encoding and retrieval without any patient/control differences in prefrontal regions. They concluded that patients with EOS have abnormalities in early visual processing streams, which impair WkM performance. Pauly et al. (2008) using a verbal n-back paradigm combined with neutral and adverse odors found that adolescent patients had hypoactivation in the DLPFC, parietal cortex, and the ACC.
Since EOS has been shown to be on a continuum with adult-onset illness (Rapoport and Inoff-Germain 2000) and as WkM continues to mature during late adolescence and early adulthood (Luciana and Nelson 2000; De Luca et al. 2003), a better understanding of the role of prefrontal regions in EOS is crucial for our understanding the neurobiology of schizophrenia. This is especially true in light of the considerable research documenting PFC abnormalities in adults with schizophrenia (Glahn et al. 2005). If EOS patients do not have abnormalities in prefrontal regions, it is possible that aberrant activity related to these structures are downstream or developmental effects of the illness (White et al. 2008). Our goal for this study was to investigate the functional connectivity of verbal WkM in EOS, with a primary interest in evaluating for the presence of aberrant PFC function.
Materials and Methods
Subjects
The participants included a total of 28 children and adolescents, including 14 patients with a schizophrenia spectrum disorder and 14 age- and sex-matched controls. The age of inclusion was between 8 and 19 years of age with the mean age of the patient and control groups was 15.1 (standard deviation [SD] 2.6) and 15.0 (SD 2.8) years, respectively, and both groups consisted of 12 boys and 2 girls (Table 1). Both the patients and controls underwent a thorough diagnostic assessment using the Kiddie-SADS-PL (Kaufman et al. 1997). Females who had started their menses underwent pregnancy testing, and none were found pregnant. Of the patients, 11 had a diagnosis of schizophrenia, 2 had schizoaffective disorder, and 1 had schizophreniform disorder. Additional clinical measures included the scale for the assessment of negative symptoms (Andreasen 1983) and the scale for the assessment of positive symptoms (Andreasen 1984).
Table 1.
Demographic and clinical characteristics for the patient and control groups
| Patients (n = 14) | Controls (n = 14) | P | |
| Demographic measures | Mean/SD | Mean/SD | |
| Age (years/SD) | 13.4/2.6 | 13.5/2.8 | NS |
| Sex (M/F) | 12/2 | 12/2 | NS |
| Parental SES | 40.4 (10.2) | 53.7 (7.4) | 0.0007 |
| Clinical measures | |||
| Psychotic symptoms (mean/SD) | 2.5/1.2 | ||
| Negative symptoms (mean/SD) | 2.6/0.8 | ||
| Disorganized symptoms (mean/SD) | 2.0/1.2 | ||
| Antipsychotic medications | Number | Mean dose | |
| Aripiprazole | 3 | 23.3 | |
| Ziprasidone | 2 | 40 | |
| Risperidone | 3 | 3.2 | |
| Quetiapine | 2 | 250 | |
| None | 2 |
NS, not significant.
The control group had no evidence of a past or present psychiatric disorder and no history of schizophrenia or psychosis in a first degree relative. Patients and controls were excluded if they had a history of substance dependence, ongoing substance abuse (within the past month), IQ less than 70, or a neurological disorder, head injury, or medical illness involving the brain. In addition, subjects were screened for any contraindications for participating in magnetic resonance imaging (MRI) or if they wore braces. Twelve of the 14 patients were on medication at the time of the scanning (Table 1). The study was approved by the Institutional Review Board at the University of Minnesota, and informed consent and assent were obtained prior to participation.
WkM Paradigm
Subjects rested comfortably in the scanner with head mobilization performed using a vacuum bag. The stimulus presentation paradigms were programmed using E-Prime (Psychology Software Tools, Inc.) and were triggered following the fourth repetition time (TR) of the scanner. Prior to scanning, subjects had 2 practice sessions, one while seated in a chair in front of a monitor and the second session in a mock scanner with back-presented stimuli, identical to that used during the scanning session. The subjects practiced until it was observed that they understood and were comfortable performing the task. Subjects were told to respond as quickly as possible but to try to make correct responses.
