Abstract
We report a patient with T-lymphoblastic leukemia/lymphoma and a t(7;8)(q22;p11). CUX1 was identified as the fusion partner of FGFR1 by fluorescence in situ hybridization and 5′ RACE-PCR. We further investigated this novel FGFR1 fusion using the interleukin-3 (IL-3) dependent Ba/F3 cell line and demonstrated IL-3 independent cell growth of CUX1-FGFR1 expressing cells. TKI258 and PKC412 potently inhibited proliferation of CUX1-FGFR1 transformed Ba/F3 cells. This growth inhibition was shown to be mediated by inhibition of CUX1-FGFR1 kinase activity for TKI258 but not PKC412. In summary, we identified a novel CUX1-FGFR1 fusion oncogene in a patient with the 8p11 myeloproliferative syndrome and demonstrated its transforming potential in the Ba/F3 cell line. Our in vitro data support the further investigation of TKI258 for the treatment of constitutively active FGFR1 fusion proteins.
Keywords: myeloid and lymphoid neoplasms with FGFR1 abnormalities (ICD-O 9967/3), 8p11 myeloproliferative syndrome (EMS), T-lymphoblastic leukemia/lymphoma, FGFR1, tyrosine kinase inhibitor, TKI258
Introduction
Myeloid and lymphoid neoplasms with FGFR1 abnormalities (ICD-O 9967/3), also called 8p11 myeloproliferative syndrome (EMS) or 8p11 stem cell leukemia/lymphoma syndrome, represent aggressive, atypical stem cell disorders. They are caused by chromosomal translocations that disrupt and constitutively activate FGFR1 (8p11) by fusion to diverse partner genes.1 To date, ten partner genes have been identified: BCR, CEP110, CPSF6, FGFR1OP, FGFR1OP2, HERV-K, LRRFIP1, MYO18A, TRIM24 (TIF1), ZMYM2 (ZNF198).2 EMS most commonly presents as a myeloproliferative neoplasm, with progression to acute myeloid leukemia within 1–2 years of diagnosis. At diagnosis, there is a strikingly high incidence of coexisting T- or B-cell lymphoblastic lymphoma/leukemia or mixed phenotype acute leukemia. The only curative treatment at the moment is allogeneic stem cell transplantation.3
Rearrangement of the FGFR1 gene is a defining cytogenetic abnormality in EMS making the FGFR1 receptor tyrosine kinase a promising target for therapy. To date, the tyrosine kinase inhibitors PKC412, SU5402 and PD173074 were shown to potently inhibit the FGFR1 kinase activity.4,5 Despite promising in vitro results, the in vivo response in the single patient tested with PKC412 was disappointing and currently none of these compounds is in clinical use.4
Another potential drug candidate is TKI258 (CHIR258, Dovitinib). TKI258 is a multitarget receptor tyrosine kinase inhibitor with activity against class III, IV and V receptor tyrosine kinases such as VEGFRs, FGFRs, PDGFRs, FLT3, KIT, and CSF-1R.6 Previous studies showed that TKI258 had a significant inhibitory activity in a representative panel of tumor xenograft models of acute myeloid leukemia, multiple myeloma, colon and prostate cancer.6–8 In addition, TKI258 has already been evaluated in a group of patients with advanced solid tumors and is considered to be a new therapeutic agent for the treatment of melanoma and gastrointestinal stromal tumors.9 Studies performed on ZNF198-FGFR1- or BCR-FGFR1-transformed Ba/F3 cells, KG1 and KG1A AML cell lines (FGFR1OP2-FGFR1) and on primary cells of EMS patients showed that TKI258 inhibited cell proliferation at low nanomolar concentrations.10 Therefore, the TKI258 inhibitor may provide a new therapeutic option for patients with EMS.
In the present study, a patient with T-lymphoblastic leukemia/lymphoma is reported in whom we identified CUX1 as a novel fusion partner of FGFR1. Our functional studies demonstrate that TKI258 has significant and specific activity against CUX1-FGFR1.
