Von Willebrand disease (VWD) is a common autosomally inherited bleeding disorder resulting from quantitative and qualitative defects of plasma von Willebrand factor (VWF). VWD can be divided into three types: type 1 includes partial quantitative deficiencies of VWF, type 3 includes virtually complete quantitative deficiencies while type 2 encompasses four subtypes of qualitative deficiency in which specific VWF ligand-binding functions are affected.1 In the last two decades there have been studies across the world attempting to elucidate the molecular basis of VWD in order to understand the mechanisms contributing to its pathogenesis more fully. Mutations have been identified throughout the VWF gene (VWF). Missense mutations resulting in alterations to protein structure or function predominate in types 1 and 2 VWD while nonsense mutations (resulting in premature protein termination), deletions or insertions, often resulting in frameshift plus splice-site mutations, predominate in type 3.
Although these common mutations have furthered our knowledge of the molecular pathogenesis of VWD, they fail to explain all cases of the disease. However, detailed analysis of individual patients or cohorts of patients with a defined phenotype is helping to enhance understanding of the disorder. Mutations associated with VWD may be missed by standard polymerase chain reaction (PCR) and DNA sequence analysis or may be identified and their potential effect misinterpreted. Recent advances in understanding of the mutation spectrum contributing to VWD along with mechanisms contributing to other inherited disorders that may play a role in VWD are described below.
Known causative mutation types requiring further investigation in the context of von Willebrand disease
Although reported as causing VWD, certain mutation types require further investigation before their contribution to disease pathogenesis can be fully ascertained.
Copy number variations comprising large deletions within VWF have been documented, but historically homozygous deletions were reported only in type 3 VWD, as heterozygous deletions (or duplications) were difficult to identify because of the presence of a normal copy of VWF. Patients homozygous for large VWF deletions, ranging in size from one exon to complete absence of VWF, have been identified, with mutations generally predicted to disrupt protein translation and prevent VWF expression.1 Rare occurrences of large heterozygous deletions causing both types 1 and 2 VWD have also been reported, identified through linkage analysis demonstrating unusual polymorphic marker inheritance2,3 or through detection of a previously characterized type 3 VWD deletion in the heterozygous form using a mutation-specific PCR assay.4 In cases in which detail has been reported, these deletions are in-frame and function in a dominant-negative manner.4,5
Table 1.
Additional molecular mechanisms identified in or potentially contributing to the pathogenesis of VWD.
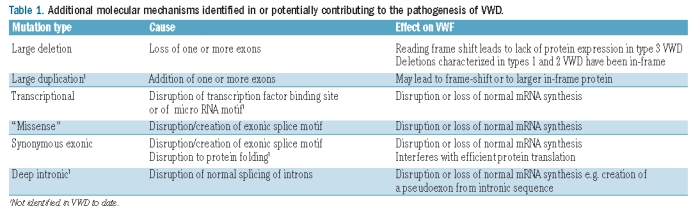
Standard PCR and DNA sequencing or mutation scanning techniques are incapable of detecting large heterozygous deletions. Significantly, techniques that can identify homozygous and heterozygous copy number variations, particularly multiplex ligation-dependent amplification (MLPA) have recently begun to identify additional deletions contributing to VWD in both heterozygous type 2 and in compound heterozygous type 3 patients.6 The role of copy number variations is, therefore, likely to be underestimated in VWD, as large deletions and exonic duplications are known to contribute to many other disorders, for example hemophilia A.7
Another region requiring further investigation is the VWF promoter. Following recent work investigating a Canadian patient with type 1 VWD, the first disease-causing promoter mutation, c.-1522_-1510del, was described.8 This mutation disrupts a transcription factor binding site consequently affecting the transcriptional expression of mutant VWF. Routine molecular analysis of VWF generally focuses on the coding exons and closely flanking intronic sequence.1 Investigation of the proximal promoter may identify additional transcriptional mutations, especially in patients with mild type 1 VWD manifesting a partial quantitative deficiency of VWF.