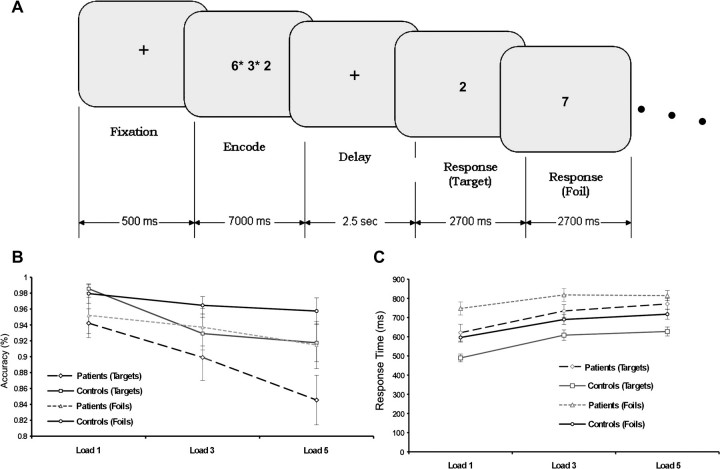
The stimuli consisted of a modified version of the Sternberg Item Recognition Paradigm (SIRP) (Sternberg 1966) (Fig. 1A) and were identical to that used by the Mind Clinical Imaging Consortium study of adults with schizophrenia (Roffman et al. 2008; Kim et al. 2009). Subjects were presented the word “Learn,” which was followed by 1, 3, or 5 digits presented simultaneously on the screen for 7 s. This was followed by a series of 16 single digits presented sequentially at a rate of 2.7 s for each digit. The participants wore a set of gloves with buttons and were instructed to push the right thumb button if the number on the screen matched a number they had seen during the learn set (target) or the left thumb button if the number did not match a number in the learn set (foil). There were 3 runs, each lasting 5 min and 58 s. Each run consisted of 2 blocks of the 1, 3, and 5 digit memory loads.
Figure 1.
(A) Timing for 1 trial of the Sternberg item recognition paradigm. Each block consisted of loads of 1, 3, or 5 digits (3 are shown in this example), followed by a delay, and a string of 16 targets or foils. (B) Behavioral results for accuracy in patients and controls. Accuracy is shown for both the targets and the foils. (C) Response time for targets and foils for the patient and control groups.
MRI Sequence
All magnetic resonance (MR) images were acquired with a 3-T Siemens MR System at the Center for Magnetic Resonance Research at the University of Minnesota. Following an initial localizer, a coronal scout image (12 slices, field of view [FoV] = 224 mm, TR = 2000 ms, time echo [TE] = 72 ms, resolution 2.3 × 1.8 × 2 mm) was obtained to locate the coronal midline. Following alignment, sagittal images were acquired along the coronal midline (12 slices, FoV = 224 mm, TR = 2040 ms, TE = 62 ms, resolution 1.2 × 0.9 × 2 mm). These sagittal slices were used to orient the volume along the anterior/posterior commissure plane.
Functional images were acquired using a gradient echo sequence with 27 axial slices and an in-plane resolution of 3.4 × 3.4 mm with a 4-mm slice thickness and a 1-mm gap. Additional sequence parameters include the following: TE = 30 ms, TR = 2000 ms, flip angle = 90°, FoV = 220 mm. A total of 177 volumes were obtained within each of the 3 runs for a total of 531 volumes.
Image Processing
The functional images were preprocessed using a combination of Analysis of Functional NeuroImages (AFNI, http://afni.nimh.nih.gov/) (Cox 1996) and FSL's FMRIB's Software Library (FSL, FMRIB Software Library; FMRIB, Functional Magnetic Resonance Imaging of the Brain; http://www.fmrib.ox.ac.uk/fsl/) (Smith et al. 2004). Following the conversion from DICOM to Nifti format, slice timing correction and motion correction were performed using AFNI (Cox 1996). Subjects who were unable to complete 3 runs of the SIRP or subjects who had greater than 2.5 mm of motion in the x, y, or z directions were excluded from the analyses.
Images were oriented to standard Montreal Neurological Institute (MNI) space utilizing FSL in a 3-stage process. First, a mean echo planar imaging (EPI) image was generated from the fMRI time series for each individual. This mean EPI image was registered to space through a 12-parameter transformation (Jenkinson and Smith 2001; Jenkinson et al. 2002). Finally, the 12-parameter transform was applied to the entire fMRI time series for each individual and each run. The data were spatially smoothed using an 8-mm full width at half-maximum Gaussian kernel (White et al. 2001). The resulting coordinates were converted to the Talairach and Tournoux standard space for anatomical mapping (Talairach and Tournoux 1988) using MNI2Tal (Mathew Brett, http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
Independent Component Analysis
Following preprocessing, a group ICA was performed on the preprocessed data (Calhoun, Adali, Pearlson, and Pekar 2001a, 2001b). The methods prescribed by this process were performed via the group ICA of fMRI (Matlab toolbox version 1.3c http://icatb.sourceforge.net). ICA is a statistical and computational data-driven technique that attempts to extract temporally related signals that are hidden within sets of random or unrelated variables. It assumes that the fMRI data are linear mixtures of independent source signals and attempts to extract maximally independent signals and their mixing coefficients. The principle behind ICA is that these independent source signals represent coherent groupings of BOLD signal change, often referred to as component maps, which are thought to be functionally relevant. Since ICA is a data-driven approach, the functional networks are generated without any assumptions about the shape of the hemodynamic time courses.