Design and Methods
Patient
A 29-year old female patient from Romania requested a second opinion on an outpatient basis. Peripheral blood examination showed anemia (Hb 10.2 g/dl), thrombocytopenia (14×109/L) and a leukocyte count of 26,280×109/L (58.9% blasts, 0.7% myelocytes, 4.6% metamyelocytes, 14.6% neutrophils, 0% eosinophils, 1.3% basophils, 14.6% lymphocytes and 5.3% monocytes). The blast immunophenotype indicated a pre-T lymphoblastic leukemia. She refused a repeat bone marrow examination. She died elsewhere of septicemia in aplasia after a first course of high-dose chemotherapy.
Cytogenetics and fluorescence in situ hybridization (FISH)
Cytogenetic and FISH analysis followed standard protocols. For FISH the following FGFR1 and TCRB (7q34) BAC clones were selected: RP11-350N15 (spanning the FGFR1 locus), RP11-1220K2 (located centromeric to TCRB) and RP11-556I13 (located telomeric to TCRB).
Molecular analysis
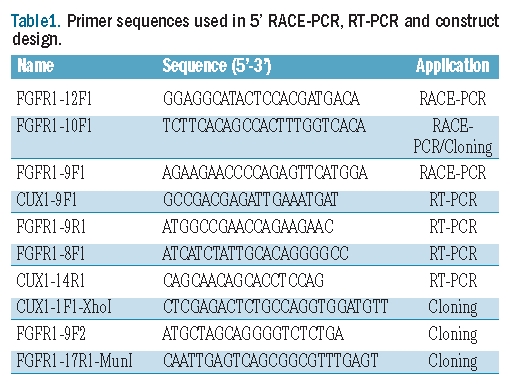
A peripheral blood sample was obtained from the patient for diagnostic cytogenetic and molecular evaluation. RNA was isolated with TRIzol Reagent (Invitrogen, Merelbeke, Belgium). 5’-RACE PCR was performed using a previously described protocol and primers.11 The final PCR product was sequenced with the ABI3100 sequencer (Applied Biosystems, Foster City, CA, USA). Fusion of CUX1 to FGFR1 was confirmed by RT-PCR using the primers CUX1-9F1 and FGFR1-9R1. The presence of the reciprocal fusion was evaluated with the primers FGFR1-8F1 and CUX1-14R1. All primer sequences are listed in Table 1.
Table 1.
Primer sequences used in 5′ RACE-PCR, RT-PCR and construct design.
Construct
The CUX1-FGFR1 fragment was amplified from the patient’s peripheral blood cDNA using Platinum Taq DNA Polymerase (Invitrogen, Merelbeke, Belgium) and subsequently cloned into the retroviral pMSCVpuro vector (Clontech, Saint-Germain-en-Laye, France).
Inhibitors
PKC412 (CGP41251/Midostaurin) and TKI258 (CHIR-258/Dovitinib) were purchased from Tocris Bioscience (Bristol, UK) and Selleck Chemicals (Houston, TX, USA), respectively. 10 mM stock solutions of the inhibitors were prepared in dimethyl sulfoxide and were stored at −80°C.
Cell culture
Viral vector production and transduction of Ba/F3 cells was performed as previously described.12 For the growth curve, 1×105 Ba/F3 cells were deprived of IL-3 and viable cells were counted on four consecutive days with a Countess Automated Cell Counter (Invitrogen, Merelbeke, Belgium). For dose-response curves, 1×105 CUX1-FGFR1-expressing Ba/F3 cells were treated with PKC412 and TKI258. The number of viable cells was determined at the start and after 48 h using the CellTiter AQueous One Solution Cell Proliferation Assay (Promega, Leiden, The Netherlands). In rescue experiments, IL-3 (2 ng/mL) was added to CUX-FGFR1-transduced Ba/F3 cells treated with PKC412 and TKI258 and the cells were incubated for 48 h.
