Abstract
As more investigations into factors affecting the quality of life of patients with multiple sclerosis (MS) are undertaken, it is becoming increasingly apparent that certain comorbidities and associated symptoms commonly found in these patients differ in incidence, pathophysiology and other factors compared with the general population. Many of these MS-related symptoms are frequently ignored in assessments of disease status and are often not considered to be associated with the disease. Research into how such comorbidities and symptoms can be diagnosed and treated within the MS population is lacking. This information gap adds further complexity to disease management and represents an unmet need in MS, particularly as early recognition and treatment of these conditions can improve patient outcomes. In this manuscript, we sought to review the literature on the comorbidities and symptoms of MS and to summarize the evidence for treatments that have been or may be used to alleviate them.
Keywords: clinical medicine, comorbidity, multiple sclerosis, neurology, review, signs and symptoms, therapy
Introduction
Multiple sclerosis (MS) is a complex neurodegenerative autoimmune disease, characterized by dissemination of inflammatory lesions in the central nervous system (CNS). The localization and severity of MS lesions within the brain and spinal cord is unpredictable and, therefore, a wide range of body systems can be adversely affected to a variable degree. Consequently, there is a myriad of symptoms and comorbidities associated with MS that can impact negatively on patient quality of life (QoL).
In relapsing MS, the primary aim of therapeutic intervention is to reduce the frequency of relapses and limit disease progression. As a result, the clinical assessments that follow diagnosis of MS are often conducted with the sole intention of monitoring these outcomes, excluding other important MS-related conditions. In raising awareness and prompting early recognition of the known symptoms and comorbidities of MS, there exists the possibility to provide treatment and improve QoL for patients. In order to highlight these issues, we sought to review the treatment options for underrecognized and often overlooked symptoms and comorbidities associated with MS.
As a consequence of the low recognition of MS-related symptoms and comorbidities, many of the pharmacological treatment options reviewed herein are not licensed specifically for the treatment of those conditions in patients with MS, and therefore report off-label use of medications.
Methods
We performed an extensive MEDLINE search covering publications on all symptoms which have been dealt with in this article combined with ‘multiple sclerosis’ during the period from 1980 to August 2010. Articles with randomized controlled studies were considered to have the highest level of evidence, followed by one or more well-documented clinical studies, such as case-controlled or cohort studies, and the lowest level of evidence came from nonrandomized historical controls, case reports or expert opinion. In this paper we focus on studies with the highest level of evidence that were available for each therapy. This is followed by a summary of the therapies that have been used to treat symptoms known to be experienced by people with MS (Table 1). Type A recommendations represent the strongest type of recommendation for each symptom.
Table 1.
Summary of therapies that have been used in the treatment of multiple sclerosis (MS)-related symptoms.
| Symptom | Type A recommendations | Pharmacological agents with limited evidence of efficacy | Other/nonpharmacological interventions |
|---|---|---|---|
| Spasticity | Baclofen, tizanidine, intrathecal baclofen (for EDSS >7) | Dantrolene, tolperisone, benzodiazepines, gabapentin, tetrahydrocannabinol, botulinum toxin | Physiotherapy (Boboath, Vojta and proprioceptive neuromuscular facilitation), cooling therapy, hydrotherapy |
| Fatigue | None | 4-aminopyridine or other potassium–channel blocker, serotonin-specific reuptake inhibitors, amantadine (for fatigue without sleepiness), modafinil (for fatigue with sleepiness) | Physiotherapy, yoga, cooling therapy, aerobic exercise, energy conservation, coping strategies (occupational therapy) |
| Depression | None | Serotonin-specific reuptake inhibitors, serotonin and noradrenaline reuptake inhibitors | Cognitive behavioural therapy |
| Psychosocial problems | None | None | Psychotherapy, stress management, relaxation techniques |
| Tremor and ataxia | None | Isoniazid, carbamazepine, topiramate | Exercise and rehabilitation, forearm cooling, deep brain stimulation, thalamotomy |
| Pain – general | None | Related to suspected cause(s) of the symptom; similar approach as taken with non-MS patients | Related to suspected cause(s) of the symptom; similar approach as taken with non-MS patients |
| Pain – trigeminal neuralgia | High-dose steroid + carbamazepine or other anticonvulsant agent | Misoprostol, baclofen, oxcarbazepine | Thermocoagulation, glycerol instillation, gamma knife radiosurgery, neuroablative procedures, microvascular decompression |
| Cognitive impairment | None | Donepezil, rivastigmine | Cognitive training techniques |
| Occulomotor | None | Memantine, gabapentin | None |
| Dysphagia | None | Anticholinesterases | Thickening agents in liquids, ‘chin tucking’ |
| Dysarthria | None | Therapies treating tremor (in rare cases) | Speech therapy, spelling boards, computer-assisted programs |
| Seizures | None | Standard antiepileptic therapies | |
| Vertigo and dizziness | None | Vestibular blocking agents | Physiotherapy, vestibular rehabilitation therapy, repositioning manoeuvres |
| Sexual problems | None | Phosphodiesterase 5 inhibitors (erectile dysfunction), topical lubricants (vaginal dryness), androgen therapy (low libido) | None |
| Sleep disorders | None | Dopaminergic agonists for restless legs syndrome; modafinil for excessive daytime sleepiness | None |
| Urogenital – urine storage | None | Antimuscarinic compounds | Incontinence pads |
| Urogenital – emptying dysfunction | None | Antimuscarinic compounds, alpha-blocking agents, antispasticity agents | Incontinence pads, clean intermittent self-catheterization, ileoviscostomy, permanent catheterization |
| Urogenital – combined dysfunction | None | Neurotoxins, botulinum toxin A, cannabinoids | Sacral nerve stimulation, hyperbaric oxygen treatment |
| Bowel dysfunction | None | Laxatives | High-fibre diet, increased fluid intake, enemas |
The medications listed here have only been investigated for the treatment of MS-related symptoms in experimental settings and sometimes not even in patients with MS. Their inclusion in this table and the broader manuscript should not be taken to imply that the authors are in any way recommending they be used in the clinical setting. EDSS, Expanded Disability Status Scale.
Although some of the pharmacological treatment options listed may have regulatory approval for individual conditions described, many are not licensed specifically for the treatment of those conditions in patients with MS, and therefore refer to off-label use of those medications.
Spasticity
Approximately 90% of patients with MS experience spasticity during their lifetime. It is often a disabling symptom and is caused by axonal degeneration or malfunction that may be combined with demyelinating plaques within specific descending spinal tracts. This leads to a disturbance of inhibitory interneuronal spinal network pathways and results predominantly in weakness of physiological flexor muscles, usually with increased (‘spastic’) muscle tone and reduced dexterity of the muscles involved. Spasticity can be classified into a tonic (with persistently elevated muscle tone) or a phasic form (with intermittently elevated muscle tone) often associated with painful cramps. In patients with severe and long-standing MS, spasticity results in contractures that impair daily activities and quality of daily life and also impact on nursing. On the other hand, spasticity may to a certain degree help to ameliorate muscle weakness which reduces stability of lower limbs [Henze et al. 2006].
Assessment
Clinical assessment of spasticity should include quantification through a validated scoring system. The most commonly used score is the Ashworth scale or the modified Ashworth scale [Bohannon and Smith, 1987; Ashworth, 1964]. These scales assess passive resistance in the joints investigated, as the examiner perceives it. The resistance is scored from 0 to 4 (Ashworth scale) or from 0 to 5 (modified Ashworth scale). The modified Ashworth scale has been demonstrated to be useful for the assessment of spasticity in clinical practice [Ghotbi et al. 2009]. Other scales are the Tardieu scale and the modified Tardieu scale [Mehrholz et al. 2005; Tardieu et al. 1954]. These assessment scales consider passive range of motion, quality of muscle reaction to passive stretch at the fastest stretching velocity and angle of muscle reaction at the point of resistance to the fastest stretching velocity when the overactive stretch reflex produces a first catch. Other authors have developed scales counting spasm frequency [Priebe et al. 1996; Penn et al. 1989].
The obvious shortcomings of all of these scales are the dependency on the examiner’s rating of spasticity on passive movement. Previous studies have shown that spasticity scales provide insufficient information about muscles involved in spastic movement, and the reliability and validity of the scales has been questioned in clinical practice [Fleuren et al. 2010; Malhotra et al. 2009]. Thus, self-reported scales of perceived spasticity (visual analogue scales or numeric rating scales) may provide additional information, but are not sufficient alone for clinical assessment [Hsieh et al. 2008; Lechner et al. 2006]. The 88-item Multiple Sclerosis Spasticity Scale (MSSS-88) has been developed by Hobart and colleagues, and validated in a large patient cohort [Hobart et al. 2006]. The main aim of this patient-based, interval-level measure is to access the impact of spasticity on quality of life in patients with MS. In addition, a new 100-point score to assess disability related to daily activities, pain, dystonia and the presence of spasticity (Rekand disability and spasticity score) has recently been proposed, but needs to be validated in larger patient cohorts [Rekand, 2010].
Specific treatment
Physiotherapy represents the mainstay in the management of spasticity. The aim of physiotherapy is to reduce abnormal sensory inputs and decrease alpha-motor neuron activity [Gracies, 2001]. Controlled studies in patients with MS have only rarely been performed.
One study examined the effect of physiotherapy in MS-related gait disturbances reporting positive results [Wiles et al. 2001]. The most common techniques (e.g. those by Bobath, Vojta and proprioceptive neuromuscular facilitation) appear to be of roughly equal efficacy, despite their different rationale [Paci, 2003]. Treadmill training with partial body weight support, combined with Vojta-type physiotherapy, was reported to reduce spasticity in MS patients [Laufens et al. 1998]. Moreover, repetitive training of isolated movements may also reduce spasticity in a paretic hand [Butefisch et al. 1995].
Other treatment modalities, such as cooling [Mecomber and Herman, 1971] and hydrotherapy [Kesiktas et al. 2004], are reported to exhibit a transient beneficial effect on elevated muscle tone. Functional electrical stimulation by means of surface electrodes applied to the thigh muscles has been shown to induce cycling leg movements and reveal a significant reduction of spasticity after each stimulation session [Krause et al. 2007]. Studies investigating the effect of transcutaneous electrical nerve stimulation (TENS; 100 Hz and 0.125 ms pulse width) in patients with MS demonstrated no apparent effect in reducing spasticity; longer applications, however, appeared to be useful in treating MS patients with pain and muscle spasm [Miller et al. 2007]. Whole-body vibration has not been demonstrated to add any additional effect on spasticity in patients with MS [Schyns et al. 2009].
Pharmacological treatment
The effect of baclofen over placebo has been demonstrated in a number of trials [Sawa and Paty, 1979; Sachais et al. 1977; Duncan et al. 1976]. While there is evidence of an antispasmodic effect, the main benefit observed is a significant reduction of spasms. Two further randomized crossover trials reported some effect on spasticity [Feldman et al. 1978; Hudgson and Weightman, 1971]. Three randomized, controlled trials and two randomized crossover trials compared the effect of tizanidine with that of placebo and demonstrated that it reduces muscle tone [Smith et al. 1994; Lapierre et al. 1987; Knutsson et al. 1982]. When compared with baclofen, however, there is some evidence of similar efficacy [Bass et al. 1988; Eyssette et al. 1988; Smolenski et al. 1981]. Baclofen and tizanidine are the most commonly used oral drugs to reduce spasticity, which is in agreement with previous recommendations from the Spasticity Management Guideline Development Panel [Haselkorn et al. 2005; Snow et al. 1990]. Continuous intrathecal infusion of baclofen via an implantable pump has been shown to reduce spasticity [Creedon, 1997; Penn et al. 1989]. Its use is generally reserved for those with drug-resistant spasticity who have by and large lost ambulation.
The efficacy of gabapentin (300–3600 mg/day) was evaluated in two placebo-controlled randomized crossover trials. In one there were significant differences between the groups on all of the eight outcome measures assessed, with few adverse effects [Cutter et al. 2000]. A second trial reported significant effects on the Ashworth scale score and the Expanded Disability Status Scale (EDSS) [Mueller et al. 1997].
Botulinium toxin is an important addition to the treatment options for spasticity. Various randomized, placebo-controlled studies demonstrated significant a reduction of spasticity in adductor muscles compared with placebo [Hyman et al. 2000; Snow et al. 1990].
The evidence of a beneficial effect of dantrolene sodium over placebo is limited [Gambi et al. 1983; Monster, 1974; Gelenberg and Poskanzer, 1973] and for this reason dantrolene is used as a second-line drug. Tolperisone is also used second-line because of a lack of available evidence of efficacy. Following the early data on dantrolene, diazepam (a benzodiazepine) was originally thought to have a similar effect, with the potential to be even better tolerated [Schmidt et al. 1976]. Benzodiazepines are now known to exhibit strong antispastic effects; however, their profound side effects, specifically sedation and dependence, limit their use in MS [Shakespeare et al. 2003; Paisley et al. 2002].
Tetrahydrocannabinol (THC) or a cannabis extract have been studied extensively as treatment modalities to reduce spasticity. In a placebo-controlled trial with THC in 630 patients, no significant reduction of spasticity was found when using the Ashworth scale. Nevertheless, the therapeutic effects on patients’ overall mobility and on the subjective impression of pain reduction with THC and with cannabis extract suggest a potential usefulness of these drugs [Zajicek et al. 2003]. These observations have been confirmed in some smaller studies [Vaney et al. 2004; Wade et al. 2004; Killestein et al. 2002]. Sativex (USAN: nabiximols), an oromucosal spray containing THC and cannabidiol (CBD), has been studied for its efficacy in the alleviation of spasticity in MS patients. The results from three randomized, placebo-controlled, double-blind parallel group studies were recently combined. This meta-analysis demonstrates that nabiximols is well tolerated and reduces spasticity [Wade et al. 2010]. Based on the available evidence, however, the use of cannabinoids cannot be recommended, except in single refractory cases as second-line therapy when the treatment is performed by physicians with a high level of experience with cannabinoids. The dose-dependent side effects are that of other THC products; the potential for development of drug dependence has not been formally studied. Furthermore, in many countries cannabinoids are illicit drugs and require a special permit to be used in medicine.
There is only limited evidence that intrathecal injection of corticosteroids (triamcinolone acetonide) is clinically effective in spasticity of spinal origin. One group has reported a collection of clinical cases in which the effect of repeated intrathecal triamcinolone injections (40 mg every third day, up to six times) was studied resulting in a significant improvement of the EDSS score and of walking distance [Hoffmann et al. 2006, 2003]. With respect to the invasive nature of treatment and the limited data available, it can be recommended to be administered only by experienced neurologists.
Fatigue
Fatigue is one of the most frequent and disabling symptoms reported in MS. Up to 90% of patients are affected [Fisk et al. 1994a; Krupp et al. 1988] and over half of patients state that it is the most troublesome symptom of the disease [Fisk et al. 1994a]. Fatigue differs from normal tiredness as it is unexpected, occurs suddenly and without any direct external reason. Symptoms are more prominent in the afternoon [Krupp et al. 1988], aggravated by heat and have a negative impact on QoL, reducing daily tasks and exercise and interfering with work, family and social life [Comi et al. 2001; Fisk et al. 1994a].
The pathophysiology of fatigue in MS is unclear as there is no relation with disease duration; it can occur at all stages of the disease [Comi et al. 2001] and is often already present at the onset of disease [Whitaker and Mitchell, 1997]. There is no relationship between fatigue and age [Krupp et al. 1995; Fisk et al. 1994a] or gender of patients [Colosimo et al. 1995]. Positive correlations between EDSS score and fatigue have been reported [Bergamaschi et al. 1997; Colosimo et al. 1995; Krupp et al. 1995], but this relationship was not confirmed in a more recent study [Bakshi et al. 2000]. As fatigue can occur at early stages of the disease it would imply that it is not related to MS severity and that the observed positive correlations derive from other comorbid factors. There appears to be an association between fatigue and depression [Brown et al. 2009; Bakshi et al. 2000], and between fatigue and self-esteem [Fragoso et al. 2009], but the underlying mechanisms are not well understood.
Increasing evidence attests that fatigue is not related to peripheral inflammatory activity [Giovannoni et al. 2001] nor to global clinical severity of the disease [Bakshi et al. 2000], but may be linked to dysfunction within the nervous system. However, imaging studies did not show any association with T2 lesion load or the number of gadolinium-enhanced lesions [Bakshi et al. 1999]. Focal lesions in the brainstem are the only measure for which a direct relationship to fatigue has been suggested [Möller et al. 1994]. Other imaging studies have provided convergent arguments suggesting a dysfunction in the striatal–thalamic–frontal system among patients experiencing fatigue [Andreasen et al. 2010; Tellez et al. 2008; Filippi et al. 2002; Roelcke et al. 1997]. Whether such abnormalities are a constant feature among patients with fatigue or characterize a specific pattern concerning only a sub-group of patients remains to be determined. A sympathetic vasomotor dysfunction has also been suggested [Flachenecker et al. 2003], but causal abnormalities probably mostly reside in the CNS.