The spatial maps generated by ICA were averaged together across the 3 scan sessions and resulted in 27 independent component (IC) spatial maps for every subject. These 27 IC spatial maps represent the regions of the brain related to a specific time course. Every voxel within a component spatial map contains a z score, with high z scores reflecting a greater contribution to the associated time course.
Component Selection
One of the strengths of ICA is its ability to find noise-related components that represent head motion, ventricle activity, eye movement, and other signal artifacts. Thus, we first evaluated each of the spatial maps and eliminate those with motion or other artifacts. These were readily identified by symmetric activations on the opposite sides of the skull, activations within the ventricles, or activation within the eye itself. This resulted in the removal of 8 components related to artifacts.
The second phase consisted of identifying and limiting the components to only those that were task related. A regression was performed on the ICA component time courses using an SPM5 general linear model (GLM) design matrix coded for encoding and retrieval for each of the 3 WkM loads. This resulted in a set of beta weights for each regressor associated with a particular subject and component. The resulting beta weights represent the degree to which the component was associated with the WkM task relative to the fixation baseline (i.e., a high beta weight represents a large task-related modulation of a component for a given regressor). Finally, only components that showed a statistically significant effect of load for either encoding or retrieval were included in the study. This resulted in the elimination of all but 13 components. These 13 components were used to assess for patient and control differences using a mixed-model repeated-measures analysis of variance (ANOVA).
GLM Analysis
Single-subject analyses were performed using FMRIB's fMRI Expert Analysis Tool FEAT (http://www.fmrib.ox.ac.uk/fsl/feat5/index.html). The time series for the behavioral conditions modeled 3 different encoding and 3 different probe conditions, these reflecting the 3 memory loads. This modeled time series was convolved with the hemodynamic response function that was modeled from a linear combination of gamma functions.
Finally, to complement the ICA analysis, a GLM was implemented using FMRIB's improved linear model. A singular value decomposition was utilized to assess the fit of each voxel to the design matrix using local autocorrelation (Woolrich et al. 2001). The 2 within-subject runs were combined using a fixed effects model. The higher level group analysis, which compared patients and controls for each of the contrasts, was performed using FMRIB's local analysis of mixed effects. The resulting images showing patient/control differences were corrected for multiple comparisons using random Gaussian fields with a significance value set at p < 0.05, corrected.
Statistical Analyses
The demographic data were assessed using chi square for categorical data and t-tests for continuous data. A 2 (diagnosis) × 2 (encode/retrieval) × 3 (load) mixed-model repeated-measures ANOVA was performed using diagnosis, task, and load as the fixed effects and subject as the random variable. The task-related beta weights for each of the individual components were entered into a 2 (diagnosis) by 3 (load) mixed-model repeated-measures ANOVA. To control for performance differences between patients and controls, a 2 (group) by 3 (load) by 3 (run) mixed-model ANOVA was performed using accuracy as a covariate.
Results
There were no differences in age or sex between the patients with schizophrenia and the control group (Table 1). There was a significant difference in the socioeconomic status (SES) and paternal and maternal education level between the 2 groups. The controls came from families with higher SES and education levels. The patients had on average mild to moderate negative and positive symptoms at the time of scanning and mild disorganized symptoms (Table 1). All but 2 of the patients were on antipsychotic medications during the scanning session.
Behavioral Results
Both patients and controls responded faster to the probes than the foils (paired t = 13.3, degrees of freedom = 80, P < 0.0001). There were significant effects of both load (F2,50 = 52.1, P < 0.0001) and diagnosis (F1,25 = 11.5, P = 0.002) for the probe response time (RT) using a 2 (group) by 3 (load) mixed-model repeated-measures ANOVA (Fig. 2). Similarly, there was also an effect of load (F2,50 = 35.7, P < 0.0001) and diagnosis (F1,25 = 10.4, P = 0.004) for the foil RT. Neither the probe nor the foil RT conditions had an interaction between load and diagnosis. Evaluating accuracy, a 2 (group) by 3 (load) mixed-model repeated-measures ANOVA found a significant effect of load for both the probes (F2,50 = 11.2, P < 0.0001) and the foils (F2,50 = 3.4, P < 0.05), without significant effects of either diagnosis or a load by diagnosis interactions.
Figure 2.