Apoptotic assay
Ba/F3 cells at a density of 5×105 were cultured for 48 h in 24-well plates in the presence of PKC412 and TKI258, or vehicle. Induction of apoptosis was evaluated by flow cytometry using Annexin-V-FLUOS Staining Kit according to the manufacturer's protocol (Roche Applied Science, Mannheim, Germany). Samples were acquired with BD FACSCanto System and data were analyzed with BD FACSDiVa software (BD Biosciences, San Jose, CA, USA).
Western Blotting
Four million cells were incubated with inhibitors for 90 min and were lysed after a wash in ice-cold PBS cells. Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories Hercules, CA, USA). Lysates were separated by SDS-PAGE electrophoresis and immunoblotted. Different antibodies were used: anti-FGFR1, anti-STAT5a (Santa Cruz Biotechnology, CA, USA), anti-RPS6K, anti-phospho-FGFR1, anti-phospho-RPS6K, anti-phospho-STAT5 (Cell Signaling Technologies, Danvers, MA, USA) and anti-alpha-tubulin (Sigma-Aldrich, St Louis, MO, USA). Detection was performed by chemiluminescence (Western Lightning-ECL, Perkin Elmer, Waltham, MA, USA) and captured using a FUJI LAS3000mini imaging system.
Results and Discussion
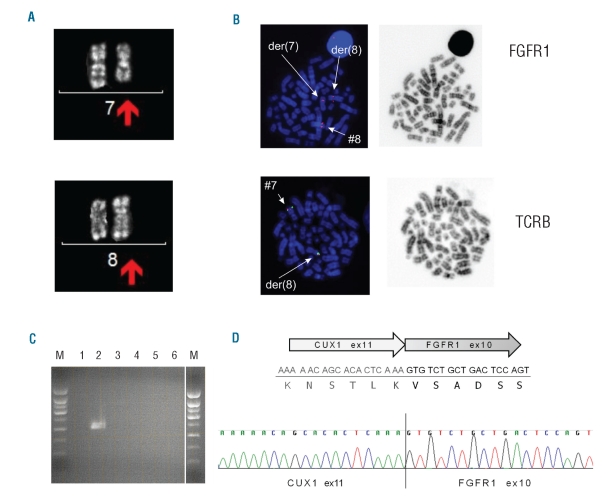
Cytogenetic analysis was performed on a diagnostic blood sample of a patient with precursor T-lymphoblastic leukemia/lymphoma, without apparent myeloproliferation or eosinophilia. A t(7;8)(q22;p11) was found (Figure 1A). Recurrent chromosomal 8p11 rearrangements are the genetic hallmark of EMS and give rise to fusions of the FGFR1 tyrosine kinase with different partner genes. Therefore, we analyzed the translocation in more detail by FISH using FGFR1 flanking probes. We could confirm the 8p11 breakpoint and 7q as the partner chromosome (Figure 1B). Using 5′-RACE PCR followed by sequencing, we showed that this translocation leads to the formation of an in-frame fusion transcript between CUX1 exon 11 (7q22) and FGFR1 exon 10 (8p11) (Figure 1D). The CUX1 and FGFR1 reference sequences were obtained from the Ensembl release 59 - Aug 2010 (transcripts IDs: CUX1 -ENST00000292538 and FGFR1 - ENST00000397091). The presence of this novel CUX1-FGFR1 fusion was further confirmed by RT-PCR and sequencing using primers in both partners (Figure 1C). The reciprocal FGFR1-CUX1 fusion transcript could not be detected in this patient. CUX1 is a homeobox family DNA binding protein which has not previously been described as a fusion partner in hematologic malignancies. Of note, Belloni et al. have reported another translocation t(7;8)(q34;p11) in a patient with the 8p11 myeloproliferative syndrome with a different 7q breakpoint and which led to a fusion between FGFR1 and TRIM24 (TIF1), transcription intermediary factor 1.13
Figure 1.