Assessment
The diagnosis and management of fatigue in MS are complicated by a number of issues, including symptom heterogeneity. The MS Council for Clinical Practice Guidelines [The Multiple Sclerosis Council for Clinical Practice Guidelines, 1998] has defined MS-related fatigue as a ‘subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual or desired activities’. In some cases fatigue may be related to depression, cognitive dysfunction or motor impairment, defining secondary fatigue. Nevertheless, for many patients, fatigue exists independently of motor weakness, cognitive or mood disorders.
The assessment of fatigue is problematic due to the subjective nature of the disorder and the fact that 13 different fatigue scales have been used in patients with MS since 1989. However, none of these scales have proved to accurately and reliably assess total fatigue in MS and tend to focus on specific components of fatigue. As a result the discrimination between cognitive and motor aspects of fatigue, which has been one objective of the fatigue impact scale [Fisk et al. 1994b, 1994a], remains poor, most of the scales having an emphasis on physical symptoms. The recently reported Fatigue Scale for Motor and Cognitive Functions (FSMC) [Penner et al. 2009] attempts to overcome these limitations. Although the FSMC has yet to be employed in large-scale studies, it has undergone validation assessments, which showed that the scale provides differential quantification and graduation of cognitive and motor fatigue.
Specific treatment
There is a current unmet need for treatments tailored to specific aspects of MS-related fatigue.
Several nonpharmacological approaches have been proposed, including physiotherapy [Wiles et al. 2001], aerobic exercise [Mostert and Kesselring, 2002], yoga [Oken et al. 2004] or cooling [Schwid et al. 2003]. The observed positive effects were somewhat weak although these approaches benefited from having no adverse side effects. More recently, a controlled trial has demonstrated that progressive resistance training can improve muscle strength and functional capacity in MS, and this is associated with improvement in fatigue, mood and quality of life [Dalgas et al. 2010, 2009]. Fatigue management and energy conservation have also been proposed as effective acute and long-term strategies to minimize fatigue in MS [Sauter et al. 2008; Mathiowetz et al. 2005].
Pharmacological treatment
Pemoline, a CNS psychostimulant which acts, at least partly, through the dopaminergic system showed a possible benefit in reducing fatigue with a daily dose of 75 mg in one study [Weinshenker et al. 1992], but another trial failed to show any efficacy at a dose of 56.25 mg/day [Krupp et al. 1995]. Side effects such as irritability, headache, insomnia, nausea, anorexia (as well as possible hepatotoxicity) severely limit the use of pemoline in clinical practice.
Trials with 4-aminopyridine, a potassium-channel blocker, have shown some improvement of motor weakness, ambulation and fatigue [Rossini et al. 2001; Bever et al. 1994; Polman et al. 1994 b; van Diemen et al. 1993], but side effects might be serious. The related molecule, 3,4-diaminopyridine, is better tolerated, but is poorly effective on fatigue [Sheean et al. 1998; Bever et al. 1996; Polman et al. 1994a]. However, this family of molecules could specifically improve symptoms for patients with temperature-sensitive or exercise-dependent fatigue [Polman et al. 1994a], and recent results suggest they improve motor disorders and walking ability [Goodman et al. 2007]. Indeed, a recent randomized, placebo-controlled study showed improvement in walking speed in approximately one-third of patients treated with fampridine, a sustained-release 4-aminopyridine preparation [Goodman et al. 2009]. In addition, the drug is now licensed in the USA to improve walking in people with all types of MS. Main side effects were paraesthesia, abdominal pain, dizziness, confusion and convulsions [Goodman et al. 2009].
Antidepressant molecules such as selective serotonin reuptake inhibitors (SSRIs) have been suggested as treatments for fatigue, but their clinical efficacy in fatigue remains to be proven. Improvements in MS-related fatigue have been shown with aspirin (1300 mg/day) in a small crossover study [Wingerchuk et al. 2005], and also with levocarnitine in a small open-label study of patients treated with immunosuppressant and immunomodulatory drugs [Lebrun et al. 2006].
Amantadine, an antiviral agent, also acts as a CNS stimulant due to its putative dopaminergic, noradrenergic and antiglutamatergic properties. This drug has been evaluated for fatigue relief in patients with MS in placebo-controlled clinical trials, mainly following a crossover design [Pucci et al. 2007; Krupp et al. 1995; Cohen and Fisher, 1989; Canadian MS Research Group, 1987; Murray, 1985]. Overall, the results from these studies have suggested amantadine may provide a mild, short-term reduction in fatigue in 20–40% of patients, although the results are inconsistent. In addition, the impact of this transient reduction on patient functioning and health-related quality of life remains to be determined. The main side effects are hyperactivity, insomnia, hallucinations and oedema.
Modafinil is a wake-promoting agent with clear impact on sleepiness. A small benefit on fatigue has been suggested in two nonrandomized studies [Rammohan et al. 2002; Zifko et al. 2002], but a double-blind, placebo-controlled, randomized trial did not confirm the benefit on a whole population of patients with fatigue [Stankoff et al. 2005]. However, a positive trend was reported for patients with excessive daytime sleepiness.
Depression
Depression is common in patients with MS. Lifetime morbidity of a depressive disorder in these patients is reported to be approximately 50% [Sadovnick et al. 1996]. A study that involved posting of Center for Epidemiologic Studies Depression Scale (CES-D) questionnaires to patients with MS [Chwastiak et al. 2002] showed that clinically significant depressive symptoms were present in 42% of patients, and moderate to severe depression in a further 29% of patients. Other studies have shown a 12-month prevalence of depression of 15% [Ziemssen, 2009] to 26% [Patten et al. 2003] in patients with MS compared with 9% in the general population, and a standardized mortality ratio for suicide of 2.3 for patients with MS compared with the general population [Fredrikson et al. 2003].
The known effects of depression can be exacerbated in MS and have been observed to result in reductions in QoL [Wang et al. 2000], physical activity [Motl et al. 2009], social interactions [O'Brien, 1993], treatment adherence [Mohr et al. 1997] and working memory capacity [Arnett et al. 1999].
As a chronic disease, MS has a significant impact on social roles (e.g. personal relationships and work), standards of living, psychological well-being and recreational activities. The impact is not just restricted to the patient but also to friends and relatives, particularly children and partners [Hakim et al. 2000]. Clinical neurologists need to be aware of the types of psychosocial problems that can be associated with MS and be vigilant for these problems in patients they have under their care.
Assessment
The assessment of depression in MS is complicated by the overlap between the signs and symptoms of the disease and those of depression itself. A study by Mohr and colleagues [Beeney and Arnett, 2008] showed that four of the nine core symptoms of depression in the Diagnostic and Statistical Manual of Mental Disorders (DSM- IV) also occur in MS: fatigue, psychomotor retardation, decreased concentration and sleep disturbance. In addition, it has been shown that only 70% of cases of depression are identified in patients with MS when employing the widely used Beck Depression Inventory (BDI) with a cutoff score of 13, leaving almost one third of patients undiagnosed [Sullivan et al. 1995]. However, despite this shortfall in diagnosis, the Goldman Consensus Statement of 2005 [Goldman Consensus Group, 2005] still considered the BDI as the most appropriate scale to use in the screening for depression in patients with MS.
Although validated in clinical studies, the use of questionnaires and other assessment tools for depression is not widely implemented in clinical practice. There is evidence that asking one question about whether the patient feels sad or depressed [Vahter et al. 2007; Avasarala et al. 2003] or two simple questions, which assess mood and loss of interest or pleasure [Mohr et al. 2007], may be sufficient to diagnose depression in patients with MS with a high level of sensitivity and specificity.
In a large systematic review of 72 studies [Dennison et al. 2009], adjustment to a diagnosis of MS and disease progression was found to depend on a wide range of factors, the most strongly correlated being perceived stress and emotional-coping strategies while uncertainty over future health was robustly associated with a worse adjustment to diagnosis. Other factors were similar to those for other major disease diagnoses such as social support, interactions with others, cognitive psychopathology, illness and symptom cognitions, perception of control and self-efficacy, positive psychology and health behaviours.
No formal screening tools are currently available for determining and rating these problems in patients with MS. Depending on the individual issues affecting each patient, depression scales and QoL instruments may be helpful, although lacking in specificity. Timely recognition of psychosocial alterations in patients can ensure the provision of appropriate social and therapeutic support which may include psychotherapy, stress management and relaxation techniques as well as vocational rehabilitation [Khan et al. 2009; Malcomson et al. 2007].
Specific treatment
Psychotherapies have been shown to have a positive impact on MS-related depression with the results of cognitive behavioural therapy (CBT) observed to be equivalent to the administration of sertraline [Mohr et al. 2001]. A review of intervention studies on depression in persons with MS showed that there is reasonable evidence that CBT is beneficial in the treatment of depression and in helping people adjust to (and cope with) living with the disease [Walker and Gonzalez, 2007].
Pharmacological treatment
No randomized, controlled studies have been performed on the efficacy of pharmacological treatment of depression in patients with MS; however, there is considerable evidence that SSRIs and selective noradrenaline reuptake inhibitors are effective in the treatment of general depression. The dual action serotonin and noradrenaline reuptake inhibitors have also been shown to be effective in the treatment of depression [Herrera-Guzmán et al. 2010]. When choosing an antidepressant for patients with MS, the side effect profile of the chosen medication must be taken into consideration, particularly where it can exacerbate a condition that may already be present in the patient (e.g. sexual dysfunction or spasticity). Use of interferon therapy had been considered to be associated with an increased incidence of depression in patients with MS [Mohr et al. 1999]. However, more recent investigations have dismissed such an association [Patten et al. 2008, 2005].
Tremor and ataxia
Tremor is estimated to occur in 75% of patients diagnosed with MS [Deuschl et al. 1998], although prevalence of this symptom has not been studied systematically or on a large scale. In patients with MS, tremor has been reported in various parts of the body including the head, neck, vocal cords, trunk and limbs [Alusi et al. 1999]. Alusi and colleagues reported that in 100 randomly selected patients with MS in the UK, tremor severity was minimal in 27%, mild in 16% and moderate to severe in 15% of patients; the severity was correlated with the degree of dysarthria, dysmetria and dysdiadochokinesia; and was associated with greater disability [Alusi et al. 2001]. In a community-based US study of 200 patients with MS, clinical tremor was found in 25% of patients and severe tremor in 3% of patients [Pittock et al. 2004]. The lower overall incidence of tremor in the US study may be related to differences in study design and/or the selection of patients. A 2005 study provided evidence that tremor amplitude was correlated with the lesion load in the contralateral pons [Feys et al. 2005b].
Three types of general tremor have been identified [Deuschl et al. 1998]:
postural tremor, which is present while a position is voluntarily maintained against gravity;
intention tremor, which is obvious when a movement is target-directed with an increased amplitude during visually guided movements towards the target; and
rest tremor, which is observed when a body part is not voluntarily activated and supported against gravity.
The first two subtypes are related to the cerebellum and its connections whereas the third subtype is indicative of pathology in the basal ganglia. Although demyelination, inflammation and axonal injury have been reported in the basal ganglia of patients with MS [Vercellino et al. 2009], rest tremor has not been reported in MS [Koch et al. 2007].
Ataxia is a group of abnormal movements, of which tremor is only one symptom. Other conditions included in this group include incoordination, delay in movement, dysmetria and dysdiadochokinesia [Ghez and Thach, 2000]. Up to 85% of people with MS may experience ataxia at some point in time and in 32% of patients the ataxia will be severe enough to decrease functional abilities [Weinshenker et al. 1996].
Ataxia can be the result of damage to the cerebellum (cerebellar ataxia), the posterior columns of the spinal cord (sensory ataxia) or dysfunction of the vestibular system (vestibular ataxia). Ataxia can also result from thalamic and parietal and frontal-lobe lesions.
Assessment
Evaluation of tremor is not specified in the functional systems scale for cerebellar function in the EDSS. Moreover, clinical practice indicates that it is not easy to quantify the severity of this symptom. Several methods have been suggested although all are rather complicated and too cumbersome for routine use in a clinic [Matsumoto et al. 2001]. A simple 0 to 10 scale has been shown to be a reliable measurement tool in patients with MS [Alusi et al. 2001; Bain et al. 1993], although it has yet to be validated in large clinical studies.
The Fahn Tremor Rating Scale (FTRS) [Fahn et al. 1988] assesses intention tremor in the terminal period of the finger-to-nose test. A modified version, without the functional disability rating scale, has been assessed for use with people with MS [Hooper et al. 1998]. It was found to be a reliable and potentially useful tool for assessing movement disorders in these patients.
Questionnaires and scales destined to evaluate QoL may be informative about tremor severity since this symptom is known to have a great impact on the daily life of the patients. However, these methods are almost exclusively subjective and their use in evaluating specific symptoms such as tremor is presently unvalidated.
The 2007 Cochrane Database Systematic Review of treatment of ataxia in MS [Mills et al. 2007] concluded that standardized, well-validated measures of ataxia need to be developed and employed in large, double-blinded, randomized, controlled trials. Items such as the International Cooperative Ataxia Rating Scale (ICARS) which has been shown to be a reliable and repeatable measure of ataxia [Storey et al. 2004; Trouillas et al. 1997], needs to be validated for specific use in MS. To date, the use of the Brief Ataxia Rating Scale or the Abnormal Involuntary Movement Scale has not been assessed in MS.
Specific treatment
Exercise and rehabilitation regimens have been shown to improve functional independence despite having a minimal impact on the level of neurologic impairment, although the results are only short lived [Brown and Kraft, 2005]. These regimens are known to have a greater impact on patients with severe MS compared with less affected patients, although, as with cognitive problems, ataxia seems to be refractory, particularly in those with severe disease [Brown and Kraft, 2005]. In addition to exercise and rehabilitation regimens, some studies have reported a beneficial effect of local forearm cooling on upper limb intention tremor, whereby sustained cooling of the forearm reduced tremor amplitude and frequency in patients with MS [Feys et al. 2005a; Albrecht et al. 1998].
Pharmacological treatment
So far, no medical treatment has reliably been proven to be efficacious for tremor in patients with MS. Most reports of a treatment effect derive from uncontrolled open-label studies and only a few randomized controlled trials have been published, each only including a small number of patients, with inconsistent results. Isoniazid failed to show any benefit in decreasing the severity of either static or kinetic cerebellar tremors when administered with propranolol and ethanol [Koller, 1984] and only minimal improvements were observed in six of eight patients who received isoniazid alone [Bozek et al. 1987]. In contrast, two small (≤5 patients) open investigations showed significant improvements in tremor in patients with MS given isoniazid [Francis et al. 1986; Sabra et al. 1982]. Cannabinoids have also been investigated for the relief of this symptom [Koch et al. 2007], although their efficacy has not been reliably demonstrated. In non-MS patients, beneficial effects on tremor have been reported for carbamazepine [Sechi et al. 1989] and an isolated case study has suggested that intrathecal baclofen may be worthy of further investigation [Weiss et al. 2003]. The use of clonazepam or propranolol has also been advocated, but there is limited supporting evidence [Perlman, 2000].
Surgical treatment
Successful alleviation of tremor in patients with MS has been achieved using deep brain stimulation (DBS) of the ventralis intermedius (VIM) thalamic nucleus [Wishart et al. 2003], possibly by restoring motor inhibition in the cerebellothalamocortical pathway [Ayache et al. 2009].
It remains unclear whether DBS or thalamotomy is the most effective strategy in MS and whether either will provide an overall improvement in QoL. A systematic literature review showed that numerous studies have attempted to assess the efficacy and safety of thalamotomy and DBS in the treatment of MS tremor, but no standardized outcome measures have been employed [Yap et al. 2007]. The review showed that both procedures initially suppressed tremor in over 90% of patients, although functional improvement was seen only in 47.8% of those who underwent thalamotomy as opposed to 85.2% of those who had DBS [Yap et al. 2007].
The long-term efficacy of thalamic stimulation versus thalamotomy for tremor suppression in MS was assessed in a randomized study and showed that a diminished effect of stimulation was observed in half of the patients, although subjective outcome assessments favoured stimulation compared with thalamotomy [Schuurman et al. 2008]. DBS (thalamic stimulation in particular) is considered a relatively safe and probably effective treatment in medication-resistant, disabling tremor associated with MS [Lyons and Pahwa, 2008].
The consensus recommendations of the German DBS Study Group [Timmermann et al. 2009] rely primarily on expert opinion and include:
extensive pre-operative characterization of tremor, ataxia with accompanying disabilities, status of the MS, comorbidities and burden of disease;
careful intraoperative testing of effects and side effects;
intensive postoperative testing and programming as well as regular re-evaluation of therapeutic effect.
The efficacy and safety of treatments for ataxia in MS are poorly documented, although neurosurgery and rehabilitation interventions may, at least, be partly beneficial.
Pain
Pain affects between 44% and 80% of people with MS and has a significant impact on their lives [Hirsh et al. 2009; O'Connor et al. 2008; Ehde et al. 2006]. Although it is not clear whether the incidence of pain is greater amongst patients with MS compared with age-matched healthy subjects, a nonsystematic literature review of pain in MS by Bermejo and colleagues showed that increased pain was associated with duration of disease and severity of MS [Bermejo et al. 2010]. They also reported that pain intensity was related to levels of fatigue and depression and that pain impacts patients’ QoL, limiting both their daily activities and their working life.