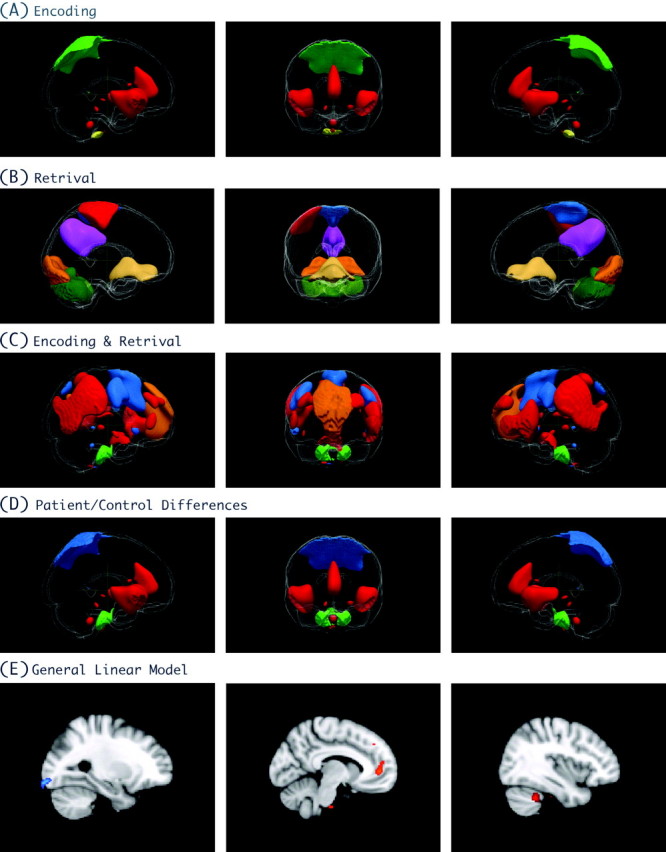
(A) Brain regions which demonstrated significant effects of load during encoding. (B) Brain regions which demonstrated significant effects of load during retrieval. (C) Brain regions which demonstrated significant effects of load during both encoding and retrieval. (D) Brain regions which demonstrated significant differences in functional connectivity between patients and controls. The different colors reflect connectivity patterns and are not suggestive of significance levels. (E) Patient/control differences in the GLM analysis. Using cluster-based correction (left), only left occipital activation was seen. After relaxing the threshold, activations emerged in the ACC (middle) and cerebellum (right).
ICA Results
The 13 components that passed the selection criteria were grouped depending on whether they were significantly related to the encoding phase, retrieval phase, or both. There were 3 ICs related solely to encoding, 6 components related solely to retrieval, and 4 components related to both encoding and retrieval. To determine which brain regions were representative of a component, a random-effects analysis in SPM5 was performed across all subjects on the raw spatial maps for each individual component using a false discovery rate (FDR) correction (FDR, P < 1 × 10−12) (Genovese et al. 2002). The FDR was utilized only to identify the specific components, and the statistical analyses of the spatial maps do not determine whether a given component is task related but merely represents a statistical visualization of the relevant regions for that component under a statistically relevant threshold.
Networks Associated with Encoding
The 3 ICs that are solely associated with encoding include a network involving 1) the occipital lobe and cerebellum; 2) the ACC and the temporal lobes bilaterally; and 3) a cerebellar network (Table 2). Of these 3 ICs, the network involving the ACC and temporal lobes demonstrated an effect of diagnosis and the network involving the occipital lobe and cerebellum had a diagnosis by load interaction. Thus, 2 out of the 3 encode-related IC networks showed differences between patients and controls. See Figure 2A for the spatial distribution of the ICs and Figure 2D for the IC networks that were different between patients and controls.
Table 2.
Brain networks associated with WkM load and their differences between patients and controls
| Brain network | Effect of load | Effect of diagnosis | Load by diagnosis interaction | |
| F2,52/P | F1,26/P | F2,52/P | ||
| Encode only | ||||
| Occipital lobe–cerebellum | 5.7/0.005 | 4.8/0.01 | ||
| Bilateral temporal–anterior cingulate | 3.5/0.04 | 3.8/0.05 | ||
| Cerebellum | 5.14/0.009 | |||
| Retrieval only | ||||
| ACC–medial frontal | 8.1/0.0008 | |||
| Occipital lobe | 6.1/0.004 | |||
| Left superior parietal–left motor–right cerebellum | 11.0/0.0001 | |||
| Medial parietal–orbitofrontal cortex | 3.2/0.05 | |||
| Anterior PFC–cerebellum–motor | 9.4/0.0003 | |||
| Occipital lobe | 6.9/0.0003 | |||
| Encoding and retrieval | Encode | Retrieval | Retrieval | |
| DLPFC–premotor–ACC–bilateral cerebellum | 15.6/<0.0001 | 35.9/<0.0001 | ||
| PFC–ACC–bilateral parietal–PCC | 4.1/0.02 | 26.3/<0.0001 | ||
| Bilateral DLPFC–bilateral parietal | 3.9/0.03 | 7.5/0.001 | ||
| Striatum–cerebellum | 6.1/0.003 | 6.9/0.002 | 4.2/0.05 |
Networks Associated with Retrieval
The 6 ICs associated solely with retrieval are shown in Table 2 and Figure 2B. These included a network involving the ACC and medial frontal lobe; 2 networks involving the occipital lobe; 2 networks involving motor function, including a network involving the left superior parietal, left motor, and right cerebellum; and a network involving the medial PFC, motor, and cerebellum. Finally, there was a network involving the medial parietal lobe and the orbitofrontal cortex. While all these components were significantly related to load, none demonstrated statistically significant differences between patients and controls.