Cytogenetic and molecular identification of CUX1 as a fusion partner of FGFR1. (A) Cytogenetic analysis of patient’s peripheral blood cells. The derivative chromosomes 7 and 8 are shown with the arrows. (B) Fluorescence in situ hybridization using RP11-350N15 (spectrum orange) spanning the FGFR1 locus (8p11), and RP11-556I13 (spectrum orange) and RP11-1220K2 (sybr green) flanking TCRB (7q34). Hybridization of the FGFR1 probe shows one normal signal on chromosome 8, one signal on der(7) and one signal on der(8). Hybridization of the TCRB probe confirms translocation of the distal part of 7q to 8p. (C) RT-PCR. Lanes 1 and 4: negative control (no cDNA), lanes 2 and 5: cDNA from the reported patient; lanes 3,6: cDNA from a case with CEP110-FGFR1. Forward primer in CUX1 and reverse primer in FGFR1 (Lane 1-3); forward primer in FGFR1 and reverse primer in CUX1 (lane 4–6). Lane M represents 1kb size standard. (D) Schematic representation of the identified fusion transcript. The DNA and protein sequence at the fusion border are presented. An electropherogram with the CUX1-FGFR1 fusion sequence of the PCR product is also shown.
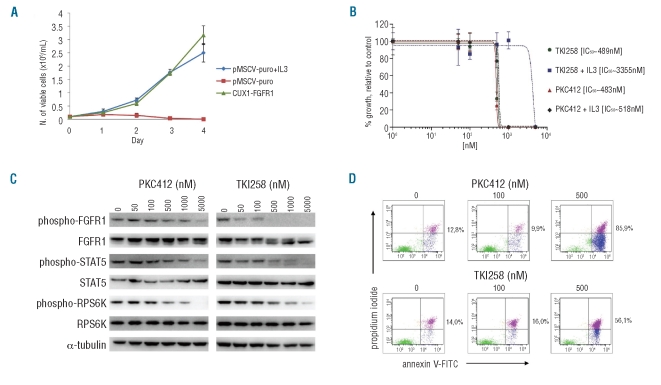
To evaluate the transforming potential of this novel CUX1-FGFR1 fusion, the fusion transcript was cloned and used to transduce Ba/F3 cells. CUX1-FGFR1-expressing Ba/F3 cells displayed IL-3 independent proliferation (Figure 2A). Western blot analysis of these transformed Ba/F3 cells demonstrated constitutive phosphorylation of CUX1-FGFR1 and its downstream effectors STAT5 and ribosomal protein S6 kinase (RPS6K) (data not shown). Together these results suggest an oncogenic character of the CUX1-FGFR1 fusion protein.
Figure 2.
In vitro assays on CUX1-FGFR1-expressing Ba/F3 cells. (A) IL-3 deprivation of Ba/F3 cells transduced with CUX1-FGFR1 resulted in transformation to growth factor independent growth. The mean growth ± SEM of 3 separate measurements over four consecutive days is presented. (B) The dose-response curves of CUX1-FGFR1-transduced Ba/F3 cells, treated with TKI258 and PKC412 for 48 h in the absence or presence of IL-3 (2ng/mL) are presented. Points represent the average results of 2 experiments performed in triplicate plotted with the curve-fitting GraphPad Prism 5 software; bars, SD. The calculated IC50 for each inhibitor is indicated. (C) Western blot analyses of CUX1-FGFR1-transformed Ba/F3 cells after treatment with PKC412 and TKI258. Phosphorylation of CUX1-FGFR1 and its downstream effectors STAT5 and RPS6K decreased with increasing inhibitor concentrations. Expression of total CUX1-FGFR1, STAT5 and RPS6K remained unaffected. (D) Effect of PKC412 and TKI258 on apoptosis of CUX1-FGFR1-expressing Ba/F3 cells after treatment for 48 h. The percentage of apoptotic plus necrotic CUX1-FGFR1-transduced Ba/F3 cells is indicated.