Mutations resulting in an unexpected effect on the von Willebrand factor protein
The consequence of an identified mutation does not always result in the expected phenotype. This has been observed in VWD with apparent missense mutations which ordinarily would be expected to affect protein composition. The novel "missense" mutation p.Gly1180 Arg identified in type 2A VWD failed to mimic the disease phenotype when expressed in vitro despite segregating with VWD in the affected family.9 The reason for this became apparent following further investigation of the patient’s RNA. The c.3538G>A nucleotide change that predicted a missense mutation was shown to disrupt a splicing motif causing in-frame deletions of exon 23 (p.Glu990_Lys1036del) and exon 26 (p.Pro1127_ Gly1180delinsArg). Additionally, the p.Gly19Arg mutation previously identified in type 1 VWD also fails to influence VWF expression in vitro but is predicted in silico to influence splicing of exon 2.10
Novel mutations previously overlooked in von Willebrand disease
In this issue of the journal, Daidone et al.11 highlight a mutation previously overlooked in VWF molecular analysis. The authors analyzed all 52 exons, flanking intronic sequence and the 5′ and 3′ untranslated regions of VWF in a family diagnosed with mild-moderate type 1 VWD. Having failed to identify a causative mutation, linkage between VWF and disease phenotype prompted the authors to analyze cDNA from the index case. The analysis identified a heterozygous c.7055_7081del mutation which mimicked disease phenotype when expressed in vitro. As a corresponding nucleotide deletion had not been observed in the genomic DNA (gDNA), it was postulated that the mutation must result from a splicing defect. Re-analysis of the gDNA highlighted the synonymous c.7056C>T (p.Gly2352Gly) alteration which was subsequently predicted, using in silico software, to create a novel splice donor site within exon 41, leading to loss of the remainder of the exon and an in-frame deletion of nine amino acids. The mutation results in p.Gly2352_Cys2360del in the B2 domain.11
This study highlights that mutations could be overlooked during standard VWF molecular analysis because they are perceived as being not disease-causative. Notably, the c.7056C>T mutation was previously observed in the Canadian type 1 VWD study, but was reported as a polymorphism.12 Many silent alterations have been reported in VWF13,14 and may warrant in silico analysis to predict any potential influence on splicing along with cDNA analysis to further investigate any predicted effect.
Synonymous alterations have also been shown to affect protein function by interfering with protein folding, which subsequently disrupts protein translation. The synonymous c.459G>A (p.Val153Val) mutation in F9 has recently been shown to cause mild hemophilia B in this manner.15
Further mutations may be under-investigated in VWF. Both copy number variations and point mutations occurring deep within intronic sequences have been shown to interfere with normal splicing patterns and result in the formation of pseudoexons in other disorders.16,17 It is also feasible that deep intronic mutations could disrupt transcription factor binding site or micro RNA motifs, particularly if occurring within terminal introns.
Concluding remarks
Studies such as the one reported by Daidone et al.11 emphasize that additional work is required in order to understand the molecular basis of VWD fully. New technologies such as next generation sequencing will highlight additional sequence variants that may contribute to VWD, but synonymous mutations are still likely to be overlooked. It is, therefore, beneficial to combine gDNA molecular analysis with information from several additional sources including disease and gene co-segregation within the family, any previous reports of the sequence variant,13,14 in silico analysis to investigate predicted effects of sequence variants on the protein and on mRNA splicing plus analysis of cDNA when possible.18–20 Mutation prediction packages [for example, Alamut® (Interactive Biosoftware, Rouen, France)] combine several tools and facilitate these analyses. Given the complexity of VWD, it is perhaps unsurprising that its molecular basis also appears complex. However, whether "overlooked" mutations are rare anomalies or significant contributors to the pathogenesis of VWD remains to be answered.
Supplementary Material
Footnotes
(Related Original Article on page 881)
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Goodeve AC. The genetic basis of von Willebrand disease. Blood Rev. 2010;24(3):123–34. doi: 10.1016/j.blre.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Johansson AM, Lanke E, Säll T, Lethagen S, Halldén C. A large deletion identified in a Swedish family with type 1 VWD. Thromb Haemost. 2011;105(4):733–4. doi: 10.1160/TH10-08-0556. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi F, Marchetti G, Guerra S, Casonato A, Gemmati D, Patracchini P, et al. A de novo and heterozygous gene deletion causing a variant of von Willebrand disease. Blood. 1990;75(3):677–83. [PubMed] [Google Scholar]