Networks Associated with both Encoding and Retrieval
The remaining 4 components (Table 2) showed significant effects of load during both the encoding and the retrieval phases of the WkM task. These 5 ICs include networks associated with 1) DLPFC, premotor, ACC, and bilateral cerebellum; 2) the PFC, bilateral parietal, and the posterior cingulate cortex (PCC); 3) bilateral DLPFC and bilateral parietal; and 4) the cerebellum and striatum (Fig. 2C). While many of these networks include brain regions typically associated with WkM performance, only the networks involving the cerebellum and striatum showed statistically significant differences between patients and controls (Figs 2D and 3C).
Figure 3.
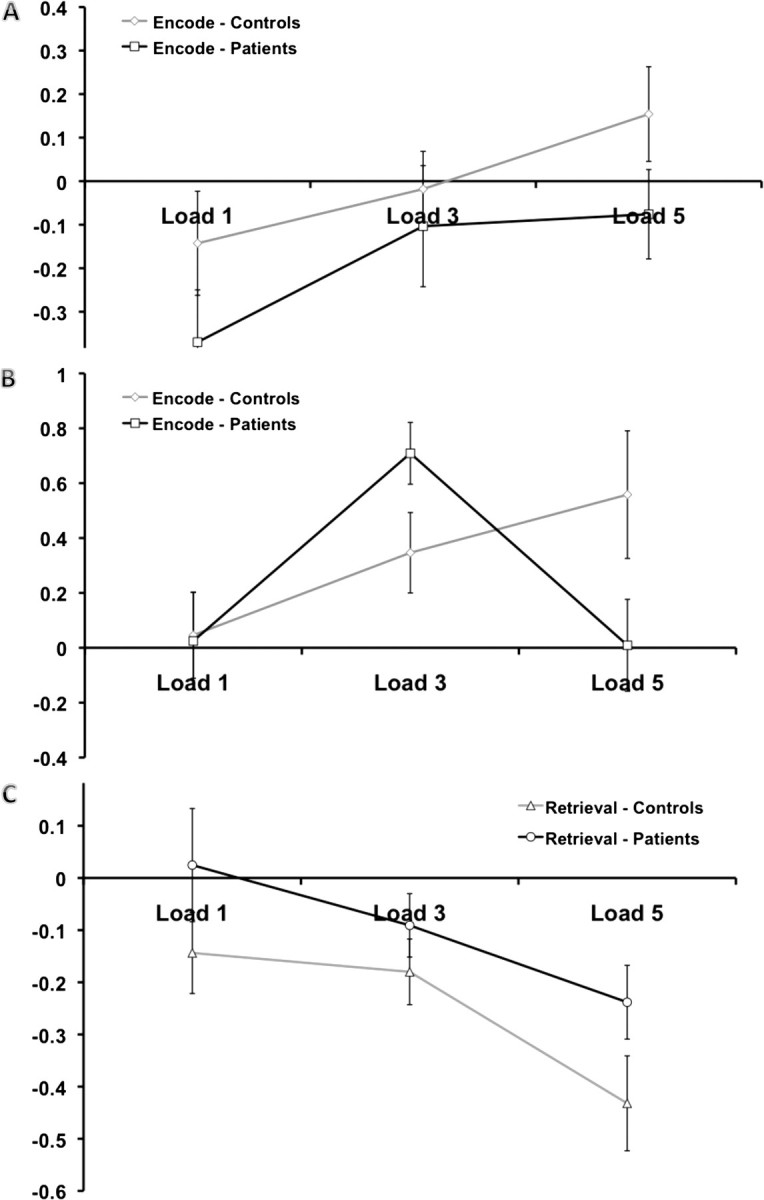
Patient/control differences in the task-related beta weights for (A) connectivity between the ACC and the temporal lobes bilaterally; (B) connectivity between the occipital lobe and the cerebellum; and (C) connectivity between the cerebellum and striatum.
Age-Related Differences
To assess whether there were age-related differences in connectivity in the 2 groups, we performed Spearman rank order correlations between age and the ICs. Interestingly, there was an inverse correlation (higher age and lower connectivity) in the EOS patients in networks involving the cerebellum and subcortical regions (r = −0.61, P = 0.02) that was not found in the control group (r = −0.04). The control group demonstrated an inverse correlation in networks involving the ACC with the bilateral temporal lobe (r = −63, P = 0.02) that was not seen in the patient group (r = 0.19).