Next, we tested the sensitivity of CUX1-FGFR1 to PKC412 and TKI258, two multitarget receptor tyrosine kinase inhibitors with reported activity against FGFR1. Treatment of the CUX1-FGFR1-expressing Ba/F3 cells with the kinase inhibitor TKI258 significantly inhibited cell growth with an IC50 of 489 nM (Figure 2B). Western blot analysis demonstrated a corresponding decrease in CUX1-FGFR1 phosphorylation with increasing doses of TKI258, while protein expression was unaffected (Figure 2C). A significant inhibition of phosphorylation was already detectable at 50 nM, with complete inhibition at 1 μM. The downstream effectors STAT5 and RPS6K also showed a decreasing phosphorylation with TKI258 concentrations equal to or higher than 500 nM (Figure 2C). Furthermore, using an Annexin-V/propidium iodide-based apoptosis assay, we could show that 48 h exposure to TKI258 induced apoptosis followed by cell death in CUX1-FGFR1-expressing Ba/F3 cells. Massive apoptosis/necrosis was recorded at 500 nM of TKI258 (Figure 2D).
PKC412 inhibited the cell growth of CUX1-FGFR1-expressing Ba/F3 cells with an IC50 of 483 nM and significant induction of apoptosis/necrosis in these cells was also recorded at 500 nM of inhibitor (Figures 2B and D). However, by Western blotting we showed that an effect of PKC412 on the phosphorylation status of CUX1-FGFR1 and its downstream effectors was only obtained at concentrations equal to or higher than 1000 nM. The inhibitory effect on the proliferation of CUX1-FGFR1-expressing cells could be rescued by addition of exogenous IL-3 for TKI258 but not for PKC412 (Figure 2B). This suggests that PKC412 inhibits proliferation in CUX1-FGFR1 transformed Ba/F3 cells by non-specific toxic effects rather than by specific inhibition of the FGFR1- fusion kinase (Figures 2B and C). Non-specific toxic effects of PKC412 at concentrations from 500 nM have also been observed in Ba/F3 transformed with other kinases.14,15 In contrast, the correlation between inhibition of growth and of phosphorylation by TKI258, and the IL-3 rescue of growth inhibition by TKI258 demonstrate that growth inhibition by TKI is specifically mediated by inhibition of FGFR1 signaling. Taken together, the in vitro data presented here suggest that TKI258 is a more potent FGFR1 inhibitor with a wider therapeutic index than PKC412, which could be used for the treatment of the novel CUX1-FGFR1 fusion as well as other constitutively active FGFR1 fusion proteins. This result is consistent with the previous findings by Chase and colleagues.10
CUX1 (cut-like homeobox 1) encodes a member of the homeodomain family of DNA binding proteins. This homeobox transcription factor contains one homeobox and three repetitive CUT DNA-binding domains as well as an N-terminal coiled-coil region. CUX1 is expressed as multiple isoforms and is cleaved by proteases such as cathepsin L. In healthy individuals, CUX1 plays a role in embryonic development, cell cycle progression and cell differentiation.16 An elevated expression of CUX1 has been reported in breast tumors and cancer cell lines, in malignant plasma cells in multiple myeloma and in acute lymphoblastic leukemia, and in pancreatic tumors. A role as an important survival factor downstream of PI3K/AKT has also been suggested.16,17 In contrast, the 7q22 region where CUX1 is located was also found to be frequently deleted in uterine leiomyomas, AML and MDS although somatic mutations of CUX1 have not been demonstrated.18,19
In summary, we report a novel translocation t(7;8)(q22;p11) in the WHO disease category of myeloid and lymphoid neoplasms with FGFR1 abnormalities. The t(7;8)(q22;p11) generates an in-frame fusion transcript between CUX1 exon 11 and FGFR1 exon 10. There are no previous reports of CUX1 as partner gene in cancer translocations. The N-terminal coiled-coil domain is retained in the fusion and likely mediates dimerization and hence constitutive tyrosine kinase activation, as demonstrated for other oncogenic fusion kinases such as BCR-ABL1 and ETV6-JAK2.20,21 Some previously identified FGFR1 fusion partners like ZMYM2 and CEP110 are also known to harbor an oligomerization domain. The involvement of exon 10 of FGFR1 is another typical feature of the 8p11 myeloproliferative syndrome. Furthermore, we demonstrated the transforming character of CUX1-FGFR1 in the Ba/F3 cell system, and established CUX1-FGFR1 as a potential target for therapy. TKI258 specifically inhibited CUX1-FGFR1 phosphorylation and CUX1-FGFR1 driven cell proliferation and survival, in contrast to PKC412, the inhibitory effect of which was not mediated by inhibition of the kinase. Our results encourage further testing of TKI258 in representative patient populations. The outcome of such clinical trials is eagerly awaited since for the moment EMS remains a disorder which cannot be treated.