A recent systematic review of pain in MS [O'Connor et al. 2008] differentiates between four distinct pain categories:
continuous central neuropathic pain (e.g. dysesthetic extremity pain);
intermittent central neuropathic pain (e.g. trigeminal neuralgia, Lhermitte’s sign);
musculoskeletal pain (e.g. lower back pain, muscle spasms or relating to tonic spasms);
mixed neuropathic and non-neuropathic pain (e.g. headache).
An additional source of pain derives from MS-specific drug therapies which, although not serious, may decrease compliance with treatment [Pardo et al. 2010].
Some of these types of pain (e.g. dysesthetic pain, trigeminal neuralgia, Lhermitte’s sign and painful tonic spasms) are directly related to the disease and associated with plaque formation in the spinal cord and brain [Nurmikko et al. 2010], whereas a study of headaches (including migraine) showed no difference in the prevalence rates between patients with MS and control subjects [Putzki et al. 2009].
Trigeminal neuralgia is the most common and most distinct paroxysmal disorder and pain syndrome in MS. The high prevalence in MS (estimates of 6.3% [Putzki et al. 2009] in patients with MS compared with an overall prevalence of 0.01–0.02% [Manzoni and Torelli, 2005]) is explained by the cause of neuralgic pain: aberrant ephaptic (nonsynaptic) transmission from somatosensory to nociceptive fibres at a site of local damage to the myelin sheath. Neuralgia is not limited to the trigeminal nerve and, although much rarer, may involve other sites of the head, neck, trunk and extremities.
Assessment
To date, there have been no systematic studies on the best diagnostic procedures for pain specifically in patients with MS. As with any patient with pain, a thorough clinical and neurological examination is required and the use of a pain diary to document triggers, intensity, concomitant features, duration and pain-relief methods employed is an important tool to aid diagnosis and future treatment plans. Several studies have shown that visual analogue scales are the most appropriate method for recording pain severity [Henze, 2005; Pöllmann et al. 2005; Solaro et al. 2003].
Specific treatment
General pain
Therapeutic management of MS-related pain syndromes is primarily focused on symptom relief and is mainly driven by the suspected causes of the pain [O'Connor et al. 2008; Beard et al. 2003]. This approach comprises both pharmacological and nonpharmacological measures similar to methods used in treating non-MS pain. The lack of controlled clinical trials, combined with a poor understanding of the pathophysiology, prevents comprehensive management criteria for MS-related pain [Nurmikko et al. 2010].
Trigeminal neuralgia
Treatment of neuralgias in patients with MS is slightly different from treatment in non-MS patients [Mumenthaler and Mattle, 2008]. High-dose steroids, usually combined with an anticonvulsant, can be particularly effective, especially when the neuralgia is associated with a relapse. Misoprostol, a prostaglandin E1 agonist, has also been found effective in patients with MS with trigeminal neuralgia [DMKG Study Group, 2003], and the muscle-relaxant and antispasmodic agent baclofen is occasionally useful instead of, or in addition to, anticonvulsive drugs such as oxcarbazepine [Leandri, 2003].
Surgical treatment
The American Academy of Neurology/European Federation of Neurological Societies reported that there was insufficient evidence to support or refute the effectiveness of surgical management of trigeminal neuralgia in MS despite some indications that surgical treatments were less effective in patients with MS compared with classical neuralgia [Gronseth et al. 2008]. They concluded that the role of surgery versus pharmacotherapy in the management of trigeminal neuralgia in patients with MS remains uncertain [Gronseth et al. 2008].
Interventional treatments involving ganglion and root level neuroablative procedures can be used [Nurmikko et al. 2010], although neurosurgical exploration of the posterior fossa to free the nerve root from a vascular loop, as performed in cases of idiopathic trigeminal neuralgia, should be avoided in patients with MS as the pathogenesis of the disorder in these patients is probably related to plaques in the brainstem and not to vascular loops encroaching the nerve. Percutaneous radiofrequency rhizotomy has been reported to result in complete pain relief in 71–81% of patients with MS, although the number of treated patients was small (≤17) [Berk et al. 2003; Kanpolat et al. 2000]. Between 30% and 50% of the patients had a recurrence of the neuralgia over a mean follow-up period of between 52 and 60 months. Successful treatment of patients with MS with trigeminal neuralgia has also been achieved using percutaneous retrogasserian glycerol rhizotomy [Kondziolka and Lunsford, 2005].
Overall, microvascular decompression has been suggested to provide the longest freedom from pain [Gronseth et al. 2008], but because of the unique characteristics of MS-related trigeminal neuralgia, it has been suggested that this technique should be used only after neuroablative procedures have been considered [Nurmikko et al. 2010]. Results of a long-term study published in 2009 on gamma knife radiosurgery showed that reasonable pain control was maintained for 5 years in over 50% of patients with MS [Zorro et al. 2009].
Cognition
Some degree of cognitive impairment is noted in approximately 45–65% of patients with MS [DeSousa et al. 2002]. The impairment may start at early stages of the disease and cognition continues to deteriorate with disease progression [Huijbregts et al. 2006]. The main symptoms of these deficits include problems with concentration, mental exhaustion and fatigue, spurious actions, learning difficulties and forgetfulness.
Clinical studies have identified that the most common cognitive deficits faced by patients with MS include reduced information processing speed and episodic memory deficits. The effects of these problems, when combined with other known cognitive deficits such as conceptual reasoning, verbal fluency and concentration difficulties, can lead to social impairment, unemployment and a reduced QoL; factors often regarded as being more disabling than motor deficits [Rogers and Panegyres, 2007; Rao et al. 1991b].
Progressive loss of neocortical grey matter has been shown to play a major role in the development of MS-associated cognitive impairment [Amato et al. 2007; Tekok-Kilic et al. 2007], and as a greater degree of grey matter loss is generally seen at later stages of the disease [Fisher et al. 2008], it is apparent that cognitive impairment is likely to increase in patients with progressing disease states. In addition, diffuse white matter damage, affecting connections between distant cortical areas, has been shown to be largely responsible for the impairment of working memory [Audoin et al. 2007] and other cognitive defects [Dineen et al. 2009].
Assessment
Several different questionnaires and testing batteries have been proposed for screening and evaluation of cognitive deficits in patients with MS [Benedict et al. 2008, 2002; Parmenter et al. 2007; Rao et al. 1991a, 1991b]. However, there is no general consensus on which questionnaires and tests are most beneficial.
Specific treatment
Management of cognitive impairment is difficult and ‘cognitive rehabilitation in MS is in its relative infancy’ [O'Brien et al. 2008], although nonpharmacological therapeutic procedures, particularly types of cognitive training, have been shown to be at least partially effective [Basso et al. 2008, 2006; Brenk et al. 2008; Gentry, 2008; Goverover et al. 2008; Chiaravalloti and DeLuca, 2002].
Pharmacological treatment
There are no approved drugs for treatment of cognitive deficits in MS. However, off-label use of donepezil in 69 patients with MS has been reported to have positive effects but could not be confirmed in a larger trial [Krupp et al. 2004]. A study with memantine was terminated early following evidence of treatment-related worsening of neurological symptoms [Villoslada et al. 2009]. Rivastigmine has shown no significant effects on cognitive problems in MS patients in smaller studies so far, but is currently being investigated in a larger multicentre study with 200 patients [Shaygannejad et al. 2008].
Oculomotor symptoms and nystagmus
Oculomotor disorders due to brain stem lesions are common in MS but are often undiagnosed as they may be associated with relatively few symptoms [Rougier and Tilikete, 2008]. In a UK survey, the most common causes of acquired nystagmus were considered to be MS and stroke [Choudhuri et al. 2007]. Diplopia due to brain stem lesions may cause considerable functional impairment and nystagmus may result in a decrease in visual acuity.
Specific treatment
There are limited treatments available for diplopia; in cases associated with a relapse, covering the (most) affected eye may give sufficient symptomatic relief. In persistent cases, prism glasses may be helpful.
Pharmacological treatment
Symptomatic relief from nystagmus has been reported with several medications in patients without MS. Results from a randomized, crossover study in 21 patients with acquired pendular nystagmus [Averbuch-Heller et al. 1997] showed that improvements were seen with gabapentin but not baclofen, although this latter medication has been shown to improve conditions of periodic alternating nystagmus [Rucker, 2008]. A placebo-controlled, crossover study in 16 patients with cerebellar atrophy [Strupp et al. 2003] demonstrated that 3,4–diaminopyridine had a positive effect in patients with downbeat nystagmus.
Two crossover studies including patients with MS compared gabapentin and memantine in the treatment of acquired nystagmus [Starck et al. 2010; Thurtell et al. 2010]. Although the number of patients was very low (11 and 3 patients, respectively), both studies indicated that each treatment could improve nystagmus. A slightly better response was observed with memantine in the study, which only included patients with MS [Starck et al. 2010]. Side effects including unsteadiness with gabapentin and lethargy with memantine were common [Thurtell et al. 2010].
Dysphagia and dysarthria
Dysphagia
Dysphagia, although not a common manifestation in early stages of MS, develops in many moderately and severely disabled patients with MS and may be potentially life threatening through aspiration or malnutrition [Calcagno et al. 2002; Thomas and Wiles, 1999; Abraham et al. 1997; Hughes et al. 1994; Daly et al. 1962]. Permanent dysphagia occurs in up to 25% of patients with MS and increases in prevalence as disability advances [De Pauw et al. 2002]. Up to two-thirds of the most disabled patients (EDSS >7.5) and those with severe brainstem dysfunction are at risk of aspiration [Calcagno et al. 2002].
Patients and their families should be questioned about coughing and choking episodes during meals or previous episodes of pneumonia to identify patients at risk or for consideration of a formal swallowing evaluation with videoflouroscopy. This evaluation can determine the site and severity of the disorder and has been shown to be a sensitive diagnostic measure for this complication of MS [Wiesner et al. 2002]. Reminding patients to eat small quantities of food slowly, to chew adequately and avoid talking whilst swallowing may often be sufficient. ‘Chin tucking’ during swallowing can significantly reduce the risk of aspiration. Furthermore, drinking a sip of iced water before taking a mouthful of food may be helpful for some patients.
Patients with significant dysphagia for liquids may benefit from the use of thickening agents during meals although this approach is contraindicated in patients with pharyngeal dysfunction. Anecdotally, anticholinesterases used in the treatment of bulbar weakness due to myasthenia gravis, may improve swallowing in patients with advanced MS, although benefits may be transient.
Dysarthria
Dysarthria, particularly ataxic or mixed spastic–ataxic dysarthria, is a common finding in disabled patients with MS. It has been noted to be associated with midbrain lesions [Blanco et al. 2008]. Speech therapy may be helpful in teaching precision during articulation and such palliative interventions can greatly improve patient QoL [Cohen et al. 2009]. In severe examples, communication devices including spelling boards and computer-assisted communication programs may be of value. Occasionally, therapies effective for the treatment of tremor can be helpful for cerebellar dysarthria.
Seizures
Seizures are not especially common in MS, as it predominantly affects the white matter. It is only with disease progression [Fisher et al. 2008] or more progressive types of the disease [Bozzali et al. 2002] that damage to the grey matter becomes more apparent. Therefore, it is not surprising that seizures are not as common in MS as in conditions that destroy grey and white matter from the onset, such as stroke.
According to a recent review, the prevalence of seizures in patients with MS ranges between 2.1% and 5.4% [Koch et al. 2008], depending on the type of study and the patient population investigated, compared with between 0.5% and 1% in the general population [Sander, 2003]. The incidence of seizures was found to be threefold compared with age-matched controls in two of three studies [Nicoletti et al. 2003; Olafsson et al. 1999], while in the third study it was not increased [Nyquist et al. 2002]. One study found the risk of seizures increased in patients with progressive forms of MS [Martínez-Juárez et al. 2009].
Approximately one third of seizures in MS are primary and one third secondary generalized seizures, which is similar to the general population. The final third of the seizures are partial seizures, simple partial seizures being twice as common as complex partial seizures. In addition, unusual forms of partial seizures and partial and generalized status epilepticus can occur [Koch et al. 2008]. To date, it is not clear whether demyelinating lesions adjacent to the cortex or frank cortical lesions are related to seizures [Nyquist et al. 2001; Sokic et al. 2001].
The prognosis of epilepsy in 599 patients with MS did not differ from that of a control population [Kinnunen and Wikstrom, 1986]. However, status epilepticus seems to be more frequent and therefore the prognosis may be worse than in non-MS seizures.
Pharmacological treatment
Most antiepileptic drugs have been investigated for the treatment of seizures in patients with MS; however, no adequate randomized, controlled trials have been performed with these medications [Koch et al. 2009]. Therefore, it is not clear which drugs are best for MS-associated seizures. It is reasonable to use the same drugs as in other partial and generalized seizures, e.g. carbamazepine, oxcarbazepine, valproic acid, lamotrigine or levetiracetam for focal seizures or valproic acid, lamotrigine, levetiracetam or topiramate for generalized seizures. The question of when to initiate treatment has not been resolved. Seizures occurring with an acute relapse are usually benign and self-limiting, whereas recurrent seizures unrelated to relapses need to be treated. Side effects can be different than in non-MS patients. They can include worsening of weakness and therefore have to be monitored carefully.
Vertigo and dizziness
Vertigo
True vertigo, defined as an illusion of movement caused by asymmetric involvement of the vestibular system, is estimated to occur in approximately 20% of patients with MS [Frohman et al. 2003] and has been reported to occur in significantly more patients compared with a control population [Rae-Grant et al. 1999]. Lesions located within the vestibular nuclei and the pons region of the brainstem, where cranial nerve VIII originates, are the most common sources of damage leading to vertigo [Karatas, 2008; Frohman et al. 2003] and the lesion location may be the reason why vertigo in patients with MS is usually accompanied by vestibular ataxia. However, the cause of vertigo may be unrelated to central pathology and could derive from common conditions such as benign paroxysmal positional vertigo (BPPV), vestibular migraine, Ménière's disease and vestibular neuritis [Neuhauser and Lempert, 2009], or medications used to treat symptoms such as spasticity [Dario and Tomei, 2004] or neuropathic pain [Hazell et al. 2002]. It is rare for acute vertigo to persist over long periods and usually remits gradually over a few weeks.
No specific studies in the treatment of vertigo in MS have been reported. In the general population, acute vertigo attacks can be relieved with vestibular blocking agents, although it is recommended that these agents are only used in the short term. It is important that the cause of the vertigo is accurately diagnosed and, if possible, the causative conditions are treated. However, the majority of patients are managed symptomatically through the use of, for example, physiotherapy, vestibular rehabilitation therapy and/or repositioning manoeuvres.
Dizziness
Dizziness is a general term for a sense of disequilibrium and is more common than central vertigo. A 1989 US investigation in primary care practices [Sloane, 1989] reported that the frequency of dizziness rises steadily with age, with females complaining of this condition more frequently than males. There are many causes of dizziness including inner ear, neurological and psychiatric disorders. Causes and treatment are generally similar as those for vertigo although, as with that symptom, there have been no systematic studies involving patients with MS.
In patients with persistent and severe dizziness it is advisable to check the patient for cerebellar signs, internuclear ophthalmoplegia, coarse, rebound or vertical nystagmus and any smooth pursuit abnormalities.
Sexual problems
Normal sexual function involves a complex series of physical and psychological factors that are easily disturbed in a chronic disease such as MS. Owing to this, evaluation of sexual disorder in patients with MS requires insight into the primary (changes in libido, sexual response and orgasm due to nervous system damage), secondary (physical disabilities such as fatigue, muscle rigidity, weakness and spasms) and tertiary (emotional, social and cultural aspects of MS) components of MS-associated sexual dysfunction [Fletcher et al. 2009]. Sexual problems are not only distressing but can have a large impact on QoL for both patients and their partners [Tepavcevic et al. 2008].
The incidence of sexual dysfunction in patients with MS varies considerably between different reports. A 2009 review reported that between 50% and 90% of men and 40% and 80% of women are affected [Kessler et al. 2009]. The most frequently reported symptoms in women are anorgasmia or hyporgasmia, decreased vaginal lubrication and reduced libido, and men most frequently experience impotence or erectile dysfunction (ED), ejaculatory and/or orgasmic dysfunction and reduced libido [Fraser et al. 2008; Tepavcevic et al. 2008; Zorzon et al. 1999].
The most prevalent sexual complaint in men with MS is ED, which has been estimated to affect up to 70% of patients [Zorzon et al. 1999; Betts et al. 1994]. Despite the high frequency, it is likely that the incidence of ED increases with disease duration as it is very uncommon for it to be among the presenting symptoms of the disease [Betts et al. 1994]. Ejaculatory disorders are reported in one study as being observed in about half of male patients [Zorzon et al. 1999]. Problems with premature ejaculation varied across studies; Zorzon and colleagues reported a similar incidence in MS as in the general male population [Zorzon et al. 1999] while in contrast Tepavcevic and colleagues reported premature ejaculation as one of the main sexual problems in men with MS [Tepavcevic et al. 2008].