- 4.Sutherland MS, Cumming AM, Bowman M, Bolton-Maggs PHB, Bowen DJ, Collins PW, et al. A novel deletion mutation is recurrent in von Willebrand disease types 1 and 3. Blood. 2009;114(5):1091–8. doi: 10.1182/blood-2008-08-173278. [DOI] [PubMed] [Google Scholar]
- 5.Casari C, Pinotti M, Lancellotti S, Adinolfi E, Casonato A, De Cristofaro R, et al. The dominant-negative von Willebrand factor gene deletion p.P1127_C1948delinsR: molecular mechanism and modulation. Blood. 2010;116(24):5371–6. doi: 10.1182/blood-2010-02-268920. [DOI] [PubMed] [Google Scholar]
- 6.Yadegari H, Driesen J, Hass M, Budde U, Pavlova A, Oldenburg J. Large deletions identified in patients with von Willebrand disease by MLPA. J Thromb Haemost. 2011;9(5):1083–6. doi: 10.1111/j.1538-7836.2011.04260.x. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann MA, Oldenburg J, Müller CR, Rost S. Characterization of duplication breakpoints in the factor VIII gene. J Thromb Haemost. 2010;8(12):2696–704. doi: 10.1111/j.1538-7836.2010.04040.x. [DOI] [PubMed] [Google Scholar]
- 8.Othman M, Chirinian Y, Brown C, Notley C, Hickson N, Hampshire D, et al. Functional characterization of a 13-bp deletion (c.-1522_-1510del13) in the promoter of the von Willebrand factor gene in type 1 von Willebrand disease. Blood. 2010;116(18):3645–52. doi: 10.1182/blood-2009-12-261131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James PD, O'Brien LA, Hegadorn CA, Notley CRP, Sinclair GD, Hough C, et al. A novel type 2A von Willebrand factor mutation located at the last nucleotide of exon 26 (3538G>A) causes skipping of 2 nonadjacent exons. Blood. 2004;104(9):2739–45. doi: 10.1182/blood-2003-12-4286. [DOI] [PubMed] [Google Scholar]
- 10.Eikenboom J, Hilbert L, Ribba AS, Hommais A, Habart D, Messenger S, et al. Expression of 14 von Willebrand factor mutations identified in patients with type 1 von Willebrand disease from the MCMDM-1VWD study. J Thromb Haemost. 2009;7(8):1304–12. doi: 10.1111/j.1538-7836.2009.03486.x. [DOI] [PubMed] [Google Scholar]
- 11.Daidone V, Gallinaro L, Cattini MG, Pontara E, Bertomoro A, Pagnan A, et al. An apparently silent nucleotide substitution (c.7056C>T) in the von Willebrand factor gene is responsible for type 1 von Willebrand disease. Haematologica. 2011;96(6):881–7. doi: 10.3324/haematol.2010.036848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James PD, Notley C, Hegadorn C, Leggo J, Tuttle A, Tinlin S, et al. The mutational spectrum of type 1 von Willebrand disease: results from a Canadian cohort study. Blood. 2007;109(1):145–54. doi: 10.1182/blood-2006-05-021105.. [DOI] [PubMed] [Google Scholar]
- 13.NCBI. db SNP. [cited 30/04/2011] Available from: http://www.ncbi.nlm.nih.gov/projects/SNP/
- 14.VWFdb. International Society on Thrombosis and Haemostasis Scientific and Standardization Committee VWF Information Homepage. [cited 30/04/2011] Available from: www.vwf.group.shef.ac.uk.
- 15.Kimchi-Sarfaty C, Simhadri VL, Kopelman D, Friedman A, Edwards N, Javaid A, et al. The synonymous V107V mutation in factor IX is not so silent and may cause hemophilia B in patients. Blood. 2010;116(21) abst 2197. [Google Scholar]
- 16.Khelifi MM, Ishmukhametova A, Khau Van Kien P, Thorel D, Méchin D, Perelman S, et al. Pure intronic rearrangements leading to aberrant pseudoexon inclusion in dystrophinopathy: a new class of mutations? Hum Mutat. 2011;32(4):467–75. doi: 10.1002/humu.21471. [DOI] [PubMed] [Google Scholar]
- 17.Castoldi E, Duckers C, Radu C, Spiezia L, Rossetto V, Tagariello G, et al. Homozygous F5 deep-intronic splicing mutation resulting in severe factor V deficiency and undetectable thrombin generation in platelet rich plasma. J Thromb Haemost. 2011;9(5):9599–68. doi: 10.1111/j.1538-7836.2011.04237.x. [DOI] [PubMed] [Google Scholar]
- 18.Bell J, Bodmer D, Sistermans E, Ramsden S. Practice guidelines for the Interpretation and Reporting of Unclassified Variants (UVs) in Clinical Molecular Genetics. [cited 30/04/2011] Available from: http://cmgsweb.shared.hosting.zen.co.uk/BPGs/Best_Practice_Guidelines.htm.
- 19.Houdayer C, Dehainault C, Mattler C, Michaux D, Caux-Moncoutier V, Pagès-Berhouet S, et al. Evaluation of in silico splice tools for decision-making in molecular diagnosis. Hum Mutat. 2008;29(7):975–82. doi: 10.1002/humu.20765. [DOI] [PubMed] [Google Scholar]
- 20.Keeney S, Bowen D, Cumming A, Enayat S, Goodeve A, Hill M. The molecular analysis of von Willebrand disease: a guideline from the UK Haemophilia Centre Doctors' Organisation Haemophilia Genetics Laboratory Network. Haemophilia. 2008;14(5):1099–111. doi: 10.1111/j.1365-2516.2008.01813.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