Supplementary Material
Acknowledgments
The authors would like to thank Monique Rubens for excellent technical support with cytogenetic and FISH analysis.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Funding: this work was supported by grants from the KU Leuven (concerted action grant to JC and PV), FWO-Vlaanderen and the Interuniversity Attraction Poles (IAP) granted by the Federal Office for Scientific, Technical and Cultural Affairs, Belgium (JC and PV). PV is a senior clinical investigator of FWO-Vlaanderen.
References
- 1.Bain BJ, Gilliland DG, Horny HP, Vardiman JW. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB or FGFR1. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid tissues. Lyon: IARC; 2008. pp. 67–74. [Google Scholar]
- 2.Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Hum Pathol. 2010;41(4):461–76. doi: 10.1016/j.humpath.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald D, Aguiar RC, Mason PJ, Goldman JM, Cross NC. A new myeloproliferative disorder associated with chromosomal translocations involving 8p11: a review. Leukemia. 1995;9(10):1628–30. [PubMed] [Google Scholar]
- 4.Chen J, Deangelo DJ, Kutok JL, Williams IR, Lee BH, Wadleigh M, et al. PKC412 inhibits the zinc finger 198-fibroblast growth factor receptor 1 fusion tyrosine kinase and is active in treatment of stem cell myeloproliferative disorder. Proc Natl Acad Sci USA. 2004;101(40):14479–84. doi: 10.1073/pnas.0404438101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grand EK, Chase AJ, Heath C, Rahemtulla A, Cross NC. Targeting FGFR3 in multiple myeloma: inhibition of t(4;14)-positive cells by SU5402 and PD173074. Leukemia. 2004;18(5):962–6. doi: 10.1038/sj.leu.2403347. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Lopes de Menezes D, Vora J, Harris A, Ye H, Nordahl L, et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin Cancer Res. 2005;11(10):3633–41. doi: 10.1158/1078-0432.CCR-04-2129. [DOI] [PubMed] [Google Scholar]
- 7.Lopes de Menezes DE, Peng J, Garrett EN, Louie SG, Lee SH, Wiesmann M, et al. CHIR-258: a potent inhibitor of FLT3 kinase in experimental tumor xenograft models of human acute myelogenous leukemia. Clin Cancer Res. 2005;11(14):5281–91. doi: 10.1158/1078-0432.CCR-05-0358. [DOI] [PubMed] [Google Scholar]
- 8.Xin X, Abrams TJ, Hollenbach PW, Rendahl KG, Tang Y, Oei YA, et al. CHIR-258 is efficacious in a newly developed fibroblast growth factor receptor 3-expressing orthotopic multiple myeloma model in mice. Clin Cancer Res. 2006;12(16):4908–15. doi: 10.1158/1078-0432.CCR-06-0957. [DOI] [PubMed] [Google Scholar]
- 9.Sarker D, Molife R, Evans TR, Hardie M, Marriott C, Butzberger-Zimmerli P, et al. A phase I pharmacokinetic and pharmacodynamic study of TKI258, an oral, multitargeted receptor tyrosine kinase inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2008;14(7):2075–81. doi: 10.1158/1078-0432.CCR-07-1466. [DOI] [PubMed] [Google Scholar]