Outside of clinical studies, it has been suggested that problems of sexual dysfunction in patients with MS may be underestimated and can occur even in the absence of severe disability [Demirkiran et al. 2006]. Fraser and colleagues investigated the relationship between sexual dysfunction and other disabilities in MS [Fraser et al. 2008]. In men, the only statistically significant correlation was a positive relationship with lower-limb and bladder disability. In contrast, in women significant positive correlations were seen between sexual dysfunction and all other MS disabilities, with the strongest relationship being with fatigue. In neither gender was sexual dysfunction seen to be related to duration of disease.
Pharmacological treatment
Treatments for sexual dysfunction in patients with MS of both genders are, in the main, the same as for the general population and largely depend on the aetiology of the problem. Oral phosphodiesterase 5 (PDE5) inhibitors can be prescribed for patients with ED and there is evidence to suggest that sildenafil can be effective in both sexes at doses up to 100 mg, although the data in men are more robust [Dachille et al. 2008]. A variety of topical lubricants, gels and creams are available to overcome vaginal dryness, and androgen therapy with such compounds as methyltestosterone or dehydroepiandrosterone can help to increase libido, particularly in women with low androgen concentrations [Shifren, 2004]. However, long-term use of these latter compounds is not advised due to their side-effect profile.
Sleep disorders and sleepiness
Excessive daytime sleepiness and/or sleep disorders are frequent problems reported by patients with MS. These disorders could also contribute to the feelings of fatigue in MS. Using the Pittsburgh sleep quality index, it has been suggested that fatigue is associated with disruption of sleep microstructure and poor subjective sleep quality [Kaynak et al. 2006]. Excessive daytime sleepiness has been reported in 32% of patients with MS and was mainly associated with insomnia [Stanton et al. 2006]. The pathophysiology of sleepiness, as well as its relationship with fatigue, is not known: in addition to insomnia, the high prevalence of restless legs syndrome (RLS), mainly observed in disabled patients with sensory and pyramidal signs, might contribute to poor sleep quality and/or excessive sleepiness [Manconi et al. 2008, 2007; Moreira et al. 2008].
Pharmacological treatment
In a randomized placebo-controlled study of modafinil as a treatment for fatigue in patients with MS, nearly half of the patients had an ESS (Epworth Sleepiness Scale) score above 10, attesting to clinical sleepiness and, although fatigue was reduced in patients receiving modafinil, improvements were not significantly better than with placebo [Stankoff et al. 2005]. Patients with RLS may respond to low doses of dopaminergic agonists. In a recent unpublished study, a central hypersomnia associated with sleepiness was found in some patients [Stankoff et al. unpublished data] corroborating previous description of patients with MS and narcolepsy/cataplexy [Sandyk, 1995]. Such patients, who remain to be described more precisely, should respond better to molecules that promote wakefulness, such as modafinil.
Urinary dysfunction
The complex innervations of the bladder from both the autonomic and somatic neural pathways (Figure 1) [Birder et al. 2010; Drake et al. 2010] mean that bladder dysfunctions are frequent complaints in MS. However, the risk of progression to renal failure is low and therefore treatment of these disorders should be medically manageable in most patients. The nature of the underlying disease also means that symptoms are liable to change over time: in a small study of 22 patients with well-documented MS, 54% of the patients developed significant changes in urodynamic parameters over a mean follow-up interval of 42 months [Ciancio et al. 2001].
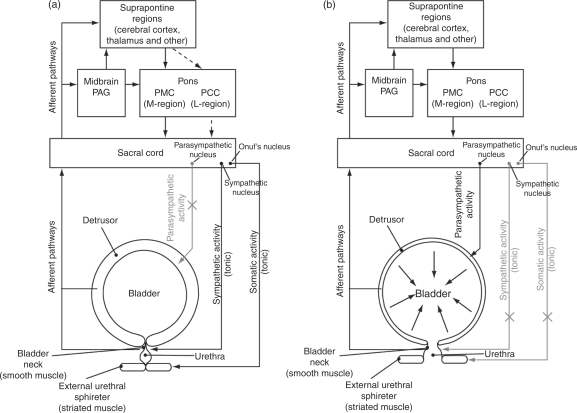
Figure 1.
Neural control of the urinary bladder. Urinary bladder control is governed by a complex neural network involving the brainstem and spinal cord. At all times, afferent signals from the bladder and urethra transmit to the lumbosacral spinal cord, mainly via the pelvic nerve but also via the hypogastric and pudendal nerves. The afferent impulses are transmitted from the spinal cord to voluntary control centres within the CNS via the dorsal and/or spinothalmic tract. Within these afferent signals, the Gracile nucleus relays to the thalamus and cortex, while the periaqueductal grey (PAG) communicates with multiple sites including the pontine micturition centre (PMC) and the thalamus. After processing of afferent signals, switching mechanism within the pons (the PMC and potentially the pontine continence centre [PCC]) and spinal cord relay appropriate efferent signals to the bladder via the parasympathetic (pelvic) nerves, sympathetic (mainly hypogastric) nerves, and somatic (pudendal) nerves. (a) Urinary filling and storage: the detrusor remains relaxed due to a lack of parasympathetic activity and the bladder outlet is closed due to excitatory sympathetic and somatic ('guarding') reflexes that stimulate tonic contraction of the internal and external urethral sphincters, respectively. The PCC has a putative role as an 'off' switch during filling and storage, directing the urethral sphincter to contract and preventing parasympathetic bladder contractions. As the bladder fills, there is a concurrent increase in afferent signals to the brainstem and spinal cord. (b) Urinary voiding (micturition): this occurs when afferent signals from the bladder exceed a certain threshold and activate the PAG, which subsequently excites the PMC to promote an efferent promicturition response. Consequently, parasympathetic activity causes the detrusor (and bladder as a whole) to contract, whilst suppression of sympathetic and somatic activity causes relaxation of the internal and external urinary sphincters, respectively.
Bladder dysfunction in MS takes three major forms: storage, emptying and combined dysfunction [Kalb and Holland, 2008; Goodwin and Fowler, 1997] and will affect more than 75% of patients with MS during the course of the disease [Betts, 1999]. Storage dysfunction results from an overactive detrusor muscle (hyperreflexia) that contracts when small amounts of urine are collected, resulting in symptoms of urinary urgency, urinary frequency, nocturia and urge incontinence. Detrusor hyporeflexia is often indicative of pontine lesions [Araki et al. 2003] and severity of symptoms often correlates with the degree of spinal cord involvement [Kalsi and Fowler, 2005]. Symptoms of emptying dysfunction include uncontrolled leakage of urine (‘dribbling’), urinary hesitancy and incontinence due to overfilling of the bladder.
Combined urinary dysfunction (detrusor–external sphincter dyssynergia) is caused by a lack of coordination between the contraction of the detrusor muscle and the external sphincter resulting in a lack of simultaneous relaxation. The symptoms of this type of dysfunction are urinary urgency combined with hesitancy when voiding, and incomplete voiding (often resulting in the need to urinate again soon after). In a retrospective study, 174 of 249 patients (70%) had combined urinary dysfunction symptoms with observations of upper urinary tract deterioration being rare [Onal et al. 2009]. Detrusor–sphincter dyssynergia is indicative of cervical spinal cord lesions [Araki et al. 2003].
Assessment
The UK consensus statement on the management of the bladder in MS [Fowler et al. 2009], stated that successful management could be achieved based on a simple algorithm which includes testing for a urinary tract infection and measurement of the post micturition residual urine volume. Obtaining a detailed urinary history, often combined with the keeping of a micturition diary, can also be valuable in determining the source of MS-related bladder dysfunction. This is in contrast with guidelines from other countries that recommend cystometry.
Uroflowmetric tests can help to assess whether urethral obstructions are present and, although not routinely performed, a urodynamic evaluation can provide useful information relating to detrusor–external sphincter dyssynergia or detrusor arreflexia.
Pharmacological treatment
Different treatment approaches have been tried depending upon the type of urogenital dysfunction diagnosed. The treatment of urine storage problems in patients with MS has been attempted with antimuscarinic drugs such as oxybutynin [Gajewski and Awad, 1986], trospium chloride [Shvarts et al. 2009], propantheline [Gajewski and Awad, 1986], imipramine (particularly for nocturia) [Wall, 1990] and solifenacin succinate [Kaplan et al. 2010]. Efficacy results were varied but treatment was often associated with significant adverse effects commonly associated with anticholinergic therapies such as dry mouth and constipation and, less frequently, impairment of accommodation with blurred vision [Fowler et al. 1992]. The use of darifenacin for overactive bladder has been found to preserve cognitive function compared with anticholinergic drugs, with no impairment of memory or other cognitive functions in three randomized controlled trials [Kay and Ebinger, 2008].
Treatment of emptying dysfunction is also complicated by side effects. Alpha-blocking agents (such as phenoixybenzamine) have been shown to result in some improvements but can cause hypotension [Vereecken et al. 1983], while antispasticity agents (such as baclofen) may result in increased fatigue and muscle weakness [Fowler et al. 1992]. The main option for the treatment of incontinence is administration of antimuscarinic medications, in combination (if necessary) with clean intermittent self-catheterization [Fowler et al. 2009] and administration of desmopressin [Panicker and Haslam, 2009; Kalsi and Fowler, 2005]. In patients unwilling or unable to administer self-catheterization, ileovesicostomy (or suprapubic cystostomy) has been shown to be an effective alternative [Atan et al. 1999; Gudziak et al. 1999]. In severe cases, the only viable alternative is permanent catheterization.
For patients suffering from combined urinary dysfunction problems, selected use of combinations of the outlined treatment options for storage and emptying dysfunctions can be employed on an individual patient basis.
Experimental treatment options for combined urinary dysfunction have included use of neurotoxins as second-line therapy for detrusor overactivity [Radziszewski et al. 2009], sacral nerve stimulation [Lavano et al. 2004; Ruud Bosch and Groen, 1996], intradetrusor injection of botulinum toxin type A [Panicker and Haslam, 2009; Kalsi et al. 2007; Kalsi and Fowler, 2005], hyperbaric oxygen treatment [Kindwall et al. 1991; Meneghetti et al. 1990] and cannabinoids [Kalsi and Fowler, 2005]. However, small studies with these treatments have given conflicting results and rigorous clinical investigations into the effectiveness of these options have yet to be performed.
Bowel dysfunction
The overall prevalence of bowel symptoms in patients with MS is approximately 68% [Hinds and Wald, 1989] with the principal symptoms being faecal incontinency and constipation which affect up to 43% and over 50% of patients, respectively [Norton and Chelvanayagam, 2010; Hinds et al. 1990]. An online survey of 155 patients with MS suggested that few strategies employed by patients resulted in successful bowel management and that there was a need for high-quality research on all aspects of managing bowel dysfunction in MS in order to improve patients’ QoL [Norton and Chelvanayagam, 2010].
The neuroanatomical basis for bowel disorders in patients with MS is less clear than that of bladder dysfunctions. Factors that could contribute to constipation include poor mobility, voluntary fluid restriction to minimize urinary incontinency, anticholinergic drugs taken for concomitant bladder symptoms and poor dietary habits. In addition, a slow colonic transit time has been seen in patients with MS [Chia et al. 1995]. Faecal incontinency may arise as a result of diminished perineal and rectal sensation, weak sphincter squeeze pressures, faecaloma leading to rectal overloading and overflow, or any combination of these factors.
Specific treatment
Although general recommendations for management of bowel dysfunction in MS include maintaining a high-fibre diet, high fluid intake, regular bowel routine and the use of enemas or laxatives, the evidence to support the efficacy of these recommendations is scant [Wiesel et al. 2001]. Biofeedback retraining has been shown to be effective treatment in some patients, particularly those with limited disability and a nonprogressive disease course [Wiesel et al. 2000]. Long-term pharmacological treatment to prevent bowel dysfunction is not recommended and can lead to habituation. However, pharmacological treatment cannot always be avoided [Preziosi and Emmanuel, 2009].
Sacral nerve stimulation has been used for the treatment of faecal incontinence [Govaert et al. 2009]. This procedure has not been systematically studied in MS, but may lead to substantial benefit in some patients.
Conclusions
Our primary aim in this review was to increase awareness of the comorbidities and symptoms of MS and to emphasize their importance and potential influence on patient QoL. For example, a study investigating QoL in patients with MS showed that worsening symptoms of fatigue, pain, depression and perceived cognitive complaints had a major impact on QoL in these individuals [Motl et al. 2010]. Depression has been reported as being the main factor influencing QoL; however, disability status, fatigue and reduced sleep quality also have an impact on physical domains of QoL [Lobentanz et al. 2004]. Physical activity has been associated with a small improvement in QoL in MS patients [Motl and Gosney, 2008], although this may be an indirect relationship [Motl et al. 2009]. Rehabilitative strategies have also been linked with improved and maintained functioning in patients with MS [Rasova et al. 2010], and should be encouraged.
The effect of these comorbidities and symptoms can have serious ramifications for people with MS and may contribute to treatment compliance issues and recovery from relapses, etc. The presence of these additional symptoms can also place a greater burden on carers, family, friends and other support networks [Rivera-Navarro et al. 2009]. Consequently, accurate and timely diagnosis, prescription of medication (if appropriate) and implementation of coping strategies will significantly contribute to the well-being and QoL of people with MS.
Acknowledgements
This manuscript was written by the authors following initial discussions at an advisory meeting sponsored by Novartis Pharma AG. The authors would like to thank Professor Jan Hillert for his valued contribution to these initial discussions. Editorial support for this manuscript was provided by Mark Hughes, PhD and Dean Clarke, PhD of Complete Medical Communications, and was funded by Novartis Pharma AG.
Funding
Source and nature of funding is disclosed in the acknowledgements section.
Conflict of interest statement
João Carlos Correia de Sa has received grants from Biogen Idec, Bayer Schering, Merck Serono, Sanofi-Aventis and Novartis. Laura Airas has received speaker honoraria from Biogen Idec, Sanofi-Aventis, EMD Serono, Inc. and Novartis; and has received research support from Biogen Idec and EMD Serono, Inc. Emmanuel Bartholome has received funding from Biogen Idec, Merck Serono, Sanofi-Aventis, Novartis and Medical Device Works for consultancy, from Biogen Idec for grants, and from Biogen Idec and Novartis for travel and expenses. Nikolaos Grigoriadis has received honoraria and research grants from Biogen Idec, Merck Serono, Novartis and Sanofi-Aventis. Heinrich Mattle has received honoraria for lectures and consulting and research support from Bayer-Schering Healthcare, Biogen Idec, Merck Serono, Novartis, Teva, Sanofi-Aventis, Pfizer and Swiss Multiple Sclerosis Society. Celia Oreja-Guevara has received personal compensation for activities with Merck Serono, Bayer Schering, Biogen, Novartis and Sanofi-Aventis as a speaker or as an advisory board member. Jonathan O’Riordan has received research funding from GW Pharma, has received payment for consultancy work from Bayer Schering and has also received honoraria from Novartis for participation in an advisory board. Finn Sellebjerg has received funding for travel, speaker honoraria, served as a consultant or received research grants from Bayer Schering, Biogen Idec, Genmab, Lundbeck, Merck Serono, Novartis, Novo Nordisk, Sanofi-Aventis, Schering Plough and Teva. Bruno Stankoff has received honoraria for advisory boards from Novartis, Bayer Schering Pharma, Sanofi-Aventis and Teva Pharmaceuticals and for consultancy and membership in steering committee from Sanofi-Aventis. Karl Vass has received honoraria for oral presentations, participation on advisory boards and travel grants from Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis, Bayer Schering, AstraTech and CSL Behring. Agata Walczak has received honoraria for advisory board from Novartis. Heinz Wiendl has received funding for travel and speaker honoraria from Bayer Schering Pharma, Biogen Idec/Elan Corporation, Sanofi-Aventis, Merck Serono, and Teva Pharmaceutical Industries Ltd; has served/serves as a consultant for Merck Serono, Medac, Inc., Sanofi-Aventis/Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, Novartis, and Novo Nordisk; and receives research support from Bayer Schering Pharma, Biogen Idec/Elan Corporation, Sanofi-Aventis, Merck Serono and Novo Nordisk. Bernd C Kieseier has received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Bayer Schering, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi-Aventis and Teva Neurosciences.