Brain Connectivity and Behavioral Data
To assess whether the differences between patients and controls were secondary to differences in performance, a 2 (group) by 3 (load) by 3 (run) mixed-model ANOVA was performed using accuracy as a covariate. In this analysis, runs were not averaged, allowing for a direct matching between the beta weights of the ICA analysis within each run with the behavioral performance for the corresponding run. The results were very similar, with diagnosis by load interactions for the networks involving the ACC and medial frontal lobe (F2,207 = 4.1, P = 0.018) and networks involving the occipital lobe and the cerebellum (F2,206 = 5.6, P = 0.004). In addition, an occipital lobe network showed an effect of diagnosis (F2,25.7 = 4.1, P = 0.05).
When using the mean RT for accurate probes as a covariate, the significant findings remained unchanged. The network between the ACC and bilateral temporal lobes showed a significant effect of diagnosis (F1,30.1 = 7.5, P = 0.007), and there was a load by diagnosis interaction for the network involving the occipital lobe and the cerebellum (F2,207 = 5.8, P = 0.004). During retrieval, the network involving the striatum and cerebellum was no longer significant; however, a network involving the cerebellum, motor strip, and frontal pole was significant (F1,26.2 = 5.5, P = 0.03).
The significant findings when using accuracy for foils as a covariate were the same as when the accuracy for probes was used as a covariate. There were diagnosis by load interactions for the networks involving the ACC and medial frontal lobe (F2,207 = 4.2, P = 0.016) and networks involving the occipital lobe and the cerebellum (F2,206 = 5.2, P = 0.006). In addition, an occipital lobe network showed an effect of diagnosis (F2,25.7 = 4.5, P = 0.04).
When using the mean RT for accurate foils as a covariate, the results also remained quite similar. The load by diagnosis interaction for the network involving the occipital lobe and the cerebellum remained significant (F2,207 = 6.1, P = 0.003), whereas the network between the ACC and bilateral temporal lobes showed a trend effect of diagnosis (F1,30.1 = 3.4, P = 0.07). During retrieval, the network involving cerebellum, motor strip, and frontal pole trended to significance (F1,26.2 = 4.0, P = 0.056).
Finally, to assess whether error rates were correlated with any of the components, we performed Spearman rank correlations between accuracy and each of the components across the different loads. The controls had a significant relationship between accuracy toward probes and the network involving the PFC, ACC, PCC, and the parietal lobe bilaterally (r = 0.18, P = 0.005), whereas this relationship was not found in the patient group (r = 0.05). Similarly, controls had a significant relationship between accuracy to foils and networks involving the ACC and medial frontal lobes (r = −0.13, P = 0.04) and the cerebellum (r = 0.15, P = 0.02). Neither of these networks were significant in the patient group.
GLM Results
With clusterwise correction for multiple testing using a corrected P value of 0.05, patients had significantly less activation in the occipital lobe (3458 voxels, P = 0.0001) during retrieval (Fig. 2, left) compared with controls. The peak activation difference between patients and controls occurred at coordinates x = −22, y = −98, z = −14. There were no differences between patients and controls during encoding. Interestingly, reducing the significance threshold to a P < 0.001 uncorrected, lower activation levels were also seen in the ACC and cerebellum (Fig. 2, middle and left).
Discussion
Functional connectivity is defined as the temporal correlation of the hemodynamic waveforms (i.e., BOLD signal for a specific voxel or regional clusters of voxels over time) between different brain regions (Friston et al. 1993). The theory is that distributed neural networks within the brain mediate the performance on a specific task. Different tasks tap both separate and common distributed networks. With EOS schizophrenia being on a continuum with adult-onset schizophrenia (Rapoport and Inoff-Germain 2000) and with the considerable evidence identifying DLPFC abnormalities in adults with schizophrenia (Glahn et al. 2005; Ragland et al. 2007), we predicted disrupted functional connectivity between the DLPFC and regions known to involve WkM networks, as has been shown in studies of adults with schizophrenia (Kim et al. 2009).
Importantly, we found that patients and controls were both tapping neural networks known to be involved in WkM performance (Table 2) (Goldman-Rakic 1999; Smith and Jonides 1999; Muller and Knight 2006). These networks were highly significant for the effect of load and included widespread networks involving the DLPFC, posterior parietal lobe, ACC and PCC, temporal lobe, and the striatum (Fig. 2). In addition, brain regions known to involve motor performance were shown to have regional connectivity during the retrieval phase of the WkM task. This was expected, since the motor response was present only during this phase of the task. Thus, there is good evidence that both EOS patients and controls were tapping neural networks known to be active during WkM tasks.