- 10.Chase A, Grand FH, Cross NC. Activity of TKI258 against primary cells and cell lines with FGFR1 fusion genes associated with the 8p11 myeloproliferative syndrome. Blood. 2007;110(10):3729–34. doi: 10.1182/blood-2007-02-074286. [DOI] [PubMed] [Google Scholar]
- 11.Walz C, Chase A, Schoch C, Weisser A, Schlegel F, Hochhaus A, et al. The t(8;17)(p11;q23) in the 8p11 myeloproliferative syndrome fuses MYO18A to FGFR1. Leukemia. 2005;19(6):1005–9. doi: 10.1038/sj.leu.2403712. [DOI] [PubMed] [Google Scholar]
- 12.Schwaller J, Frantsve J, Aster J, Williams IR, Tomasson MH, Ross TS, et al. Transformation of hematopoietic cell lines to growth-factor independence and induction of a fatal myelo- and lymphoproliferative disease in mice by retrovirally transduced TEL/JAK2 fusion genes. EMBO J. 1998;17 (18):5321–33. doi: 10.1093/emboj/17.18.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belloni E, Trubia M, Gasparini P, Micucci C, Tapinassi C, Confalonieri S, et al. 8p11 myeloproliferative syndrome with a novel t(7;8) translocation leading to fusion of the FGFR1 and TIF1 genes. Genes Chromosomes Cancer. 2005;42(3):320–5. doi: 10.1002/gcc.20144. [DOI] [PubMed] [Google Scholar]
- 14.Lierman E, Michaux L, Beullens E, Pierre P, Marynen P, Cools J, et al. FIP1L1-PDGFRalpha D842V, a novel panresistant mutant, emerging after treatment of FIP1L1-PDGFRalpha T674I eosinophilic leukemia with single agent sorafenib. Leukemia. 2009;23(5):845–51. doi: 10.1038/leu.2009.2. [DOI] [PubMed] [Google Scholar]
- 15.Weisberg E, Wright RD, Jiang J, Ray A, Moreno D, Manley PW, et al. Effects of PKC412, nilotinib, and imatinib against GIST-associated PDGFRA mutants with differential imatinib sensitivity. Gastroenterology. 2006;131(6):1734–42. doi: 10.1053/j.gastro.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sansregret L, Nepveu A. The multiple roles of CUX1: insights from mouse models and cell-based assays. Gene. 2008;412(1–2):84–94. doi: 10.1016/j.gene.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Ripka S, Neesse A, Riedel J, Bug E, Aigner A, Poulsom R, et al. CUX1: target of Akt signalling and mediator of resistance to apoptosis in pancreatic cancer. Gut. 2010;59(8):1101–10. doi: 10.1136/gut.2009.189720. [DOI] [PubMed] [Google Scholar]
- 18.Hindersin S, Niemeyer CM, Germing U, Gobel U, Kratz CP. Mutation analysis of CUTL1 in childhood myeloid neoplasias with monosomy 7. Leuk Res. 2007;31(9):1323–4. doi: 10.1016/j.leukres.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Patrikis MI, Bryan EJ, Thomas NA, Rice GE, Quinn MA, Baker MS, et al. Mutation analysis of CDP, TP53, and KRAS in uterine leiomyomas. Mol Carcinog. 2003;37(2):61–4. doi: 10.1002/mc.10127. [DOI] [PubMed] [Google Scholar]
- 20.Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278(5341):1309–12. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 21.McWhirter JR, Galasso DL, Wang JY. A coiled-coil oligomerization domain of Bcr is essential for the transforming function of Bcr-Abl oncoproteins. Mol Cell Biol. 1993;13(12):7587–95. doi: 10.1128/mcb.13.12.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.