References
- Abraham S., Scheinberg L.C., Smith C.R., LaRocca N.G. (1997) Neurologic impairment and disability status in outpatients with multiple sclerosis reporting dysphagia symptomatology. Neurorehabil Neural Repair 11: 7–13 [Google Scholar]
- Albrecht H., Schwecht M., Pollmann W., Parag D., Erasmus L.P., Konig N. (1998) Local ice application in therapy of kinetic limb ataxia. Clinical assessment of positive treatment effects in patients with multiple sclerosis. Nervenarzt 69: 1066–1073 [DOI] [PubMed] [Google Scholar]
- Alusi S.H., Glickman S., Aziz T.Z., Bain P.G. (1999) Tremor in multiple sclerosis. J Neurol Neurosurg Psychiatry 66: 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alusi S.H., Worthington J., Glickman S., Bain P.G. (2001) A study of tremor in multiple sclerosis. Brain 124: 720–730 [DOI] [PubMed] [Google Scholar]
- Amato M.P., Portaccio E., Goretti B., Zipoli V., Battaglini M., Bartolozzi M.L., et al. (2007) Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol 64: 1157–1161 [DOI] [PubMed] [Google Scholar]
- Andreasen A.K., Jakobsen J., Soerensen L., Andersen H., Petersen T., Bjarkam C.R., et al. (2010) Regional brain atrophy in primary fatigued patients with multiple sclerosis. Neuroimage 50: 608–615 [DOI] [PubMed] [Google Scholar]
- Araki I., Matsui M., Ozawa K., Takeda M., Kuno S. (2003) Relationship of bladder dysfunction to lesion site in multiple sclerosis. J Urol 169: 1384–1387 [DOI] [PubMed] [Google Scholar]
- Arnett P.A., Higginson C.I., Voss W.D., Bender W.I., Wurst J.M., Tippin J.M. (1999) Depression in multiple sclerosis: relationship to working memory capacity. Neuropsychology 13: 546–556 [DOI] [PubMed] [Google Scholar]
- Ashworth B. (1964) Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 192: 540–542 [PubMed] [Google Scholar]
- Atan A., Konety B.R., Nangia A., Chancellor M.B. (1999) Advantages and risks of ileovesicostomy for the management of neuropathic bladder. Urology 54: 636–640 [DOI] [PubMed] [Google Scholar]
- Audoin B., Guye M., Reuter F., Au Duong M.V., Confort-Gouny S., Malikova I., et al. (2007) Structure of WM bundles constituting the working memory system in early multiple sclerosis: a quantitative DTI tractography study. Neuroimage 36: 1324–1330 [DOI] [PubMed] [Google Scholar]
- Avasarala J.R., Cross A.H., Trinkaus K. (2003) Comparative assessment of Yale Single Question and Beck Depression Inventory Scale in screening for depression in multiple sclerosis. Mult Scler 9: 307–310 [DOI] [PubMed] [Google Scholar]
- Averbuch-Heller L., Tusa R.J., Fuhry L., Rottach K.G., Ganser G.L., Heide W., et al. (1997) A double-blind controlled study of gabapentin and baclofen as treatment for acquired nystagmus. Ann Neurol 41: 818–825 [DOI] [PubMed] [Google Scholar]
- Ayache S.S., Ahdab R., Neves D.O., Nguyen J.P., Lefaucheur J.P. (2009) Thalamic stimulation restores defective cerebellocortical inhibition in multiple sclerosis tremor. Mov Disord 24: 467–469 [DOI] [PubMed] [Google Scholar]
- Bain P.G., Findley L.J., Atchison P., Behari M., Vidailhet M., Gresty M., et al. (1993) Assessing tremor severity. J Neurol Neurosurg Psychiatry 56: 868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi R., Miletich R.S., Henschel K., Shaikh Z.A., Janardhan V., Wasay M., et al. (1999) Fatigue in multiple sclerosis: cross-sectional correlation with brain MRI findings in 71 patients. Neurology 53: 1151–1153 [DOI] [PubMed] [Google Scholar]
- Bakshi R., Shaikh Z.A., Miletich R.S., Czarnecki D., Dmochowski J., Henschel K., et al. (2000) Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult Scler 6: 181–185 [DOI] [PubMed] [Google Scholar]
- Bass B., Weinshenker B., Rice G.P., Noseworthy J.H., Cameron M.G., Hader W., et al. (1988) Tizanidine versus baclofen in the treatment of spasticity in patients with multiple sclerosis. Can J Neurol Sci 15: 15–19 [DOI] [PubMed] [Google Scholar]
- Basso M.R., Ghormley C., Lowery N., Combs D., Bornstein R.A. (2008) Self-generated learning in people with multiple sclerosis: an extension of Chiaravalloti and DeLuca (2002). J Clin Exp Neuropsychol 30: 63–69 [DOI] [PubMed] [Google Scholar]
- Basso M.R., Lowery N., Ghormley C., Combs D., Johnson J. (2006) Self-generated learning in people with multiple sclerosis. J Int Neuropsychol Soc 12: 640–648 [DOI] [PubMed] [Google Scholar]
- Beard S., Hunn A., Wight J. (2003) Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technol Assess 7: iii–iii ix-iii, 111 [DOI] [PubMed] [Google Scholar]
- Beeney J.E., Arnett P.A. (2008) Endorsement of self-report neurovegetative items of depression is associated with multiple sclerosis disease symptoms. J Int Neuropsychol Soc 14: 1057–1062 [DOI] [PubMed] [Google Scholar]
- Benedict R.H., Duquin J.A., Jurgensen S., Rudick R.A., Feitcher J., Munschauer F.E., et al. (2008) Repeated assessment of neuropsychological deficits in multiple sclerosis using the Symbol Digit Modalities Test and the MS Neuropsychological Screening Questionnaire. Mult Scler 14: 940–946 [DOI] [PubMed] [Google Scholar]
- Benedict R.H., Fischer J.S., Archibald C.J., Arnett P.A., Beatty W.W., Bobholz J., et al. (2002) Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol 16: 381–397 [DOI] [PubMed] [Google Scholar]
- Bergamaschi R., Romani A., Versino M., Poli R., Cosi V. (1997) Clinical aspects of fatigue in multiple sclerosis. Funct Neurol 12: 247–251 [PubMed] [Google Scholar]
- Berk C., Constantoyannis C., Honey C.R. (2003) The treatment of trigeminal neuralgia in patients with multiple sclerosis using percutaneous radiofrequency rhizotomy. Can J Neurol Sci 30: 220–223 [DOI] [PubMed] [Google Scholar]
- Bermejo P.E., Oreja-Guevara C., Díez-Tejedor E. (2010) [Pain in multiple sclerosis: prevalence, mechanisms, types and treatment]. Rev Neurol 50: 101–108 [PubMed] [Google Scholar]
- Betts C.D. (1999) Bladder And Sexual Dysfunction in Multiple Sclerosis. In: Fowler C.J. (ed.). Neurology of Bladder, Bowel, and Sexual Dysfunction: Blue Books of Practical Neurology, Butterworth-Heinemann: Boston, MA, 289–308 [Google Scholar]
- Betts C.D., Jones S.J., Fowler C.G., Fowler C.J. (1994) Erectile dysfunction in multiple sclerosis. Associated neurological and neurophysiological deficits, and treatment of the condition. Brain 117: 1303–1310 [DOI] [PubMed] [Google Scholar]
- Bever C.T., Jr, Anderson P.A., Leslie J., Panitch H.S., Dhib-Jalbut S., Khan O.A., et al. (1996) Treatment with oral 3,4 diaminopyridine improves leg strength in multiple sclerosis patients: results of a randomized, double-blind, placebo-controlled, crossover trial. Neurology 47: 1457–1462 [DOI] [PubMed] [Google Scholar]
- Bever C.T., Jr, Young D., Anderson P.A., Krumholz A., Conway K., Leslie J., et al. (1994) The effects of 4-aminopyridine in multiple sclerosis patients: results of a randomized, placebo-controlled, double-blind, concentration-controlled, crossover trial. Neurology 44: 1054–1059 [DOI] [PubMed] [Google Scholar]
- Birder L., de Groat W., Mills I., Morrison J., Thor K., Drake M. (2010) Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn 29: 128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco Y., Compta Y., Graus F., Saiz A. (2008) Midbrain lesions and paroxysmal dysarthria in multiple sclerosis. Mult Scler 14: 694–697 [DOI] [PubMed] [Google Scholar]
- Bohannon R.W., Smith M.B. (1987) Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67: 206–207 [DOI] [PubMed] [Google Scholar]
- Bozek C.B., Kastrukoff L.F., Wright J.M., Perry T.L., Larsen T.A. (1987) A controlled trial of isoniazid therapy for action tremor in multiple sclerosis. J Neurol 234: 36–39 [DOI] [PubMed] [Google Scholar]
- Bozzali M., Cercignani M., Sormani M.P., Comi G., Filippi M. (2002) Quantification of brain gray matter damage in different MS phenotypes by use of diffusion tensor MR imaging. AJNR Am J Neuroradiol 23: 985–988 [PMC free article] [PubMed] [Google Scholar]
- Brenk A., Laun K., Haase C.G. (2008) Short-term cognitive training improves mental efficiency and mood in patients with multiple sclerosis. Eur Neurol 60: 304–309 [DOI] [PubMed] [Google Scholar]
- Brown R.F., Valpiani E.M., Tennant C.C., Dunn S.M., Sharrock M., Hodgkinson S., et al. (2009) Longitudinal assessment of anxiety, depression, and fatigue in people with multiple sclerosis. Psychol Psychother 82: 41–56 [DOI] [PubMed] [Google Scholar]
- Brown T.R., Kraft G.H. (2005) Exercise and rehabilitation for individuals with multiple sclerosis. Phys Med Rehabil Clin N Am 16: 513–555 [DOI] [PubMed] [Google Scholar]
- Butefisch C., Hummelsheim H., Denzler P., Mauritz K.H. (1995) Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J Neurol Sci 130: 59–68 [DOI] [PubMed] [Google Scholar]
- Calcagno P., Ruoppolo G., Grasso M.G., De Vincentiis M., Paolucci S. (2002) Dysphagia in multiple sclerosis—prevalence and prognostic factors. Acta Neurol Scand 105: 40–43 [DOI] [PubMed] [Google Scholar]
- Canadian MS Research Group (1987) A randomized controlled trial of amantadine in fatigue associated with multiple sclerosis. Can J Neurol Sci 14: 273–278 [DOI] [PubMed] [Google Scholar]
- Chia Y.W., Fowler C.J., Kamm M.A., Henry M.M., Lemieux M.C., Swash M. (1995) Prevalence of bowel dysfunction in patients with multiple sclerosis and bladder dysfunction. J Neurol 242: 105–108 [DOI] [PubMed] [Google Scholar]
- Chiaravalloti N.D., DeLuca J. (2002) Self-generation as a means of maximizing learning in multiple sclerosis: an application of the generation effect. Arch Phys Med Rehabil 83: 1070–1079 [DOI] [PubMed] [Google Scholar]
- Choudhuri I., Sarvananthan N., Gottlob I. (2007) Survey of management of acquired nystagmus in the United Kingdom. Eye (Lond) 21: 1194–1197 [DOI] [PubMed] [Google Scholar]
- Chwastiak L., Ehde D.M., Gibbons L.E., Sullivan M., Bowen J.D., Kraft G.H. (2002) Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry 159: 1862–1868 [DOI] [PubMed] [Google Scholar]
- Ciancio S.J., Mutchnik S.E., Rivera V.M., Boone T.B. (2001) Urodynamic pattern changes in multiple sclerosis. Urology 57: 239–245 [DOI] [PubMed] [Google Scholar]
- Cohen R.A., Fisher M. (1989) Amantadine treatment of fatigue associated with multiple sclerosis. Arch Neurol 46: 676–680 [DOI] [PubMed] [Google Scholar]
- Cohen S.M., Elackattu A., Noordzij J.P., Walsh M.J., Langmore S.E. (2009) Palliative treatment of dysphonia and dysarthria. Otolaryngol Clin North Am 42: 107–121 x [DOI] [PubMed] [Google Scholar]
- Colosimo C., Millefiorini E., Grasso M.G., Vinci F., Fiorelli M., Koudriavtseva T., et al. (1995) Fatigue in MS is associated with specific clinical features. Acta Neurol Scand 92: 353–355 [DOI] [PubMed] [Google Scholar]
- Comi G., Leocani L., Rossi P., Colombo B. (2001) Physiopathology and treatment of fatigue in multiple sclerosis. J Neurol 248: 174–179 [DOI] [PubMed] [Google Scholar]
- Creedon S.D. (1997) Intrathecal baclofen for severe spacticity: a meta-analysis. Int J Rehabil Health 3: 171–185 [Google Scholar]
- Cutter N.C., Scott D.D., Johnson J.C., Whiteneck G. (2000) Gabapentin effect on spasticity in multiple sclerosis: a placebo-controlled, randomized trial. Arch Phys Med Rehabil 81: 164–169 [DOI] [PubMed] [Google Scholar]
- Dachille G., Ludovico G.M., Pagliarulo G., Vestita G. (2008) Sexual dysfunctions in multiple sclerosis. Minerva Urol Nefrol 60: 77–79 [PubMed] [Google Scholar]
- Dalgas U., Stenager E., Jakobsen J., Petersen T., Hansen H.J., Knudsen C., et al. (2009) Resistance training improves muscle strength and functional capacity in multiple sclerosis. Neurology 73: 1478–1484 [DOI] [PubMed] [Google Scholar]
- Dalgas U., Stenager E., Jakobsen J., Petersen T., Hansen H.J., Knudsen C., et al. (2010) Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler 16: 480–490 [DOI] [PubMed] [Google Scholar]
- Daly D.D., Code C.F., Andersen H.A. (1962) Disturbances of swallowing and esophageal motility in patients with multiple sclerosis. Neurology 12: 250–256 [DOI] [PubMed] [Google Scholar]
- Dario A., Tomei G. (2004) A benefit–risk assessment of baclofen in severe spinal spasticity. Drug Saf 27: 799–818 [DOI] [PubMed] [Google Scholar]
- De Pauw A., Dejaeger E., D'hooghe B., Carton H. (2002) Dysphagia in multiple sclerosis. Clin Neurol Neurosurg 104: 345–351 [DOI] [PubMed] [Google Scholar]
- Demirkiran M., Sarica Y., Uguz S., Yerdelen D., Aslan K. (2006) Multiple sclerosis patients with and without sexual dysfunction: are there any differences? Mult Scler 12: 209–214 [DOI] [PubMed] [Google Scholar]
- Dennison L., Moss-Morris R., Chalder T. (2009) A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev 29: 141–153 [DOI] [PubMed] [Google Scholar]
- DeSousa E.A., Albert R.H., Kalman B. (2002) Cognitive impairments in multiple sclerosis: a review. Am J Alzheimers Dis Other Demen 17: 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G., Bain P., Brin M. (1998) Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 13(Suppl. 3): 2–23 [DOI] [PubMed] [Google Scholar]
- Dineen R.A., Vilisaar J., Hlinka J., Bradshaw C.M., Morgan P.S., Constantinescu C.S., et al. (2009) Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 132: 239–249 [DOI] [PubMed] [Google Scholar]
- DMKG Study Group (2003) Misoprostol in the treatment of trigeminal neuralgia associated with multiple sclerosis. J Neurol 250: 542–545 [DOI] [PubMed] [Google Scholar]
- Drake M.J., Fowler C.J., Griffiths D., Mayer E., Paton J.F., Birder L. (2010) Neural control of the lower urinary and gastrointestinal tracts: supraspinal CNS mechanisms. Neurourol Urodyn 29: 119–127 [DOI] [PubMed] [Google Scholar]
- Duncan G.W., Shahani B.T., Young R.R. (1976) An evaluation of baclofen treatment for certain symptoms in patients with spinal cord lesions. A double-blind, cross-over study. Neurology 26: 441–446 [DOI] [PubMed] [Google Scholar]
- Ehde D.M., Osborne T.L., Hanley M.A., Jensen M.P., Kraft G.H. (2006) The scope and nature of pain in persons with multiple sclerosis. Mult Scler 12: 629–638 [DOI] [PubMed] [Google Scholar]
- Eyssette M., Rohmer F., Serratrice G., Warter J.M., Boisson D. (1988) Multi-centre, double-blind trial of a novel antispastic agent, tizanidine, in spasticity associated with multiple sclerosis. Curr Med Res Opin 10: 699–708 [DOI] [PubMed] [Google Scholar]
- Fahn S., Tolosa E., Marin C. (1988) Clinical rating scale in tremor. In: Jankovic A., Tolosa E. (eds). Parkinson's Disease and Movement Disorders, Urban and Schwarzenberg: Munich, 225–234 [Google Scholar]
- Feldman R.G., Kelly-Hayes M., Conomy J.P., Foley J.M. (1978) Baclofen for spasticity in multiple sclerosis. Double-blind crossover and three-year study. Neurology 28: 1094–1098 [DOI] [PubMed] [Google Scholar]