There were 3 networks that showed differences in functional connectivity between patients and controls (Figs 2D and 3). The first difference occurred during encoding and involved network connections between the ACC and the temporal lobe (Fig. 3A). The second abnormal circuit, also present during encoding, involved connectivity between the occipital lobe and the cerebellum. The differences in this second network demonstrated a load by diagnosis interaction, as shown in Figure 3B. During retrieval, a network connecting the cerebellum and striatum demonstrated statistically significant differences between patients and controls (Fig. 3C).
Interestingly, none of these networks that showed differences between patients and controls included regions in the DLPFC. Haenschel et al. (2007) also did not find differences in the DLPFC in adolescent patients performing a visuospatial WkM task; however, Pauly et al. (2008) used a verbal n-back paradigm combined with neutral and adverse odors and reported hypoactivation in the DLPFC, parietal cortex, and the ACC in adolescents with schizophrenia. It is possible that the added component of adverse and neutral odors altered the task resulting in differences in prefrontal activation patterns (Compton et al. 2006). To the best of our knowledge, this is the first study evaluating functional connectivity in adolescents with schizophrenia.
Structural imaging studies of childhood-onset schizophrenia, referring to those who have the onset of schizophrenia before 13 years of age, have identified important differences in the developmental neurobiology of schizophrenia. More pronounced differences occur in parietal lobe regions, which progress to prefrontal regions as the children go from early to later adolescence (Thompson et al. 2001; Gogtay 2008).
Also during this period of development, functional imaging studies have demonstrated differences in the functional activation patterns between children, adolescents, and adults. Interestingly, during verbal WkM tasks, there is evidence that children tend to rely less on cerebellar and parietal regions (O'Hare et al. 2008) and that frontal and parietal regions are less well developed (Crone et al. 2006) compared with adults. In addition, there is evidence of a shift from more diffuse patterns of activity to localized patterns, as brain maturation occurs (Durston et al. 2006). Thus, the combination of less GM differences in younger patients, coupled with alternate networks and more diffuse activation patterns associated with development, may result in smaller effect sizes and thus less readily detectable signals in children and adolescents with schizophrenia. Finally, there is evidence that dopamine homeostasis is different in adolescents than in adults, notably involving the PFC (Wahlstrom et al. 2007).
There are several limitations to the current study, the first being the relatively small sample size. However, all subjects performed 3 separate runs with performance rates much greater than chance. In addition, we found significant activations within WkM networks as well as patient/control differences in several brain networks. All but 2 of the patients were on antipsychotic medications at the time of the scan, which may alter functional connectivity. However, there is evidence in adults that antipsychotic medications tend to normalize functional connectivity (Stephan et al. 2001). Finally, some of the differences may be related to differences in general intelligence, “g.” However, since IQ is adversely affected by the development of schizophrenia, it can be difficult to tease apart illness versus differences in g.
In summary, we found evidence of disrupted connectivity in networks involving the anterior cingulate, cerebellum, striatum, and occipital lobe in patients with EOS. We did not find evidence for aberrant connectivity in regions of the PFC in this study. Since WkM performance continues to develop through adolescence and into early adulthood (Luciana and Nelson 2000; De Luca et al. 2003), one possible explanation is that aberrant PFC function tends to be a downstream effect that occurs with continued development. Alternatively, the PFC may be inherently noisier in earlier development and thus is less likely to show differences. If the former is true, however, it would suggest that aberrant PFC is a developmental downstream effect of an earlier insult, perhaps involving the limbic system (White et al. 2008). Future studies should evaluate larger populations with EOS across different developmental periods. Differences in the patterns of functional connectivity between children and adolescents and adults with schizophrenia may provide a window into the neurodevelopmental course of the illness.
Funding
Mental Illness and Neuroscience Discovery Research Network (NIMH K08 MH068540, NIBIB R01 EB000840); NARSAD through the Essel Foundation.
Acknowledgments
Conflict of Interest: None of the authors have any conflicts of interest related to this work.