- Feys P., Helsen W., Liu X., Mooren D., Albrecht H., Nuttin B., et al. (2005a) Effects of peripheral cooling on intention tremor in multiple sclerosis. J Neurol Neurosurg Psychiatry 76: 373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys P., Maes F., Nuttin B., Helsen W., Malfait V., Nagels G., et al. (2005b) Relationship between multiple sclerosis intention tremor severity and lesion load in the brainstem. Neuroreport 16: 1379–1382 [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M.A., Colombo B., Falini A., Codella M., Scotti G., et al. (2002) Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage 15: 559–567 [DOI] [PubMed] [Google Scholar]
- Fisher E., Lee J.C., Nakamura K., Rudick R.A. (2008) Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol 64: 255–265 [DOI] [PubMed] [Google Scholar]
- Fisk J.D., Pontefract A., Ritvo P.G., Archibald C.J., Murray T.J. (1994a) The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 21: 9–14 [PubMed] [Google Scholar]
- Fisk J.D., Ritvo P.G., Ross L., Haase D.A., Marrie T.J., Schlech W.F. (1994b) Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis 18(Suppl. 1): S79–S83 [DOI] [PubMed] [Google Scholar]
- Flachenecker P., Rufer A., Bihler I., Hippel C., Reiners K., Toyka K.V., et al. (2003) Fatigue in MS is related to sympathetic vasomotor dysfunction. Neurology 61: 851–853 [DOI] [PubMed] [Google Scholar]
- Fletcher S.G., Castro-Borrero W., Remington G., Treadaway K., Lemack G.E., Frohman E.M. (2009) Sexual dysfunction in patients with multiple sclerosis: a multidisciplinary approach to evaluation and management. Nat Clin Pract Urol 6: 96–107 [DOI] [PubMed] [Google Scholar]
- Fleuren J.F., Voerman G.E., Erren-Wolters C.V., Snoek G.J., Rietman J.S., Hermens H.J., et al. (2010) Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 81: 46–52 [DOI] [PubMed] [Google Scholar]
- Fowler C.J., Panicker J.N., Drake M., Harris C., Harrison S.C., Kirby M., et al. (2009) A UK consensus on the management of the bladder in multiple sclerosis. J Neurol Neurosurg Psychiatry 80: 470–477 [DOI] [PubMed] [Google Scholar]
- Fowler C.J., van Kerrebroeck P.E., Nordenbo A., Van Poppel H. (1992) Treatment of lower urinary tract dysfunction in patients with multiple sclerosis. Committee of the European Study Group of SUDIMS (Sexual and Urological Disorders in Multiple Sclerosis). J Neurol Neurosurg Psychiatry 55: 986–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso Y.D., da Silva E.O., Finkelsztejn A. (2009) Correlation between fatigue and self-esteem in patients with multiple sclerosis. Arq Neuropsiquiatr 67: 818–821 [DOI] [PubMed] [Google Scholar]
- Francis D.A., Grundy D., Heron J.R. (1986) The response to isoniazid of action tremor in multiple sclerosis and its assessment using polarised light goniometry. J Neurol Neurosurg Psychiatry 49: 87–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C., Mahoney J., McGurl J. (2008) Correlates of sexual dysfunction in men and women with multiple sclerosis. J Neurosci Nurs 40: 312–317 [DOI] [PubMed] [Google Scholar]
- Fredrikson S., Cheng Q., Jiang G.X., Wasserman D. (2003) Elevated suicide risk among patients with multiple sclerosis in Sweden. Neuroepidemiology 22: 146–152 [DOI] [PubMed] [Google Scholar]
- Frohman E.M., Kramer P.D., Dewey R.B., Kramer L., Frohman T.C. (2003) Benign paroxysmal positioning vertigo in multiple sclerosis: diagnosis, pathophysiology and therapeutic techniques. Mult Scler 9: 250–255 [DOI] [PubMed] [Google Scholar]
- Gajewski J.B., Awad S.A. (1986) Oxybutynin versus propantheline in patients with multiple sclerosis and detrusor hyperreflexia. J Urol 135: 966–968 [DOI] [PubMed] [Google Scholar]
- Gambi D., Rossini P.M., Calenda G., Rosetti S., Langoni A., Dantrolene A. (1983) Dantrolene sodium in the treatment of spasticity caused by multiple sclerosis or degenerative myelopathies: a double-blind, cross-over study in comparison with placebo. Curr Ther Res Clin Exp 33: 835–840 [Google Scholar]
- Gelenberg A.J., Poskanzer D.C. (1973) The effect of dantrolene sodium on spasticity in multiple sclerosis. Neurology 23: 1313–1315 [DOI] [PubMed] [Google Scholar]
- Gentry T. (2008) PDAs as cognitive aids for people with multiple sclerosis. Am J Occup Ther 62: 18–27 [DOI] [PubMed] [Google Scholar]
- Ghez C., Thach W.T. (2000) The Cerebellum, McGraw-Hill Health Professions: New York, . [Google Scholar]
- Ghotbi N., Ansari N.N., Naghdi S., Hasson S., Jamshidpour B., Amiri S. (2009) Inter-rater reliability of the Modified Modified Ashworth Scale in assessing lower limb muscle spasticity. Brain Inj 23: 815–819 [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Thompson A.J., Miller D.H., Thompson E.J. (2001) Fatigue is not associated with raised inflammatory markers in multiple sclerosis. Neurology 57: 676–681 [DOI] [PubMed] [Google Scholar]
- Goldman Consensus Group (2005) The Goldman Consensus statement on depression in multiple sclerosis. Mult Scler 11: 328–337 [DOI] [PubMed] [Google Scholar]
- Goodman A.D., Brown T.R., Krupp L.B., Schapiro R.T., Schwid S.R., Cohen R., et al. (2009) Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet 373: 732–738 [DOI] [PubMed] [Google Scholar]
- Goodman A.D., Cohen J.A., Cross A., Vollmer T., Rizzo M., Cohen R., et al. (2007) Fampridine-SR in multiple sclerosis: a randomized, double-blind, placebo-controlled, dose-ranging study. Mult Scler 13: 357–368 [DOI] [PubMed] [Google Scholar]
- Goodwin R.J., Fowler C.G. (1997) Bladder, bowel and sexual dysfunction: recent advances. In: Thompson A.J., Polman C.H., Hohlfeld R. (eds). Multiple Sclerosis: Clinical Challenges and Controversies, Martin Dunitz: London, 265–281 [Google Scholar]
- Govaert B., van Gemert W.G., Baeten C.G. (2009) Neuromodulation for functional bowel disorders. Best Pract Res Clin Gastroenterol 23: 545–553 [DOI] [PubMed] [Google Scholar]
- Goverover Y., Chiaravalloti N., DeLuca J. (2008) Self-generation to improve learning and memory of functional activities in persons with multiple sclerosis: meal preparation and managing finances. Arch Phys Med Rehabil 89: 1514–1521 [DOI] [PubMed] [Google Scholar]
- Gracies J.M. (2001) Pathophysiology of impairment in patients with spasticity and use of stretch as a treatment of spastic hypertonia. Phys Med Rehabil Clin N Am 12: 747–768 vi [PubMed] [Google Scholar]
- Gronseth G., Cruccu G., Alksne J., Argoff C., Brainin M., Burchiel K., et al. (2008) Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology 71: 1183–1190 [DOI] [PubMed] [Google Scholar]
- Gudziak M.R., Tiguert R., Puri K., Gheiler E.L., Triest J.A. (1999) Management of neurogenic bladder dysfunction with incontinent ileovesicostomy. Urology 54: 1008–1011 [DOI] [PubMed] [Google Scholar]
- Hakim E.A., Bakheit A.M., Bryant T.N., Roberts M.W., Intosh-Michaelis S.A., Spackman A.J., et al. (2000) The social impact of multiple sclerosis—a study of 305 patients and their relatives. Disabil Rehabil 22: 288–293 [DOI] [PubMed] [Google Scholar]
- Haselkorn J.K., Balsdon R.C., Fry W.D., Herndon R.M., Johnson B., Little J.W., et al. (2005) Overview of spasticity management in multiple sclerosis. Evidence-based management strategies for spasticity treatment in multiple sclerosis. J Spinal Cord Med 28: 167–199 [PubMed] [Google Scholar]
- Hazell P., O'Connell D., Heathcote D., Henry D. (2002) Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev CD002317–CD002317 [DOI] [PubMed] [Google Scholar]
- Henze T. (2005) Managing specific symptoms in people with multiple sclerosis. Int MS J 12: 60–68 [PubMed] [Google Scholar]
- Henze T., Rieckmann P., Toyka K.V. (2006) Symptomatic treatment of multiple sclerosis. Multiple Sclerosis Therapy Consensus Group (MSTCG) of the German Multiple Sclerosis Society. Eur Neurol 56: 78–105 [DOI] [PubMed] [Google Scholar]
- Herrera-Guzmán I., Herrera-Abarca J.E., Gudayol-Ferré E., Herrera-Guzmán D., Gómez-Carbajal L., Peña-Olvira M., et al. (2010) Effects of selective serotonin reuptake and dual serotonergic–noradrenergic reuptake treatments on attention and executive functions in patients with major depressive disorder. Psychiatry Res 177: 323–329 [DOI] [PubMed] [Google Scholar]
- Hinds J.P., Eidelman B.H., Wald A. (1990) Prevalence of bowel dysfunction in multiple sclerosis. A population survey. Gastroenterology 98: 1538–1542 [DOI] [PubMed] [Google Scholar]
- Hinds J.P., Wald A. (1989) Colonic and anorectal dysfunction associated with multiple sclerosis. Am J Gastroenterol 84: 587–595 [PubMed] [Google Scholar]
- Hirsh A.T., Turner A.P., Ehde D.M., Haselkorn J.K. (2009) Prevalence and impact of pain in multiple sclerosis: physical and psychologic contributors. Arch Phys Med Rehabil 90: 646–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobart J.C., Riazi A., Thompson A.J., Styles I.M., Ingram W., Vickery P.J., et al. (2006) Getting the measure of spasticity in multiple sclerosis: the Multiple Sclerosis Spasticity Scale (MSSS-88). Brain 129: 224–234 [DOI] [PubMed] [Google Scholar]
- Hoffmann V., Kuhn W., Schimrigk S., Islamova S., Hellwig K., Lukas C., et al. (2006) Repeat intrathecal triamcinolone acetonide application is beneficial in progressive MS patients. Eur J Neurol 13: 72–76 [DOI] [PubMed] [Google Scholar]
- Hoffmann V., Schimrigk S., Islamova S., Hellwig K., Lukas C., Brune N., et al. (2003) Efficacy and safety of repeated intrathecal triamcinolone acetonide application in progressive multiple sclerosis patients. J Neurol Sci 211: 81–84 [DOI] [PubMed] [Google Scholar]
- Hooper J., Taylor R., Pentland B., Whittle I.R. (1998) Rater reliability of Fahn's tremor rating scale in patients with multiple sclerosis. Arch Phys Med Rehabil 79: 1076–1079 [DOI] [PubMed] [Google Scholar]
- Hsieh J.T., Wolfe D.L., Miller W.C., Curt A. (2008) Spasticity outcome measures in spinal cord injury: psychometric properties and clinical utility. Spinal Cord 46: 86–95 [DOI] [PubMed] [Google Scholar]
- Hudgson P., Weightman D. (1971) Baclofen in the treatment of spasticity. Br Med J 4: 15–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J.C., Enderby P.M., Langton Hewer R. (1994) Dysphagia and multiple sclerosis: a study and discussion of its nature and impact. Clin Rehabil 8: 18–26 [Google Scholar]
- Huijbregts S.C., Kalkers N.F., de Sonneville L.M., de Groot V., Polman C.H. (2006) Cognitive impairment and decline in different MS subtypes. J Neurol Sci 245: 187–194 [DOI] [PubMed] [Google Scholar]
- Hyman N., Barnes M., Bhakta B., Cozens A., Bakheit M., Kreczy-Kleedorfer B., et al. (2000) Botulinum toxin (Dysport) treatment of hip adductor spasticity in multiple sclerosis: a prospective, randomised, double blind, placebo controlled, dose ranging study. J Neurol Neurosurg Psychiatry 68: 707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb R.C., Holland N. (2008) Urinary Dysfunction and MS: A Consumer Guide to Clinical Practice Guidelines, National Multiple Sclerosis Society [Google Scholar]
- Kalsi V., Fowler C.J. (2005) Therapy Insight: bladder dysfunction associated with multiple sclerosis. Nat Clin Pract Urol 2: 492–501 [DOI] [PubMed] [Google Scholar]
- Kalsi V., Gonzales G., Popat R., Apostolidis A., Elneil S., Dasgupta P., et al. (2007) Botulinum injections for the treatment of bladder symptoms of multiple sclerosis. Ann Neurol 62: 452–457 [DOI] [PubMed] [Google Scholar]
- Kanpolat Y., Berk C., Savas A., Bekar A. (2000) Percutaneous controlled radiofrequency rhizotomy in the management of patients with trigeminal neuralgia due to multiple sclerosis. Acta Neurochir (Wien) 142: 685–689 [DOI] [PubMed] [Google Scholar]
- Kaplan S.A., Goldfischer E.R., Steers W.D., Gittelman M., Andoh M., Forero-Schwanhaeuser S. (2010) Solifenacin treatment in men with overactive bladder: effects on symptoms and patient-reported outcomes. Aging Male 13: 100–107 [DOI] [PubMed] [Google Scholar]
- Karatas M. (2008) Central vertigo and dizziness: epidemiology, differential diagnosis, and common causes. Neurologist 14: 355–364 [DOI] [PubMed] [Google Scholar]
- Kay G.G., Ebinger U. (2008) Preserving cognitive function for patients with overactive bladder: evidence for a differential effect with darifenacin. Int J Clin Pract 62: 1792–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaynak H., Altintas A., Kaynak D., Uyanik O., Saip S., Agaoglu J., et al. (2006) Fatigue and sleep disturbance in multiple sclerosis. Eur J Neurol 13: 1333–1339 [DOI] [PubMed] [Google Scholar]
- Kesiktas N., Paker N., Erdogan N., Gulsen G., Bicki D., Yilmaz H. (2004) The use of hydrotherapy for the management of spasticity. Neurorehabil Neural Repair 18: 268–273 [DOI] [PubMed] [Google Scholar]
- Kessler T.M., Fowler C.J., Panicker J.N. (2009) Sexual dysfunction in multiple sclerosis. Expert Rev Neurother 9: 341–350 [DOI] [PubMed] [Google Scholar]
- Khan F., Ng L., Turner-Stokes L. (2009) Effectiveness of vocational rehabilitation intervention on the return to work and employment of persons with multiple sclerosis. Cochrane Database Syst Rev CD007256–CD007256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killestein J., Hoogervorst E.L., Reif M., Kalkers N.F., van Loenen A.C., Staats P.G., et al. (2002) Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 58: 1404–1407 [DOI] [PubMed] [Google Scholar]
- Kindwall E.P., McQuillen M.P., Khatri B.O., Gruchow H.W., Kindwall M.L. (1991) Treatment of multiple sclerosis with hyperbaric oxygen. Results of a national registry. Arch Neurol 48: 195–199 [DOI] [PubMed] [Google Scholar]
- Kinnunen E., Wikstrom J. (1986) Prevalence and prognosis of epilepsy in patients with multiple sclerosis. Epilepsia 27: 729–733 [DOI] [PubMed] [Google Scholar]
- Knutsson E., Martensson A., Gransberg L. (1982) Antiparetic and antispastic effects induced by tizanidine in patients with spastic paresis. J Neurol Sci 53: 187–204 [DOI] [PubMed] [Google Scholar]
- Koch M., Mostert J., Heersema D., De Keyser J. (2007) Tremor in multiple sclerosis. J Neurol 254: 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M., Uyttenboogaart M., Polman S., De Keyser J. (2008) Seizures in multiple sclerosis. Epilepsia 49: 948–953 [DOI] [PubMed] [Google Scholar]
- Koch M.W., Polman S.K., Uyttenboogaart M., De Keyser J. (2009) Treatment of seizures in multiple sclerosis. Cochrane Database Syst Rev CD007150–CD007150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller W.C. (1984) Pharmacologic trials in the treatment of cerebellar tremor. Arch Neurol 41: 280–281 [DOI] [PubMed] [Google Scholar]
- Kondziolka D., Lunsford L.D. (2005) Percutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia: technique and expectations. Neurosurg Focus 18: E7–E7 [DOI] [PubMed] [Google Scholar]
- Krause P., Szecsi J., Straube A. (2007) FES cycling reduces spastic muscle tone in a patient with multiple sclerosis. NeuroRehabilitation 22: 335–337 [PubMed] [Google Scholar]
- Krupp L.B., Alvarez L.A., LaRocca N.G., Scheinberg L.C. (1988) Fatigue in multiple sclerosis. Arch Neurol 45: 435–437 [DOI] [PubMed] [Google Scholar]
- Krupp L.B., Christodoulou C., Melville P., Scherl W.F., MacAllister W.S., Elkins L.E. (2004) Donepezil improved memory in multiple sclerosis in a randomized clinical trial. Neurology 63: 1579–1585 [DOI] [PubMed] [Google Scholar]
- Krupp L.B., Coyle P.K., Doscher C., Miller A., Cross A.H., Jandorf L., et al. (1995) Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology 45: 1956–1961 [DOI] [PubMed] [Google Scholar]
- Lapierre Y., Bouchard S., Tansey C., Gendron D., Barkas W.J., Francis G.S. (1987) Treatment of spasticity with tizanidine in multiple sclerosis. Can J Neurol Sci 14: 513–517 [DOI] [PubMed] [Google Scholar]
- Laufens G., Poltz W., Reimann G., Schmiegelt F., Stempski S. (1998) Laufband- und Vojta-Physiotherapie an ausgewählten MS-Patienten – Ein Vergleich der Soforteffekte. Phys Rehab Kur Med 8: 174–177 [Google Scholar]
- Lavano A., Volpentesta G., Aloisi M., Veltri C., Piragine G., Signorelli C.D. (2004) Use of chronic sacral nerve stimulation in neurological voiding disorders. J Neurosurg Sci 48: 157–159 [PubMed] [Google Scholar]
- Leandri M. (2003) Therapy of trigeminal neuralgia secondary to multiple sclerosis. Expert Rev Neurother 3: 661–671 [DOI] [PubMed] [Google Scholar]
- Lebrun C., Alchaar H., Candito M., Bourg V., Chatel M. (2006) Levocarnitine administration in multiple sclerosis patients with immunosuppressive therapy-induced fatigue. Mult Scler 12: 321–324 [DOI] [PubMed] [Google Scholar]
- Lechner H.E., Frotzler A., Eser P. (2006) Relationship between self- and clinically rated spasticity in spinal cord injury. Arch Phys Med Rehabil 87: 15–19 [DOI] [PubMed] [Google Scholar]
- Lobentanz I.S., Asenbaum S., Vass K., Sauter C., Klosch G., Kollegger H., et al. (2004) Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neurol Scand 110: 6–13 [DOI] [PubMed] [Google Scholar]
- Lyons K.E., Pahwa R. (2008) Deep brain stimulation and tremor. Neurotherapeutics 5: 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcomson K.S., Dunwoody L., Lowe-Strong A.S. (2007) Psychosocial interventions in people with multiple sclerosis: a review. J Neurol 254: 1–13 [DOI] [PubMed] [Google Scholar]
- Malhotra S., Pandyan A.D., Day C.R., Jones P.W., Hermens H. (2009) Spasticity, an impairment that is poorly defined and poorly measured. Clin Rehabil 23: 651–658 [DOI] [PubMed] [Google Scholar]
- Manconi M., Fabbrini M., Bonanni E., Filippi M., Rocca M., Murri L., et al. (2007) High prevalence of restless legs syndrome in multiple sclerosis. Eur J Neurol 14: 534–539 [DOI] [PubMed] [Google Scholar]
- Manconi M., Rocca M.A., Ferini-Strambi L., Tortorella P., Agosta F., Comi G., et al. (2008) Restless legs syndrome is a common finding in multiple sclerosis and correlates with cervical cord damage. Mult Scler 14: 86–93 [DOI] [PubMed] [Google Scholar]
- Manzoni G.C., Torelli P. (2005) Epidemiology of typical and atypical craniofacial neuralgias. Neurol Sci 26(Suppl. 2): s65–s67 [DOI] [PubMed] [Google Scholar]
- Martínez-Juárez I.E., López-Meza E., González-Aragón M.C., Ramírez-Bermúdez J., Corona T. (2009) Epilepsy and multiple sclerosis: Increased risk among progressive forms. Epilepsy Res 84: 250–253 [DOI] [PubMed] [Google Scholar]
- Mathiowetz V.G., Finlayson M.L., Matuska K.M., Chen H.Y., Luo P. (2005) Randomized controlled trial of an energy conservation course for persons with multiple sclerosis. Mult Scler 11: 592–601 [DOI] [PubMed] [Google Scholar]
- Matsumoto J., Morrow D., Kaufman K., Davis D., Ahlskog J.E., Walker A., et al. (2001) Surgical therapy for tremor in multiple sclerosis: an evaluation of outcome measures. Neurology 57: 1876–1882 [DOI] [PubMed] [Google Scholar]
- Mecomber S.A., Herman R.M. (1971) Effects of local hypothermia on reflex and voluntary activity. Phys Ther 51: 271–281 [DOI] [PubMed] [Google Scholar]
- Mehrholz J., Wagner K., Meissner D., Grundmann K., Zange C., Koch R., et al. (2005) Reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in adult patients with severe brain injury: a comparison study. Clin Rehabil 19: 751–759 [DOI] [PubMed] [Google Scholar]
- Meneghetti G., Sparta S., Rusca F., Facco E., Martini A., Comacchio F., et al. (1990) Hyperbaric oxygen therapy in the treatment of multiple sclerosis. A clinical and electrophysiological study in a 2 year follow-up. Riv Neurol 60: 67–71 [PubMed] [Google Scholar]
- Miller L., Mattison P., Paul L., Wood L. (2007) The effects of transcutaneous electrical nerve stimulation (TENS) on spasticity in multiple sclerosis. Mult Scler 13: 527–533 [DOI] [PubMed] [Google Scholar]
- Mills R.J., Yap L., Young C.A. (2007) Treatment for ataxia in multiple sclerosis. Cochrane Database Syst Rev CD005029–CD005029 [DOI] [PubMed] [Google Scholar]
- Mohr D.C., Boudewyn A.C., Goodkin D.E., Bostrom A., Epstein L. (2001) Comparative outcomes for individual cognitive-behavior therapy, supportive–expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol 69: 942–949 [PubMed] [Google Scholar]
- Mohr D.C., Goodkin D.E., Likosky W., Gatto N., Baumann K.A., Rudick R.A. (1997) Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Arch Neurol 54: 531–533 [DOI] [PubMed] [Google Scholar]
- Mohr D.C., Hart S.L., Julian L., Tasch E.S. (2007) Screening for depression among patients with multiple sclerosis: two questions may be enough. Mult Scler 13: 215–219 [DOI] [PubMed] [Google Scholar]
- Mohr D.C., Likosky W., Dwyer P., Van Der W.J., Boudewyn A.C., Goodkin D.E. (1999) Course of depression during the initiation of interferon beta-1a treatment for multiple sclerosis. Arch Neurol 56: 1263–1265 [DOI] [PubMed] [Google Scholar]
- Möller A., Wiedemann G., Rohde U., Backmund H., Sonntag A. (1994) Correlates of cognitive impairment and depressive mood disorder in multiple sclerosis. Acta Psychiatr Scand 89: 117–121 [DOI] [PubMed] [Google Scholar]
- Monster A.W. (1974) Spasticity and the effect of dantrolene sodium. Arch Phys Med Rehabil 55: 373–383 [PubMed] [Google Scholar]
- Moreira N.C., Damasceno R.S., Medeiros C.A., Bruin P.F., Teixeira C.A., Horta W.G., et al. (2008) Restless leg syndrome, sleep quality and fatigue in multiple sclerosis patients. Braz J Med Biol Res 41: 932–937 [DOI] [PubMed] [Google Scholar]
- Mostert S., Kesselring J. (2002) Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult Scler 8: 161–168 [DOI] [PubMed] [Google Scholar]
- Motl R.W., Gosney J.L. (2008) Effect of exercise training on quality of life in multiple sclerosis: a meta-analysis. Mult Scler 14: 129–135 [DOI] [PubMed] [Google Scholar]
- Motl R.W., McAuley E., Snook E.M., Gliottoni R.C. (2009) Physical activity and quality of life in multiple sclerosis: intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med 14: 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl R.W., Suh Y., Weikert M. (2010) Symptom cluster and quality of life in multiple sclerosis. J Pain Symptom Manage 42(4): 212–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M.E., Gruenthal M., Olson W.L., Olson W.H. (1997) Gabapentin for relief of upper motor neuron symptoms in multiple sclerosis. Arch Phys Med Rehabil 78: 521–524 [DOI] [PubMed] [Google Scholar]
- Multiple Sclerosis Council for Clinical Practice Guidelines (1998) Fatigue and Multiple Sclerosis: Evidence-Based Management Strategies, Washington DC: Paralyzed Veterans Of America.
- Mumenthaler M., Mattle H. (2008) Neurologie, Thieme Verlag: Stuttgart, . [Google Scholar]
- Murray T.J. (1985) Amantadine therapy for fatigue in multiple sclerosis. Can J Neurol Sci 12: 251–254 [DOI] [PubMed] [Google Scholar]
- Neuhauser H.K., Lempert T. (2009) Vertigo: epidemiologic aspects. Semin Neurol 29: 473–481 [DOI] [PubMed] [Google Scholar]
- Nicoletti A., Sofia V., Biondi R., Lo F.S., Reggio E., Patti F., et al. (2003) Epilepsy and multiple sclerosis in Sicily: a population-based study. Epilepsia 44: 1445–1448 [DOI] [PubMed] [Google Scholar]
- Norton C., Chelvanayagam S. (2010) Bowel problems and coping strategies in people with multiple sclerosis. Br J Nurs 19: 220–226 [DOI] [PubMed] [Google Scholar]
- Nurmikko T.J., Gupta S., Maclver K. (2010) Multiple sclerosis-related central pain disorders. Curr Pain Headache Rep 14: 189–195 [DOI] [PubMed] [Google Scholar]
- Nyquist P.A., Cascino G.D., McClelland R.L., Annegers J.F., Rodriguez M. (2002) Incidence of seizures in patients with multiple sclerosis: a population-based study. Mayo Clin Proc 77: 910–912 [DOI] [PubMed] [Google Scholar]
- Nyquist P.A., Cascino G.D., Rodriguez M. (2001) Seizures in patients with multiple sclerosis seen at Mayo Clinic, Rochester, Minn, 1990–1998. Mayo Clin Proc 76: 983–986 [DOI] [PubMed] [Google Scholar]
- O'Brien A.R., Chiaravalloti N., Goverover Y., DeLuca J. (2008) Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil 89: 761–769 [DOI] [PubMed] [Google Scholar]
- O'Brien M.T. (1993) Multiple sclerosis: the relationship among self-esteem, social support, and coping behavior. Appl Nurs Res 6: 54–63 [DOI] [PubMed] [Google Scholar]
- O'Connor A.B., Schwid S.R., Herrmann D.N., Markman J.D., Dworkin R.H. (2008) Pain associated with multiple sclerosis: systematic review and proposed classification. Pain 137: 96–111 [DOI] [PubMed] [Google Scholar]
- Oken B.S., Kishiyama S., Zajdel D., Bourdette D., Carlsen J., Haas M., et al. (2004) Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology 62: 2058–2064 [DOI] [PubMed] [Google Scholar]
- Olafsson E., Benedikz J., Hauser W.A. (1999) Risk of epilepsy in patients with multiple sclerosis: a population-based study in Iceland. Epilepsia 40: 745–747 [DOI] [PubMed] [Google Scholar]
- Onal B., Siva A., Buldu I., Demirkesen O., Cetinel B. (2009) Voiding dysfunction due to multiple sclerosis: a large scale retrospective analysis. Int Braz J Urol 35: 326–333 [DOI] [PubMed] [Google Scholar]
- Paci M. (2003) Physiotherapy based on the Bobath concept for adults with post-stroke hemiplegia: a review of effectiveness studies. J Rehabil Med 35: 2–7 [DOI] [PubMed] [Google Scholar]
- Paisley S., Beard S., Hunn A., Wight J. (2002) Clinical effectiveness of oral treatments for spasticity in multiple sclerosis: a systematic review. Mult Scler 8: 319–329 [DOI] [PubMed] [Google Scholar]
- Panicker J., Haslam C. (2009) Lower urinary tract dysfunction in MS: management in the community. Br J Community Nurs 14: 474–480 [DOI] [PubMed] [Google Scholar]
- Pardo G., Boutwell C., Conner J., Denney D., Oleen-Burkey M. (2010) Effect of oral antihistamine on local injection site reactions with self-administered glatiramer acetate. J Neurosci Nurs 42: 40–46 [DOI] [PubMed] [Google Scholar]
- Parmenter B.A., Weinstock-Guttman B., Garg N., Munschauer F., Benedict R.H. (2007) Screening for cognitive impairment in multiple sclerosis using the Symbol digit Modalities Test. Mult Scler 13: 52–57 [DOI] [PubMed] [Google Scholar]
- Patten S.B., Beck C.A., Williams J.V., Barbui C., Metz L.M. (2003) Major depression in multiple sclerosis: a population-based perspective. Neurology 61: 1524–1527 [DOI] [PubMed] [Google Scholar]
- Patten S.B., Francis G., Metz L.M., Lopez-Bresnahan M., Chang P., Curtin F. (2005) The relationship between depression and interferon beta-1a therapy in patients with multiple sclerosis. Mult Scler 11: 175–181 [DOI] [PubMed] [Google Scholar]
- Patten S.B., Williams J.V., Metz L.M. (2008) Anti-depressant use in association with interferon and glatiramer acetate treatment in multiple sclerosis. Mult Scler 14: 406–411 [DOI] [PubMed] [Google Scholar]
- Penn R.D., Savoy S.M., Corcos D., Latash M., Gottlieb G., Parke B., et al. (1989) Intrathecal baclofen for severe spinal spasticity. N Engl J Med 320: 1517–1521 [DOI] [PubMed] [Google Scholar]
- Penner I.K., Raselli C., Stocklin M., Opwis K., Kappos L., Calabrese P. (2009) The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler 15: 1509–1517 [DOI] [PubMed] [Google Scholar]
- Perlman S.L. (2000) Cerebellar ataxia. Curr Treat Options Neurol 2: 215–224 [DOI] [PubMed] [Google Scholar]
- Pittock S.J., McClelland R.L., Mayr W.T., Rodriguez M., Matsumoto J.Y. (2004) Prevalence of tremor in multiple sclerosis and associated disability in the Olmsted County population. Mov Disord 19: 1482–1485 [DOI] [PubMed] [Google Scholar]
- Pöllmann W., Feneberg W., Steinbrecher A., Haupts M.R., Henze T. (2005) [Therapy of pain syndromes in multiple sclerosis—an overview with evidence-based recommendations]. Fortschr Neurol Psychiatr 73: 268–285 [DOI] [PubMed] [Google Scholar]
- Polman C.H., Bertelsmann F.W., de Waal R., van Diemen H.A., Uitdehaag B.M., van Loenen A.C., et al. (1994a) 4-Aminopyridine is superior to 3,4-diaminopyridine in the treatment of patients with multiple sclerosis. Arch Neurol 51: 1136–1139 [DOI] [PubMed] [Google Scholar]
- Polman C.H., Bertelsmann F.W., van Loenen A.C., Koetsier J.C. (1994b) 4-aminopyridine in the treatment of patients with multiple sclerosis. Long-term efficacy and safety. Arch Neurol 51: 292–296 [DOI] [PubMed] [Google Scholar]
- Preziosi G., Emmanuel A. (2009) Neurogenic bowel dysfunction: pathophysiology, clinical manifestations and treatment. Expert Rev Gastroenterol Hepatol 3: 417–423 [DOI] [PubMed] [Google Scholar]
- Priebe M.M., Sherwood A.M., Thornby J.I., Kharas N.F., Markowski J. (1996) Clinical assessment of spasticity in spinal cord injury: a multidimensional problem. Arch Phys Med Rehabil 77: 713–716 [DOI] [PubMed] [Google Scholar]
- Pucci E., Branas P., D'Amico R., Giuliani G., Solari A., Taus C. (2007) Amantadine for fatigue in multiple sclerosis. Cochrane Database Syst Rev CD002818–CD002818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzki N., Pfriem A., Limmroth V., Yaldizli O., Tettenborn B., Diener H.C., et al. (2009) Prevalence of migraine, tension-type headache and trigeminal neuralgia in multiple sclerosis. Eur J Neurol 16: 262–267 [DOI] [PubMed] [Google Scholar]
- Radziszewski P., Crayton R., Zaborski J., Czlonkowska A., Borkowski A., Bossowska A., et al. (2009) Multiple sclerosis produces significant changes in urinary bladder innervation which are partially reflected in the lower urinary tract functional status-sensory nerve fibers role in detrusor overactivity. Mult Scler 15: 860–868 [DOI] [PubMed] [Google Scholar]
- Rae-Grant A.D., Eckert N.J., Bartz S., Reed J.F. (1999) Sensory symptoms of multiple sclerosis: a hidden reservoir of morbidity. Mult Scler 5: 179–183 [DOI] [PubMed] [Google Scholar]
- Rammohan K.W., Rosenberg J.H., Lynn D.J., Blumenfeld A.M., Pollak C.P., Nagaraja H.N. (2002) Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study. J Neurol Neurosurg Psychiatry 72: 179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.M., Leo G.J., Bernardin L., Unverzagt F. (1991a) Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41: 685–691 [DOI] [PubMed] [Google Scholar]
- Rao S.M., Leo G.J., Ellington L., Nauertz T., Bernardin L., Unverzagt F. (1991b) Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 41: 692–696 [DOI] [PubMed] [Google Scholar]
- Rasova K., Feys P., Henze T., van Tongeren H., Cattaneo D., Jonsdottir J., et al. (2010) Emerging evidence-based physical rehabilitation for multiple sclerosis—towards an inventory of current content across Europe. Health Qual Life Outcomes 8: 76–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekand T. (2010) Clinical assessment and management of spasticity: a review. Acta Neurol Scand Suppl: 190: 62–66 [DOI] [PubMed] [Google Scholar]