References
- Andreasen NC. The scale for the assessment of negative symptoms (Sans) Iowa City(IA): The University of Iowa; 1983. [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (Saps) Iowa City (IA): The University of Iowa; 1984. [Google Scholar]
- Andreasen NC. Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science. 1997;275:1586–1593. doi: 10.1126/science.275.5306.1586. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW., 2nd Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci. 1992;4:125–133. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Rezai K, Alliger R, Swayze VW, 2nd, Flaum M, Kirchner P, Cohen G, O'Leary DS. Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Assessment with Xenon 133 single-photon emission computed tomography and the tower of London. Arch Gen Psychiatry. 1992;49:943–958. doi: 10.1001/archpsyc.1992.01820120031006. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu JC. Hypofrontality in schizophrenia as assessed by pet. Am J Psychiatry. 1987;144:122–123. doi: 10.1176/ajp.144.1.122. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Kiehl KA, Astur R, Pekar JJ, Pearlson GD. A method for multitask Fmri data fusion applied to schizophrenia. Hum Brain Mapp. 2006;27:598–610. doi: 10.1002/hbm.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, McGinty V, Pekar J, Watson T, Pearlson G. Fmri activation in a visual-perception task: network of areas detected using the general linear model and independent components analysis. Neuroimage. 2001;14:1080–1088. doi: 10.1006/nimg.2001.0921. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional Mri data using independent component analysis. Hum Brain Mapp. 2001a;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. Spatial and temporal independent component analysis of functional Mri data containing a pair of task-related waveforms. Hum Brain Mapp. 2001b;13:43–53. doi: 10.1002/hbm.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Carter CS, Heckers S, Nichols T, Pine DS, Strother S. Optimizing the design and analysis of clinical functional magnetic resonance imaging research studies. Biol Psychiatry. 2008;64:842–849. doi: 10.1016/j.biopsych.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Compton MT, McKenzie Mack L, Esterberg ML, Bercu Z, Kryda AD, Quintero L, Weiss PS, Walker EF. Associations between olfactory identification and verbal memory in patients with schizophrenia, first-degree relatives, and non-psychiatric controls. Schizophr Res. 2006;86:154–166. doi: 10.1016/j.schres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Cox RW. Afni: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci USA. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C. Normative data from the cantab. I: development of executive function over the lifespan. J Clin Exp Neuropsychol. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A Shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Franzen G, Ingvar DH. Absence of activation in frontal structures during psychological testing of chronic schizophrenics. J Neurol Neurosurg Psychiatry. 1975;38:1027–1032. doi: 10.1136/jnnp.38.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (Pet) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Synopsis of function and dysfunction of the frontal lobe. Acta Psychiatr Scand. 1999;395(Suppl):51–57. doi: 10.1111/j.1600-0447.1999.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull. 2008;34:30–36. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46:650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, Linden DE. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2007;64:1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-Sads-Pl): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, Ford JM, Gollub RL, White T, Wible C, et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an Fbirn and Mcic study. Hum Brain Mapp. 2009;30:3795–3811. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Nelson C. Neurodevelopmental assessment of cognitive function using cantab: validation and future goals. In: Ernst, Rumsey, editors. Functional neuroimaging in child psychiatry. Cambridge (UK): Cambridge University Press; 2000. pp. 379–397. [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by Fmri. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Muller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139:51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER. Neurodevelopmental changes in verbal working memory load-dependency: an Fmri investigation. Neuroimage. 2008;42:1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly K, Seiferth NY, Kellermann T, Backes V, Vloet TD, Shah NJ, Schneider F, Habel U, Kircher TT. Cerebral dysfunctions of emotion-cognition interactions in adolescent-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2008;47:1299–1310. doi: 10.1097/CHI.0b013e318184ff16. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimaging of cognitive disability in schizophrenia: search for a pathophysiological mechanism. Int Rev Psychiatry. 2007;19:417–427. doi: 10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Inoff-Germain G. Update on childhood-onset schizophrenia. Curr Psychiatry Rep. 2000;2:410–415. doi: 10.1007/s11920-000-0024-4. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Gollub RL, Calhoun VD, Wassink TH, Weiss AP, Ho BC, White T, Clark VP, Fries J, Andreasen NC, et al. Mthfr 677c –> T genotype disrupts prefrontal function in schizophrenia through an interaction with comt 158val –> Met. Proc Natl Acad Sci USA. 2008;105:17573–17578. doi: 10.1073/pnas.0803727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as Fsl. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Magnotta VA, White T, Arndt S, Flaum M, O'Leary DS, Andreasen NC. Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by Fmri during a simple motor task. Psychol Med. 2001;31:1065–1078. doi: 10.1017/s0033291701004330. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Stuttgart (Germany), New York: Thieme Verlag; 1988. [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol. 2001;56:5–15. doi: 10.1037/0003-066x.56.1.5. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Hooper CJ, Vrshek-Schallhorn S, Oetting WS, Brott MJ, Luciana M. Variations in the catechol O-methyltransferase polymorphism and prefrontally guided behaviors in adolescents. Biol Psychiatry. 2007;61:626–632. doi: 10.1016/j.biopsych.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Weinberger D, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr Bull. 1988;14:157–168. doi: 10.1093/schbul/14.2.157. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- White T, Cullen K, Rohrer LM, Karatekin C, Luciana M, Schmidt M, Hongwanishkul D, Kumra S, Charles Schulz S, Lim KO. Limbic structures and networks in children and adolescents with schizophrenia. Schizophr Bull. 2008;34:18–29. doi: 10.1093/schbul/sbm110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, O'Leary D, Magnotta V, Arndt S, Flaum M, Andreasen NC. Anatomic and functional variability: the effects of filter size in group Fmri data analysis. Neuroimage. 2001;13:577–588. doi: 10.1006/nimg.2000.0716. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of Fmri data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]