- Rivera-Navarro J., Benito-León J., Oreja-Guevara C., Pardo J., Dib W.B., Orts E., et al. (2009) Burden and health-related quality of life of Spanish caregivers of persons with multiple sclerosis. Mult Scler 15: 1347–1355 [DOI] [PubMed] [Google Scholar]
- Roelcke U., Kappos L., Lechner-Scott J., Brunnschweiler H., Huber S., Ammann W., et al. (1997) Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology 48: 1566–1571 [DOI] [PubMed] [Google Scholar]
- Rogers J.M., Panegyres P.K. (2007) Cognitive impairment in multiple sclerosis: evidence-based analysis and recommendations. J Clin Neurosci 14: 919–927 [DOI] [PubMed] [Google Scholar]
- Rossini P.M., Pasqualetti P., Pozzilli C., Grasso M.G., Millefiorini E., Graceffa A., et al. (2001) Fatigue in progressive multiple sclerosis: results of a randomized, double-blind, placebo-controlled, crossover trial of oral 4-aminopyridine. Mult Scler 7: 354–358 [DOI] [PubMed] [Google Scholar]
- Rougier M.B., Tilikete C. (2008) [Ocular motor disorders in multiple sclerosis]. J Fr Ophtalmol 31: 717–721 [DOI] [PubMed] [Google Scholar]
- Rucker J.C. (2008) An update on acquired nystagmus. Semin Ophthalmol 23: 91–97 [DOI] [PubMed] [Google Scholar]
- Ruud Bosch J.L., Groen J. (1996) Treatment of refractory urge urinary incontinence with sacral spinal nerve stimulation in multiple sclerosis patients. Lancet 348: 717–719 [DOI] [PubMed] [Google Scholar]
- Sabra A.F., Hallett M., Sudarsky L., Mullally W. (1982) Treatment of action tremor in multiple sclerosis with isoniazid. Neurology 32: 912–913 [DOI] [PubMed] [Google Scholar]
- Sachais B.A., Logue J.N., Carey M.S. (1977) Baclofen, a new antispastic drug. A controlled, multicenter trial in patients with multiple sclerosis. Arch Neurol 34: 422–428 [DOI] [PubMed] [Google Scholar]
- Sadovnick A.D., Remick R.A., Allen J., Swartz E., Yee I.M., Eisen K., et al. (1996) Depression and multiple sclerosis. Neurology 46: 628–632 [DOI] [PubMed] [Google Scholar]
- Sander J.W. (2003) The epidemiology of epilepsy revisited. Curr Opin Neurol 16: 165–170 [DOI] [PubMed] [Google Scholar]
- Sandyk R. (1995) The pineal gland, cataplexy, and multiple sclerosis. Int J Neurosci 83: 153–163 [DOI] [PubMed] [Google Scholar]
- Sauter C., Zebenholzer K., Hisakawa J., Zeitlhofer J., Vass K. (2008) A longitudinal study on effects of a six-week course for energy conservation for multiple sclerosis patients. Mult Scler 14: 500–505 [DOI] [PubMed] [Google Scholar]
- Sawa G.M., Paty D.W. (1979) The use of baclofen in treatment of spasticity in multiple sclerosis. Can J Neurol Sci 6: 351–354 [DOI] [PubMed] [Google Scholar]
- Schmidt R.T., Lee R.H., Spehlmann R. (1976) Comparison of dantrolene sodium and diazepam in the treatment of spasticity. J Neurol Neurosurg Psychiatry 39: 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurman P.R., Bosch D.A., Merkus M.P., Speelman J.D. (2008) Long-term follow-up of thalamic stimulation versus thalamotomy for tremor suppression. Mov Disord 23: 1146–1153 [DOI] [PubMed] [Google Scholar]
- Schwid S.R., Petrie M.D., Murray R., Leitch J., Bowen J., Alquist A., et al. (2003) A randomized controlled study of the acute and chronic effects of cooling therapy for MS. Neurology 60: 1955–1960 [DOI] [PubMed] [Google Scholar]
- Schyns F., Paul L., Finlay K., Ferguson C., Noble E. (2009) Vibration therapy in multiple sclerosis: a pilot study exploring its effects on tone, muscle force, sensation and functional performance. Clin Rehabil 23: 771–781 [DOI] [PubMed] [Google Scholar]
- Sechi G.P., Zuddas M., Piredda M., Agnetti V., Sau G., Piras M.L., et al. (1989) Treatment of cerebellar tremors with carbamazepine: a controlled trial with long-term follow-up. Neurology 39: 1113–1115 [DOI] [PubMed] [Google Scholar]
- Shakespeare D.T., Boggild M., Young C. (2003) Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev CD001332–CD001332 [DOI] [PubMed] [Google Scholar]
- Shaygannejad V., Janghorbani M., Ashtari F., Zanjani H.A., Zakizade N. (2008) Effects of rivastigmine on memory and cognition in multiple sclerosis. Can J Neurol Sci 35: 476–481 [DOI] [PubMed] [Google Scholar]
- Sheean G.L., Murray N.M., Rothwell J.C., Miller D.H., Thompson A.J. (1998) An open-labelled clinical and electrophysiological study of 3,4 diaminopyridine in the treatment of fatigue in multiple sclerosis. Brain 121: 967–975 [DOI] [PubMed] [Google Scholar]
- Shifren J.L. (2004) The role of androgens in female sexual dysfunction. Mayo Clin Proc 79: S19–S24 [PubMed] [Google Scholar]
- Shvarts P.G., Kadykov A.S., Shvedkov V.V., Timerbaeva S.L., Polevaia E.V., Mulach A.N. (2009) [Experience with application of trospium chloride in patients with neurogenic detrusor overactivity]. Urologia 5: 24–29 [PubMed] [Google Scholar]
- Sloane P.D. (1989) Dizziness in primary care. Results from the National Ambulatory Medical Care Survey. J Fam Pract 29: 33–38 [PubMed] [Google Scholar]
- Smith C., Birnbaum G., Carter J.L., Greenstein J., Lublin F.D. (1994) Tizanidine treatment of spasticity caused by multiple sclerosis: results of a double-blind, placebo-controlled trial. US Tizanidine Study Group. Neurology 44: S34–S42 [PubMed] [Google Scholar]
- Smolenski C., Muff S., Smolenski-Kautz S. (1981) A double-blind comparative trial of new muscle relaxant, tizanidine (DS 103-282), and baclofen in the treatment of chronic spasticity in multiple sclerosis. Curr Med Res Opin 7: 374–383 [PubMed] [Google Scholar]
- Snow B.J., Tsui J.K., Bhatt M.H., Varelas M., Hashimoto S.A., Calne D.B. (1990) Treatment of spasticity with botulinum toxin: a double-blind study. Ann Neurol 28: 512–515 [DOI] [PubMed] [Google Scholar]
- Sokic D.V., Stojsavljevic N., Drulovic J., Dujmovic I., Mesaros S., Ercegovac M., et al. (2001) Seizures in multiple sclerosis. Epilepsia 42: 72–79 [DOI] [PubMed] [Google Scholar]
- Solaro C., Lunardi G.L., Mancardi G.L. (2003) Pain and MS. Int MS J 10: 14–19 [PubMed] [Google Scholar]
- Stankoff B., Waubant E., Confavreux C., Edan G., Debouverie M., Rumbach L., et al. (2005) Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology 64: 1139–1143 [DOI] [PubMed] [Google Scholar]
- Stanton B.R., Barnes F., Silber E. (2006) Sleep and fatigue in multiple sclerosis. Mult Scler 12: 481–486 [DOI] [PubMed] [Google Scholar]
- Starck M., Albrecht H., Pöllmann W., Dieterich M., Straube A. (2010) Acquired pendular nystagmus in multiple sclerosis: an examiner-blind cross-over treatment study of memantine and gabapentin. J Neurol 257: 322–327 [DOI] [PubMed] [Google Scholar]
- Storey E., Tuck K., Hester R., Hughes A., Churchyard A. (2004) Inter-rater reliability of the International Cooperative Ataxia Rating Scale (ICARS). Mov Disord 19: 190–192 [DOI] [PubMed] [Google Scholar]
- Strupp M., Schuler O., Krafczyk S., Jahn K., Schautzer F., Buttner U., et al. (2003) Treatment of downbeat nystagmus with 3,4-diaminopyridine: a placebo-controlled study. Neurology 61: 165–170 [DOI] [PubMed] [Google Scholar]
- Sullivan M.J., Weinshenker B., Mikail S., Bishop S.R. (1995) Screening for major depression in the early stages of multiple sclerosis. Can J Neurol Sci 22: 228–231 [DOI] [PubMed] [Google Scholar]
- Tardieu G., Shentoub S., Delarue R. (1954) [Research on a technic for measurement of spasticity.]. Rev Neurol (Paris) 91: 143–144 [PubMed] [Google Scholar]
- Tekok-Kilic A., Benedict R.H., Weinstock-Guttman B., Dwyer M.G., Carone D., Srinivasaraghavan B., et al. (2007) Independent contributions of cortical gray matter atrophy and ventricle enlargement for predicting neuropsychological impairment in multiple sclerosis. Neuroimage 36: 1294–1300 [DOI] [PubMed] [Google Scholar]
- Tellez N., Alonso J., Rio J., Tintore M., Nos C., Montalban X., et al. (2008) The basal ganglia: a substrate for fatigue in multiple sclerosis. Neuroradiology 50: 17–23 [DOI] [PubMed] [Google Scholar]
- Tepavcevic D.K., Kostic J., Basuroski I.D., Stojsavljevic N., Pekmezovic T., Drulovic J. (2008) The impact of sexual dysfunction on the quality of life measured by MSQoL-54 in patients with multiple sclerosis. Mult Scler 14: 1131–1136 [DOI] [PubMed] [Google Scholar]
- Thomas F.J., Wiles C.M. (1999) Dysphagia and nutritional status in multiple sclerosis. J Neurol 246: 677–682 [DOI] [PubMed] [Google Scholar]
- Thurtell M.J., Joshi A.C., Leone A.C., Tomsak R.L., Kosmorsky G.S., Stahl J.S., et al. (2010) Crossover trial of gabapentin and memantine as treatment for acquired nystagmus. Ann Neurol 67: 676–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann L., Deuschl G., Fogel W., Hilker R., Kupsch A., Lange M., et al. (2009) [Deep brain stimulation for tremor in multiple sclerosis: consensus recommendations of the German Deep Brain Stimulation Association]. Nervenarzt 80: 673–677 [DOI] [PubMed] [Google Scholar]
- Trouillas P., Takayanagi T., Hallett M., Currier R.D., Subramony S.H., Wessel K., et al. (1997) International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145: 205–211 [DOI] [PubMed] [Google Scholar]
- Vahter L., Kreegipuu M., Talvik T., Gross-Paju K. (2007) One question as a screening instrument for depression in people with multiple sclerosis. Clin Rehabil 21: 460–464 [DOI] [PubMed] [Google Scholar]
- van Diemen H.A., Polman C.H., van Dongen M.M., Nauta J.J., Strijers R.L., van Loenen A.C., et al. (1993) 4-Aminopyridine induces functional improvement in multiple sclerosis patients: a neurophysiological study. J Neurol Sci 116: 220–226 [DOI] [PubMed] [Google Scholar]
- Vaney C., Heinzel-Gutenbrunner M., Jobin P., Tschopp F., Gattlen B., Hagen U., et al. (2004) Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler 10: 417–424 [DOI] [PubMed] [Google Scholar]
- Vercellino M., Masera S., Lorenzatti M., Condello C., Merola A., Mattioda A., et al. (2009) Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. J Neuropathol Exp Neurol 68: 489–502 [DOI] [PubMed] [Google Scholar]
- Vereecken R.L., Van Poppel H., Boeckx G., Leruitte A. (1983) Long-term alpha-adrenergic-blocking therapy in detrusor-urethra dyssynergia. Eur Urol 9: 167–169 [DOI] [PubMed] [Google Scholar]
- Villoslada P., Arrondo G., Sepulcre J., Alegre M., Artieda J. (2009) Memantine induces reversible neurologic impairment in patients with MS. Neurology 72: 1630–1633 [DOI] [PubMed] [Google Scholar]
- Wade D.T., Collin C., Stott C., Duncombe P. (2010) Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler 16: 707–714 [DOI] [PubMed] [Google Scholar]
- Wade D.T., Makela P., Robson P., House H., Bateman C. (2004) Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler 10: 434–441 [DOI] [PubMed] [Google Scholar]
- Walker I.D., Gonzalez E.W. (2007) Review of intervention studies on depression in persons with multiple sclerosis. Issues Ment Health Nurs 28: 511–531 [DOI] [PubMed] [Google Scholar]
- Wall L.L. (1990) The management of detrusor instability. Clin Obstet Gynecol 33: 367–377 [DOI] [PubMed] [Google Scholar]
- Wang J.L., Reimer M.A., Metz L.M., Patten S.B. (2000) Major depression and quality of life in individuals with multiple sclerosis. Int J Psychiatry Med 30: 309–317 [DOI] [PubMed] [Google Scholar]
- Weinshenker B.G., Issa M., Baskerville J. (1996) Long-term and short-term outcome of multiple sclerosis: a 3-year follow-up study. Arch Neurol 53: 353–358 [DOI] [PubMed] [Google Scholar]
- Weinshenker B.G., Penman M., Bass B., Ebers G.C., Rice G.P. (1992) A double-blind, randomized, crossover trial of pemoline in fatigue associated with multiple sclerosis. Neurology 42: 1468–1471 [DOI] [PubMed] [Google Scholar]
- Weiss N., North R.B., Ohara S., Lenz F.A. (2003) Attenuation of cerebellar tremor with implantation of an intrathecal baclofen pump: the role of gamma-aminobutyric acidergic pathways. Case report. J Neurosurg 99: 768–771 [DOI] [PubMed] [Google Scholar]
- Whitaker J.N., Mitchell G.W. (1997) Clinical features of multiple sclerosis. In: Raine C.S., McFarland H.F., Tourtellotte W.W. (eds). Multiple Sclerosis: Clinical and Pathogenic Basis, Chapman & Hall: London, 3–19 [Google Scholar]
- Wiesel P.H., Norton C., Glickman S., Kamm M.A. (2001) Pathophysiology and management of bowel dysfunction in multiple sclerosis. Eur J Gastroenterol Hepatol 13: 441–448 [DOI] [PubMed] [Google Scholar]
- Wiesel P.H., Norton C., Roy A.J., Storrie J.B., Bowers J., Kamm M.A. (2000) Gut focused behavioural treatment (biofeedback) for constipation and faecal incontinence in multiple sclerosis. J Neurol Neurosurg Psychiatry 69: 240–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner W., Wetzel S.G., Kappos L., Hoshi M.M., Witte U., Radue E.W., et al. (2002) Swallowing abnormalities in multiple sclerosis: correlation between videofluoroscopy and subjective symptoms. Eur Radiol 12: 789–792 [DOI] [PubMed] [Google Scholar]
- Wiles C.M., Newcombe R.G., Fuller K.J., Shaw S., Furnival-Doran J., Pickersgill T.P., et al. (2001) Controlled randomised crossover trial of the effects of physiotherapy on mobility in chronic multiple sclerosis. J Neurol Neurosurg Psychiatry 70: 174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk D.M., Benarroch E.E., O'Brien P.C., Keegan B.M., Lucchinetti C.F., Noseworthy J.H., et al. (2005) A randomized controlled crossover trial of aspirin for fatigue in multiple sclerosis. Neurology 64: 1267–1269 [DOI] [PubMed] [Google Scholar]
- Wishart H.A., Roberts D.W., Roth R.M., McDonald B.C., Coffey D.J., Mamourian A.C., et al. (2003) Chronic deep brain stimulation for the treatment of tremor in multiple sclerosis: review and case reports. J Neurol Neurosurg Psychiatry 74: 1392–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap L., Kouyialis A., Varma T.R. (2007) Stereotactic neurosurgery for disabling tremor in multiple sclerosis: thalamotomy or deep brain stimulation? Br J Neurosurg 21: 349–354 [DOI] [PubMed] [Google Scholar]
- Zajicek J., Fox P., Sanders H., Wright D., Vickery J., Nunn A., et al. (2003) Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet 362: 1517–1526 [DOI] [PubMed] [Google Scholar]
- Ziemssen T. (2009) Multiple sclerosis beyond EDSS: depression and fatigue. J Neurol Sci 277(Suppl. 1): S37–S41 [DOI] [PubMed] [Google Scholar]
- Zifko U.A., Rupp M., Schwarz S., Zipko H.T., Maida E.M. (2002) Modafinil in treatment of fatigue in multiple sclerosis. Results of an open-label study. J Neurol 249: 983–987 [DOI] [PubMed] [Google Scholar]
- Zorro O., Lobato-Polo J., Kano H., Flickinger J.C., Lunsford L.D., Kondziolka D. (2009) Gamma knife radiosurgery for multiple sclerosis-related trigeminal neuralgia. Neurology 73: 1149–1154 [DOI] [PubMed] [Google Scholar]
- Zorzon M., Zivadinov R., Bosco A., Bragadin L.M., Moretti R., Bonfigli L., et al. (1999) Sexual dysfunction in multiple sclerosis: a case–control study. I. Frequency and comparison of groups. Mult Scler 5: 418–427 [DOI] [PubMed] [Google Scholar]